Valorization of Winery By-Products as Bio-Fillers for Biopolymer-Based Composites
Abstract
:1. Introduction
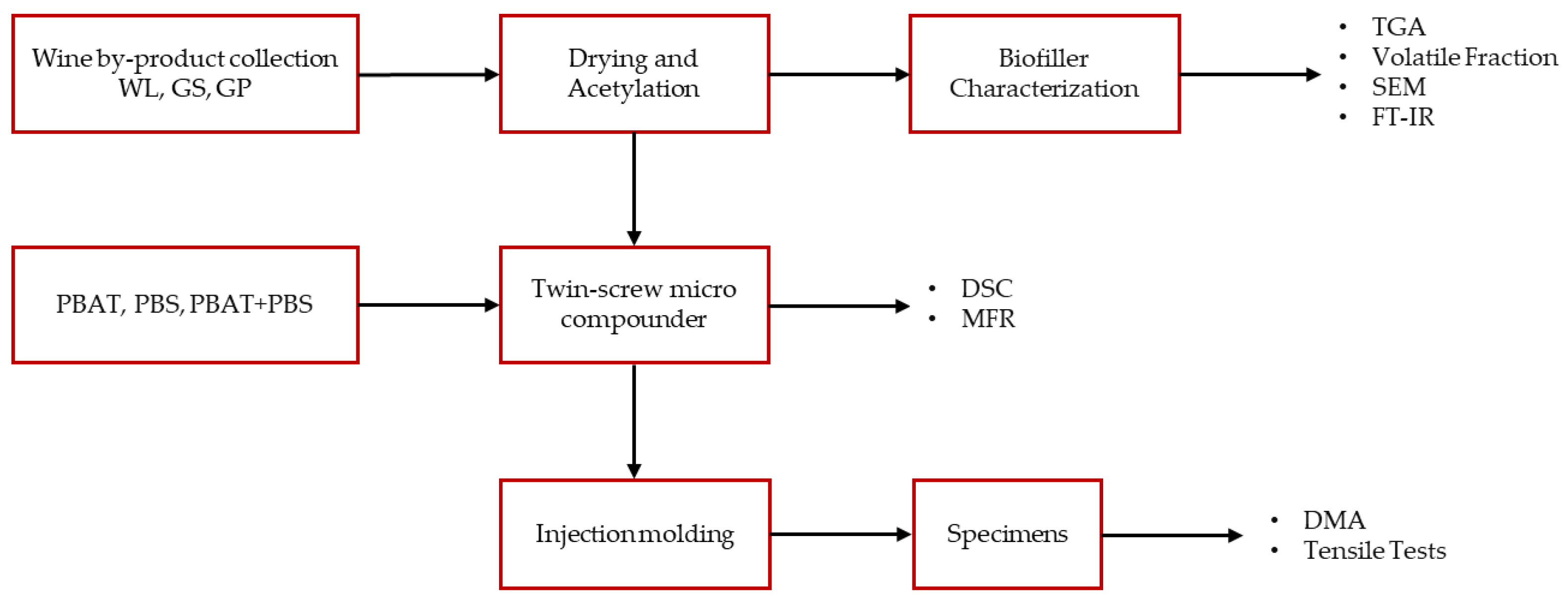
2. Materials and Methods
2.1. Materials
2.2. Wine By-Products’ Functionalization
2.3. Bio-Filler Characterization
2.3.1. Volatile and Not-Volatile Solid Fractions
2.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
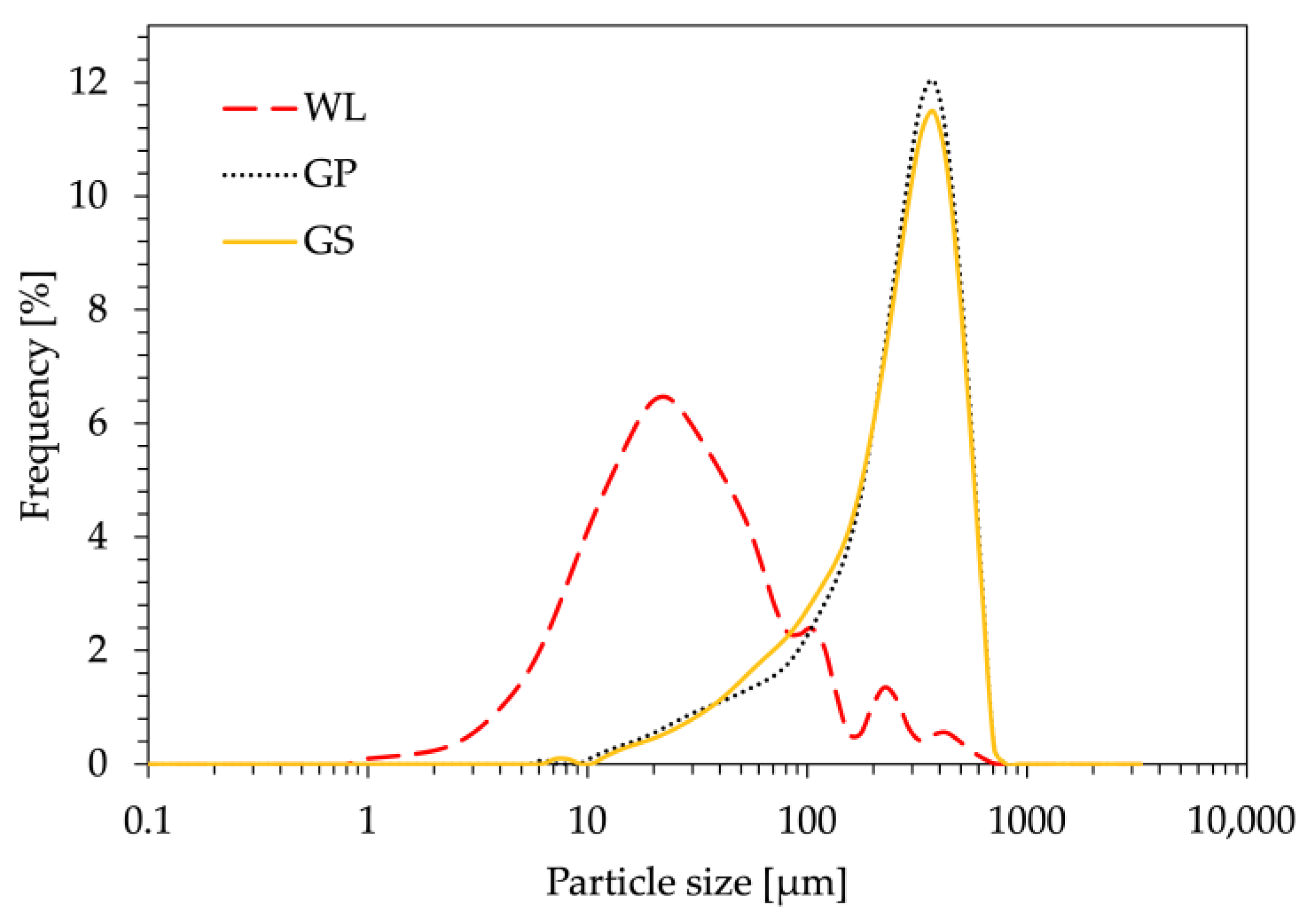
2.3.3. Granulometry
2.3.4. Density Pycnometry
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Thermogravimetric Analysis (TGA)
2.4. PBAT/PBS-Based Composites Preparation
2.5. PBAT/PBS-Based Composites Characterization
2.5.1. Differential Scanning Calorimetry (DSC)
2.5.2. Hygroscopic Analysis
2.5.3. Mass Melt Flow Rate (MFR)
2.5.4. Dynamic Mechanical Analysis (DMA)
2.5.5. Tensile Tests
3. Results and Discussions
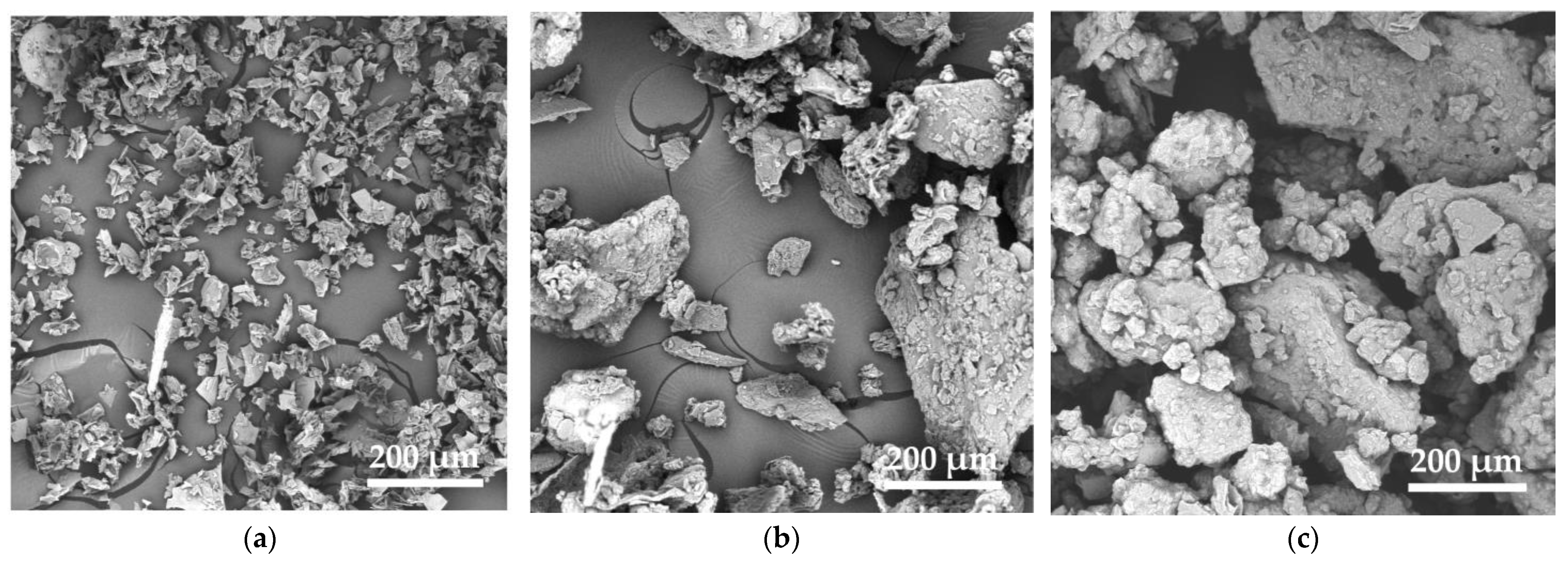
3.1. Bio-Fillers Characterization
3.2. Bio-Composites Characterization
3.2.1. Thermal and Viscosity Characterization
3.2.2. Hygroscopic Gravimetric Analysis
3.2.3. Tensile Properties
3.2.4. Dynamic Mechanical Analysis (DMA)
4. Conclusions
- The incorporation of bio-fillers did not significantly alter the thermal properties of the resulting composites, ensuring their stability across varying temperature conditions;
- The compounding of bio-fillers decreased the viscosity of the composites, most significantly for WL, probably due to its inherent dimensions, without compromising the processability of the bio-composites;
- All bio-composites exhibited increased water affinity, with GS-based composites showing the highest affinity. Acetylated samples demonstrated slightly lower water absorption, attributed to reduced hydrophilicity;
- Tensile testing revealed a noticeable stiffening effect in the bio-composites, especially those filled with WL. The Young’s modulus reached approximately 1.5 GPa, with PBS matrices exhibiting a higher stiffening effect compared to PBAT. Specifically, in PBAT-based composites, there was a 108% improvement with the addition of WL and a 162% improvement with acetylated WL, while in the case of PBS-based composites, there was an approximately 65% increase in Young’s modulus with both WL and acetylated WL;
- The stiffening effects of the three different bio-fillers were further evidenced by an increase in the storage modulus across a wide temperature range, highlighting the positive effect of acetylation at higher temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nikiema, J.; Asiedu, Z. A Review of the Cost and Effectiveness of Solutions to Address Plastic Pollution. Environ. Sci. Pollut. Res. 2022, 29, 24547–24573. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing Environmental Plastic Pollution by Designing Polymer Materials for Managed End-of-Life. Nat. Rev. Mater. 2022, 7, 104–116. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U. Novel Biopolymer-Based Sustainable Composites for Food Packaging Applications: A Narrative Review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Jahandideh, A.; Ashkani, M.; Moini, N. Chapter 8—Biopolymers in Textile Industries. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–218. ISBN 978-0-12-819240-5. [Google Scholar]
- Silva, R.G.C.; Ferreira, T.F.; Borges, É.R. Identification of Potential Technologies for 1,4-Butanediol Production Using Prospecting Methodology. J. Chem. Technol. Biotechnol. 2020, 95, 3057–3070. [Google Scholar] [CrossRef]
- Skoog, E.; Shin, J.H.; Saez-Jimenez, V.; Mapelli, V.; Olsson, L. Biobased Adipic Acid—The Challenge of Developing the Production Host. Biotechnol. Adv. 2018, 36, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Assessment of Aliphatic–Aromatic Copolyester Biodegradable Mulch Films. Part II: Laboratory Simulated Conditions. Chemosphere 2008, 71, 1607–1616. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Thermo-Mechanical Properties and Creep Modelling of Wine Lees Filled Polyamide 11 (PA11) and Polybutylene Succinate (PBS) Bio-Composites. Compos. Sci. Technol. 2020, 188, 107974. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Togliatti, E.; Marozzi, M.; Rizzi, F.M.A.; Pugliese, D.; Cavazza, A.; Pitirollo, O.; Grimaldi, M.; Milanese, D. Flexible PBAT-Based Composite Filaments for Tunable FDM 3D Printing. ACS Appl. Bio Mater. 2022, 5, 3219–3229. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the Wine Wastes on the Thermal Stability, Mechanical Properties, and Biodegradation’s Rate of Poly(3-Hydroxybutyrate). J. Appl. Polym. Sci. 2021, 138, 49713. [Google Scholar] [CrossRef]
- Nanni, A.; Ricci, A.; Versari, A.; Messori, M. Wine Derived Additives as Poly(Butylene Succinate) (PBS) Natural Stabilizers for Different Degradative Environments. Polym. Degrad. Stab. 2020, 182, 109381. [Google Scholar] [CrossRef]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.; Messori, M. Effect of the Wine Lees Wastes as Cost-Advantage and Natural Fillers on the Thermal and Mechanical Properties of Poly(3-Hydroxybutyrate-Co-Hydroxyhexanoate) (PHBH) and Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) (PHBV). J. Appl. Polym. Sci. 2020, 137, 48869. [Google Scholar] [CrossRef]
- Laverde, V.; Marin, A.; Benjumea, J.M.; Rincón Ortiz, M. Use of Vegetable Fibers as Reinforcements in Cement-Matrix Composite Materials: A Review. Constr. Build. Mater. 2022, 340, 127729. [Google Scholar] [CrossRef]
- Hammami, D.; Khlif, M.; Tounsi, F.; Bradai, C. Effect of Maleic Anhydride–Grafted Polypropylene Coupling Agent on Mechanical Properties of HDPE Composites Filled with Grape Leaves Fiber. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Abdulla, A.C.L.; Kalladi, A.J.; Sunojkumar, P. Biopolymer Blend Composite Films Based on Polyvinyl Alcohol/Chitosan/Grape Seed Extract via Green Approach for Flexible Optoelectronic Devices. J. Thermoplast. Compos. Mater. 2024, 08927057241238986. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Techawinyutham, W.; Rangappa, S.M.; Siengchin, S. Lignocellulose Based Biofiller Reinforced Biopolymer Composites from Fruit Peel Wastes as Natural Pigment. Int. J. Biol. Macromol. 2024, 257, 128767. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Misra, M.; Drzal, L.T.; Robledo, D.; Freile-Pelegrin, Y. Preparation and Characterization of Biodegradable Agar/Poly(Butylene Adipate-Co-Terephatalate) Composites. Polym. Eng. Sci. 2009, 49, 1117–1126. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites. Materials 2023, 16, 6843. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of Vineyard By-Products to Obtain Composted Digestate and Biochar Suitable for Nursery Grapevine (Vitis vinifera L.) Production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Troncozo, M.I.; Lješević, M.; Beškoski, V.P.; Anđelković, B.; Balatti, P.A.; Saparrat, M.C.N. Fungal Transformation and Reduction of Phytotoxicity of Grape Pomace Waste. Chemosphere 2019, 237, 124458. [Google Scholar] [CrossRef]
- Olaru, N.; Olaru, L.; Vasile, C.; Ander, P. Surface Modified Cellulose Obtained by Acetylation without Solvents of Bleached and Unbleached Kraft Pulps. Polimery 2011, 56, 834–840. [Google Scholar] [CrossRef]
- ISO E. 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization of Standardization: Geneva, Switzerland, 2012.
- Mago, G.; Kalyon, D.M.; Fisher, F.T. Nanocomposites of Polyamide-11 and Carbon Nanostructures: Development of Microstructure and Ultimate Properties Following Solution Processing. J. Polym. Sci. B Polym. Phys. 2011, 49, 1311–1321. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Baydar, N.G.; Akkurt, M. Oil Content and Oil Quality Properties of Some Grape Seeds. Turk. J. Agric. For. 2001, 25, 163–168. [Google Scholar]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, M.; Shekhawat, D.; Lee, S.-Y.; Park, S.-J. The Role of Fillers to Enhance the Mechanical, Thermal, and Wear Characteristics of Polymer Composite Materials: A Review. Compos. Part. A Appl. Sci. Manuf. 2023, 175, 107775. [Google Scholar] [CrossRef]
- Delgado de la Torre, M.P.; Priego-Capote, F.; Luque de Castro, M.D. Characterization and Comparison of Wine Lees by Liquid Chromatography–Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, D.; Ozcalik, O.; Altiok, E.; Bayraktar, O. Characterization and Recovery of Tartaric Acid from Wastes of Wine and Grape Juice Industries. J. Therm. Anal. Calorim. 2008, 94, 767–771. [Google Scholar] [CrossRef]
- Saywell, L.G. Clarification of Wine. Ind. Eng. Chem. 1934, 26, 981–982. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; Montagna, L.S.; de Farias, M.A.; Magalhães, J.A.; Tada, D.B.; Passador, F.R.; Machado, J.P.B.; Lemes, A.P. Cytotoxicity and Physico-Chemical Evaluation of Acetylated and Pegylated Cellulose Nanocrystals. J. Nanoparticle Res. 2018, 20, 206. [Google Scholar] [CrossRef]
- Nanni, A.; Cancelli, U.; Montevecchi, G.; Masino, F.; Messori, M.; Antonelli, A. Functionalization and Use of Grape Stalks as Poly(Butylene Succinate) (PBS) Reinforcing Fillers. Waste Manag. 2021, 126, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Giubilini, A.; Sciancalepore, C.; Messori, M.; Bondioli, F. New Biocomposite Obtained Using Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBH) and Microfibrillated Cellulose. J. Appl. Polym. Sci. 2020, 137, 48953. [Google Scholar] [CrossRef]
- Giubilini, A.; Sciancalepore, C.; Messori, M.; Bondioli, F. Valorization of Oat Hull Fiber from Agri-Food Industrial Waste as Filler for Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Mater. Cycles Waste Manag. 2021, 23, 402–408. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, L.; Nie, J.; Wang, H.; Yang, D. Study on Poly(Lactic Acid)/Natural Fibers Composites. J. Appl. Polym. Sci. 2012, 125, E526–E533. [Google Scholar] [CrossRef]
- Väisänen, T.; Das, O.; Tomppo, L. A Review on New Bio-Based Constituents for Natural Fiber-Polymer Composites. J. Clean. Prod. 2017, 149, 582–596. [Google Scholar] [CrossRef]
- Kai, W.; He, Y.; Inoue, Y. Fast Crystallization of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Talc and Boron Nitride as Nucleating Agents. Polym. Int. 2005, 54, 780–789. [Google Scholar] [CrossRef]
- Botta, L.; Titone, V.; Mistretta, M.C.; La Mantia, F.P.; Modica, A.; Bruno, M.; Sottile, F.; Lopresti, F. PBAT Based Composites Reinforced with Microcrystalline Cellulose Obtained from Softwood Almond Shells. Polymers 2021, 13, 2643. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.P.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liang, S.; McDonald, A.G. Thermophysical Properties and Biodegradation Behavior of Green Composites Made from Polyhydroxybutyrate and Potato Peel Waste Fermentation Residue. Ind. Crops Prod. 2015, 69, 91–103. [Google Scholar] [CrossRef]
- Lammi, S.; Gastaldi, E.; Gaubiac, F.; Angellier-Coussy, H. How Olive Pomace Can Be Valorized as Fillers to Tune the Biodegradation of PHBV Based Composites. Polym. Degrad. Stab. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and Characterization of Environmentally Friendly Composites from Poly(Butylene Succinate) (PBS) and Almond Shell Flour with Different Compatibilizers. Compos. B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]





| Biopolymer | Supplier Code | Melting Temperature (°C) | Density (g/cm3) | Melt Flow Index (190 °C; 2.16 kg) (g/10 min) |
|---|---|---|---|---|
| PBAT | PBE 006 | 110–115 | 1.26 | 4–6 |
| PBS | PBE 003 | 115 | 1.26 | 4–6 |
| Sample A | Sample B | Sample C | |
|---|---|---|---|
| Composition (v%) | A0: 100% PBAT | B0: 50% PBAT, 50% PBS | C0: 100% PBS |
| A1: 70% PBAT, 30% WL | B1: 30% PBAT, 30% PBS, 40% WL | C1: 70% PBS, 30% WL | |
| A2: 70% PBAT, 30% GP | B2: 30% PBAT, 30% PBS, 40% GP | C2: 70% PBS, 30% GP | |
| A3: 70% PBAT, 30% GS | B3: 30% PBAT, 30% PBS, 40% GS | C3: 70% PBS, 30% GS | |
| A4: 70% PBAT, 30% Ac. WL | C4: 70% PBS, 30% Ac. WL | ||
| A5: 70% PBAT, 30% Ac. GS | C5: 70% PBS, 30% Ac. GS |
| Bio-Filler | D10 [μm] | D50 [μm] | D90 [μm] | ρ [g/cm3] |
|---|---|---|---|---|
| WL | 7.7 ± 0.2 | 26.9 ± 1.5 | 149.7 ± 35.8 | 1.76 |
| GP | 65.3 ± 5.9 | 283.0 ± 5.7 | 496.5 ± 2.1 | 1.38 |
| GS | 60.9 ± 10.1 | 270.5 ± 9.2 | 491.0 ± 4.2 | 1.31 |
| Bio-Filler | Snv (%) | Sv (%) | N2 Atmosphere | Air | ||
|---|---|---|---|---|---|---|
| T10wt. % (°C) | Residual Mass (%) | T10wt. % (°C) | Residual Mass (%) | |||
| WL | 50.4 | 49.6 | 188 | 48 | 199 | 53 |
| GP | 6.6 | 93.4 | 232 | 20 | 243 | 7 |
| GS | 14.0 | 86.0 | 245 | 16 | 255 | 6 |
| Code | Tc (°C) | Hc (J/g) | Tg (°C) | Tm (°C) | Hm (J/g) | Xc (%) | MFR (g/10 min) |
|---|---|---|---|---|---|---|---|
| A0 | 72.8 | 16.8 | −37.9 | 119.8 | 10.6 | 9.3 | 5.2 |
| A1 | 76.0 | 11.1 | −38.4 | 118.2 | 4.6 | 5.8 | 10.9 |
| A2 | 75.8 | 11.1 | −37.5 | 119.5 | 6.0 | 7.5 | 7.0 |
| A3 | 73.7 | 10.1 | −38.9 | 119.6 | 6.7 | 8.4 | 9.1 |
| A4 | 79.3 | 10.0 | −38.6 | 121.1 | 5.4 | 6.7 | 11.4 |
| A5 | 78.9 | 12.8 | −39.1 | 121.3 | 7.3 | 9.2 | 9.8 |
| B0 | 88.7 | 45.6 | −38.7 | 113.6 | 45,6 | 40.7 | 5.6 |
| B1 | 72.7 | 19.6 | −36.6 | 110.8 | 17.2 | 42.5 | 11.1 |
| B2 | 78.6 | 22.9 | −38.9 | 112.1 | 20.8 | 51.5 | 10.1 |
| B3 | 78.7 | 21.4 | −37.8 | 112.4 | 20.5 | 50.9 | 8.8 |
| C0 | 87.4 | 63.4 | −38.4 | 114.4 | 63.7 | 57.9 | 5.7 |
| C1 | 72.8 | 40.8 | −38.4 | 112.1 | 40.8 | 53.6 | 9.3 |
| C2 | 80.3 | 42.6 | −38.0 | 113.6 | 41.3 | 53.6 | 8.3 |
| C3 | 79.8 | 44.7 | −38.4 | 113.3 | 41.6 | 54.1 | 8.9 |
| C4 | 83.8 | 47.8 | −39.1 | 113.4 | 45.6 | 59.3 | 10.4 |
| C5 | 80.3 | 44.6 | −39.1 | 113.2 | 44.8 | 58.1 | 9.2 |
| Samples | E (MPa) | UTS (MPa) | εb (mm/mm) |
|---|---|---|---|
| A0 | 124 ± 5 | 17.5 ± 1.0 | 3.23 ± 0.65 |
| B0 | 416 ± 11 | 24.5 ± 3.1 | 2.15 ± 0.22 |
| C0 | 841 ± 29 | 39.4 ± 3.9 | 1.83 ± 0.13 |
| A4 | 259 ± 10 | 12.1 ± 2.8 | 0.52 ± 0.09 |
| A5 | 325 ± 2 | 13.7 ± 1.2 | 0.72 ± 0.03 |
| C4 | 1381 ± 6 | 25.2 ± 0.4 | 0.11 ± 0.01 |
| C5 | 1406 ± 22 | 25.4 ± 0.7 | 0.13 ± 0.01 |
| Code | E’(0) (MPa) | E’(25) (MPa) | E’(50) (MPa) | E’(75) (MPa) |
|---|---|---|---|---|
| A0 | 92 | 53 | 21 | 10 |
| A1 | 226 | 110 | 54 | 26 |
| A2 | 180 | 119 | 55 | 29 |
| A3 | 153 | 96 | 42 | 20 |
| A4 | 331 | 222 | 113 | 52 |
| A5 | 163 | 115 | 58 | 28 |
| B0 | 163 | 115 | 58 | 28 |
| B1 | 629 | 298 | 152 | 83 |
| B2 | 269 | 183 | 100 | 55 |
| B3 | 451 | 333 | 208 | 124 |
| C0 | 588 | 450 | 330 | 222 |
| C1 | 1045 | 630 | 41 | 252 |
| C2 | 751 | 572 | 449 | 310 |
| C3 | 741 | 601 | 417 | 278 |
| C4 | 1040 | 827 | 627 | 460 |
| C5 | 720 | 578 | 455 | 333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagi, F.; Giubilini, A.; Veronesi, P.; Nigro, G.; Messori, M. Valorization of Winery By-Products as Bio-Fillers for Biopolymer-Based Composites. Polymers 2024, 16, 1344. https://doi.org/10.3390/polym16101344
Biagi F, Giubilini A, Veronesi P, Nigro G, Messori M. Valorization of Winery By-Products as Bio-Fillers for Biopolymer-Based Composites. Polymers. 2024; 16(10):1344. https://doi.org/10.3390/polym16101344
Chicago/Turabian StyleBiagi, Filippo, Alberto Giubilini, Paolo Veronesi, Giovanni Nigro, and Massimo Messori. 2024. "Valorization of Winery By-Products as Bio-Fillers for Biopolymer-Based Composites" Polymers 16, no. 10: 1344. https://doi.org/10.3390/polym16101344
APA StyleBiagi, F., Giubilini, A., Veronesi, P., Nigro, G., & Messori, M. (2024). Valorization of Winery By-Products as Bio-Fillers for Biopolymer-Based Composites. Polymers, 16(10), 1344. https://doi.org/10.3390/polym16101344








