Dual Semi-Interpenetrating Networks of Water-Soluble Macromolecules and Supramolecular Polymer-like Chains: The Role of Component Interactions
Abstract
:1. Introduction
2. Materials and Methods
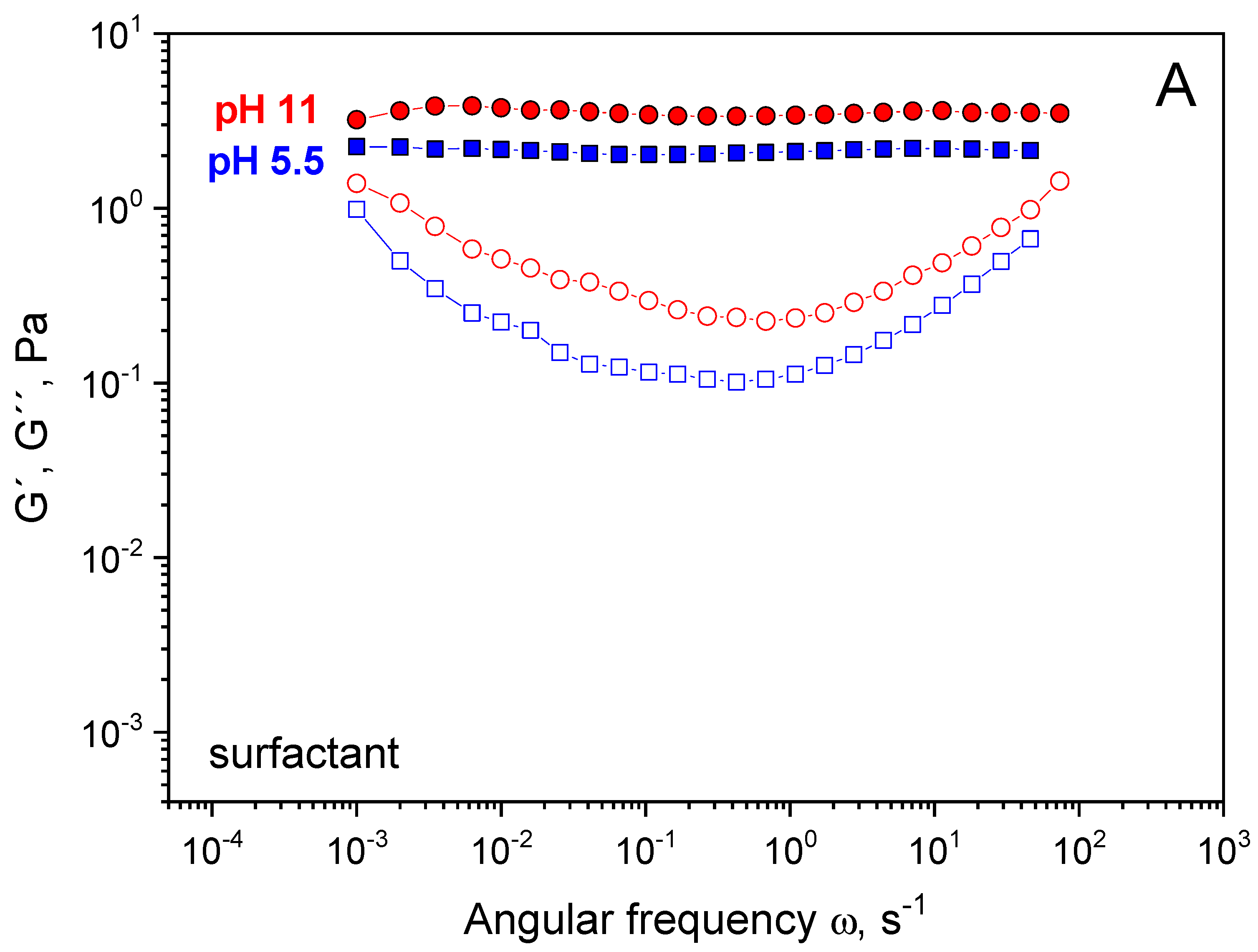
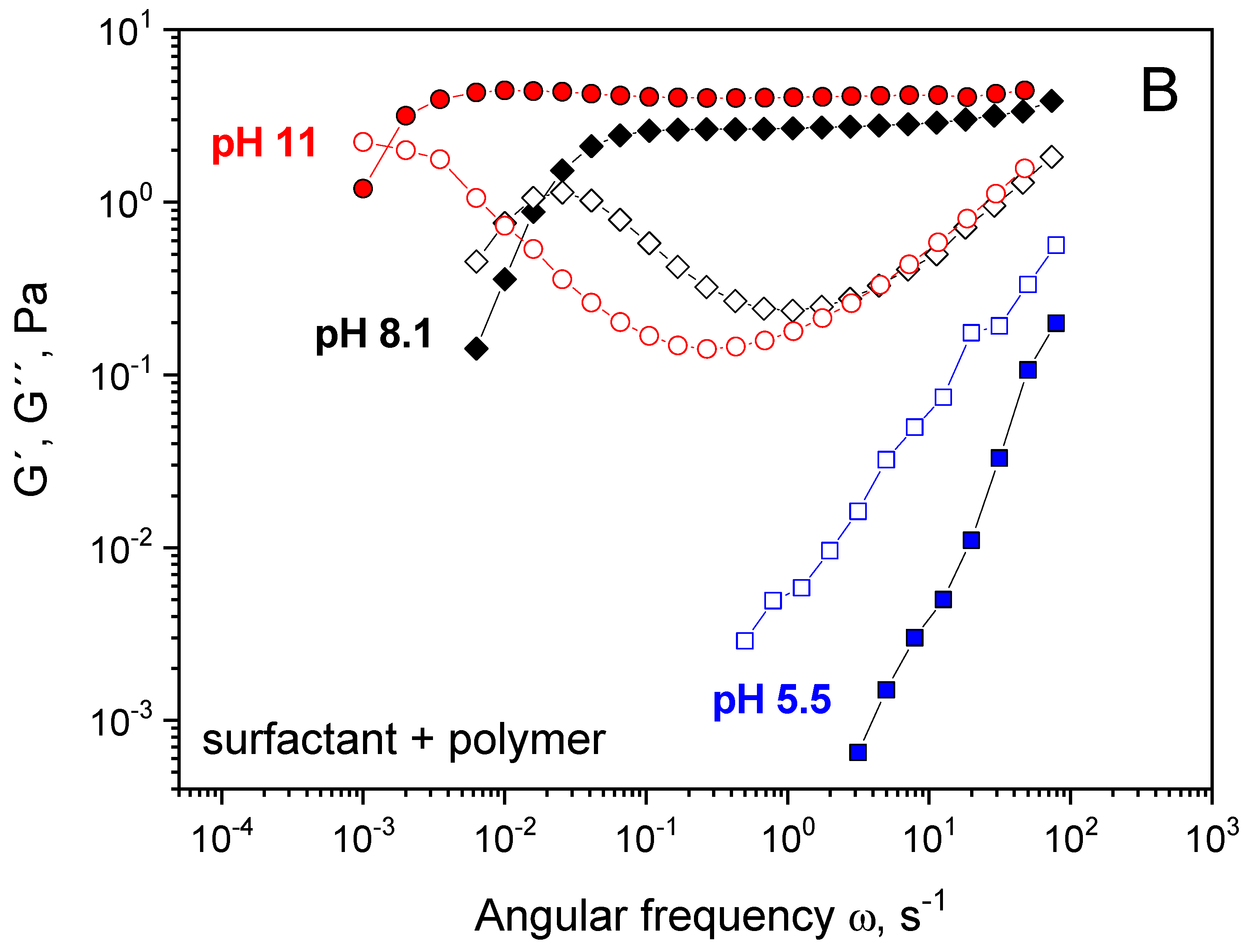
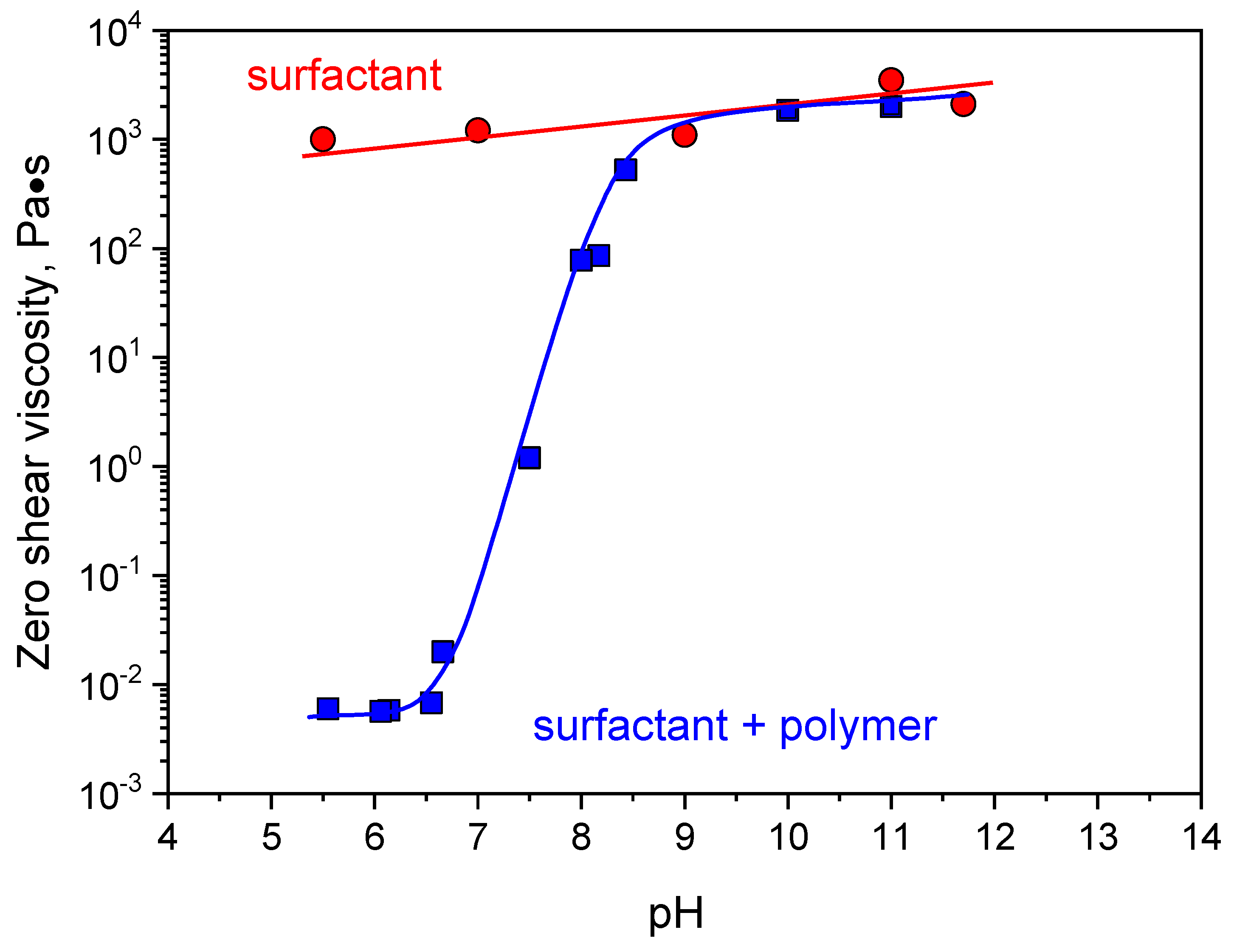
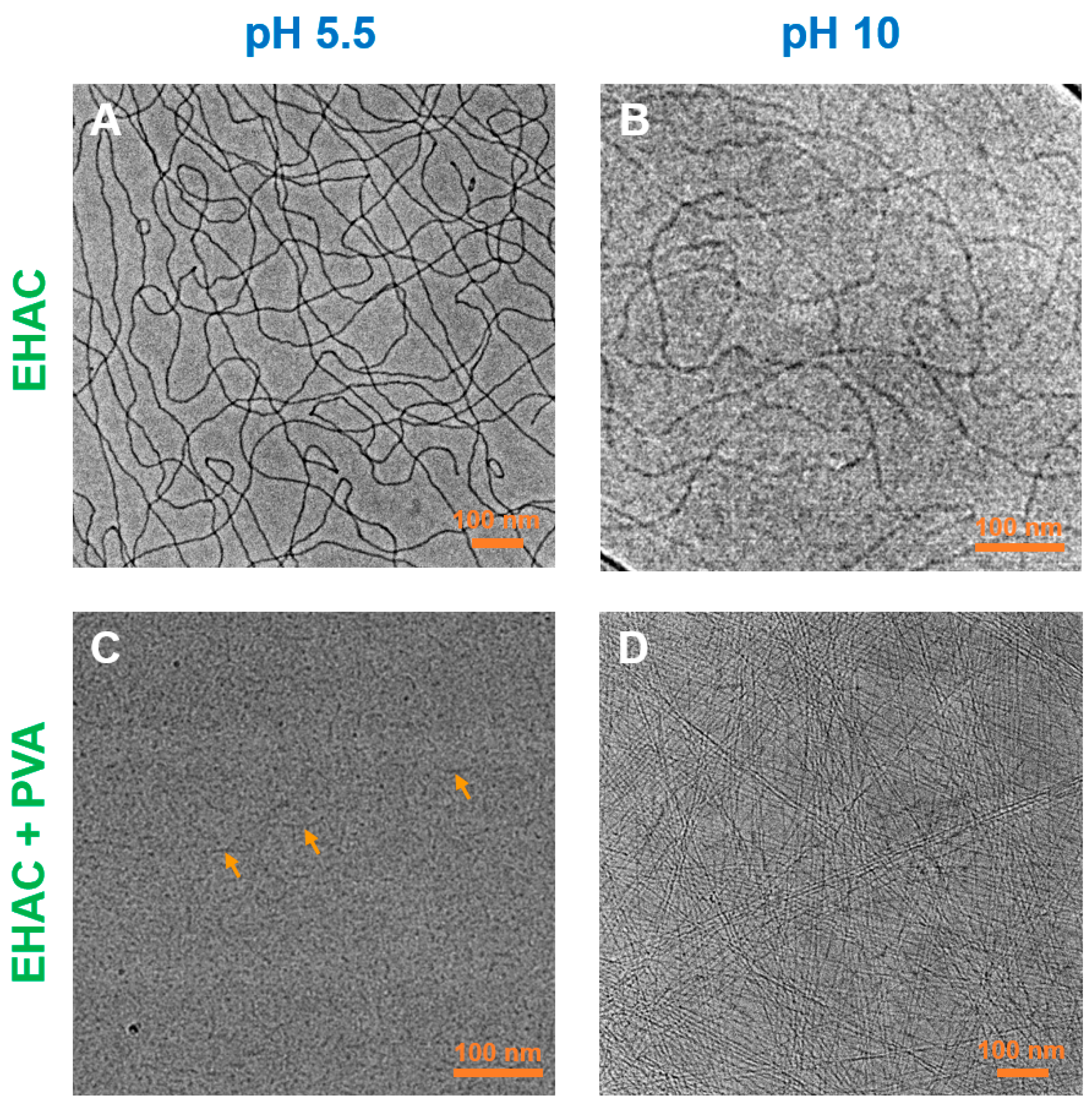
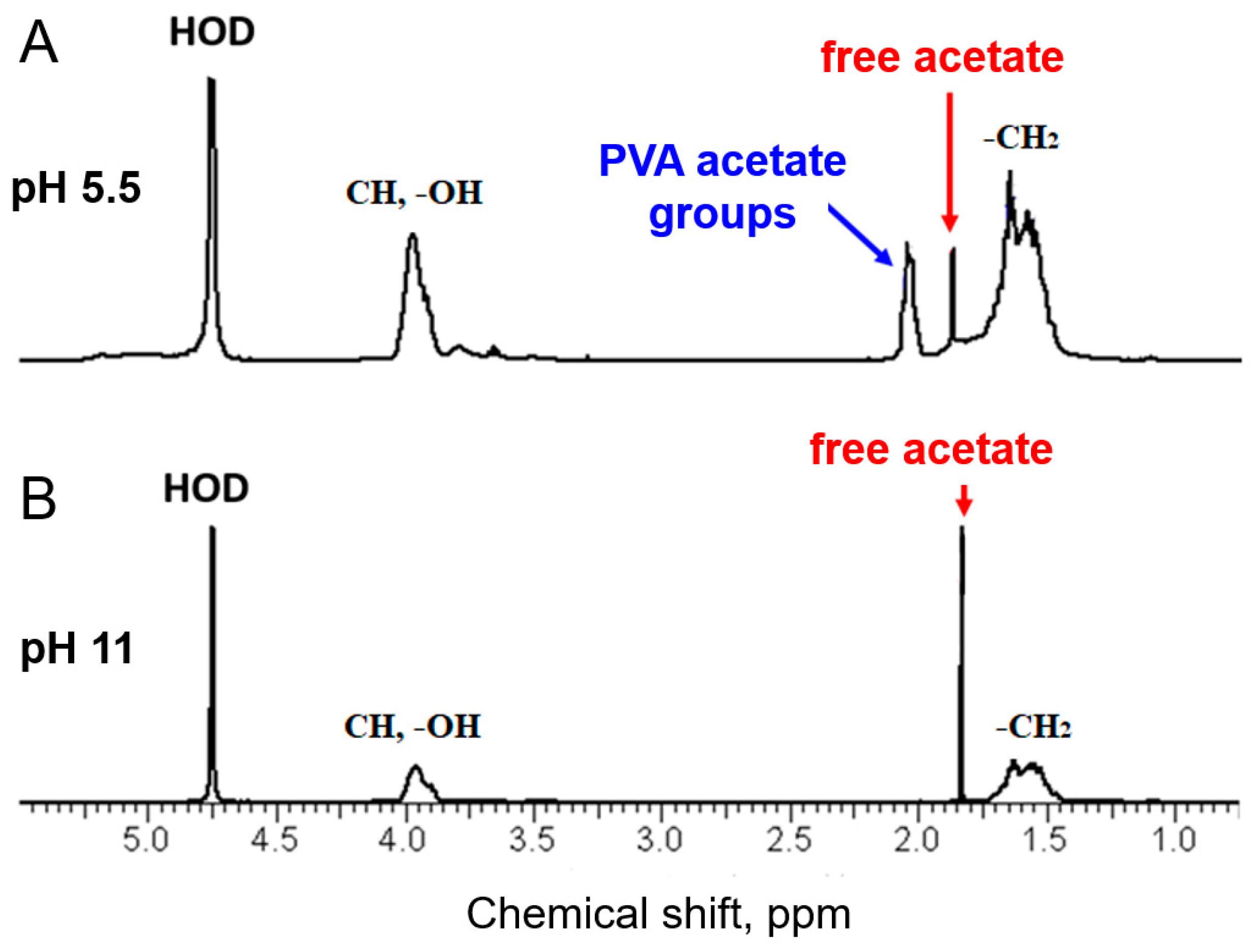
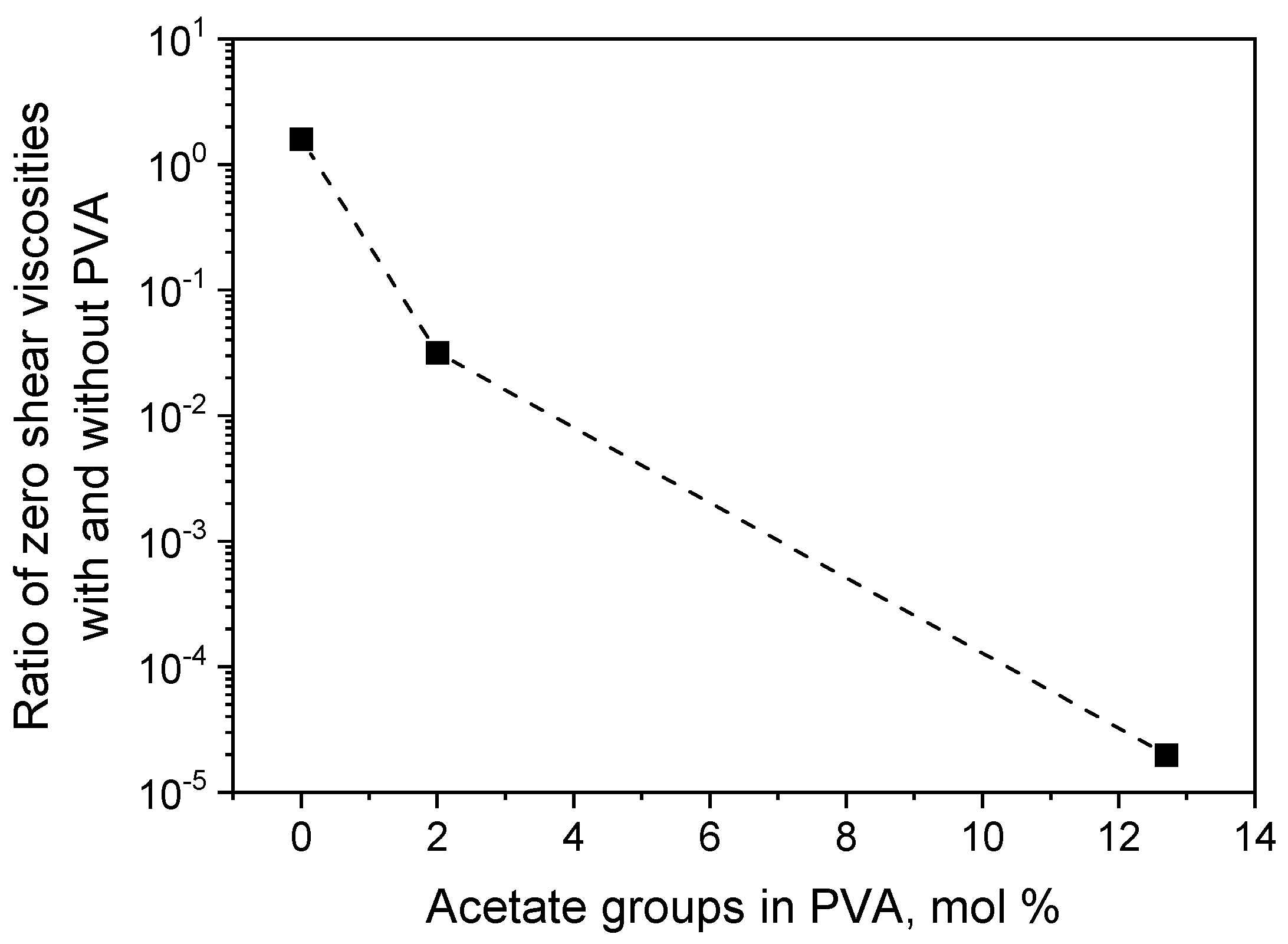
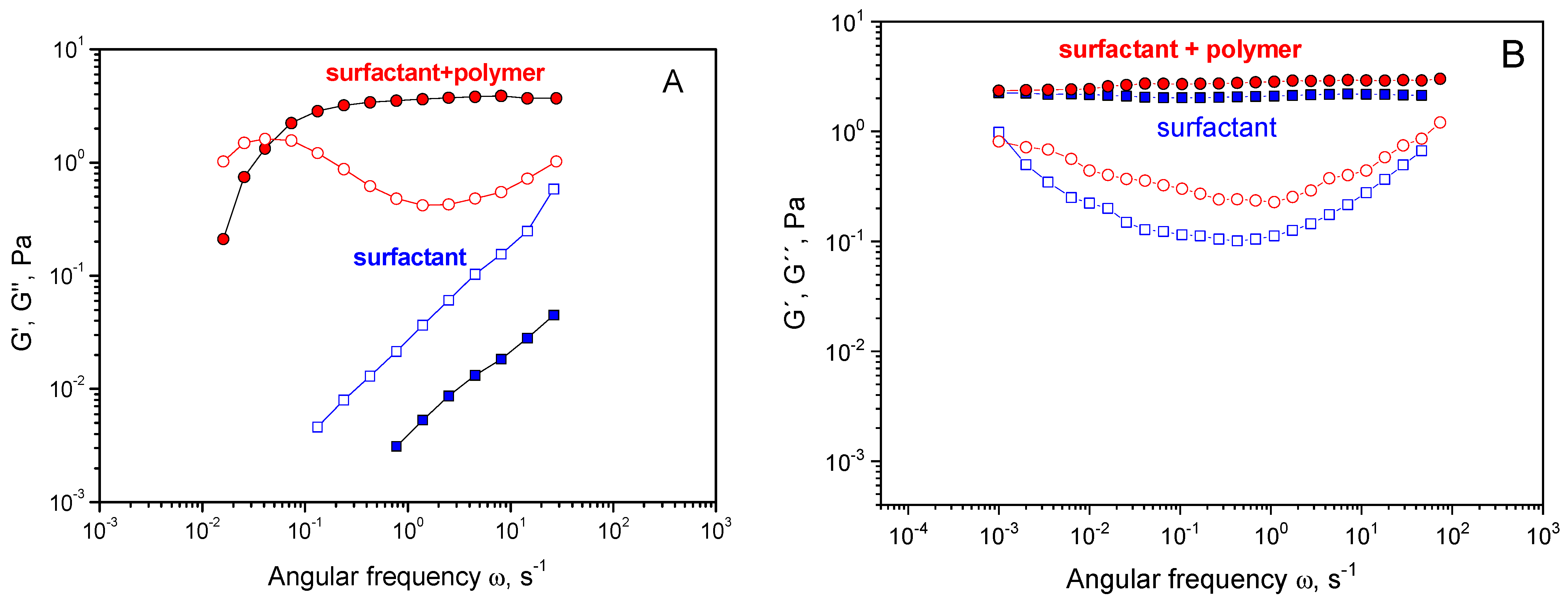
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreiss, C.A.; Feng, Y. Wormlike Micelles: Advances in Systems, Characterisation and Applications; The Royal Society of Chemistry: London, UK, 2017; ISBN 9781782629788. [Google Scholar]
- Dreiss, C.A. Wormlike Micelles: Where Do We Stand? Recent Developments, Linear Rheology and Scattering Techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z.; Dreiss, C.A. Smart Wormlike Micelles: Design, Characteristics and Applications; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662459492. [Google Scholar]
- Linet Rose, J.; Tata, B.V.R.; Talmon, Y.; Aswal, V.K.; Hassan, P.A.; Sreejith, L. Micellar Solution with PH Responsive Viscoelasticity and Colour Switching Property. RSC Adv. 2015, 5, 11397–11404. [Google Scholar] [CrossRef]
- Zana, R.; Kaler, E.W. Giant Micelles: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7308-4. [Google Scholar]
- Molchanov, V.S.; Philippova, O.E. Dominant Role of Wormlike Micelles in Temperature-Responsive Viscoelastic Properties of Their Mixtures with Polymeric Chains. J. Colloid Interface Sci. 2013, 394, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, A.L.; Molchanov, V.S.; Philippova, O.E. Polymer-like Wormlike Micelles of Ionic Surfactants: Structure and Rheological Properties. Polym. Sci.-Ser. A 2019, 61, 215–225. [Google Scholar] [CrossRef]
- Siriwatwechakul, W.; LaFleur, T.; Prud’homme, R.K.; Sullivan, P. Effects of Organic Solvents on the Scission Energy of Rodlike Micelles. Langmuir 2004, 20, 8970–8974. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Ospennikov, A.S.; Kuznetsova, E.K.; Kuklin, A.I.; Aliev, T.M.; Novikov, V.V.; Philippova, O.E. Universal Character of Breaking of Wormlike Surfactant Micelles by Additives of Different Hydrophobicity. Nanomaterials 2022, 12, 4445. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart Wormlike Micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Falbe, J. Surfactants in Consumer Products: Theory, Technology and Application; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.F. Design of Eco-Friendly Fabric Softeners: Structure, Rheology and Interaction with Cellulose Nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef]
- Kim, J.H.; Domach, M.M.; Tilton, R.D. Pyrene Micropartitioning and Solubilization by Sodium Dodecyl Sulfate Complexes with Poly(Ethylene Glycol). J. Phys. Chem. B 1999, 103, 10582–10590. [Google Scholar] [CrossRef]
- Simon, M.; Krause, P.; Chiappisi, L.; Noirez, L.; Gradzielski, M. Structural Control of Polyelectrolyte/Microemulsion Droplet Complexes (PEMECs) with Different Polyacrylates. Chem. Sci. 2019, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Le Garrec, D.; Ranger, M.; Leroux, J.C. Micelles in Anticancer Drug Delivery. Am. J. Drug Deliv. 2004, 2, 15–42. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S. A Comprehensive Review of Beneficial Applications of Viscoelastic Surfactants in Wellbore Hydraulic Fracturing Fluids. Fuel 2023, 338, 127228. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Osiptsov, A.A.; Philippova, O.E. Novel Trends in the Development of Surfactant-Based Hydraulic Fracturing Fluids: A Review. Gels 2021, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Association of Nonionic Polymers with Micelles, Bilayers, and Microemulsions. J. Chem. Phys. 1989, 90, 1980–1994. [Google Scholar] [CrossRef]
- Brackman, J.C.; Engberts, J.B.F.N. Influence of Polymers on the Micellization of Cetyltrimethylammonium Salts. Langmuir 1991, 7, 2097–2102. [Google Scholar] [CrossRef]
- Brackman, J.C.; Engberts, J.B.F.N. Polymer-Induced Breakdown of Rodlike Micelles. A Striking Transition of a Non-Newtonian to a Newtonian Fluid. J. Am. Chem. Soc. 1990, 112, 872–873. [Google Scholar] [CrossRef]
- Lin, Z.; Eads, C.D. Polymer-Induced Structural Transitions in Oleate Solutions: Microscopy, Rheology, and Nuclear Magnetic Resonance Studies. Langmuir 1997, 13, 2647–2654. [Google Scholar] [CrossRef]
- Li, X.; Len, Z.; Cai, J.; Seriven, L.E.; Davis, H.T. Polymer-Induced Microstructural Transitions in Surfactant Solutions. J. Phys. Chem. 1995, 99, 10865–10878. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Makarov, A.V.; Kuklin, A.I.; Iliopoulos, I.; Philippova, O.E. Role of Charge of Micellar Worms in Modulating Structure and Rheological Properties of Their Mixtures with Nonionic Polymer. Macromolecules 2018, 51, 213–221. [Google Scholar] [CrossRef]
- Flood, C.; Dreiss, C.A.; Croce, V.; Cosgrove, T.; Karlsson, G.G. Wormlike Micelles Mediated by Polyelectrolyte. Langmuir 2005, 21, 7646–7652. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, A.V.; Mityuk, D.Y.; Muravlev, D.A.; Philippova, O.E. Viscoelastic Solutions of Wormlike Micelles of a Cationic Surfactant and a Stiff-Chain Anionic Polyelectrolyte. Polym. Sci.-Ser. A 2019, 61, 765–772. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Kuklin, A.I.; Torocheshnikov, V.N.; Orekhov, A.S.; Roland, S.; Miquelard-Garnier, G.; Matsarskaia, O.; Iliopoulos, I.; Philippova, O.E. Double Dynamic Hydrogels Formed by Wormlike Surfactant Micelles and Cross-Linked Polymer. J. Colloid Interface Sci. 2022, 611, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Roland, S.; Miquelard-Garnier, G.; Shibaev, A.V.; Aleshina, A.L.; Chennevière, A.; Matsarskaia, O.; Sollogoub, C.; Philippova, O.E.; Iliopoulos, I. Dual Transient Networks of Polymer and Micellar Chains: Structure and Viscoelastic Synergy. Polymers 2021, 13, 4255. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, N. Poly(Vinyl Alcohol): Review of Its Promising Applications and Insights into Biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Vega, I.; Fernández, E.; Mijangos, C.; D’Accorso, N.; Lopez, D. Potential Applications of Poly(Vinyl Alcohol)-Congo Red Aqueous Solutions and Hydrogels as Liquids for Hydraulic Fracturing. J. Appl. Polym. Sci. 2008, 110, 695–700. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Aleshina, A.L.; Arkharova, N.A.; Orekhov, A.S.; Kuklin, A.I.; Philippova, O.E. Disruption of Cationic/Anionic Viscoelastic Surfactant Micellar Networks by Hydrocarbon as a Basis of Enhanced Fracturing Fluids Clean-Up. Nanomaterials 2020, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, A.I.; Islamov, A.K.; Gordeliy, V.I. Scientific Reviews: Two-Detector System for Small-Angle Neutron Scattering Instrument. Neutron News 2005, 16, 16–18. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Soloviov, D.V.; Rogachev, A.V.; Utrobin, P.K.; Kovalev, Y.S.; Balasoiu, M.; Ivankov, O.I.; Sirotin, A.P.; Murugova, T.N.; Petukhova, T.B.; et al. New Opportunities Provided by Modernized Small-Angle Neutron Scattering Two-Detector System Instrument (YuMO). J. Phys. Conf. Ser. 2011, 291, 012013. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Solovjeva, T.M.; Ivankov, O.I.; Soloviov, D.V.; Rogachev, A.V.; Kuklin, A.I. SAS Program for Two-Detector System: Seamless Curve from Both Detectors. Proc. J. Phys. Conf. Ser. 2017, 848, 012020. [Google Scholar] [CrossRef]
- SasView. Available online: http://www.sasview.org/ (accessed on 18 September 2023).
- Iancu, C.V.; Tivol, W.F.; Schooler, J.B.; Dias, D.P.; Henderson, G.P.; Murphy, G.E.; Wright, E.R.; Li, Z.; Yu, Z.; Briegel, A.; et al. Electron Cryotomography Sample Preparation Using the Vitrobot. Nat. Protoc. 2007, 1, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.R.; Kaler, E.W. Highly Viscoelastic Wormlike Micellar Solutions Formed by Cationic Surfactants with Long Unsaturated Tails. Langmuir 2001, 17, 300–306. [Google Scholar] [CrossRef]
- de Souza, R.N.; Jora, M.Z.; Duarte, L.G.T.A.; Clinckspoor, K.J.; Atvars, T.D.Z.; Sabadini, E. A New Interpretation of the Mechanism of Wormlike Micelle Formation Involving a Cationic Surfactant and Salicylate. J. Colloid Interface Sci. 2019, 552, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Shishkhanova, K.B.; Molchanov, V.S.; Baranov, A.N.; Kharitonova, E.P.; Orekhov, A.S.; Arkharova, N.A.; Philippova, O.E. A PH-Triggered Reinforcement of Transient Network of Wormlike Micelles by Halloysite Nanotubes of Different Charge. J. Mol. Liq. 2023, 370, 121032. [Google Scholar] [CrossRef]
- Khouri, S.J.; Alsaad, D.; Altwaiq, A.M. Salicylic Acid Solubility and Thermodynamic Dissociation Constant at Various Temperatures in Water: Variable Ionic Strength Titrimetric Analysis. J. Solution Chem. 2024. [Google Scholar] [CrossRef]
- Cardiel, J.J.; Tonggu, L.; Dohnalkova, A.C.; De La Iglesia, P.; Pozzo, D.C.; Wang, L.; Shen, A.Q. Worming Their Way into Shape: Toroidal Formations in Micellar Solutions. ACS Nano 2013, 7, 9704–9713. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, A.V.; Abrashitova, K.A.; Kuklin, A.I.; Orekhov, A.S.; Vasiliev, A.L.; Iliopoulos, I.; Philippova, O.E. Viscoelastic Synergy and Microstructure Formation in Aqueous Mixtures of Nonionic Hydrophilic Polymer and Charged Wormlike Surfactant Micelles. Macromolecules 2017, 50, 339–348. [Google Scholar] [CrossRef]
- Negm, N.A.; Mohamed, A.S.; Ahmed, S.M.; El-Raouf, M.A. Polymer-Cationic Surfactant Interaction: 1. Surface and Physicochemical Properties of Polyvinyl Alcohol (PVA)-S-Alkyl Isothiouronium Bromide Surfactant Mixed Systems. J. Surfactants Deterg. 2015, 18, 245–250. [Google Scholar] [CrossRef]
- Croce, V.; Cosgrove, T.; Maitland, G.; Hughes, T. Rheology, Cryogenic Transmission Electron Spectroscopy, and Small-Angle Neutron Scattering of Highly Viscoelastic Wormlike Micellar Solutions. Langmuir 2003, 19, 8536–8541. [Google Scholar] [CrossRef]
- Sommer, C.; Pedersen, J.S.; Egelhaaf, S.U.; Cannavacciuolo, L.; Kohlbrecher, J.; Schurtenberger, P. Wormlike Micelles as “Equilibrium Polyelectrolytes”: Light and Neutron Scattering Experiments. Langmuir 2002, 18, 2495–2505. [Google Scholar] [CrossRef]
- Cannavacciuolo, L.; Sommer, C.; Pedersen, J.S.; Schurtenberger, P. Size, FLexibility, and Scattering Functions of Semiflexible Polyelectrolytes with Excluded Volume Effects: Monte Carlo simulations and Neutron Scattering Experiments. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 5409–5419. [Google Scholar] [CrossRef]
- Budhlall, B.M.; Landfester, K.; Sudol, E.D.; Dimonie, V.L.; Klein, A.; El-Aasser, M.S. Characterization of Partially Hydrolyzed Poly(Vinyl Alcohol). Effect of Poly(Vinyl Alcohol) Molecular Architecture on Aqueous Phase Conformation. Macromolecules 2003, 36, 9477–9484. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Denisova, Y.I.; Krentsel, L.B.; Shandryuk, G.A.; Litmanovich, A.D.; Litmanovich, E.A.; Bondarenko, G.N.; Kudryavtsev, Y.V. Effect of Chain Structure on the Rheological Properties of Vinyl Acetate-Vinyl Alcohol Copolymers in Solution and Bulk. Macromolecules 2014, 47, 4790–4804. [Google Scholar] [CrossRef]
- Merekalova, N.D.; Bondarenko, G.N.; Denisova, Y.I.; Krentsel, L.B.; Litmanovich, A.D.; Kudryavtsev, Y.V. Effect of Chain Structure on Hydrogen Bonding in Vinyl Acetate–Vinyl Alcohol Copolymers. J. Mol. Struct. 2017, 1134, 475–481. [Google Scholar] [CrossRef]
- Squillace, O.; Fong, R.; Shepherd, O.; Hind, J.; Tellam, J.; Steinke, N.-J.; Thompson, R.L. Influence of PVAc/PVA Hydrolysis on Additive Surface Activity. Polymers 2020, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Shashkina, J.A.; Philippova, O.E.; Zaroslov, Y.D.; Khokhlov, A.R.; Pryakhina, T.A.; Blagodatskikh, I.V. Rheology of Viscoelastic Solutions of Cationic Surfactant. Effect of Added Associating Polymer. Langmuir 2005, 21, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Couillet, I.; Hughes, T.; Maitland, G. Synergistic Effects in Aqueous Solutions of Mixed Wormlike Micelles and Hydrophobically Modified Polymers. Macromolecules 2005, 38, 5271–5282. [Google Scholar] [CrossRef]
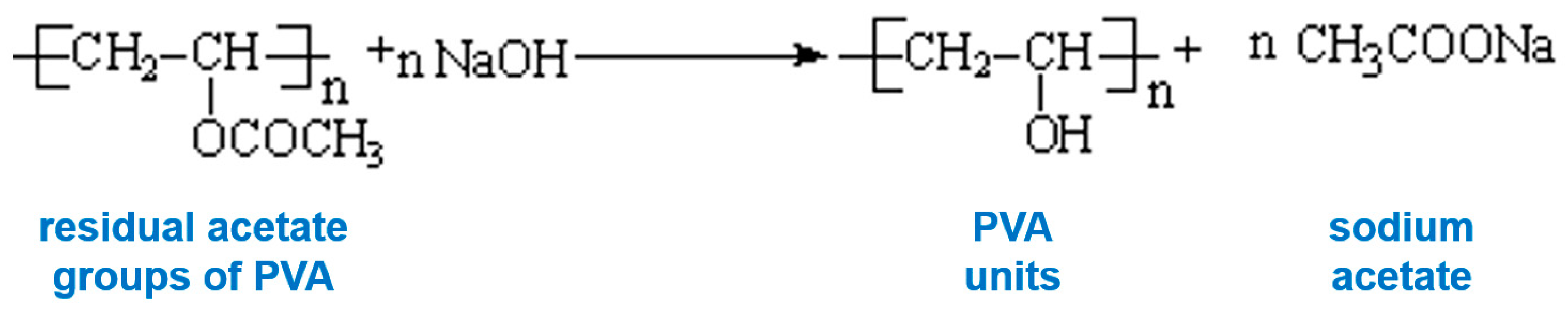
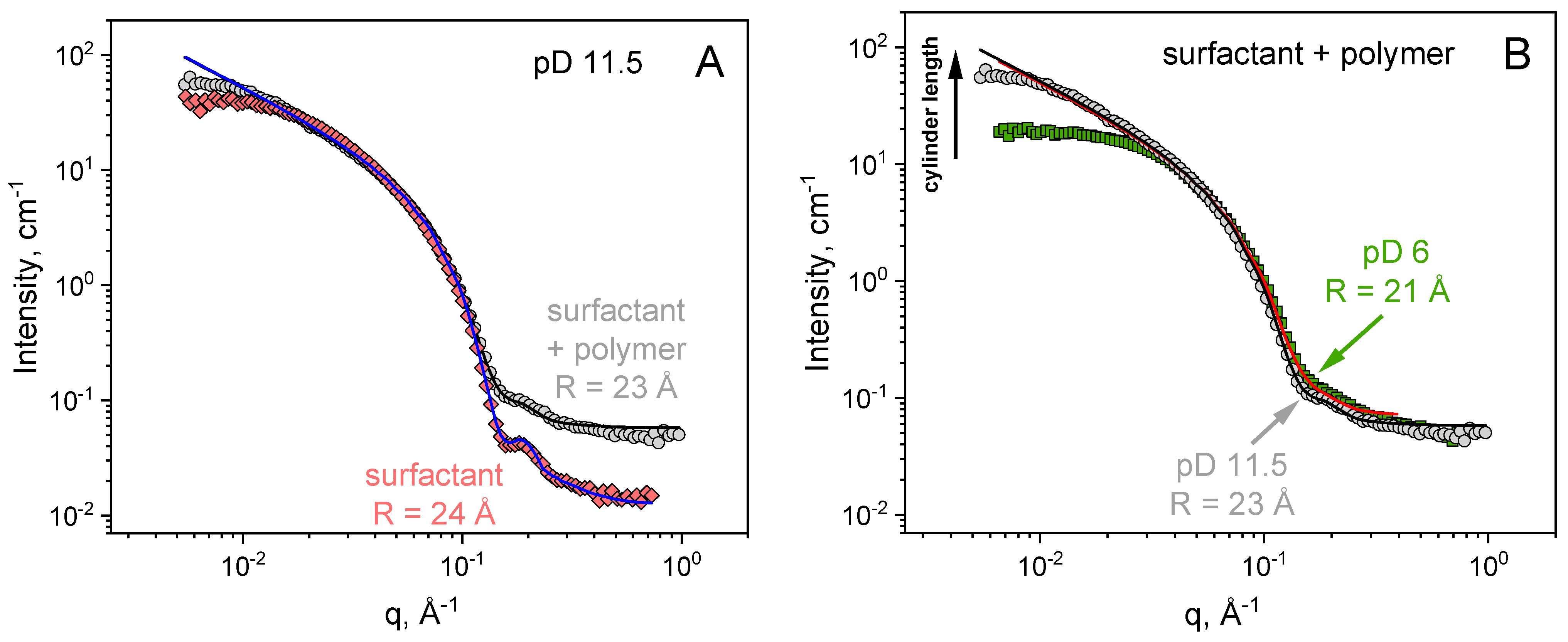

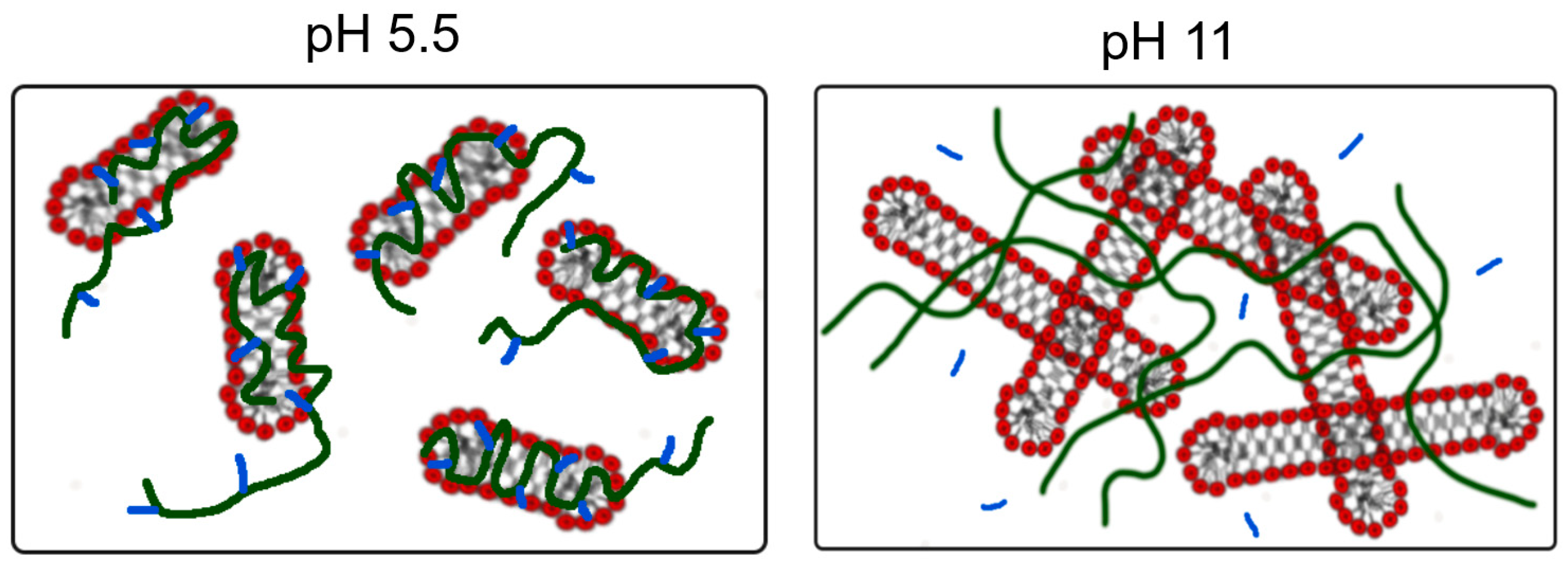
| pD | EHAC Concentration, mM | PVA Concentration, monomol/L | Radius of Cylinder R, Å | Radius Polydispersity ΔR/R |
|---|---|---|---|---|
| 6 | 26 | 24 | 0.15 | |
| 6 | 26 | 890 | 21 | 0.25 |
| 11.5 | 26 | 24 | 0.15 | |
| 11.5 | 26 | 890 | 23 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, A.L.; Kwiatkowski, A.L.; Kuklin, A.I.; Chesnokov, Y.M.; Philippova, O.E.; Shibaev, A.V. Dual Semi-Interpenetrating Networks of Water-Soluble Macromolecules and Supramolecular Polymer-like Chains: The Role of Component Interactions. Polymers 2024, 16, 1430. https://doi.org/10.3390/polym16101430
Makarova AL, Kwiatkowski AL, Kuklin AI, Chesnokov YM, Philippova OE, Shibaev AV. Dual Semi-Interpenetrating Networks of Water-Soluble Macromolecules and Supramolecular Polymer-like Chains: The Role of Component Interactions. Polymers. 2024; 16(10):1430. https://doi.org/10.3390/polym16101430
Chicago/Turabian StyleMakarova, Anna L., Alexander L. Kwiatkowski, Alexander I. Kuklin, Yuri M. Chesnokov, Olga E. Philippova, and Andrey V. Shibaev. 2024. "Dual Semi-Interpenetrating Networks of Water-Soluble Macromolecules and Supramolecular Polymer-like Chains: The Role of Component Interactions" Polymers 16, no. 10: 1430. https://doi.org/10.3390/polym16101430
APA StyleMakarova, A. L., Kwiatkowski, A. L., Kuklin, A. I., Chesnokov, Y. M., Philippova, O. E., & Shibaev, A. V. (2024). Dual Semi-Interpenetrating Networks of Water-Soluble Macromolecules and Supramolecular Polymer-like Chains: The Role of Component Interactions. Polymers, 16(10), 1430. https://doi.org/10.3390/polym16101430







