Stable Structure and Fast Ion Diffusion: A Flexible MoO2@Carbon Hollow Nanofiber Film as a Binder-Free Anode for Sodium-Ion Batteries with Superior Kinetics and Excellent Rate Capability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
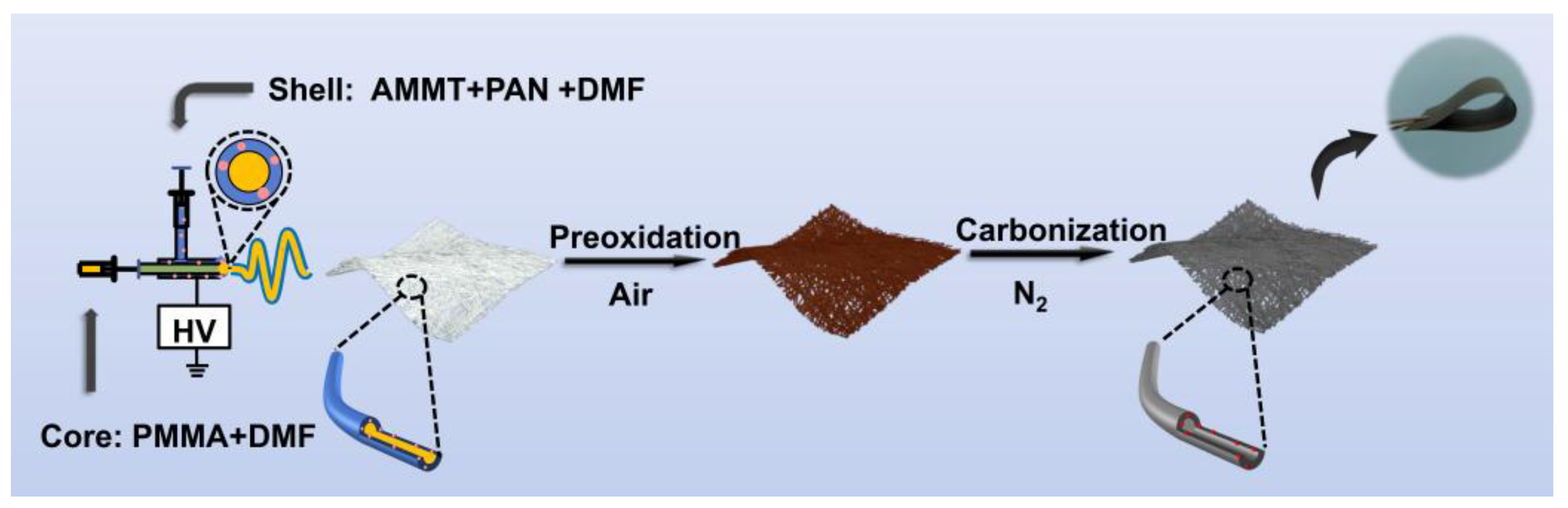
2.2. Synthesis of MoO2@HCNFs and MoO2@CNFs
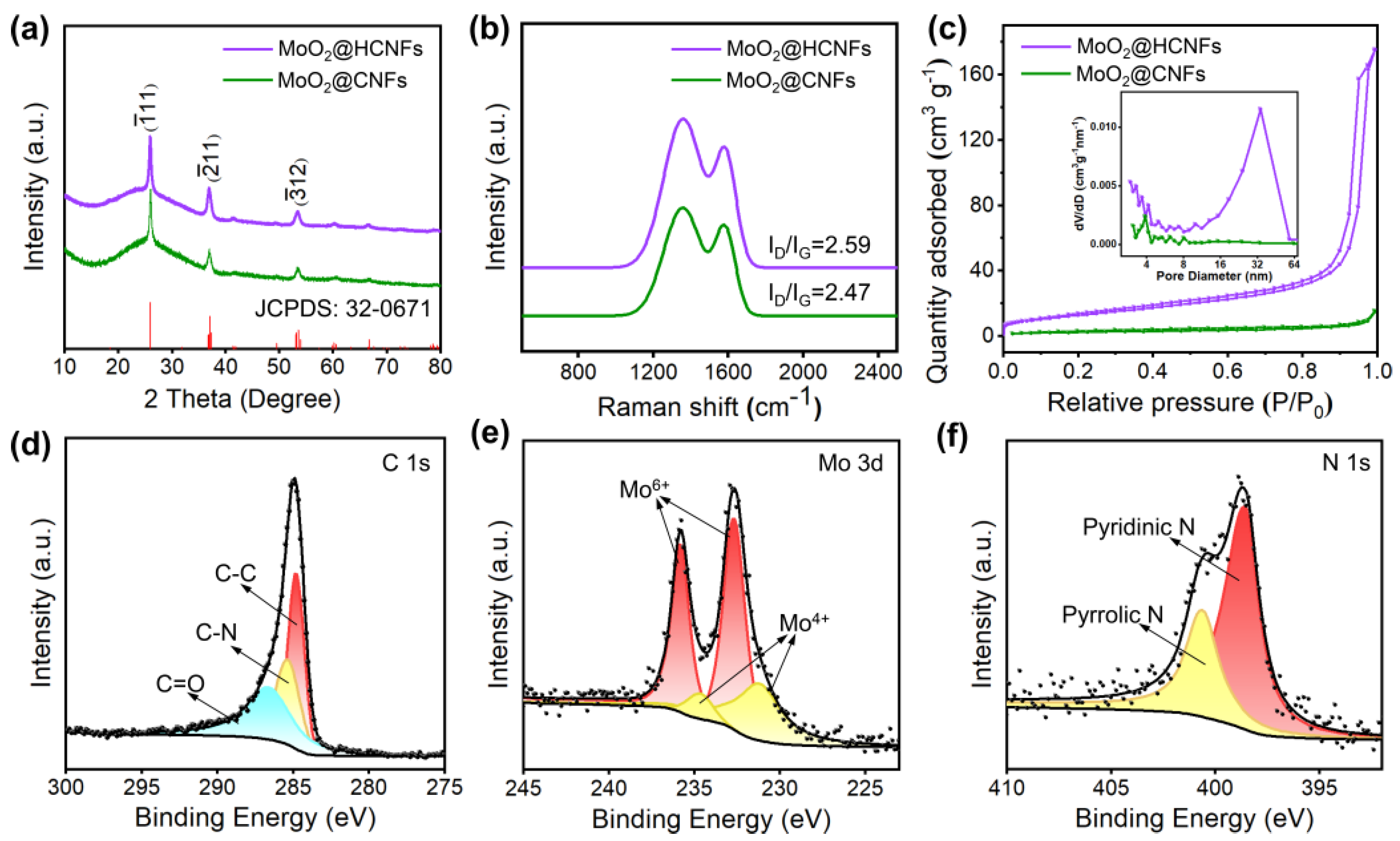
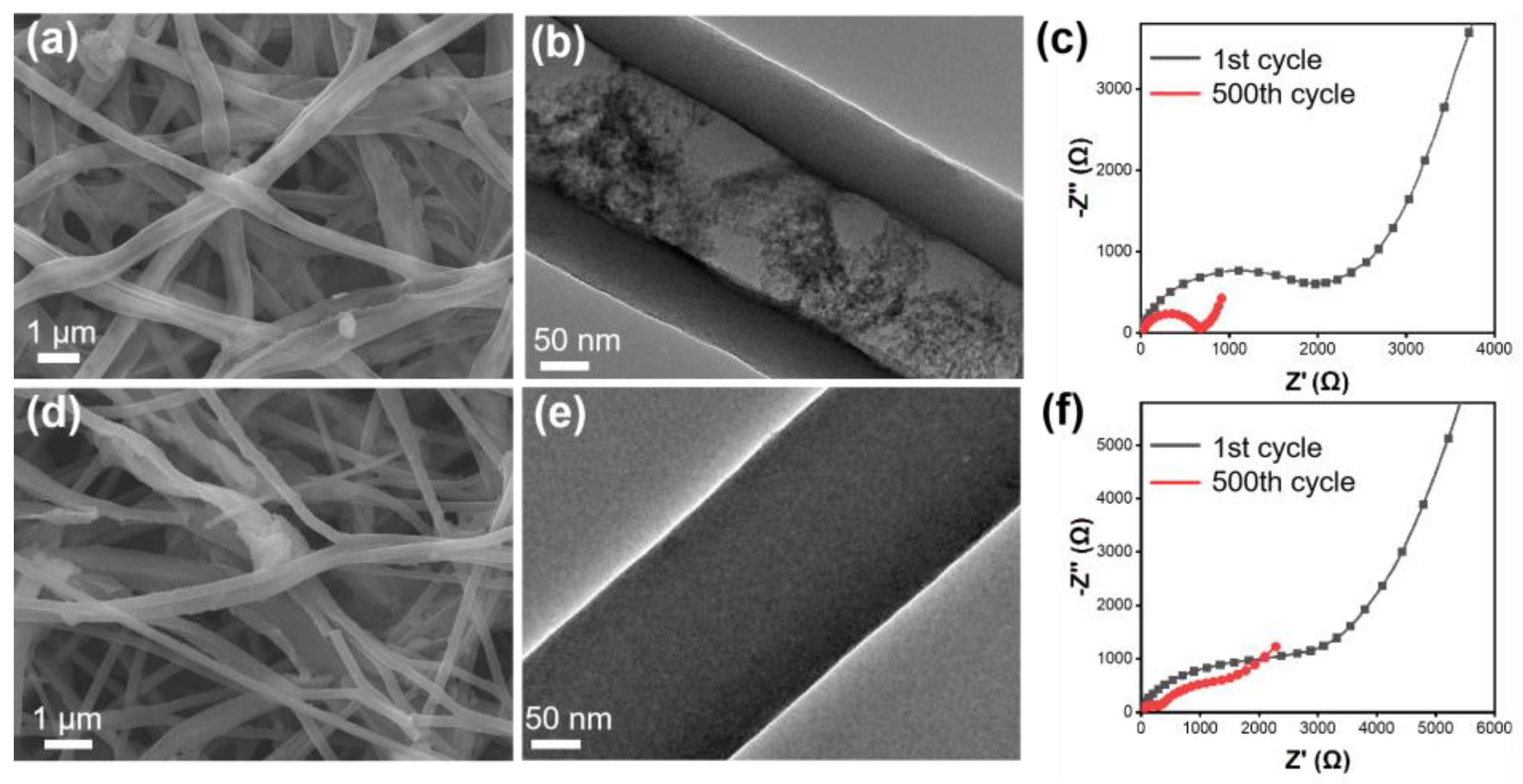
2.3. Material Characterization
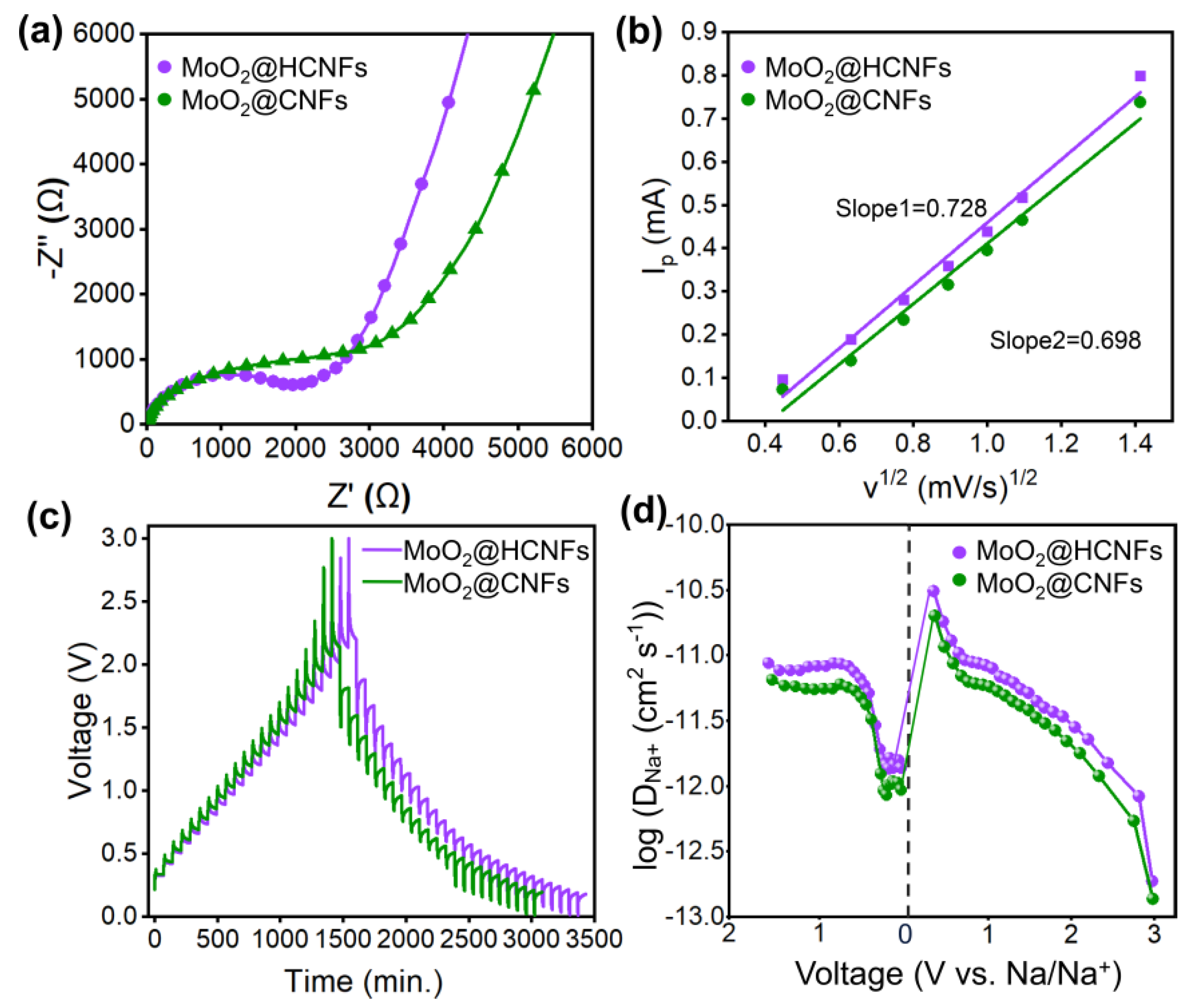
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Zhao, X.-X.; Fu, W.; Zhang, H.-X.; Guo, J.-Z.; Gu, Z.-Y.; Wang, X.-T.; Yang, J.-L.; Lü, H.-Y.; Wu, X.-L.; Ang, E.H. Pearl-structure-enhanced NASICON cathode toward ultrastable sodium-ion batteries. Adv. Sci. 2023, 10, 2301308. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H.; Wang, X. The anode materials for lithium-ion and sodium-ion batteries based on conversion reactions: A review. ChemElectroChem 2023, 10, e202201151. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Su, D.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. Electrode materials for sodium-ion batteries: Considerations on crystal structures and sodium storage mechanisms. Electrochem. Energy Rev. 2018, 1, 200–237. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and sodium-ion batteries: 50 years of research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced anode materials for rechargeable sodium-ion batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.K.; Bhatnagar, A. A review on anode materials for lithium/sodium-ion batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Yang, C.; Ou, X.; Xiong, X.; Zheng, F.; Hu, R.; Chen, Y.; Liu, M.; Huang, K. V5S8-graphite hybrid nanosheets as a high rate-capacity and stable anode material for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 107–113. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-B.; Zhang, B.; Peng, T.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.; Wu, X.-W.; Zheng, J.-C. MoS2/SnS@C hollow hierarchical nanotubes as superior performance anode for sodium-ion batteries. Nano Energy 2021, 90, 106568. [Google Scholar] [CrossRef]
- Lian, X.; Xu, N.; Ma, Y.; Hu, F.; Wei, H.; Chen, H.-Y.; Wu, Y.; Li, L.; Li, D.; Peng, S. In-situ formation of Co1−xS hollow polyhedrons anchored on multichannel carbon nanofibers as self-supporting anode for lithium/sodium-ion batteries. Chem. Eng. J. 2021, 421, 127755. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.-Y.; Lou, X.W. Bullet-like Cu9S5 hollow particles coated with nitrogen-doped carbon for sodium-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 7744–7748. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Chen, Y.-M.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Yu, L.; Hu, H.; Wu, H.B.; Lou, X.W. Complex hollow nanostructures: Synthesis and energy-related applications. Adv. Mater. 2017, 29, 1604563. [Google Scholar] [CrossRef]
- Bai, X.; Wu, N.; Yu, G.; Li, T. Recent advances in anode materials for sodium-ion batteries. Inorganics 2023, 11, 289. [Google Scholar] [CrossRef]
- Rojo, T.; Hu, Y.-S.; Forsyth, M.; Li, X. Sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1800880. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, L.; Yuan, Q. Application of new COF materials in secondary battery anode materials. Molecules 2023, 28, 5953. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhang, Y. Recent advances in carbon anodes for sodium-ion batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Zhang, B. Approaching microsized alloy anodes via solid electrolyte interphase design for advanced rechargeable batteries. Adv. Energy Mater. 2023, 13, 2300123. [Google Scholar] [CrossRef]
- Yao, S.; Ramakrishna, S.; Chen, G. Recent advances in metal-organic frameworks based on electrospinning for energy storage. Adv. Fiber Mater. 2023, 5, 1592–1617. [Google Scholar] [CrossRef]
- Tan, S.; Yang, H.; Zhang, Z.; Xu, X.; Xu, Y.; Zhou, J.; Zhou, X.; Pan, Z.; Rao, X.; Gu, Y.; et al. The progress of hard carbon as an anode material in sodium-ion batteries. Molecules 2023, 28, 3134. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Man, Y.; Yang, J.; Xie, J.; Xu, M. MoO2 nanosheets embedded in amorphous carbon matrix for sodium-ion batteries. R. Soc. Open Sci. 2017, 4, 170892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, T.; Shi, H.; Wang, T.; Zhang, M.; Cao, G. Single nozzle electrospinning synthesized MoO2@C core shell nanofibers with high capacity and long-term stability for lithium-ion storage. Adv. Mater. Interfaces 2017, 4, 1600816. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.; Marone, F.; Stampanoni, M.; Wood, V. Visualization and quantification of electrochemical and mechanical degradation in li ion batteries. Science 2013, 342, 716–720. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Z.; Li, X.; Kong, F.; Tao, S.; Qian, B. MoO2 nanosheets embedded into carbon nanofibers with a self-standing structure for lithium ion and sodium ion batteries. Ceram. Int. 2021, 47, 26839–26846. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Nithya, C.; Karvembu, R. High-performance sodium ion capacitor based on MoO2@rGO nanocomposite and goat hair derived carbon electrodes. ACS Appl. Energy Mater. 2018, 1, 841–850. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Zhang, H.; Zhu, Z.; Zhang, M. The double effects of sulfur-doping on MoO2/C nanofibers with high properties for Na-ion batteries. Appl. Surf. Sci. 2018, 455, 343–348. [Google Scholar] [CrossRef]
- Liang, J.; Gao, X.; Guo, J.; Chen, C.; Fan, K.; Ma, J. Electrospun MoO2@NC nanofibers with excellent Li+/Na+ storage for dual applications. Sci. China Mater. 2018, 61, 30–38. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Li, W.; Li, J.; Ren, S.; Wang, M.; Han, G.; Yu, J.; Zhang, Y.; Zhao, H. Doped carbon dots enable highly efficient multiple-color room temperature phosphorescence. Adv. Opt. Mater. 2023, 12, 2301962. [Google Scholar] [CrossRef]
- Meng, X.; Song, Y.; Jing, Q.; Zhao, H. Self-precipitation of highly purified red emitting carbon dots as red phosphors. J. Phys. Chem. Lett. 2023, 14, 9176–9182. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Su, X.; Dong, F.; Wang, Z. Two-phase Solvothermal Synthesis of MoO2/RGO Nanocomposites for Lithium-Ion Battery Anodes. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 3 February 2021. [Google Scholar]
- Huang, J.; Xu, Z.; Cao, L.; Zhang, Q.; Ouyang, H.; Li, J. Tailoring MoO2/graphene oxide nanostructures for stable, high-density sodium-ion battery anodes. Energy Technol. 2015, 3, 1108–1114. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Zhang, M.; Huang, H.; Li, S.; Han, X.; Liu, Z.; Yang, J.; Xiao, W.; Liang, J.; et al. Ultrafine MoO2-carbon microstructures enable ultralong-life power-type sodium ion storage by enhanced pseudocapacitance. Adv. Energy Mater. 2017, 7, 1602880. [Google Scholar] [CrossRef]
- Li, X.; Han, Z.; Yang, W.; Li, Q.; Li, H.; Xu, J.; Li, H.; Liu, B.; Zhao, H.; Li, S.; et al. 3D ordered porous hybrid of ZnSe/N-doped carbon with anomalously high Na+ mobility and ultrathin solid electrolyte interphase for sodium-ion batteries. Adv. Funct. Mater. 2021, 31, 2106194. [Google Scholar] [CrossRef]
- Yin, J.; Hai, P.; Gao, Y.; Gan, Z.; Wu, C.; Cheng, Y.; Xu, X. Theory-driven designed TiO2@MoO2 heterojunction: Balanced crystallinity and nanostructure toward desirable kinetics and high-rate sodium-ion storage. Nano Res. 2023, 16, 4941–4949. [Google Scholar] [CrossRef]
- Tao, W.; Chen, J.; Xu, C.; Liu, S.; Fakudze, S.; Wang, J.; Wang, C. Nanostructured MoS2 with interlayer controllably regulated by ionic liquids/cellulose for high-capacity and durable sodium storage properties. Small 2023, 19, 2207397. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, J.; Huang, G.; Dong, C.-L.; Huang, Y.-C.; Lu, P.; Tang, H.; Liu, Y.; Shen, S.; Yang, D. Red phosphorus grafted high-index (116) faceted anatase TiO2 for Z-scheme photocatalytic pure water splitting. Adv. Funct. Mater. 2023, 34, 2311623. [Google Scholar] [CrossRef]
- Zeng, F.; Liu, H.; Pan, Y.; Yu, M.; Qu, Y.; Yuan, C. Encapsulating N-doped carbon nanorod bundles/MoO2 nanoparticles via surface growth of ultrathin MoS2 nanosheets for ultrafast and ultralong cycling sodium storage. ACS Appl. Mater. Interfaces 2020, 12, 6205–6216. [Google Scholar] [CrossRef]
- Ni, X.P.; Chen, H.F.; Liu, C.L.; Zeng, F.L.; Yu, H.Y.; Ju, A.Q. A freestanding nitrogen-doped carbon nanofiber/MoS2 nanoflowers with expanded interlayer for long cycle-life lithium-ion batteries. J. Alloys Compd. 2020, 818, 152835. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Wang, H.; Zhao, Y.; Guo, J.; Zhao, J.; Beidaghi, M.; Gao, L. Electrochemical performances of MoO2/C nanocomposite for sodium ion storage: An insight into rate dependent charge/discharge mechanism. Electrochim. Acta 2017, 240, 379–387. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.D.; Yang, Y.; Liu, Z.H.; Wang, H.E.; Sui, J.H.; Cai, W. Fresh MoO2 as a better electrode for pseudocapacitive sodium-ion storage. New J. Chem. 2018, 42, 14721–14724. [Google Scholar] [CrossRef]
- Luo, Y.; Ding, X.; Ma, X.; Liu, D.; Fu, H.; Xiong, X. Constructing MoO2@MoS2 heterostructures anchored on graphene nanosheets as a high-performance anode for sodium ion batteries. Electrochim. Acta 2021, 388, 138612. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, N.; Gao, M.; Zhong, J.; Gu, C.; Zhang, Y.; Liu, B. Stable Structure and Fast Ion Diffusion: A Flexible MoO2@Carbon Hollow Nanofiber Film as a Binder-Free Anode for Sodium-Ion Batteries with Superior Kinetics and Excellent Rate Capability. Polymers 2024, 16, 1452. https://doi.org/10.3390/polym16111452
Feng N, Gao M, Zhong J, Gu C, Zhang Y, Liu B. Stable Structure and Fast Ion Diffusion: A Flexible MoO2@Carbon Hollow Nanofiber Film as a Binder-Free Anode for Sodium-Ion Batteries with Superior Kinetics and Excellent Rate Capability. Polymers. 2024; 16(11):1452. https://doi.org/10.3390/polym16111452
Chicago/Turabian StyleFeng, Na, Mingzhen Gao, Junyu Zhong, Chuantao Gu, Yuanming Zhang, and Bing Liu. 2024. "Stable Structure and Fast Ion Diffusion: A Flexible MoO2@Carbon Hollow Nanofiber Film as a Binder-Free Anode for Sodium-Ion Batteries with Superior Kinetics and Excellent Rate Capability" Polymers 16, no. 11: 1452. https://doi.org/10.3390/polym16111452
APA StyleFeng, N., Gao, M., Zhong, J., Gu, C., Zhang, Y., & Liu, B. (2024). Stable Structure and Fast Ion Diffusion: A Flexible MoO2@Carbon Hollow Nanofiber Film as a Binder-Free Anode for Sodium-Ion Batteries with Superior Kinetics and Excellent Rate Capability. Polymers, 16(11), 1452. https://doi.org/10.3390/polym16111452







