Degree of Cure, Microstructures, and Properties of Carbon/Epoxy Composites Processed via Frontal Polymerization
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Mixture Preparation
2.3. Specimen Preparation
2.4. Tensile Test
2.5. Microstructural Examination
2.6. Differential Scanning Calorimetry (DSC)
- H = total heat of reaction for the partially cured samples
- Ho = total heat of reaction for the uncured samples
3. Reaction Kinetics
4. Results and Discussion
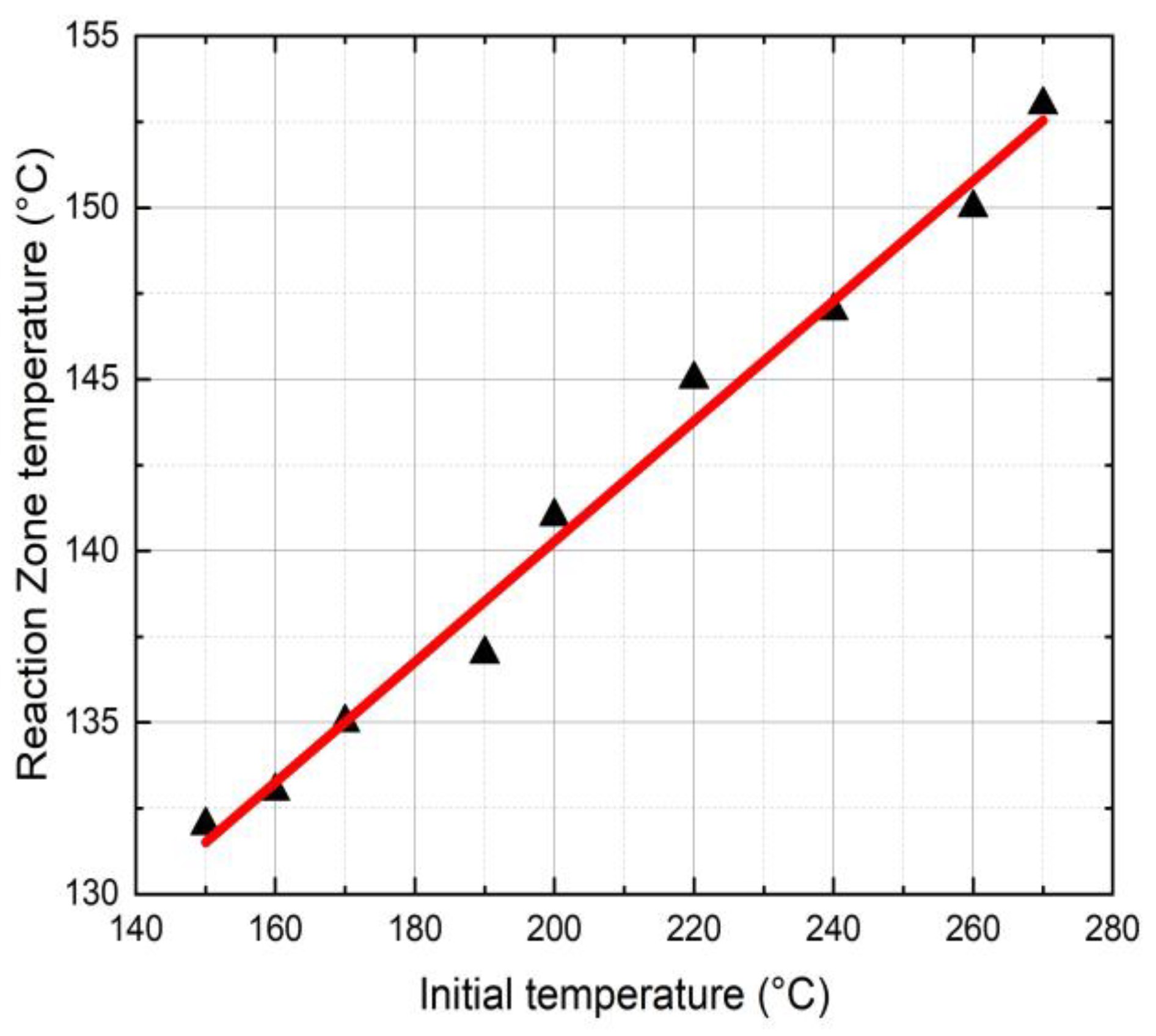
4.1. Temperature Distribution
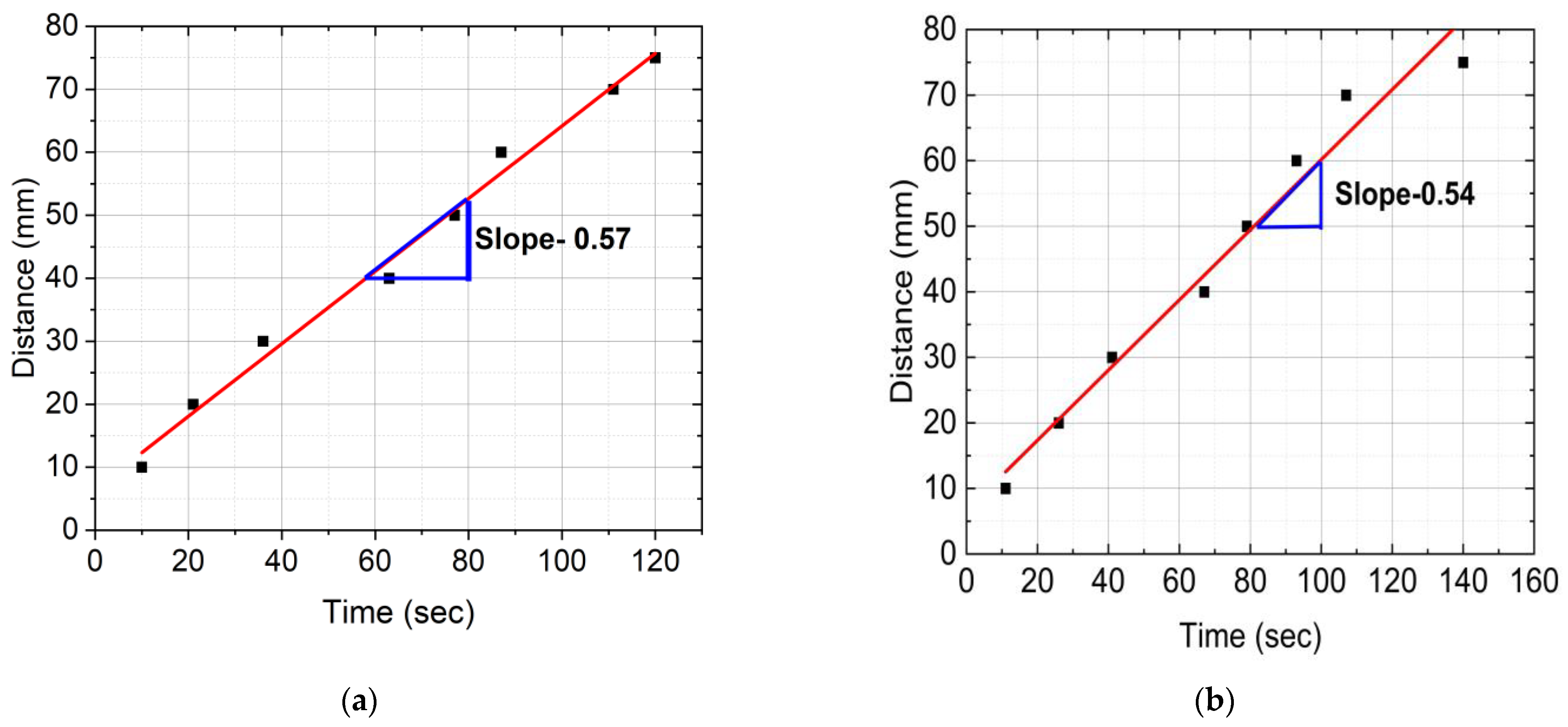
4.2. Frontal Velocity
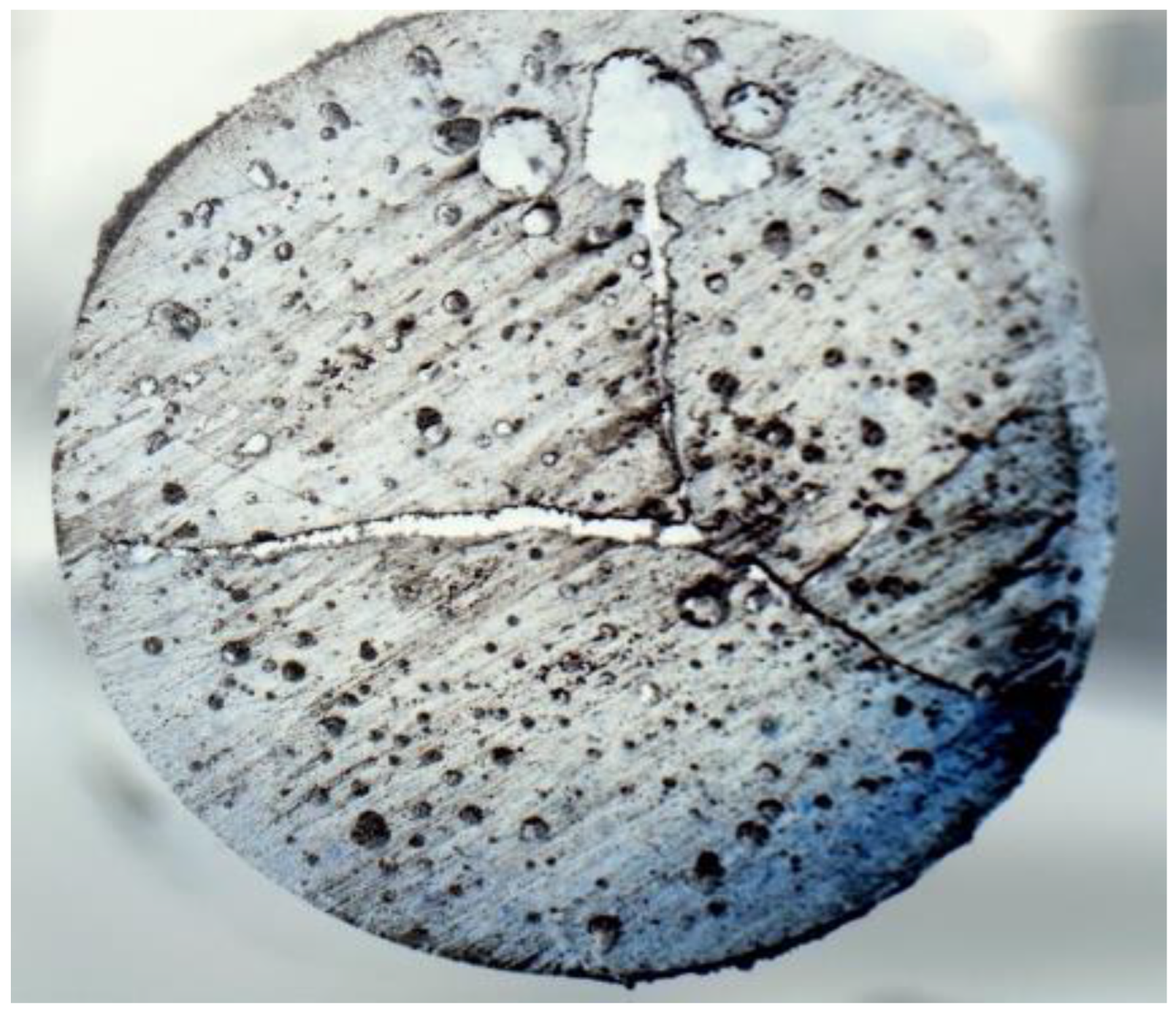
4.3. Void Contents
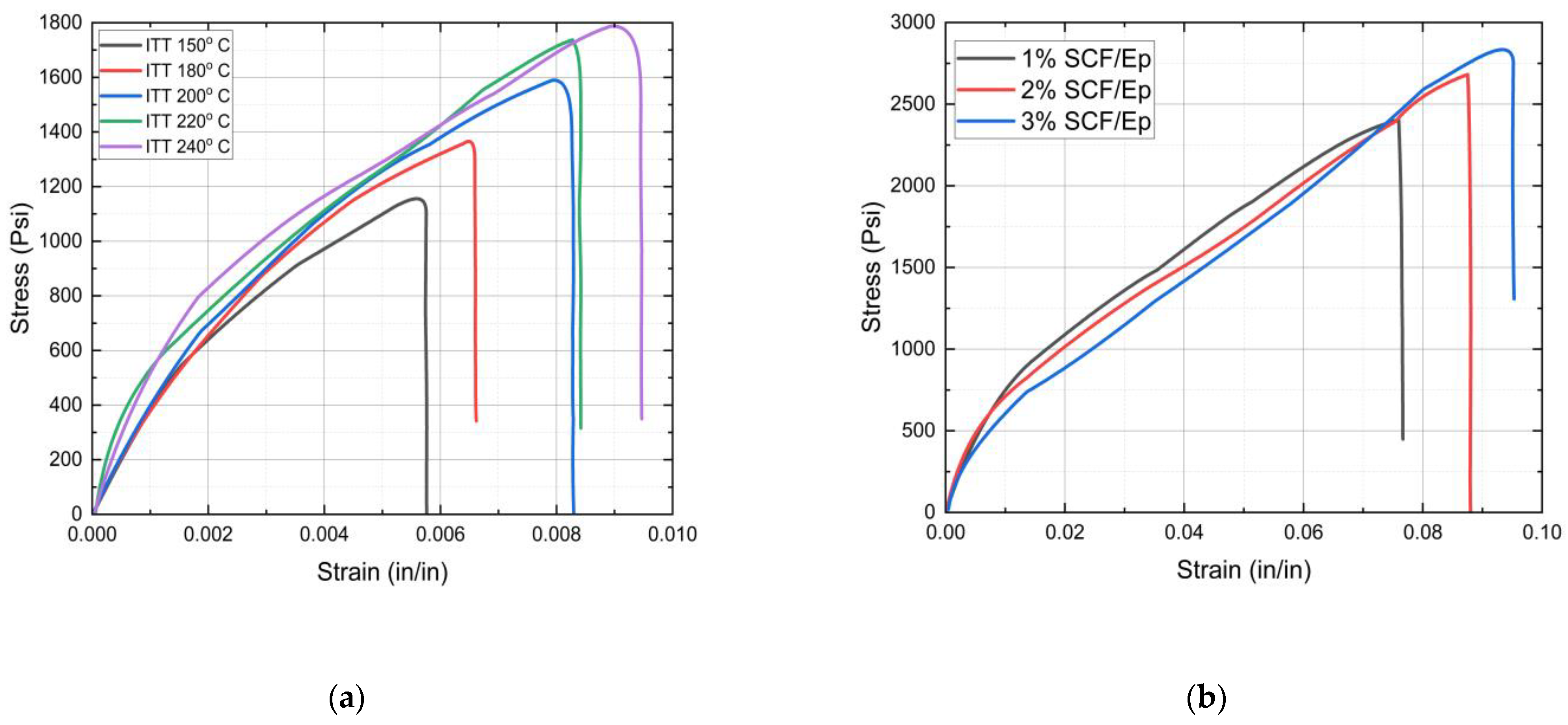
4.4. Tensile Properties
4.5. SEM of the Fractured Surface
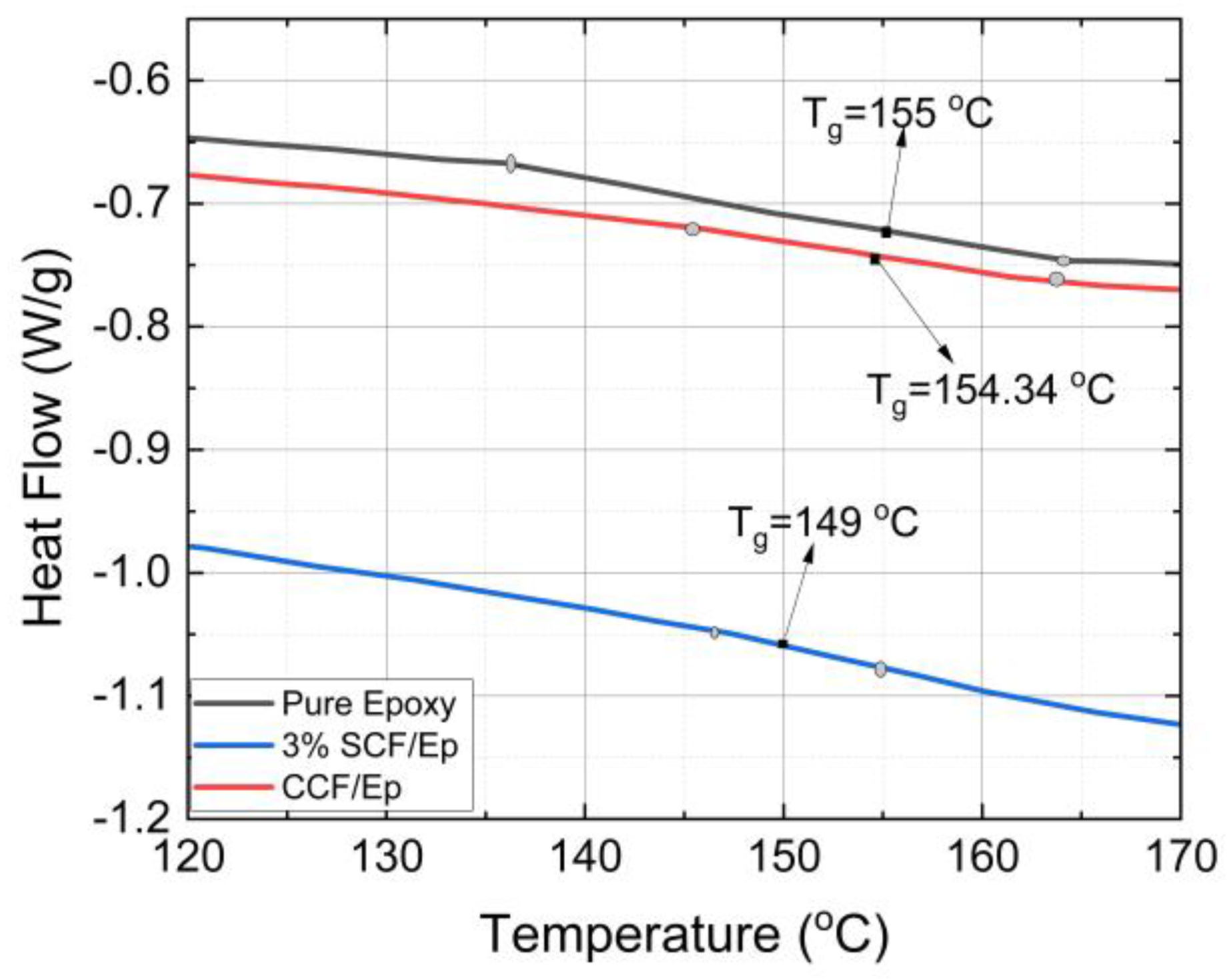
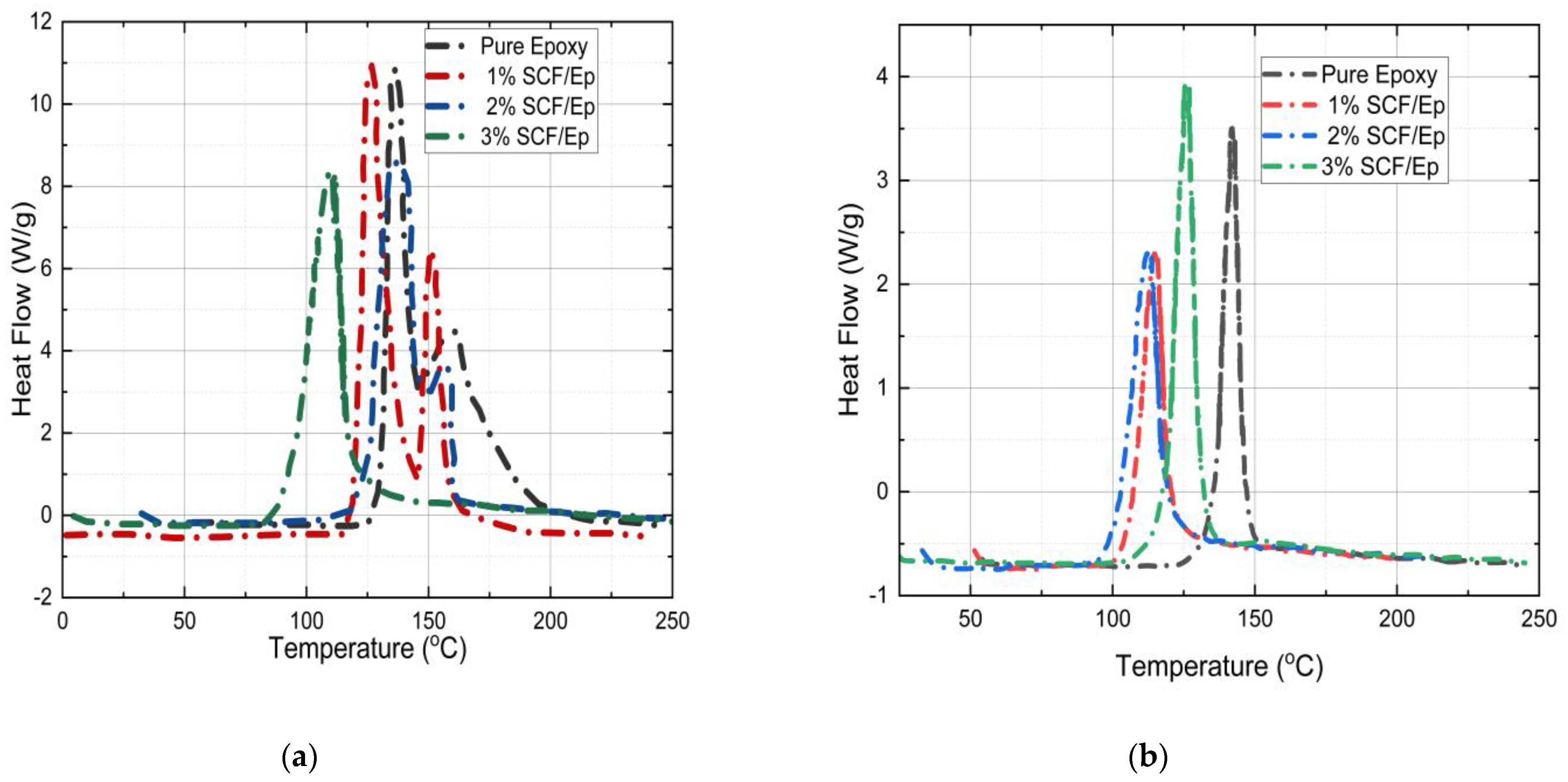
4.6. Glass Transition Temperature and Degree of Cure
5. Conclusions
- The initial triggering temperature influences the FP reaction temperature and mechanical properties of neat epoxy resin. A triggering temperature of 270 °C shows a maximum reaction temperature of 153 °C.
- The amount of SCF contents influences reaction temperature, glass transition temperature, total heat generation, degree of cure, frontal velocity, and mechanical properties of composites. The glass transition temperature and degree of cure in neat resin are seen to be higher in comparison to SCF/Ep composites. At smaller SCF loadings in SCF/Ep composites, the reduction in epoxy content leads to lower reaction zone temperatures and slower frontal velocities.
- The frontal velocity in CCF/Ep composites is seen to be higher in comparison to SCF/Ep and pure epoxy. This is because of uninterrupted exothermic reaction in resin.
- The tensile strength in neat epoxy (FP-cured) is seen to be comparatively inferior to the same cured by the conventional autoclave method. This is mostly due to large void contents, internal cracks, and a poor degree of cure in FP samples. An efficient FP sample preparation method is highly desirable to achieve improved microstructures and mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Shen, H.-X.; Liu, C.; Wang, C.-F.; Zhu, L.; Chen, S. Advances in Frontal Polymerization Strategy: From Fundamentals to Applications. Prog. Polym. Sci. 2022, 127, 101514. [Google Scholar] [CrossRef]
- Alzate-Sanchez, D.M.; Cencer, M.M.; Rogalski, M.; Kersh, M.; Sottos, N.; Moore, J.S. Anisotropic Foams Via Frontal Polymerization. Adv. Mater. 2022, 34, 2105821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, C.; Liu, R.; Li, W.; Qiu, J.; Wang, S. Catalyzed frontal polymerization-aided 3D printing of epoxy thermosets. Addit. Manuf. Lett. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Wang, B.; Fan, S.; Chen, J.; Yang, W.; Liu, W.; Li, Y. A review on prediction and control of curing process-induced deformation of continuous fiber-reinforced thermosetting composite structures. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107321. [Google Scholar] [CrossRef]
- Huang, C.; Joosten, M.W. 3D printed continuous fibre-reinforced composites: Design and characterisation of advanced pseudo-ductile hybrid laminates. Compos. Part A Appl. Sci. Manuf. 2021, 146, 106403. [Google Scholar] [CrossRef]
- He, X.; Ding, Y.; Lei, Z.; Welch, S.; Zhang, W.; Dunn, M.; Yu, K. 3D printing of continuous fiber-reinforced thermoset composites. Addit. Manuf. 2021, 40, 101921. [Google Scholar] [CrossRef]
- Additively Manufactured Continuous Carbon Fiber-Reinforced Thermoplastic for Topology Optimized Unmanned Aerial Vehicle Structures|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1359836821002316?token=83493F2F71819D01B48FCEC1A22E2AC228212CDB413BC579C742FAF71B1E48266B2AE4AC01F98D74269035E01B38EC56&originRegion=us-east-1&originCreation=20220921185752 (accessed on 21 September 2022).
- Wang, B.; Zhang, Z.; Pei, Z.; Qiu, J.; Wang, S. Current progress on the 3D printing of thermosets. Adv. Compos. Hybrid Mater. 2020, 3, 462–472. [Google Scholar] [CrossRef]
- Ziaee, M.; Naseri, I.; Johnson, J.W.; Franklin, K.A.; Yourdkhani, M. Frontal Polymerization and Three-Dimensional Printing of Thermoset Polymers with Tunable Thermomechanical Properties. ACS Appl. Polym. Mater. 2023, 5, 1715–1724. [Google Scholar] [CrossRef]
- Gai, T.; Geubelle, P.H. Impact of Boundary Heat Losses on Frontal Polymerization. J. Phys. Chem. B 2020, 124, 6404–6411. [Google Scholar] [CrossRef]
- Gao, Y.; Dearborn, M.A.; Vyas, S.; Kumar, A.; Hemmer, J.; Wang, Z.; Wu, Q.; Alshangiti, O.; Moore, J.S.; Esser-Kahn, A.P.; et al. Manipulating Frontal Polymerization and Instabilities with Phase-Changing Microparticles. J. Phys. Chem. B 2021, 125, 7537. [Google Scholar] [CrossRef]
- Gao, Y.; Dearborn, M.A.; Hemmer, J.; Wang, Z.; Esser-Kahn, A.P.; Geubelle, P.H. Controllable Frontal Polymerization and Spontaneous Patterning Enabled by Phase-Changing Particles. Small 2021, 17, 2102217. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.M.; Wu, Q.; Alshangiti, O.; Moore, J.S.; Sottos, N.R. Rapid Synthesis of Elastomers and Thermosets with Tunable Thermomechanical Properties. ACS Macro Lett. 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Naseri, I.; Yourdkhani, M. Rapid and Energy-Efficient Frontal Curing of Multifunctional Composites Using Integrated Nanostructured Heaters. ACS Appl. Mater. Interfaces 2022, 14, 50215–50224. [Google Scholar] [CrossRef] [PubMed]
- Groce, B.R. Free-Standing 3D Printing of Epoxy-Vinyl Ether Structures Using Radical-Induced Cationic Frontal Polymerization. ACS Appl. Polym. Mater. 2024, 6, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.D.; Dean, L.M.; Rudebusch, G.E.; Sottos, N.R.; White, S.R.; Moore, J.S. Alkyl Phosphite Inhibitors for Frontal Ring-Opening Metathesis Polymerization Greatly Increase Pot Life. ACS Macro Lett. 2017, 6, 609. [Google Scholar] [CrossRef]
- Sarmah, A.; Desai, S.K.; Tezel, G.B.; Vashisth, A.; Mustafa, M.M.; Arole, K.; Crowley, A.G.; Green, M.J. Rapid Manufacturing via Selective Radio-Frequency Heating and Curing of Thermosetting Resins. Adv. Eng. Mater. 2022, 24, 2101351. [Google Scholar] [CrossRef]
- Ivanoff, D.G.; Sung, J.; Butikofer, S.M.; Moore, J.S.; Sottos, N.R. Cross-Linking Agents for Enhanced Performance of Thermosets Prepared Via Frontal Ring-Opening Metathesis Polymerization. Macromolecules 2020, 53, 8360. [Google Scholar] [CrossRef]
- Liu, Q.; Abueidda, D.; Vyas, S.; Gao, Y.; Koric, S.; Geubelle, P.H. Adaptive Data-Driven Deep-Learning Surrogate Model for Frontal Polymerization in Dicyclopentadiene. J. Phys. Chem. B 2024, 128, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Husted, K.E.L.; Shieh, P.; Lundberg, D.J.; Kristufek, S.L.; Johnson, J.A. Molecularly Designed Additives for Chemically Deconstructable Thermosets without Compromised Thermomechanical Properties. ACS Macro Lett. 2021, 10, 805. [Google Scholar] [CrossRef]
- Bi, L.; Godwin, B.; Baran, M.J.; Nazir, R.; Wulff, J.E. A Cleavable Crosslinking Strategy for Commodity Polymer Functionalization and Generation of Reprocessable Thermosets. Angew. Chem. Int. Ed. Engl. 2023, 62, e202304708. [Google Scholar] [CrossRef]
- Volpert, V.; Volpert, V. Propagation of frontal polymerization—Crystallization waves. Eur. J. Appl. Math. 1994, 5, 201–215. [Google Scholar] [CrossRef]
- Pojman, J.A. Frontal Polymerization. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 957–980. [Google Scholar] [CrossRef]
- Pojman, J.A. Photoinitiated Frontal Polymerization for Rapid Repair and for Studying Spherically Propagating Thermal Fronts. 2006. 8p. Available online: https://radtech.org/proceedings/2006/papers/110.pdf (accessed on 21 September 2022).
- Bomze, D.; Knaack, P.; Liska, R. Successful Radical Induced Cationic Frontal Polymerization of Epoxy-Based Monomers by C–C Labile Compounds. Polym. Chem. 2015, 6, 8161. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Li, W.; Liu, Y.; Luo, H.; Zeng, L.; Qiu, J.; Wang, S. Direct writing of continuous carbon fibers/epoxy thermoset composites with high-strength and low energy-consumption. Addit. Manuf. 2021, 47, 102348. [Google Scholar] [CrossRef]
- Malik, M.S.; Schlögl, S.; Wolfahrt, M.; Sangermano, M. Review on Uv-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Viner, V.G. Study of Thermal Frontal Polymerization Utilizing Reactive and Non-Reactive Additives. Ph.D. Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 2009. [Google Scholar]
- Chen, T.; Li, Y.; Yang, S.-Y.; Wang, C.-F.; Chen, S. Synthesis of Versatile Poly(PMMA-B-VI) Macromonomer-Based Hydrogels Via Infrared Laser Ignited Frontal Polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1210. [Google Scholar] [CrossRef]
- van de Werken, N.; Tekinalp, H.; Khanbolouki, P.; Ozcan, S.; Williams, A.; Tehrani, M. Additively manufactured carbon fiber-reinforced composites: State of the art and perspective. Addit. Manuf. 2020, 31, 100962. [Google Scholar] [CrossRef]
- Nawafleh, N.; Celik, E. Additive manufacturing of short fiber reinforced thermoset composites with unprecedented mechanical performance. Addit. Manuf. 2020, 33, 101109. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Kunc, V.; Velez-Garcia, G.M.; Duty, C.E.; Love, L.J.; Naskar, A.K.; Blue, C.A.; Ozcan, S. Highly oriented carbon fiber–polymer composites via additive manufacturing. Compos. Sci. Technol. 2014, 105, 144–150. [Google Scholar] [CrossRef]
- Luo, T.; Ma, Y.; Cui, X. Review on Frontal Polymerization Behavior for Thermosetting Resins: Materials, Modeling and Application. Polymers 2024, 16, 185. [Google Scholar] [CrossRef]
- Cai, H.; Ye, J.; Xi, J.; Hong, Y.; Shi, Y.; Wang, Y.; He, W. A novel numerical method to evaluate the thermal conductivity of the unidirectional fiber-reinforced composites. Polym. Compos. 2022, 43, 6216–6233. [Google Scholar] [CrossRef]
- Topkaya, T.; Gao, Y.; Geubelle, P. Frontal Polymerization in Short-Fiber-Reinforced Thermoset Composites. ACS Appl. Polym. Mater. 2022, 4, 6880–6886. [Google Scholar] [CrossRef]
- Uitz, O.; Koirala, P.; Tehrani, M.; Seepersad, C.C. Fast, low-energy additive manufacturing of isotropic parts via reactive extrusion. Addit. Manuf. 2021, 41, 101919. [Google Scholar] [CrossRef]
- Nimbagal, V.; Banapurmath, N.R.; Umarfarooq, M.A.; Revankar, S.; Sajjan, A.M.; Soudagar, M.E.M.; Shahapurkar, K.; Alamir, M.A.; Alarifi, I.M.; Elfasakhany, A. Mechanical and fracture properties of carbon nano fibers/short carbon fiber epoxy composites. Polym. Compos. 2023, 44, 3977–3989. [Google Scholar] [CrossRef]
- Bomze, D.; Knaack, P.; Koch, T.; Jin, H.; Liska, R. Radical induced cationic frontal polymerization as a versatile tool for epoxy curing and composite production. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3751–3759. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. Thermal and Mechanical Interfacial Behaviors of Graphene Oxide-Reinforced Epoxy Composites Cured by Thermal Latent Catalyst. Materials 2019, 12, 1354. [Google Scholar] [CrossRef] [PubMed]
- Arabli, V.; Aghili, A. Graphene oxide/vinyl ester resin nanocomposite: The effect of graphene oxide, curing kinetics, modeling, mechanical properties and thermal stability. RSC Adv. 2016, 6, 22331–22340. [Google Scholar] [CrossRef]
- Salahuddin, N.; Rehab, A.; Yussif El-Deeb, I.; Elmokadem, R. Effect of graphene oxide on photo- and thermal curing of chalcone–based benzoxazine resins. Polym. Bull. 2022, 79, 3175–3191. [Google Scholar] [CrossRef]
- Tüzün, F.N.; Gürü, M.; Akyüz, Y. Comparison of the curing kinetic behavior for two epoxy resin systems containing EPIKOTE 828–EPIKURE 3090 and DURATEK KLM 606A–DURATEK KLM 606B. Polym. Compos. 2007, 28, 762–770. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Y.; Qian, X.; Zhao, Y.; Li, M.; Li, P. Excessive radical induced cationic frontal polymerization lead to a decline of material properties. Authorea 2022. [Google Scholar] [CrossRef]
- Groce, B.R.; Ferguson, M.M.; Pojman, J.A. Thermally initiated cationic frontal polymerization of epoxies and vinyl ethers through a lone onium salt. J. Polym. Sci. 2023, 61, 3149–3158. [Google Scholar] [CrossRef]
- Gary, D.P.; Ngo, D.; Bui, A.; Pojman, J.A. Charge transfer complexes as dual thermal/photo initiators for free-radical frontal polymerization. J. Polym. Sci. 2022, 60, 1624–1630. [Google Scholar] [CrossRef]
- Suslick, B.A.; Hemmer, J.; Groce, B.R.; Stawiasz, K.J.; Geubelle, P.H.; Malucelli, G.; Mariani, A.; Moore, J.S.; Pojman, J.A.; Sottos, N.R. Frontal Polymerizations: From Chemical Perspectives to Macroscopic Properties and Applications. Chem. Rev. 2023, 123, 3237–3298. [Google Scholar] [CrossRef] [PubMed]
- Valette, V.; Lecamp, L.; Astruc, J.; Burel, F.; Kebir, N. Elaboration of epoxy foam by radical induced cationic frontal polymerization (RICFP): A proof of concept. J. Photochem. Photobiol. Chem. 2023, 442, 114811. [Google Scholar] [CrossRef]
- Reda, T.; Pojman, J.; Rongy, L. Critical Role of Layer Thickness in Frontal Polymerization. J. Phys. Chem. B 2022, 126, 3607–3618. [Google Scholar] [CrossRef] [PubMed]
- Zewde, B.; Pitliya, P.; Raghavan, D. The role of surface modified TiO2 nanoparticles on the mechanical and thermal properties of CTBN toughened epoxy nanocomposite. J. Mater. Sci. 2016, 20, 9314–9329. [Google Scholar] [CrossRef]
- Barral, L.; Cano, J.; López, J.; López-Bueno, I.; Nogueira, P.; Abad, M.J.; Ramírez, C. Blends of an epoxy/cycloaliphatic amine resin with poly(ether imide). Polymer 2000, 41, 2657–2666. [Google Scholar] [CrossRef]
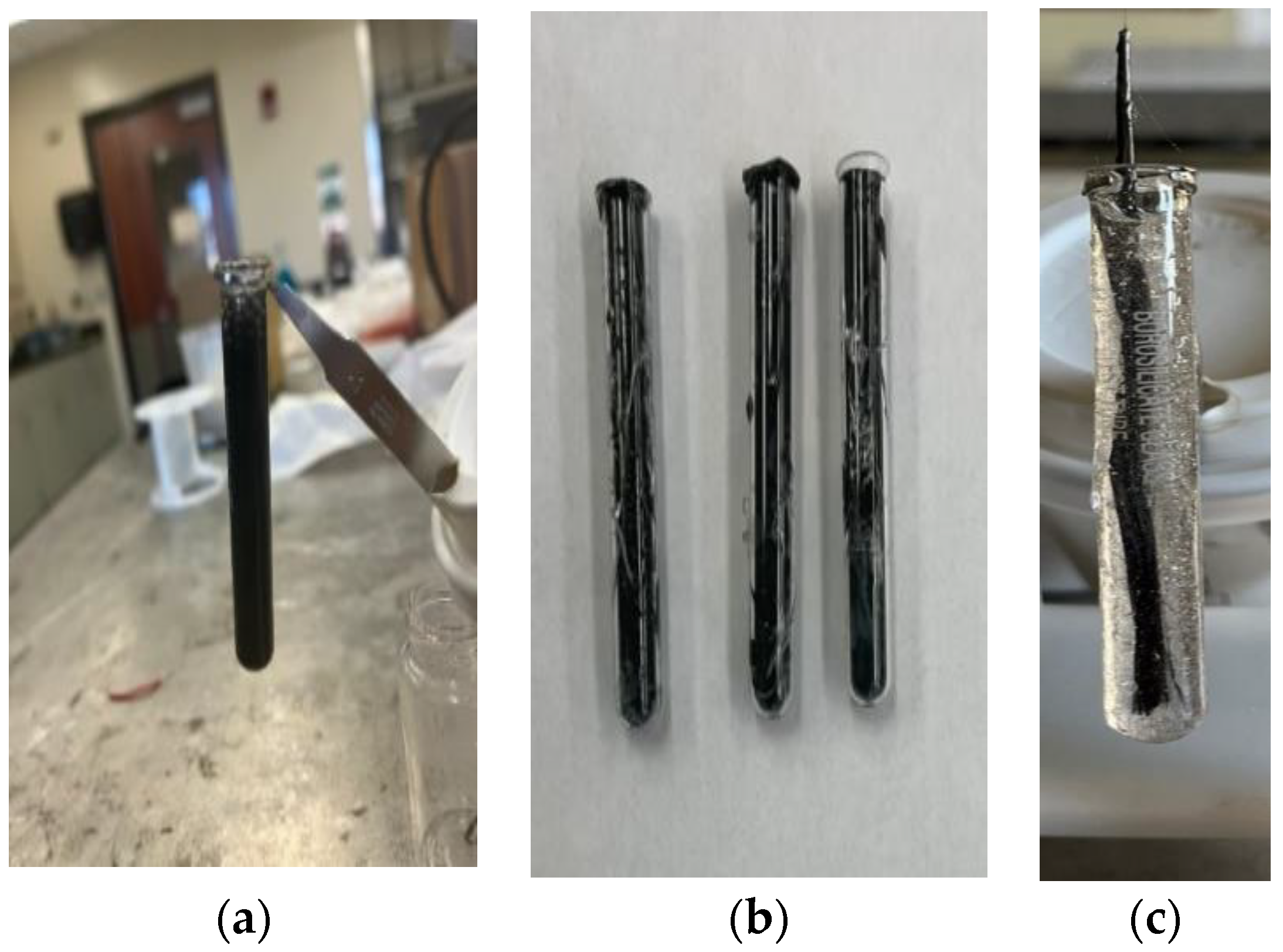







| Initiation Temperature | 150 °C | 200 °C | 240 °C |
|---|---|---|---|
| Failure strain (in/in) | 0.0061 ± 0.000377 | 0.0079 ± 0.000432 | 0.0093 ± 0.000113 |
| Failure stress (PSI) | 1130 ± 30.81 | 1521 ± 88.99 | 1701 ± 73.54 |
| SCF Percentage | 1% | 2% | 3% |
|---|---|---|---|
| Failure strain (in/in) | 0.07509 ± 0.000643 | 0.08117 ± 0.004566 | 0.084 ± 0.007257 |
| Failure stress (PSI) | 2381 ± 15.30 | 2520 ± 122.01 | 2782 ± 116.67 |
| Sample Type | Pure Epoxy | 1% SCF/Ep | 2% SCF/Ep | 3% SCF/Ep |
|---|---|---|---|---|
| The area under the curve (ΔH) for uncured resin | 278 J/g | 230 J/g | 219 J/g | 189 J/g |
| The area under the curve (ΔH) for cured resin | 42 J/g | 39 J/g | 45 J/g | 50 J/g |
| Degree of cure (αc) | 85% | 83% | 79% | 73% |
| Tg | 155 °C | 149 °C | 148 °C | 149 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shams, A.T.; Papon, E.A.; Shinde, P.S.; Bara, J.; Haque, A. Degree of Cure, Microstructures, and Properties of Carbon/Epoxy Composites Processed via Frontal Polymerization. Polymers 2024, 16, 1493. https://doi.org/10.3390/polym16111493
Shams AT, Papon EA, Shinde PS, Bara J, Haque A. Degree of Cure, Microstructures, and Properties of Carbon/Epoxy Composites Processed via Frontal Polymerization. Polymers. 2024; 16(11):1493. https://doi.org/10.3390/polym16111493
Chicago/Turabian StyleShams, Aurpon Tahsin, Easir Arafat Papon, Pravin S. Shinde, Jason Bara, and Anwarul Haque. 2024. "Degree of Cure, Microstructures, and Properties of Carbon/Epoxy Composites Processed via Frontal Polymerization" Polymers 16, no. 11: 1493. https://doi.org/10.3390/polym16111493





