Physicochemical Properties of Cellulose Nanocrystals Extracted from Postconsumer Polyester/Cotton-Blended Fabrics and Their Effects on PVA Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Discolouration
2.3. CNC Preparation
2.4. Yield Calculation
2.5. Composite Film Preparation
2.6. Material Characterisations
2.6.1. Scanning Electron Microscopy
2.6.2. Transmission Electron Microscopy
2.6.3. Hydrodynamic Properties
2.6.4. X-ray Diffraction
2.6.5. Fourier-Transform Infrared Analysis
2.6.6. Thermogravimetric Analysis
2.6.7. Tensile Measurements
3. Results and Discussion
3.1. Pretreatment of the Postconsumer Waste
3.2. Characteristics of the Extracted CNCs
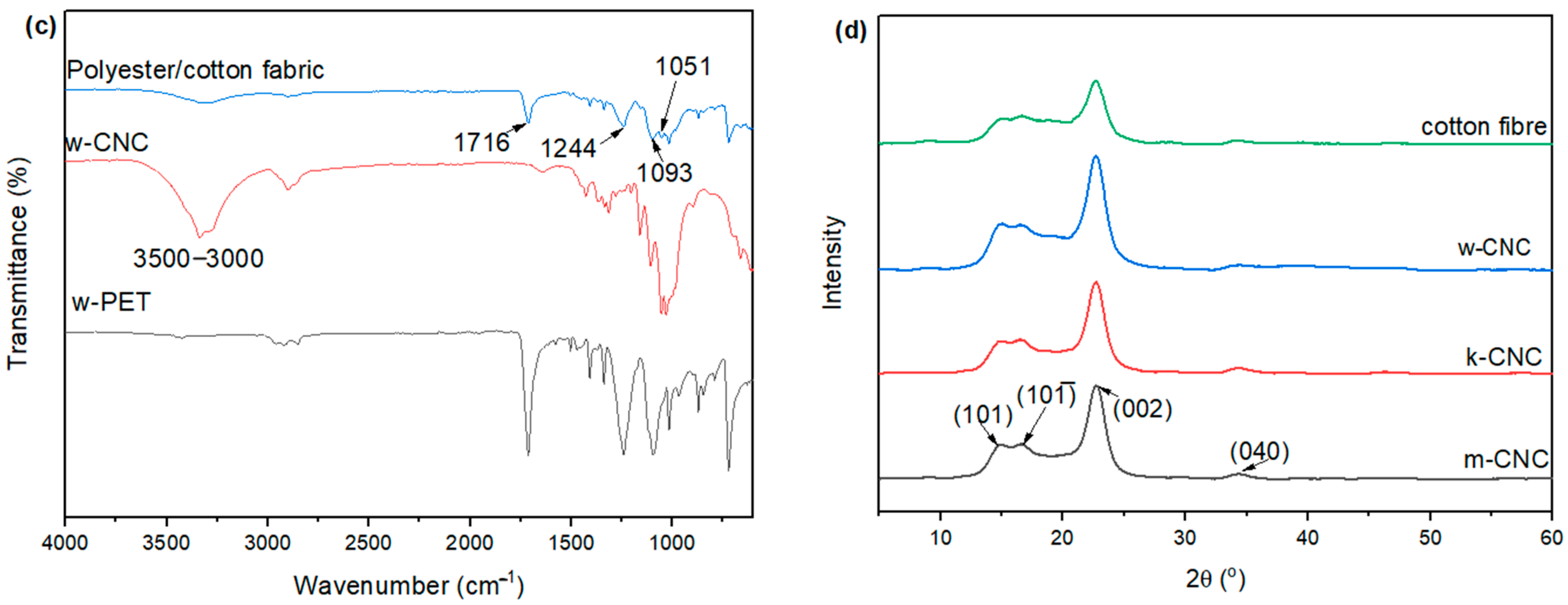
3.3. XRD Patterns of CNC from Bleached Postconsumer Waste
3.4. FTIR of CNC Extracted from the Postconsumer Waste

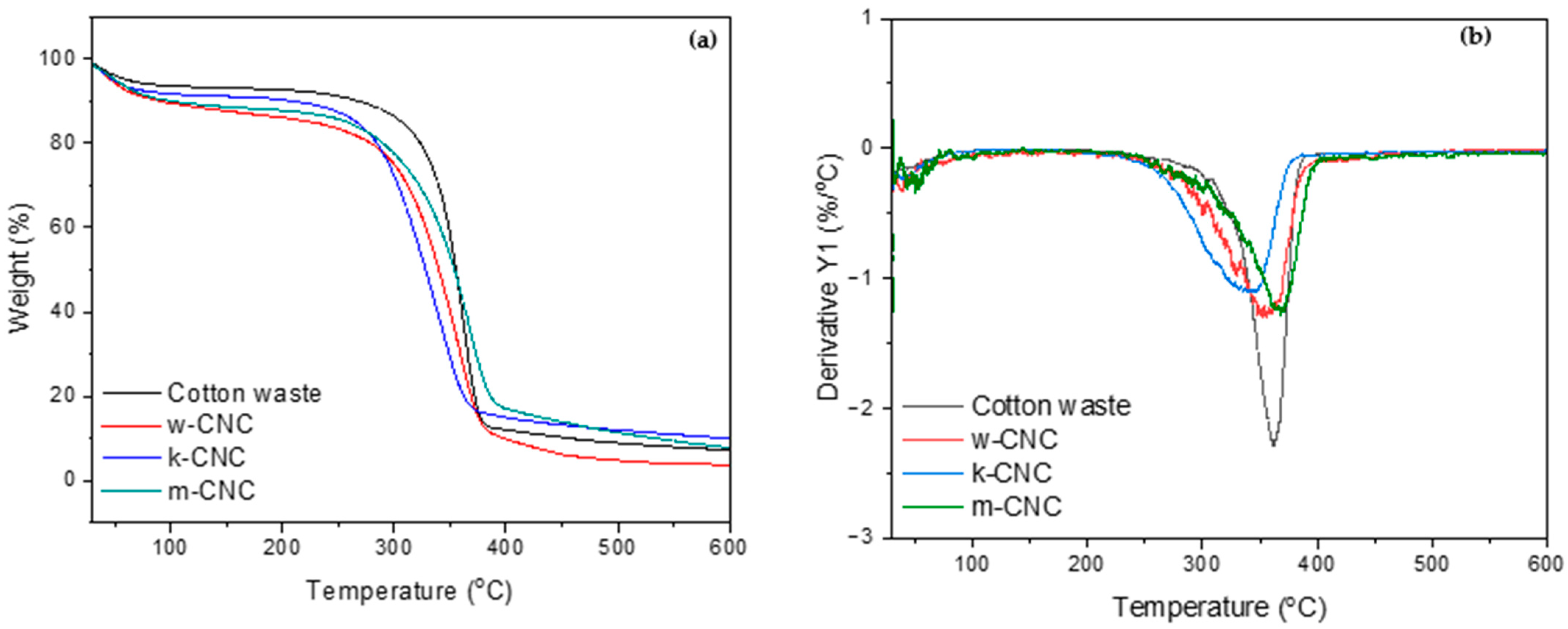
3.5. Thermal Properties of CNCs from Bleached Postconsumer Waste
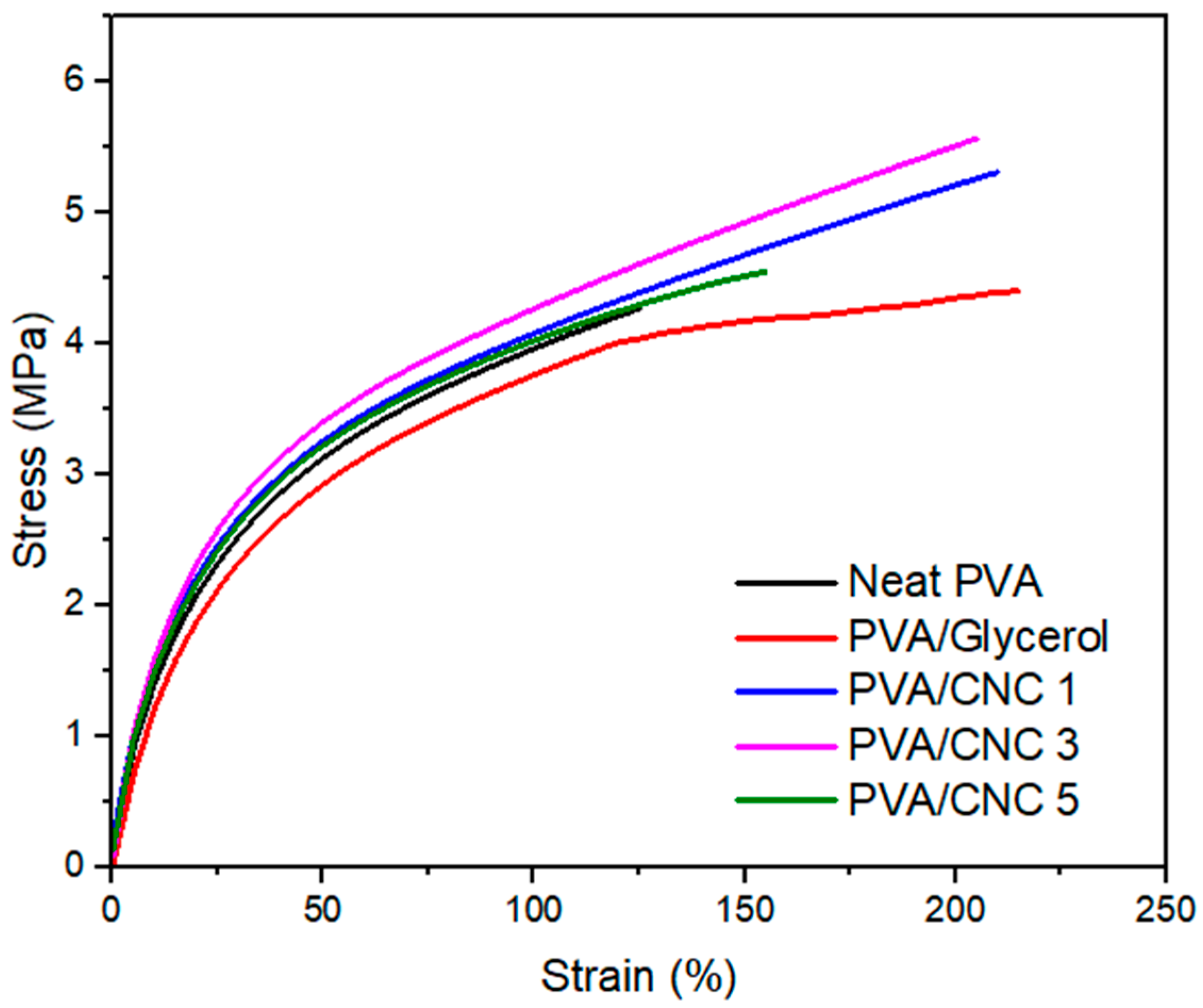
3.6. Mechanical Properties of PVA/CNC Composite Films
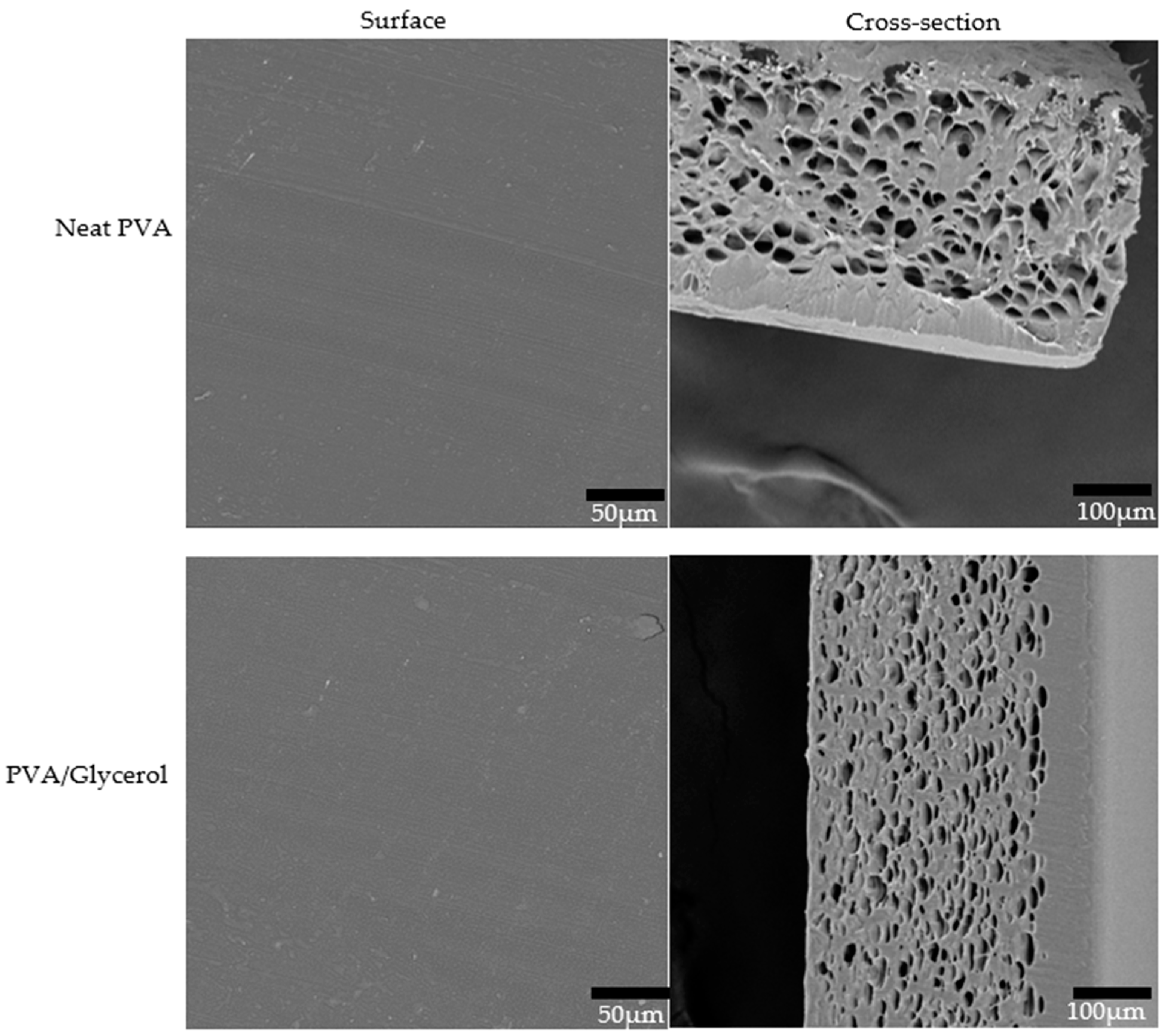
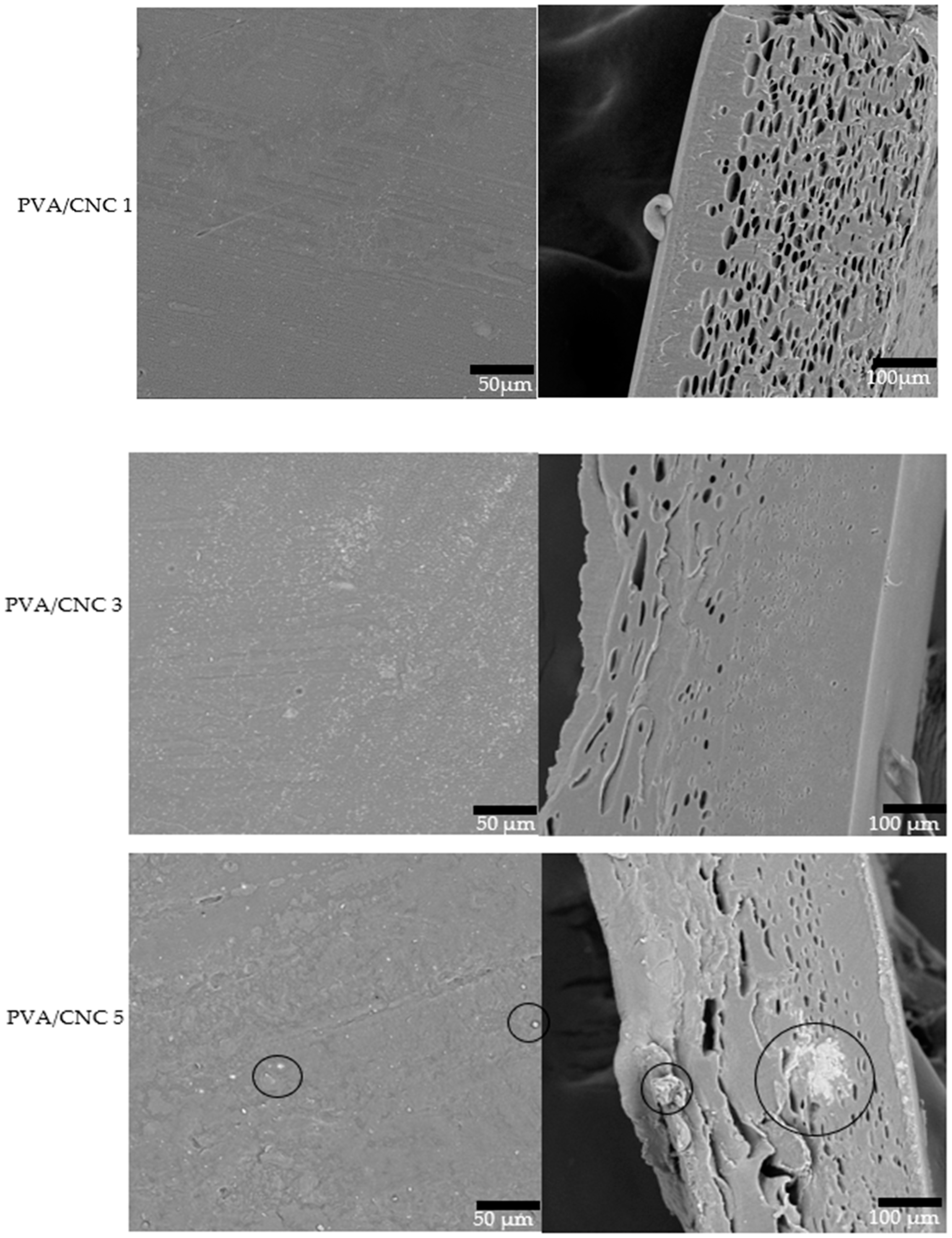
3.7. Morphological Properties of PVA/CNC Composite Films
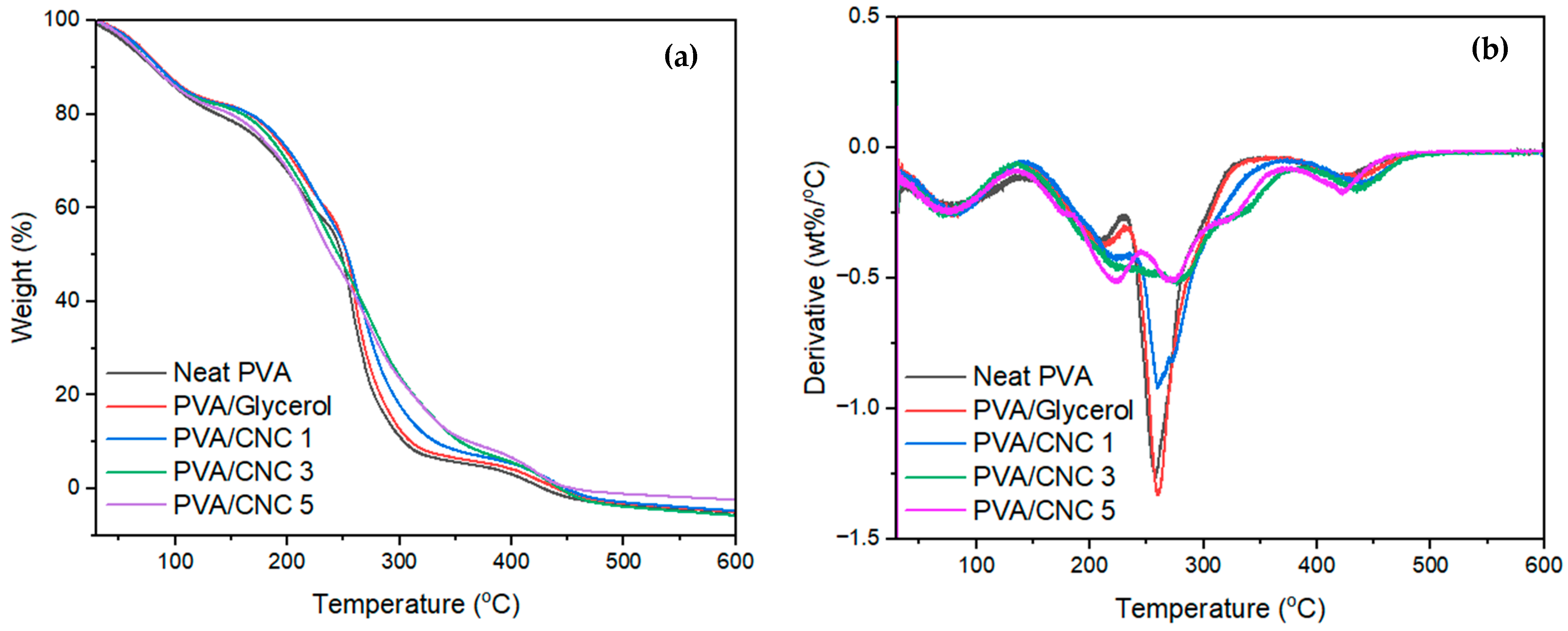
3.8. Thermal Properties of the Fabricated PVC/CNC Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanzetto, A.B.; Beltrami, L.V.R.; Zattera, A.J. Textile waste as precursors in nanocrystalline cellulose synthesis. Cellulose 2021, 28, 6967–6981. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Facts and Figures about Materials, Waste and Recycling. 2021. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/textiles-material-specific-data (accessed on 3 March 2023).
- Kahoush, M.; Kadi, N. Towards sustainable textile sector: Fractionation and separation of cotton/polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar] [CrossRef]
- Zou, Y.; Reddy, N.; Yang, Y. Reusing polyester/cotton blend fabrics for composites. Compos. Part B Eng. 2011, 42, 763–770. [Google Scholar] [CrossRef]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukošiūtė, S.-I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Yang, K.; Wang, M.; Wang, X.; Shan, J.; Zhang, J.; Tian, G.; Yang, D.; Ma, J. Polyester/Cotton-Blended Textile Waste Fiber Separation and Regeneration via a Green Chemistry Approach. ACS Sustain. Chem. Eng. 2024, 12, 4530–4538. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Ling, C.; Hou, W.; Yan, Z. Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method. Waste Manag. 2018, 82, 139–146. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z.; Zhang, M.; Zhang, B.; Dai, J. Separation and characterization of waste cotton/polyester blend fabric with hydrothermal method. Fibers Polym. 2018, 19, 742–750. [Google Scholar] [CrossRef]
- Matsumura, M.; Inagaki, J.; Yamada, R.; Tashiro, N.; Ito, K.; Sasaki, M. Material Separation from Polyester/Cotton Blended Fabrics Using Hydrothermal Treatment. ACS Omega 2024, 9, 13125–13133. [Google Scholar] [CrossRef] [PubMed]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Ling, C.; Shi, S.; Hou, W.; Yan, Z. Separation of waste polyester/cotton blended fabrics by phosphotungstic acid and preparation of terephthalic acid. Polym. Degrad. Stab. 2019, 161, 157–165. [Google Scholar] [CrossRef]
- Quartinello, F.; Vajnhandl, S.; Volmajer Valh, J.; Farmer, T.J.; Vončina, B.; Lobnik, A.; Herrero Acero, E.; Pellis, A.; Guebitz, G.M. Synergistic chemo-enzymatic hydrolysis of poly (ethylene terephthalate) from textile waste. Microb. Biotechnol. 2017, 10, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- Haule, L.V.; Carr, C.; Rigout, M. Preparation and physical properties of regenerated cellulose fibres from cotton waste garments. J. Clean. Prod. 2016, 112, 4445–4451. [Google Scholar] [CrossRef]
- Ruiz-Caldas, M.-X.; Carlsson, J.; Sadiktsis, I.; Jaworski, A.; Nilsson, U.; Mathew, A.P. Cellulose Nanocrystals from Postconsumer Cotton and Blended Fabrics: A Study on Their Properties, Chemical Composition, and Process Efficiency. ACS Sustain. Chem. Eng. 2022, 10, 3787–3798. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, Z.; Farooq, A.; Zhang, M.; Li, M.; Liu, L. Efficient recovery of the dyed cotton–polyester fabric: Cellulose nanocrystal extraction and its application in composite films. Cellulose 2021, 28, 3235–3248. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Z.; Yang, A.; Li, J.; Wang, L.; Chen, H.; Zheng, X.; Liu, Y. Nanocellulose Extracted from Waste Polyester/Cotton Fabric by Chemical-Mechanical Separation Technology. In Journal of Physics: Conference Series, 2020 Sustainability Innovation & Fashion Technology International Conference, Shanghai, China, 15–17 October 2020; IOP Publishing: Bristol, UK, 2021; Volume 1790, p. 012074. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Chen, H.; Hu, J.; Cui, L. Preparation of different morphologies cellulose nanocrystals from waste cotton fibers and its effect on PLLA/PDLA composites films. Compos. Part B Eng. 2021, 217, 108934. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef]
- Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose nanocrystals derived from textile waste through acid hydrolysis and oxidation as reinforcing agent of soy protein film. Polymers 2020, 12, 958. [Google Scholar] [CrossRef]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and kinetics study of spherical cellulose nanocrystal extracted from cotton cloth waste by acid hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Culsum, N.T.U.; Melinda, C.; Leman, I.; Wibowo, A.; Budhi, Y.W. Isolation and characterization of cellulose nanocrystals (CNCs) from industrial denim waste using ammonium persulfate. Mater. Today Commun. 2021, 26, 101817. [Google Scholar] [CrossRef]
- Maciel, M.M.Á.D.; de Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Obtainment and characterization of nanocellulose from an unwoven industrial textile cotton waste: Effect of acid hydrolysis conditions. Int. J. Biol. Macromol. 2019, 126, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Pandi, N.; Sonawane, S.H.; Kishore, K.A. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Zhong, T.; Dhandapani, R.; Liang, D.; Wang, J.; Wolcott, M.P.; Van Fossen, D.; Liu, H. Nanocellulose from recycled indigo-dyed denim fabric and its application in composite films. Carbohydr. Polym. 2020, 240, 116283. [Google Scholar] [CrossRef]
- Rowe, A.A.; Tajvidi, M.; Gardner, D.J. Thermal stability of cellulose nanomaterials and their composites with polyvinyl alcohol (PVA). J. Therm. Anal. Calorim. 2016, 126, 1371–1386. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Guseinov, S.S.; Barannikov, V.P.; Zakharov, A.G. Thermal stability of polyvinyl alcohol/nanocrystalline cellulose composites. Carbohydr. Polym. 2015, 130, 440–447. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Crosslinked poly (vinyl alcohol) composite films with cellulose nanocrystals: Mechanical and thermal properties. J. Appl. Polym. Sci. 2018, 135, 45710. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Mokhtar, W.N.A.W.; Razab, M.K.A.A.; Noor, A.a.M.; Zaudin, N.A.C.; Rasat, M.S.M.; Amin, M.A.M.; Ismardi, A. Effect of cellulose nanocrystals (CNC) on thermal properties of polyvinyl alcohol (PVA)/CNC biocomposites. In AIP Conference Proceedings, International Conference on Bioengineering and Technology (IConBET2021), Kelantan, Malaysia, 24–25 May 2021; AIP Publishing: Melville, NY, USA, 2022; Volume 2454. [Google Scholar] [CrossRef]
- ASTM D629-08; Standard Test Methods for Quantitative Analysis of Textiles. ASTM International: West Conshohocken, PA, USA, 2015.
- Li, R.; Yang, J.; Zhang, G.; Zhu, P. Decolorization of dark-colored waste cotton fabric using redox decoloring agents. RSC Adv. 2022, 12, 17689–17700. [Google Scholar] [CrossRef]
- Hermans, P.; Hermans, J.; Vermaas, D.; Weidinger, A. Deformation mechanism of cellulose gels. IV. General relationship between orientation of the crystalline and that of the amorphous portion. J. Polym. Sci. 1948, 3, 1–9. [Google Scholar] [CrossRef]
- Gusev, G. Hermans-Weidinger X-ray diffraction technique for determining polymer crystallinity and the use of the Ruland ratio. Polym. Sci. USSR 1978, 20, 1295–1297. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Berichte 2011, 89, 118–131. [Google Scholar]
- Patterson, A. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y.; Pettersson, P.O.; Torget, R.W. Heterogeneous aspects of acid hydrolysis of α-cellulose. In Biotechnology for Fuels and Chemicals: The Twenty-Fourth Symposium; Springer: Totowa, NJ, USA, 2003; pp. 505–514. [Google Scholar] [CrossRef]
- Hu, J. Structure and mechanics of woven fabrics. In Structure and Mechanics of Textile Fibre Assemblies; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Eberhardt, B.; Meissner, M.; Strasser, W. Knit fabrics. In Cloth Modeling and Animation; AK Peters/CRC Press: Boca Raton, FL, USA, 2000; pp. 123–144. [Google Scholar]
- Mohamed, S.H.; Hossain, M.S.; Mohamad Kassim, M.H.; Ahmad, M.I.; Omar, F.M.; Balakrishnan, V.; Zulkifli, M.; Yahaya, A.N.A. Recycling waste cotton cloths for the isolation of cellulose nanocrystals: A sustainable approach. Polymers 2021, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, Z.J.; Chen, M.; Couillard, M.; Leng, T.; Liu, L.; Zou, S.; Baxa, U.; Clogston, J.D.; Hamad, W.Y.; Johnston, L.J. Characterization challenges for a cellulose nanocrystal reference material: Dispersion and particle size distributions. J. Nanopart. Res. 2018, 20, 98. [Google Scholar] [CrossRef]
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liao, Y.P.; Lu, J.; Li, L.; Liu, X.; Kim, J.; Ahmed, A. The crystallinity and aspect ratio of cellulose nanomaterials determine their pro-inflammatory and immune adjuvant effects in vitro and in vivo. Small 2019, 15, 1901642. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, H.; Abdalkarim, S.Y.H.; Li, Y.; Chen, X.; Yang, X.; Zhou, Y.; Zhang, L. A comprehensive investigation on cellulose nanocrystals with different crystal structures from cotton via an efficient route. Carbohydr. Polym. 2022, 276, 118766. [Google Scholar] [CrossRef]
- Csiszár, E.; Nagy, S. A comparative study on cellulose nanocrystals extracted from bleached cotton and flax and used for casting films with glycerol and sorbitol plasticisers. Carbohydr. Polym. 2017, 174, 740–749. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Preparation and physicochemical characterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, C.; French, A.D.; Xia, G.; Han, G.; Wang, Q.; Wu, Q. Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 2012, 19, 1173–1187. [Google Scholar] [CrossRef]
- Haouache, S.; Jimenez-Saelices, C.; Cousin, F.; Falourd, X.; Pontoire, B.; Cahier, K.; Jérome, F.; Capron, I. Cellulose nanocrystals from native and mercerized cotton. Cellulose 2022, 29, 1567–1581. [Google Scholar] [CrossRef]
- Srichola, P.; Witthayolankowit, K.; Sukyai, P.; Sampoompuang, C.; Lobyam, K.; Kampakun, P.; Toomtong, R. Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites. Polymers 2023, 15, 3324. [Google Scholar] [CrossRef] [PubMed]
- Hamad, W.Y.; Hu, T.Q. Structure–process–yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Dien, B.; Thompson, S.; Condon, B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose 2019, 26, 5959–5979. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Putaux, J.-L.; Mariano, M.; Ogawa, Y.; Otaguro, H.; Pasquini, D.; Dufresne, A. Comprehensive morphological and structural investigation of cellulose I and II nanocrystals prepared by sulphuric acid hydrolysis. Rsc Adv. 2016, 6, 76017–76027. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- D’Acierno, F.; Hamad, W.Y.; Michal, C.A.; MacLachlan, M.J. Thermal degradation of cellulose filaments and nanocrystals. Biomacromolecules 2020, 21, 3374–3386. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.-M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Yeganeh, N.; Rezaei, M. The investigation of thermal decomposition pathway and products of poly (vinyl alcohol) by TG-FTIR. J. Appl. Polym. Sci. 2015, 132, 42117. [Google Scholar] [CrossRef]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]


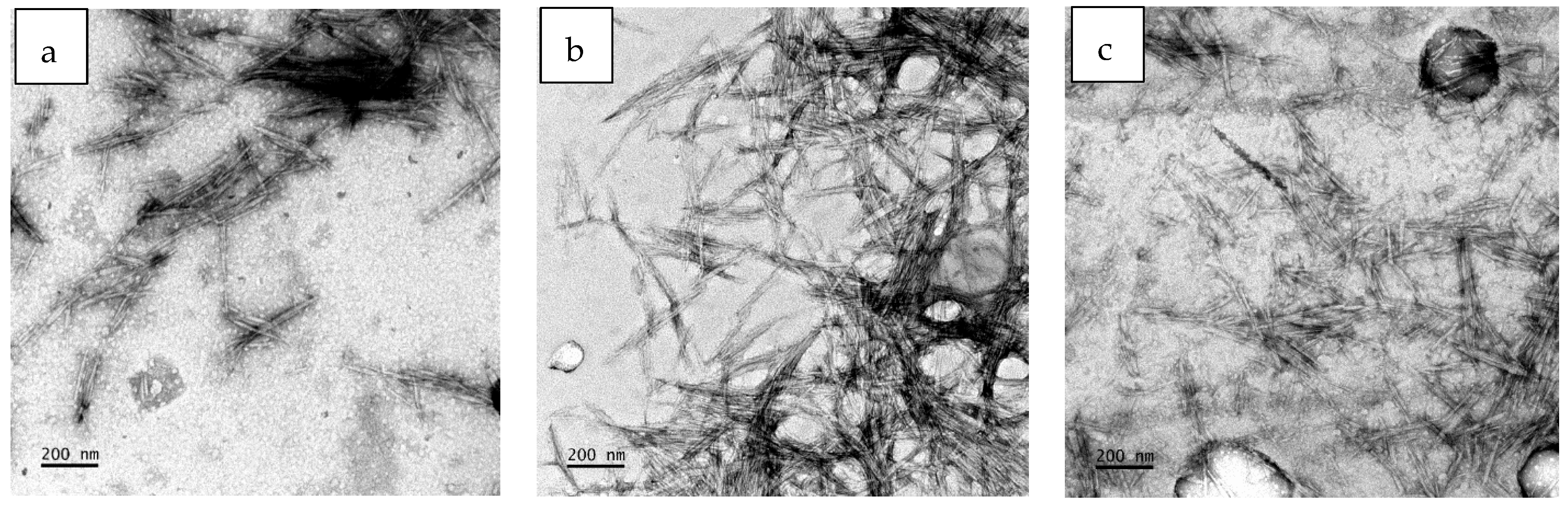
| Sample | Hydrodynamic Size (nm) | CNC Size (nm) | Zeta Potential (mV) | Polydispersity Index (PDI) | Yield (%) | PET Recovered (wt%) | ||
|---|---|---|---|---|---|---|---|---|
| Length | Width | g CNC per g Sample | g CNC per g Cotton | |||||
| WCNC | 158.4 ± 0.85 | 135–358 | 6.30–15.96 | −33.2 ± 0.55 | 0.277 ± 0.015 | 44.8 | 69.5 | 99.8 |
| KCNC | 163.1 ± 1.50 | 78–194 | 5.40–9.66 | −31.5 ± 0.65 | 0.217 ± 0.019 | 53.5 | 66.3 | 98.7 |
| MCNC | 153.8 ± 2.01 | 165–295 | 12.23–16.25 | −31.8 ± 1.37 | 0.244 ± 0.006 | 22.4 | 38.1 | 88.3 |
| Sample | Crystallinity Index | d-Spacing @ Peak 002 (Å) | Average Crystallite Size (nm) |
|---|---|---|---|
| Cotton fibre | 70.3 | 3.89 | 5.55 |
| w-CNC | 86.1 | 3.92 | 3.52 |
| k-CNC | 89.9 | 3.91 | 3.81 |
| m-CNC | 74.5 | 3.90 | 3.85 |
| Sample | Onset Degradation Temperature (°C) | Offset Degradation Temperature (°C) | Maximum Degradation Temperature (°C) | Char Residue @ 600 °C (%) |
|---|---|---|---|---|
| Cotton waste | 249 | 387 | 361 | 7.2 |
| w-CNC | 246 | 400 | 353 | 3.7 |
| k-CNC | 233 | 381 | 344 | 9.5 |
| m-CNC | 237 | 403 | 366 | 8.4 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) |
|---|---|---|---|
| Neat PVA | 4.23 ± 0.12 | 121.6 ± 5.23 | 20.86 ± 0.34 |
| PVA/Glycerol | 4.03 ± 0.86 | 215.3 ± 2.56 | 18.96 ± 0.17 |
| PVA/CNC 1 | 5.36 ± 0.63 | 214.6 ± 3.51 | 25.25 ± 0.23 |
| PVA/CNC 3 | 5.58 ± 0.71 | 205.9 ± 6.93 | 29.69 ± 0.05 |
| PVA/CNC 5 | 4.57 ± 0.27 | 157.9 ± 11.7 | 27.04 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloyi, R.B.; Sithole, B.B.; Chunilall, V. Physicochemical Properties of Cellulose Nanocrystals Extracted from Postconsumer Polyester/Cotton-Blended Fabrics and Their Effects on PVA Composite Films. Polymers 2024, 16, 1495. https://doi.org/10.3390/polym16111495
Baloyi RB, Sithole BB, Chunilall V. Physicochemical Properties of Cellulose Nanocrystals Extracted from Postconsumer Polyester/Cotton-Blended Fabrics and Their Effects on PVA Composite Films. Polymers. 2024; 16(11):1495. https://doi.org/10.3390/polym16111495
Chicago/Turabian StyleBaloyi, Rivalani Baloyi, Bruce Bishop Sithole, and Viren Chunilall. 2024. "Physicochemical Properties of Cellulose Nanocrystals Extracted from Postconsumer Polyester/Cotton-Blended Fabrics and Their Effects on PVA Composite Films" Polymers 16, no. 11: 1495. https://doi.org/10.3390/polym16111495






