Synthesis, Characterization, and Evaluation of Silver Nanoparticle-Loaded Carboxymethyl Chitosan with Sulfobetaine Methacrylate Hydrogel Nanocomposites for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNPs
2.3. Synthesis of AgNPs-Loaded CMCS-SB Hydrogel
2.4. Characterizations
2.5. Swelling and Water Absorption
2.6. Release of Silver from AgNPs/CMCS-SB Nanocomposites
2.7. Antibacterial Study
2.8. In-Vitro Cytotoxicity Study
3. Results and Discussion
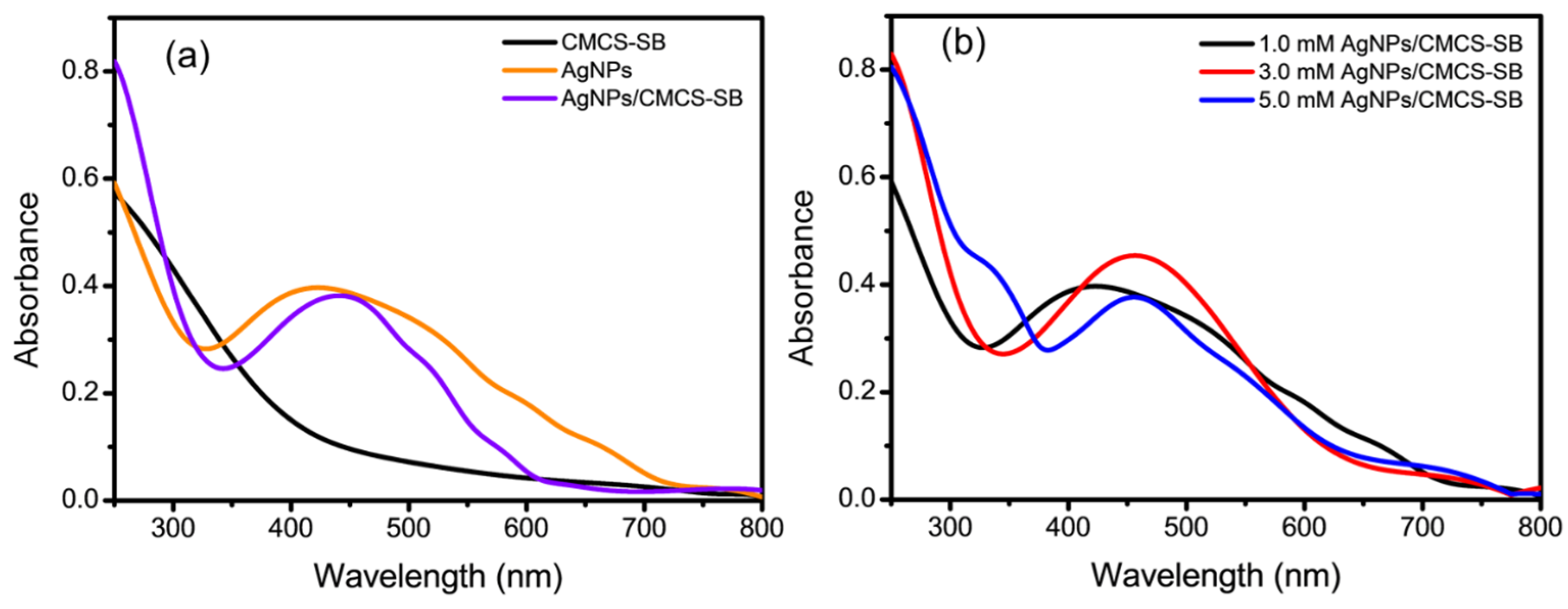
3.1. Optical Properties of AgNPs/CMCS-SB Nanocomposite
3.2. Swelling and Water Absorption of AgNPs/CMCS-SB Nanocomposite
3.3. Release of Silver from Hydrogels
3.4. XDR Pattern of AgNPs/CMCS-SB Nanocomposite
3.5. FTIR Analysis of AgNPs/CMCS-SB Nanocomposite
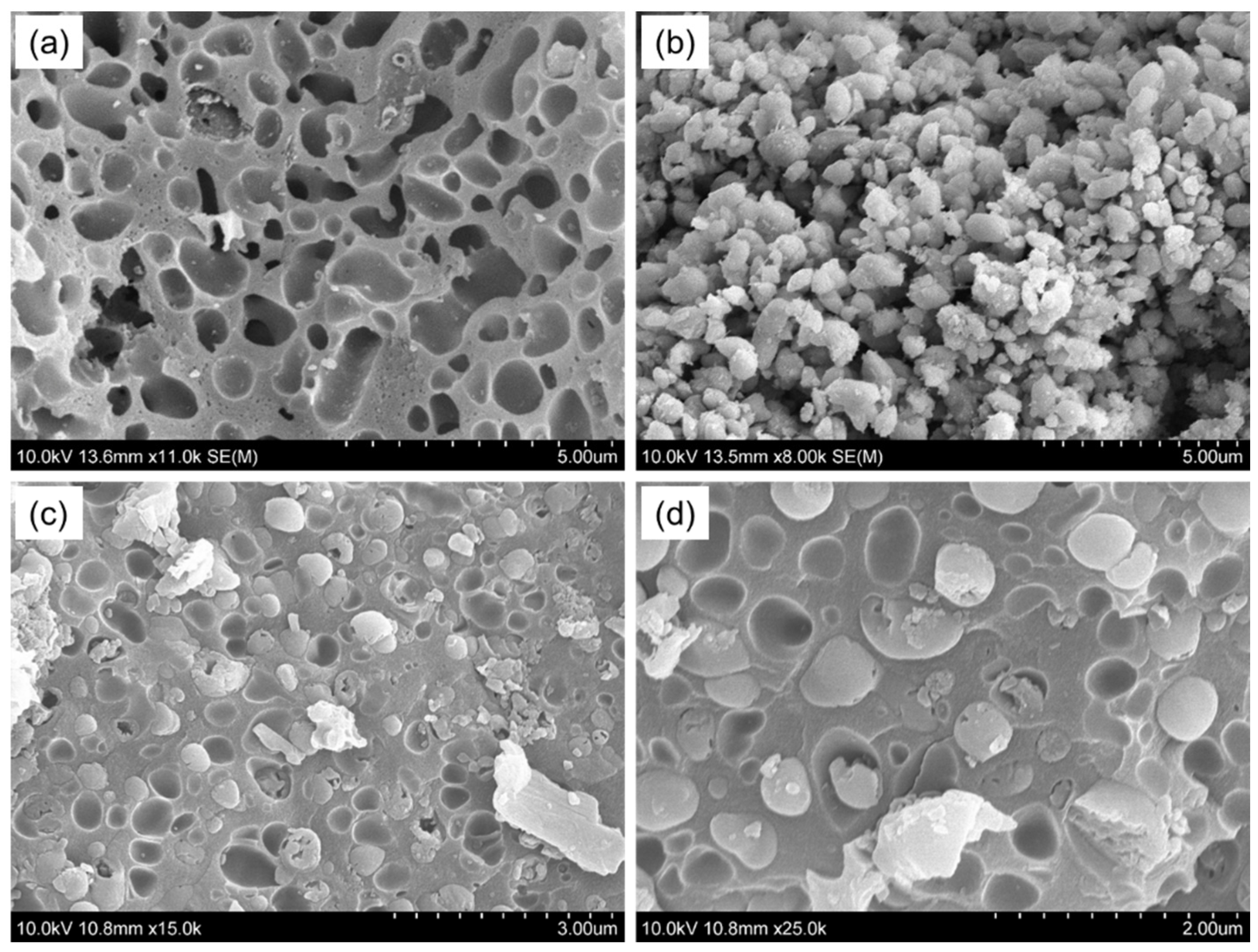
3.6. SEM Analysis of AgNPs/CMCS-SB Nanocomposite
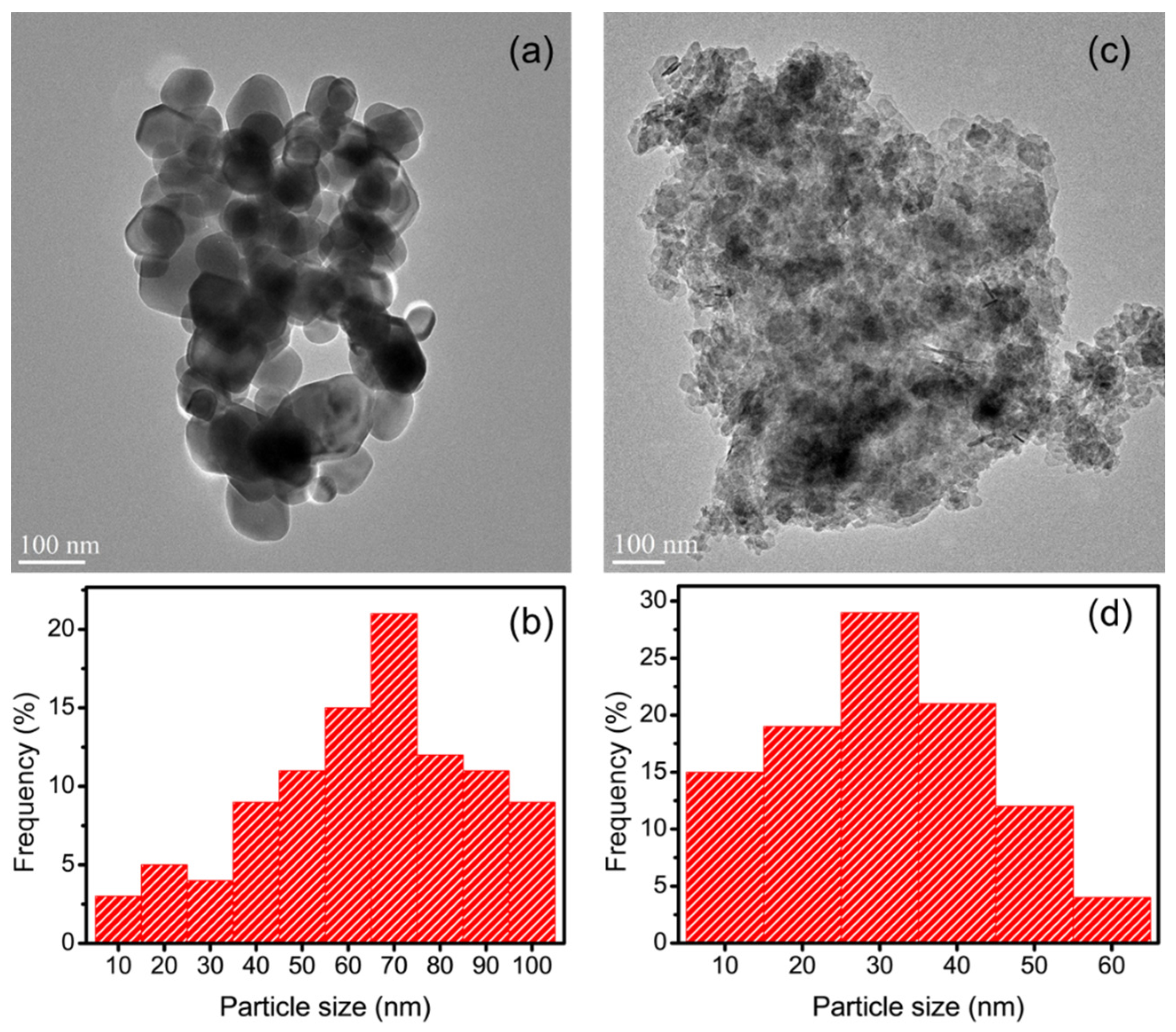
3.7. TEM Analysis of AgNPs/CMCS-SB Nanocomposite
3.8. Antibacterial Properties of AgNPs/CMCS-SB Nanocomposite
3.9. In-Vitro Cytotoxicity and Imaging of AgNPs/CMCS-SB Nanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, J.; Wu, H.; Wang, H.; Lu, X.; Shahbazi, M.A.; Wang, S. A Triple-Network Carboxymethyl Chitosan-Based Hydrogel for Hemostasis of Incompressible Bleeding on Wet Wound Surfaces. Carbohydr. Polym. 2023, 303, 120434. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of Carboxymethyl Chitosan in Cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent Advances in Carboxymethyl Chitosan-Based Materials for Biomedical Applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef] [PubMed]
- Singha, I.; Basu, A. Chitosan Based Injectable Hydrogels for Smart Drug Delivery Applications. Sens. Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Sun, H.; Chang, M.Y.Z.; Cheng, W.I.; Wang, Q.; Commisso, A.; Capeling, M.; Wu, Y.; Cheng, C. Biodegradable Zwitterionic Sulfobetaine Polymer and Its Conjugate with Paclitaxel for Sustained Drug Delivery. Acta Biomater. 2017, 64, 290–300. [Google Scholar] [CrossRef]
- Sun, H.; Yan, L.; Zhang, R.; Lovell, J.F.; Wu, Y.; Cheng, C. A Sulfobetaine Zwitterionic Polymer-Drug Conjugate for Multivalent Paclitaxel and Gemcitabine Co-Delivery. Biomater. Sci. 2021, 9, 5000–5010. [Google Scholar] [CrossRef]
- SchAnemann, E.; Koc, J.; Karthaüser, J.F.; Ozcan, O.; Schanzenbach, D.; Schardt, L.; Rosenhahn, A.; Laschewsky, A. Sulfobetaine Methacrylate Polymers of Unconventional Polyzwitterion Architecture and Their Antifouling Properties. Biomacromolecules 2021, 22, 1494–1508. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.; Le Thi, P.; Oh, D.H.; Park, K.D. Sulfobetaine Methacrylate Hydrogel-Coated Anti-Fouling Surfaces for Implantable Biomedical Devices. Biomater. Res. 2018, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, S.; Chen, L.; Chen, L.; Zhao, L.; Feng, J. Highly Antifouling Double Network Hydrogel Based on Poly(Sulfobetaine Methacrylate) and Sodium Alginate with Great Toughness. J. Mater. Sci. Technol. 2021, 85, 235–244. [Google Scholar] [CrossRef]
- Fathi, M.; Zangabad, P.S.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-Responsive Chitosan-Based Nanocarriers for Cancer Therapy. BioImpacts 2017, 7, 269–277. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable Wound Dressing Based on Carboxymethyl Chitosan Triple-Network Hydrogel for Effective Wound Antibacterial and Hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels 2023, 9, 708. [Google Scholar] [CrossRef]
- Xiang, J.; Bai, Y.; Huang, Y.; Lang, S.; Li, J.; Ji, Y.; Peng, B.; Liu, G. A Zwitterionic Silver Nanoparticle-Incorporating Injectable Hydrogel with a Durable and Efficient Antibacterial Effect for Accelerated Wound Healing. J. Mater. Chem. B 2022, 10, 7979–7994. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Feng, J.; Zhang, D.; Li, C.; Shi, Q.S.; Xie, X. In Situ Synthesis of Amply Antimicrobial Silver Nanoparticle (AgNP) by Polyzwitterionic Copolymers Bearing Hydroxyl Groups. React. Funct. Polym. 2020, 153, 104609. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Trivinõ, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of Silver Nanoparticle Synthesis by Chemical Reduction and Evaluation of Its Antimicrobial and Toxic Activity. Biomater. Res. 2019, 23, 1–15. [Google Scholar] [CrossRef]
- Li, Q.; Ai, R.; Fan, J.; Fu, X.; Zhu, L.; Zhou, Q.; Chen, L.; Ma, W.; Li, Y.; Liu, L. AgNPs-Loaded Chitosan/Sodium Alginate Hydrogel Film by in-Situ Green Reduction with Tannins for Enhancing Antibacterial Activity. Mater. Today Commun. 2024, 38, 107927. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl Alcohol/Carboxymethyl Chitosan Hydrogel Loaded with Silver Nanoparticles Exhibited Antibacterial and Self-Healing Properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant Mediated Fabrication of Silver Nanoparticles, Process Optimization, and Impact on Tomato Plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Ferraria, A.M.; do Rego, A.M.B. Unraveling the Reaction Mechanism of Silver Ions Reduction by Chitosan from so Far Neglected Spectroscopic Features. Carbohydr. Polym. 2017, 174, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Alawam, K.A.; Binshaya, A.K.S.; Alduraywish, S.A. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules 2023, 28, 3241. [Google Scholar] [CrossRef]
- Huang, S.; Yu, Z.; Zhang, Y.; Qi, C.; Zhang, S. In Situ Green Synthesis of Antimicrobial Carboxymethyl Chitosan-Nanosilver Hybrids with Controlled Silver Release. Int. J. Nanomed. 2017, 12, 3181–3191. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Constantin, M.; Lupei, M.; Bucatariu, S.M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease. Polymers 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindra, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; MohanaRaju, K. Fabrication of Porous Chitosan Films Impregnated with Silver Nanoparticles: A Facile Approach for Superior Antibacterial Application. Colloids Surf. B Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent Polymers: From Long-Established, Microplastics Generating Systems, to Sustainable, Biodegradable and Future Proof Alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- Amiri, N.; Ghaffari, S.; Hassanpour, I.; Chae, T.; Jalili, R.; Kilani, R.T.; Ko, F.; Ghahary, A.; Lange, D. Antibacterial Thermosensitive Silver–Hydrogel Nanocomposite Improves Wound Healing. Gels 2023, 9, 542. [Google Scholar] [CrossRef]
- Salem, S.S.; Hashem, A.H.; Sallam, A.A.M.; Doghish, A.S.; Al-Askar, A.A.; Arishi, A.A.; Shehabeldine, A.M. Synthesis of Silver Nanocomposite Based on Carboxymethyl Cellulose: Antibacterial, Antifungal and Anticancer Activities. Polymers 2022, 14, 3352. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Ghori, M.Z.; Henkel, B.; Strunskus, T.; Schürmann, U.; Deng, M.; Kienle, L.; Faupel, F. Tuning Silver Ion Release Properties in Reactively Sputtered Ag/TiOx Nanocomposites. Appl. Phys. A Mater. Sci. Process. 2017, 123, 470. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Hadizadeh, A.; Dehghan, S.; Hadizadeh, S. Enhanced Antibacterial Activity of Porous Chitosan-Based Hydrogels Crosslinked with Gelatin and Metal Ions. Sci. Rep. 2024, 14, 7505. [Google Scholar] [CrossRef]
- Suresh, R.; Deepa, M.; Sudha, P.N.; Gomathi, T.; Pavithra, S.; Moganavally, P. Synthesis, Characterization, Biological and Catalytic Activity of Carboxymethyl Chitosan Schiff Base Metal Complexes. Indian J. Geo-Mar. Sci. 2022, 51, 423–431. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef] [PubMed]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veera Babu, N.; Veerabhadram, G. A Novel Green One-Step Synthesis of Silver Nanoparticles Using Chitosan: Catalytic Activity and Antimicrobial Studies. Appl. Nanosci. 2014, 4, 113–119. [Google Scholar] [CrossRef]
- Anand Ganesh, V.; Kundukad, B.; Cheng, D.; Radhakrishnan, S.; Ramakrishna, S.; Van Vliet, K.J. Engineering Silver-Zwitterionic Composite Nanofiber Membrane for Bacterial Fouling Resistance. J. Appl. Polym. Sci. 2019, 136, 47580. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic Potential of Green Synthesized Silver Nanoparticles Loaded PVA Hydrogel Patches for Wound Healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Nadtoka, O.; Kutsevol, N.; Naumenko, A.; Virych, P. Photochemical Synthesis and Characterization of Hydrogel–Silver Nanoparticle Composites. Res. Chem. Intermed. 2019, 45, 4069–4080. [Google Scholar] [CrossRef]
- Nadtoka, O.; Virych, P.; Bezugla, T.; Doroschuk, V.; Lelyushok, S.; Pavlenko, V.; Yeshchenko, O.; Kutsevol, N. Antibacterial Hybrid Hydrogels Loaded with Nano Silver. Appl. Nanosci. 2022, 12, 629–636. [Google Scholar] [CrossRef]
- Hajj, F.E.; Hasan, A.; Nakhleh, J.; Osta, M.; Darwish, G.; Karam, P.; Nassereddine, M. Nanosilver loaded GelMA hydrogel for antimicrobial coating of biomedical implants. In Proceedings of the 2015 International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 16–18 September 2015; pp. 189–192. [Google Scholar]
- Murali Mohan, Y.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Mohana Raju, K. Controlling of Silver Nanoparticles Structure by Hydrogel Networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Amalraj, J.; Santos, L.S. Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights. Polymers 2023, 15, 3370. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.S.; Huang, K.C.; Lee, H.H. Fabrication and Sintering Effect on the Morphologies and Conductivity of Nano-Ag Particle Films by the Spin Coating Method. Nanotechnology 2005, 16, 779–784. [Google Scholar] [CrossRef]
- Mekkawy, A.I.; El-Mokhtar, M.A.; Nafady, N.A.; Yousef, N.; Hamad, M.; El-Shanawany, S.M.; Ibrahim, E.H.; Elsabahy, M. In Vitro and in Vivo Evaluation of Biologically Synthesized Silver Nanoparticles for Topical Applications: Effect of Surface Coating and Loading into Hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef]
- Henríquez, C.M.G.; del Carmen Pizarro Guerra, G.; Vallejos, M.A.S.; de la Fuente, S.D.R.; Flores, M.T.U.; Jimenez, L.M.R. In Situ Silver Nanoparticle Formation Embedded into a Photopolymerized Hydrogel with Biocide Properties. J. Nanostructure Chem. 2014, 4, 119–132. [Google Scholar] [CrossRef]
- Cha, H.R.; Ramesh Babu, V.; Krishna Rao, K.S.V.; Kim, Y.H.; Mei, S.; Joo, W.H.; Lee, Y.I. Fabrication of Amino Acid Based Silver Nanocomposite Hydrogels from Pva-Poly(Acrylamide-Co-Acryloyl Phenylalanine) and Their Antimicrobial Studies. Bull. Korean Chem. Soc. 2012, 33, 3191–3195. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef] [PubMed]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Sayed, M.M.E.; Mohsen, D.; Fagir, M.H.; El Dein, D.K. Green Synthesis of Silver Nanoparticles Loaded Hydrogel for Wound Healing; Systematic Review. Gels 2023, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, J.; Wang, Z.; Bai, Z.; Qu, P.; Song, Z.; Wang, W. Fabrication of Silver Nanoparticles/Gelatin Hydrogel System for Bone Regeneration and Fracture Treatment. Drug Deliv. 2021, 28, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Fagir, M.H.; El Dein, D.K. Employing of Curcumin–Silver Nanoparticle-Incorporated Sodium Alginate-Co-Acacia Gum Film Hydrogels for Wound Dressing. Gels 2023, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Kalil, H.; Liu, G.; Sossey-Alaoui, K.; Bayachou, M. Alginate-Based Hydrogel Platform Embedding Silver Nanoparticles and Cisplatin: Characterization of the Synergistic Effect on a Breast Cancer Cell Line. Front. Mol. Biosci. 2023, 10, 1242838. [Google Scholar] [CrossRef]
- Dong, Q.; Zu, D.; Kong, L.; Chen, S.; Yao, J.; Lin, J.; Lu, L.; Wu, B.; Fang, B. Construction of Antibacterial Nano-Silver Embedded Bioactive Hydrogel to Repair Infectious Skin Defects. Biomater. Res. 2022, 26, 36. [Google Scholar] [CrossRef]





| Sample | Swelling Rate % | Water Absorption % | Water Solubility % |
|---|---|---|---|
| CMCS-SB | 138.19 ± 14.83 | 9.38 ± 0.72 | 7.16 ± 0.48 |
| 1.0 mM AgNPs/CMCS-SB | 148.37 ± 15.63 | 9.48 ± 0.66 | 8.99 ± 0.41 |
| 3.0 mM AgNPs/CMCS-SB | 172.26 ± 18.14 | 11.04 ± 0.54 | 10.06 ± 0.37 |
| 5.0 mM AgNPs/CMCS-SB | 159.17 ± 16.59 | 10.57 ± 0.33 | 9.67 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandoss, S.; Velu, K.S.; Manoharadas, S.; Ahmad, N.; Palanisamy, S.; You, S.; Akhtar, M.S.; Lee, Y.R. Synthesis, Characterization, and Evaluation of Silver Nanoparticle-Loaded Carboxymethyl Chitosan with Sulfobetaine Methacrylate Hydrogel Nanocomposites for Biomedical Applications. Polymers 2024, 16, 1513. https://doi.org/10.3390/polym16111513
Mohandoss S, Velu KS, Manoharadas S, Ahmad N, Palanisamy S, You S, Akhtar MS, Lee YR. Synthesis, Characterization, and Evaluation of Silver Nanoparticle-Loaded Carboxymethyl Chitosan with Sulfobetaine Methacrylate Hydrogel Nanocomposites for Biomedical Applications. Polymers. 2024; 16(11):1513. https://doi.org/10.3390/polym16111513
Chicago/Turabian StyleMohandoss, Sonaimuthu, Kuppu Sakthi Velu, Salim Manoharadas, Naushad Ahmad, Subramanian Palanisamy, SangGuan You, Muhammad Saeed Akhtar, and Yong Rok Lee. 2024. "Synthesis, Characterization, and Evaluation of Silver Nanoparticle-Loaded Carboxymethyl Chitosan with Sulfobetaine Methacrylate Hydrogel Nanocomposites for Biomedical Applications" Polymers 16, no. 11: 1513. https://doi.org/10.3390/polym16111513
APA StyleMohandoss, S., Velu, K. S., Manoharadas, S., Ahmad, N., Palanisamy, S., You, S., Akhtar, M. S., & Lee, Y. R. (2024). Synthesis, Characterization, and Evaluation of Silver Nanoparticle-Loaded Carboxymethyl Chitosan with Sulfobetaine Methacrylate Hydrogel Nanocomposites for Biomedical Applications. Polymers, 16(11), 1513. https://doi.org/10.3390/polym16111513







