Enhanced Thermal and Mechanical Properties of Cardanol Epoxy/Clay-Based Nanocomposite through Girard’s Reagent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
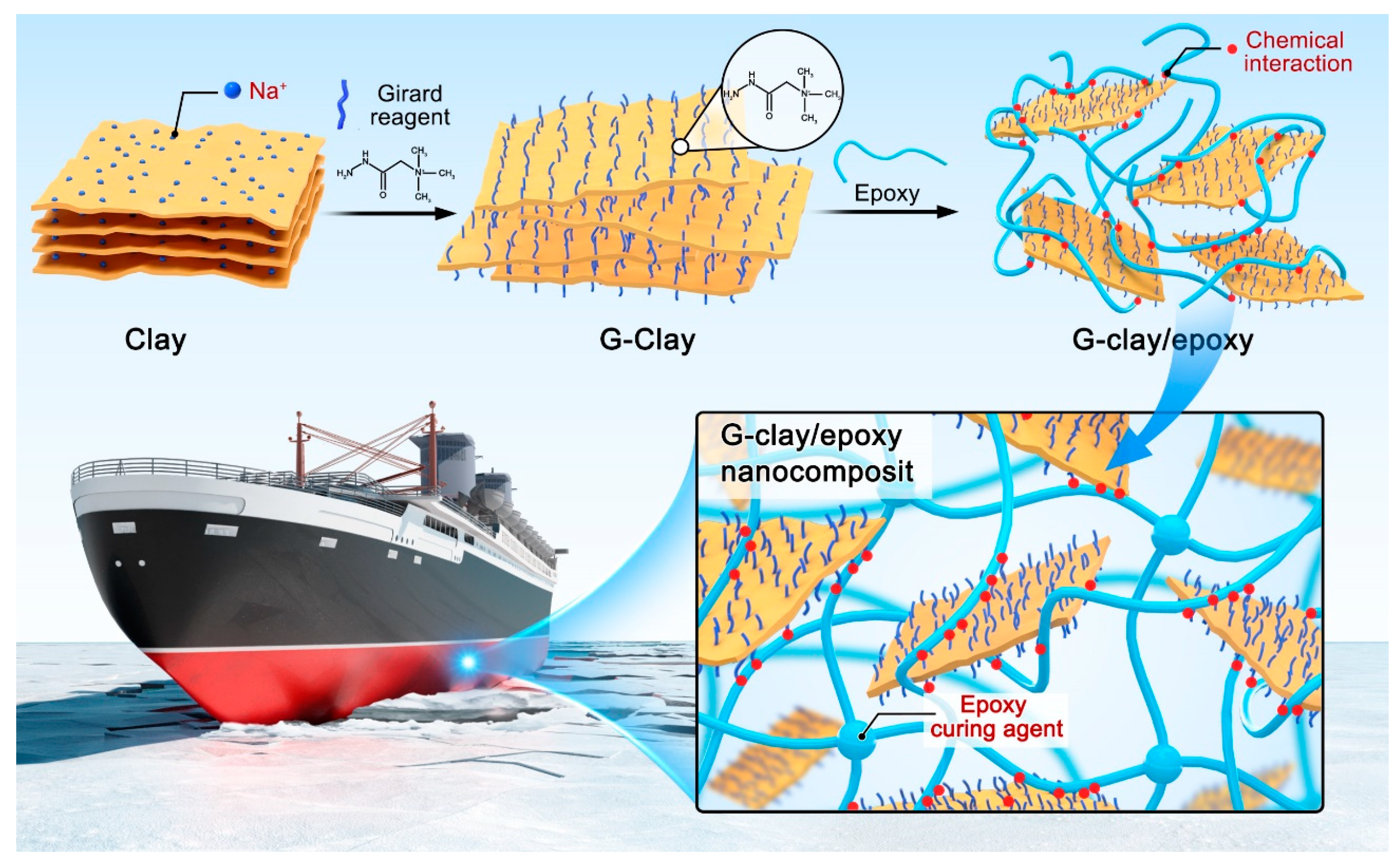
2.2. Preparation of G-clay
2.3. Preparation of G-clay Nanocomposites
2.4. Characterization
3. Results
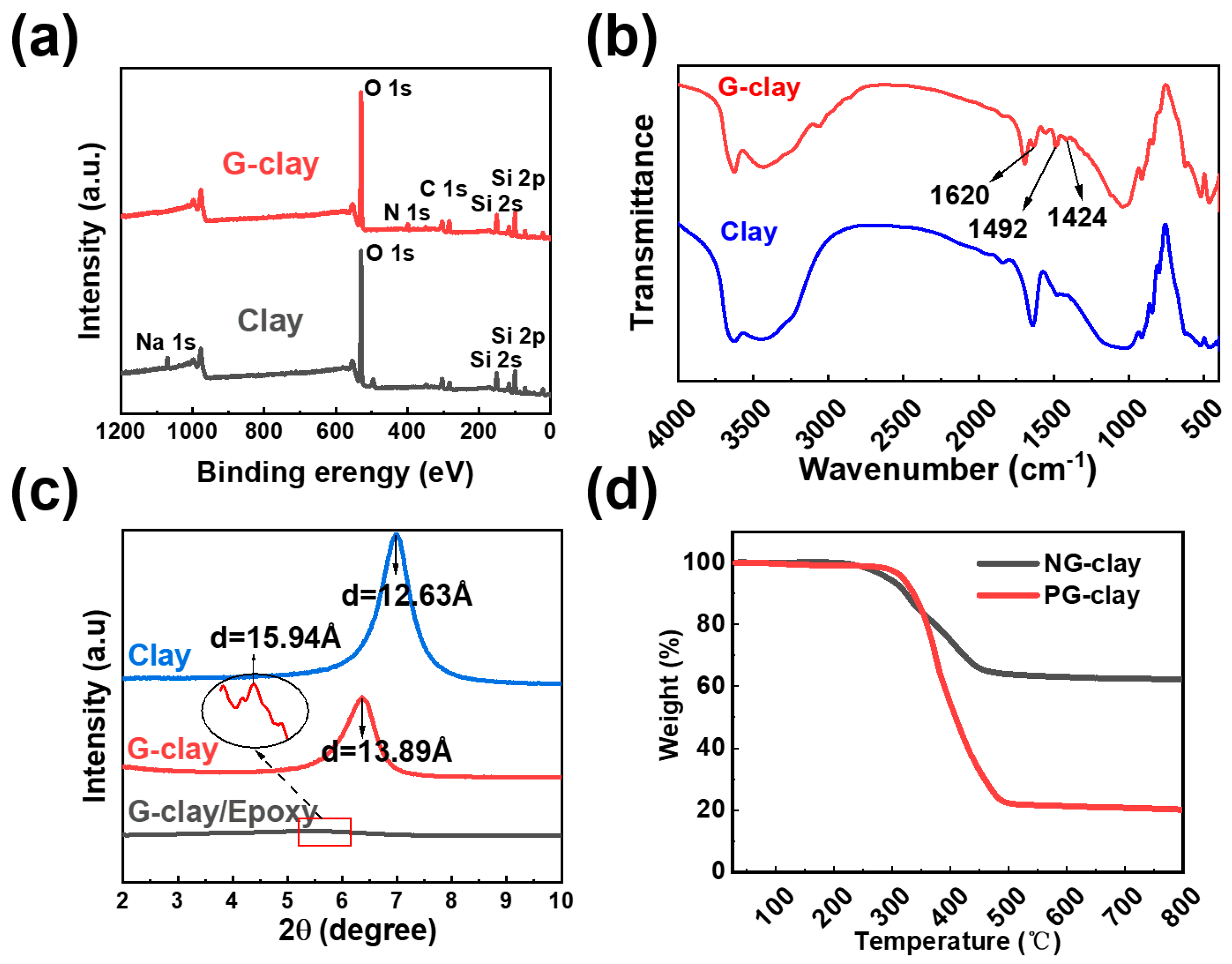
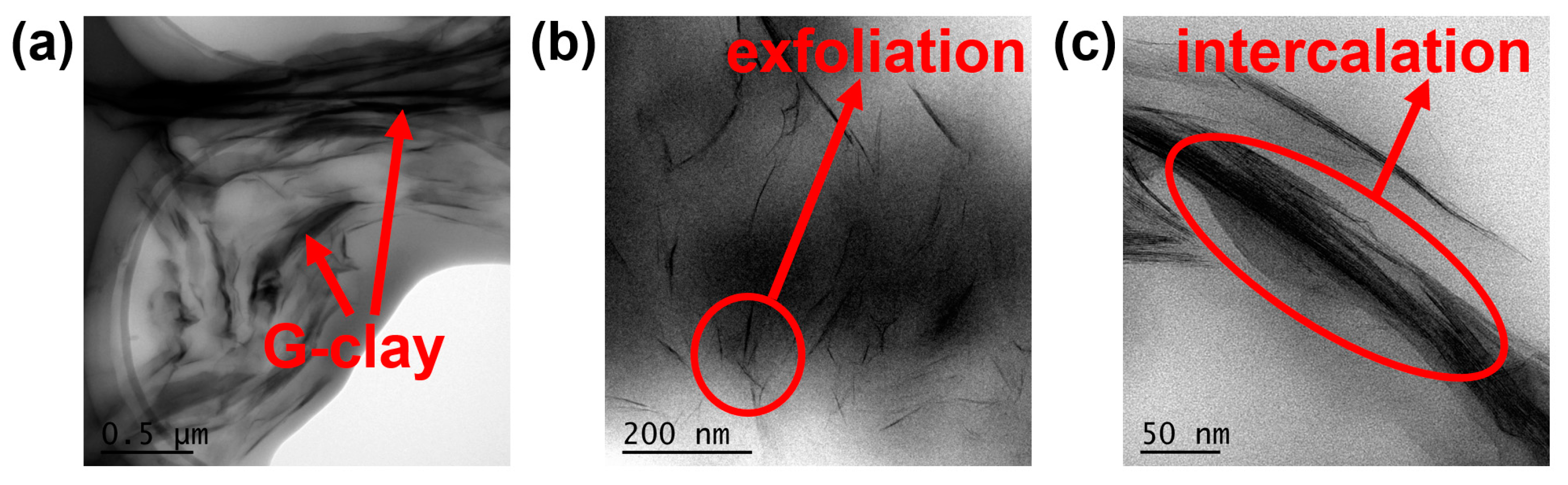
3.1. Preparation and Characterization of G-clay/Epoxy Nanocomposites
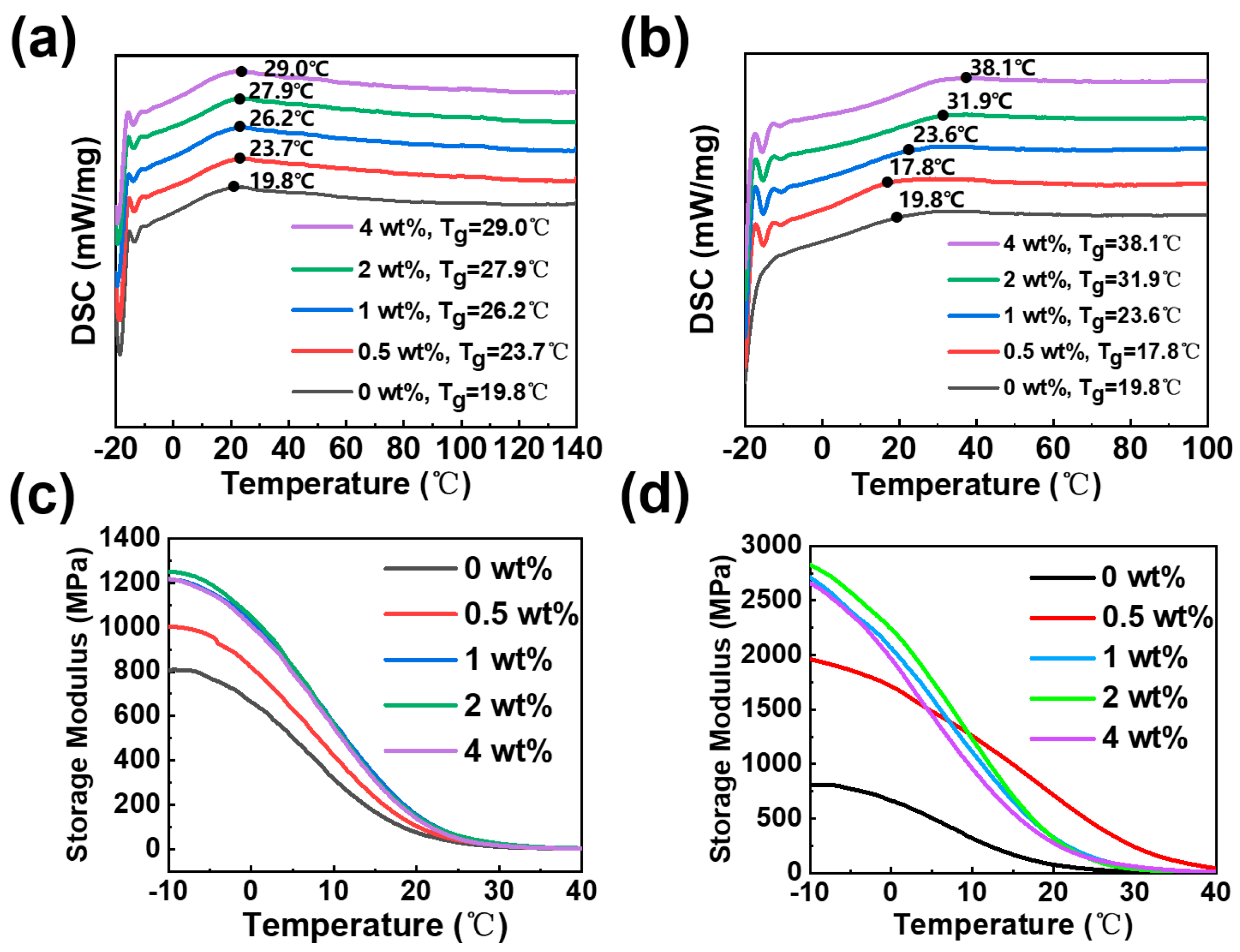
3.2. Effect of Organic Modifiers on Thermo-Mechanical Properties of Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gour, R.S.; Kodgire, V.V.; Badiger, M.V. Toughening of Epoxy Novolac Resin Using Cardanol Based Flexibilizers. J. Appl. Polym. Sci. 2016, 133, 43318. [Google Scholar] [CrossRef]
- Łukaszczyk, J.; Janicki, B.; Kaczmarek, M. Synthesis and Properties of Isosorbide Based Epoxy Resin. Eur. Polym. J. 2011, 47, 1601–1606. [Google Scholar] [CrossRef]
- Chen, J.; Chu, N.; Zhao, M.; Jin, F.; Park, S. Synthesis and Application of Thermal Latent Initiators of Epoxy Resins: A Review. J. Appl. Polym. Sci. 2020, 137, 49592. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An Anisotropically High Thermal Conductive Boron Nitride/Epoxy Composite Based on Nacre-Mimetic 3D Network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Q.; Li, J.; Lin, K.; Chen, D.; Zhou, C.; Wu, T.; Luo, X.; Liu, Y. A Novel and Green Method to Synthesize a Epoxidized Biomass Eucommia Gum as the Nanofiller in the Epoxy Composite Coating with Excellent Anticorrosive Performance. Chem. Eng. J. 2020, 379, 122323. [Google Scholar] [CrossRef]
- Roy, R.; Komarneni, S.; Roy, D.M. Multi-Phasic Ceramic Composites Made by Sol-Gel Technique. MRS Online Proc. Libr. 1984, 32, 347. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Selected Biotrends in Development of Epoxy Resins and Their Composites. Polym. Bull. 2014, 71, 3035–3049. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Chisholm, B.J.; Webster, D.C. Novel Bio-based Epoxy Resins from Eugenol as an Alternative to BPA Epoxy and High Throughput Screening of the Cured Coatings. Polymer 2021, 233, 124191. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking Water Contaminants from Epoxy Resin-Coated Pipes: A Field Study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef]
- Qian, Z.; Xiao, Y.; Zhang, X.; Li, Q.; Wang, L.; Fu, F.; Diao, H.; Liu, X. Bio-based Epoxy Resins Derived from Diphenolic Acid via Amidation Showing Enhanced Performance and Unexpected Autocatalytic Effect on Curing. Chem. Eng. J. 2022, 435, 135022. [Google Scholar] [CrossRef]
- Greco, A.; Brunetti, D.; Renna, G.; Mele, G.; Maffezzoli, A. Plasticizer for Poly(Vinyl Chloride) from Cardanol as a Renewable Resource Material. Polym. Degrad. Stabil. 2010, 95, 2169–2174. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.; Wang, L.; Xing, W.; Song, L.; Hu, Y. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Liu, G.; Jin, C.; Huo, S.; Kong, Z.; Chu, F. Preparation and Properties of Novel Bio-based Epoxy Resin Thermosets from Lignin Oligomers and Cardanol. Int. J. Biol. Macromol. 2021, 193, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Cha, S.H.; Kim, D.H. Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives. Polymers 2022, 14, 5205. [Google Scholar] [CrossRef] [PubMed]
- Darroman, E.; Durand, N.; Boutevin, B.; Caillol, S. Improved Cardanol Derived Epoxy Coatings. Prog. Org. Coat. 2016, 91, 9–16. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, L.; Liu, C.; Lan, X.; Zhao, H. Engineering the Interface in Graphene Oxide/Epoxy Composites Using Bio-based Epoxy-Graphene Oxide Nanomaterial to Achieve Superior Anticorrosion Performance. J. Colloid Interface Sci. 2021, 587, 755–766. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Gangireddy, C.S.R.; Wang, J.; Pan, Y.; Xing, W.; Song, L.; Hu, Y. Cardanol Derived Benzoxazine in Combination with Boron-Doped Graphene toward Simultaneously Improved Toughening and Flame Retardant Epoxy Composites. Compos. Part A 2019, 116, 13–23. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Guo, W.; Song, L.; Hu, Y. Cardanol as a Versatile Platform for Fabrication of Bio-based Flame-Retardant Epoxy Thermosets as DGEBA Substitutes. Chem. Eng. J. 2021, 421, 129738. [Google Scholar] [CrossRef]
- Jaillet, F.; Nouailhas, H.; Auvergne, R.; Ratsimihety, A.; Boutevin, B.; Caillol, S. Synthesis and Characterization of Novel Vinylester Prepolymers from Cardanol. Eur. J. Lipid Sci. Technol. 2014, 116, 928–939. [Google Scholar] [CrossRef]
- Jaillet, F.; Darroman, E.; Ratsimihety, A.; Auvergne, R.; Boutevin, B.; Caillol, S. New Biobased Epoxy Materials from Cardanol. Eur. J. Lipid Sci. Technol. 2014, 116, 63–73. [Google Scholar] [CrossRef]
- Shenogina, N.B.; Tsige, M.; Patnaik, S.S.; Mukhopadhyay, S.M. Molecular Modeling Approach to Prediction of Thermo-Mechanical Behavior of Thermoset Polymer Networks. Macromolecules 2012, 45, 5307–5315. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing Thermomechanical Properties of Crosslinked Epoxy by Molecular Dynamic Simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Fourcade, D.; Ritter, B.S.; Walter, P.; Schönfeld, R.; Mülhaupt, R. Renewable Resource-Based Epoxy Resins Derived from Multifunctional Poly(4-Hydroxybenzoates). Green Chem. 2013, 15, 910–918. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Kefayati, A.R.; Chitsazzadeh, M. Fabrication and Mechanical Properties of Clay/Epoxy Nanocomposite and Its Polymer Concrete. Mater. Des. 2012, 40, 443–452. [Google Scholar] [CrossRef]
- Lam, C.K.; Lau, K.T. Optimization Effect of Micro Hardness by Nanoclay Clusters in Nanoclay/Epoxy Composites. J. Thermoplast. Compos. 2009, 22, 213–225. [Google Scholar] [CrossRef]
- Subramaniyan, A.K.; Sun, C.T. Interlaminar Fracture Behavior of Nanoclay Reinforced Glass Fiber Composites. J. Compos. Mater. 2008, 42, 2111–2122. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Water Barrier Properties of Nanoclay Filled Sisal Fibre Reinforced Epoxy Composites. Compos. Part A 2011, 42, 385–393. [Google Scholar] [CrossRef]
- Hamidi, Y.K.; Aktas, L.; Altan, M.C. Effect of Nanoclay Content on Void Morphology in Resin Transfer Molded Composites. J. Thermoplast. Compos. 2008, 21, 141–163. [Google Scholar] [CrossRef]
- Sharifi, M.; Ebrahimi, M.; Jafarifard, S. Preparation and Characterization of a High Performance Powder Coating Based on Epoxy/Clay Nanocomposite. Prog. Org. Coat. 2017, 106, 69–76. [Google Scholar] [CrossRef]
- Monsif, M.; Zerouale, A.; Kandri, N.I.; Bertani, R.; Bartolozzi, A.; Bresolin, B.M.; Zorzi, F.; Tateo, F.; Zappalorto, M.; Quaresimin, M.; et al. Multifunctional Epoxy/Nanocomposites Based on Natural Moroccan Clays with High Antimicrobial Activity: Morphological, Thermal and Mechanical Properties. J. Nanomater. 2019, 2019, 2810901. [Google Scholar] [CrossRef]
- Vo, V.S.; Nguyen, V.H.; Mahouche-Chergui, S.; Carbonnier, B.; Naili, S. Estimation of Effective Elastic Properties of Polymer/Clay Nanocomposites: A Parametric Study. Compos. Part B 2018, 152, 139–150. [Google Scholar] [CrossRef]
- Caminade, A.M.; Beraa, A.; Laurent, R.; Delavaux-Nicot, B.; Hajjaji, M. Dendrimers and Hyper-Branched Polymers Interacting with Clays: Fruitful Associations for Functional Materials. J. Mater. Chem. A 2019, 7, 19634–19650. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Chen, H.; Wu, J. Synthesis of Disordered and Highly Exfoliated Epoxy/Clay Nanocomposites Using Organoclay with Catalytic Function via Acetone−Clay Slurry Method. Chem. Mater. 2004, 16, 4864–4866. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy Clay Nanocomposites—Processing, Properties and Applications: A Review. Compos. Part B 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Zaman, I.; Le, Q.H.; Kuan, H.C.; Kawashima, N.; Luong, L.; Gerson, A.; Ma, J. Interface-Tuned Epoxy/Clay Nanocomposites. Polymer 2011, 52, 497–504. [Google Scholar] [CrossRef]
- Fu, T.; Yu, L.; Wang, Z.; Yu, W.; Zhao, C.; Zhong, S.; Cui, J.; Shao, K.; Na, H. Epoxy Resin/Exfoliated Clay Hybrid Materials with High Thermal Properties. Polym. Compos. 2009, 30, 948–954. [Google Scholar] [CrossRef]
- Vo, V.S.; Mahouche-Chergui, S.; Nguyen, V.H.; Naili, S.; Carbonnier, B. Crucial Role of Covalent Surface Functionalization of Clay Nanofillers on Improvement of the Mechanical Properties of Bioepoxy Resin. ACS Sustain. Chem. Eng. 2019, 7, 15211–15220. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Ramezanzadeh, B.; Pakdel, A.S.; Shahbazi, M. A Physico-Mechanical Investigation of a Novel Hyperbranched Polymer-Modified Clay/Epoxy Nanocomposite Coating. Prog. Org. Coat. 2016, 99, 263–273. [Google Scholar] [CrossRef]
- Yang, L.; Phua, S.L.; Teo, J.K.H.; Toh, C.L.; Lau, S.K.; Ma, J.; Lu, X. A Biomimetic Approach to Enhancing Interfacial Interactions: Polydopamine-Coated Clay as Reinforcement for Epoxy Resin. ACS Appl. Mater. Interfaces 2011, 3, 3026–3032. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Li, X.; Liu, T.; Dai, Z.; Zhao, H.; Wang, L. Achieving High Thermal and Mechanical Properties of Epoxy Nanocomposites via Incorporation of Dopamine Interfaced Clay. Polym. Compos. 2018, 3, 2407–2414. [Google Scholar] [CrossRef]
- Blesic, M.; Gunaratne, H.Q.N.; Jacquemin, J.; Nockemann, P.; Olejarz, S.; Seddon, K.R.; Strauss, C.R. Tunable Thermomorphism and Applications of Ionic Liquid Analogues of Girard’s Reagents. Green Chem. 2014, 16, 4115–4121. [Google Scholar] [CrossRef]
- Pedrosa, J.; Alves, L.; Neto, C.; Rasteriro, M.; Ferreira, P. Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking. Polymers 2022, 14, 3309. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, K.; Hao, Y.; Zhang, L.; Sun, Y.; Zhang, L.; Jiang, B. Advanced Mg2+/Li+ Separation Nanofiltration Membranes Fabricated with Girard’s Reagent T Based on Functional End-Capping Strategy. J. Membr. Sci. 2024, 695, 122483. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, W.; Chen, J.; Chen, X.; Zhao, J.; Wei, F.; Jian, R.; Zheng, X.; Xu, Y. In-Situ Intercalation of Montmorillonite/Urushiol Titanium Polymer Nanocomposite for Anti-Corrosion and Anti-Aging of Epoxy Coatings. Prog. Org. Coat. 2022, 165, 106738. [Google Scholar] [CrossRef]
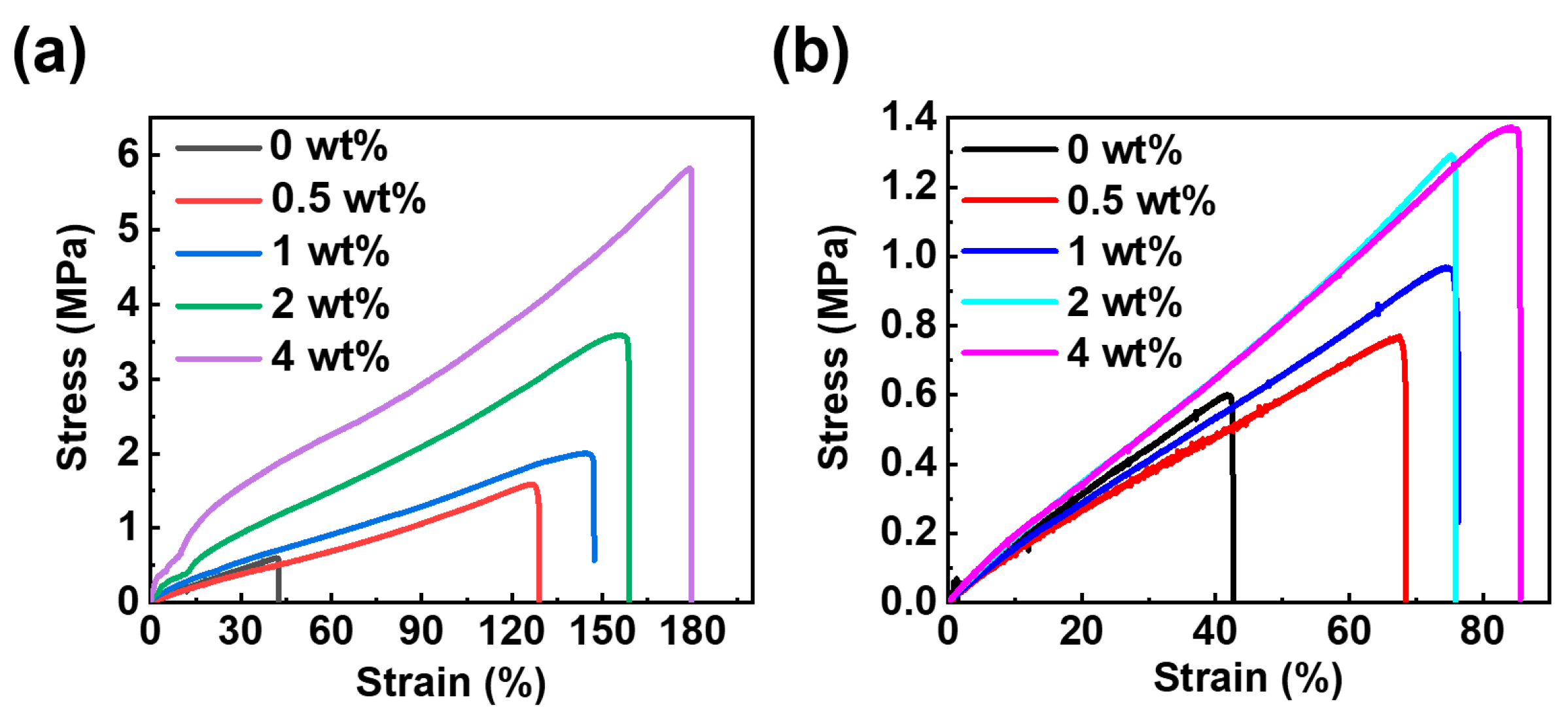
| Content of NG-clay (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 0 | 0.60 ± 0.06 | 13.76 ± 3.26 | 41.79 ± 1.32 |
| 0.5 | 0.77 ± 0.17 | 28.34 ± 5.58 | 67.50 ± 2.28 |
| 1 | 0.96 ± 0.24 | 38.93 ± 6.65 | 74.75 ± 2.36 |
| 2 | 1.29 ± 0.22 | 48.11 ± 8.24 | 75.19 ± 1.68 |
| 4 | 1.37 ± 0.28 | 60.50 ± 8.53 | 84.26 ± 3.56 |
| Content of PG-clay (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 0 | 0.60 ± 0.06 | 13.76 ± 3.26 | 41.79 ± 1.32 |
| 0.5 | 1.58 ± 0.05 | 100.50 ± 5.46 | 126.91 ± 11.82 |
| 1 | 2.00 ± 0.11 | 161.91 ± 7.91 | 144.35 ± 22.07 |
| 2 | 3.59 ± 0.23 | 304.90 ± 8.86 | 155.25 ± 17.33 |
| 4 | 5.82 ± 0.54 | 546.90 ± 12.53 | 178.82 ± 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Jia, L.; Lan, Q.; Wu, D. Enhanced Thermal and Mechanical Properties of Cardanol Epoxy/Clay-Based Nanocomposite through Girard’s Reagent. Polymers 2024, 16, 1528. https://doi.org/10.3390/polym16111528
Xu J, Jia L, Lan Q, Wu D. Enhanced Thermal and Mechanical Properties of Cardanol Epoxy/Clay-Based Nanocomposite through Girard’s Reagent. Polymers. 2024; 16(11):1528. https://doi.org/10.3390/polym16111528
Chicago/Turabian StyleXu, Ji, Lingxiao Jia, Qixin Lan, and Daheng Wu. 2024. "Enhanced Thermal and Mechanical Properties of Cardanol Epoxy/Clay-Based Nanocomposite through Girard’s Reagent" Polymers 16, no. 11: 1528. https://doi.org/10.3390/polym16111528




