Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants
Abstract
:1. Introduction
2. The Fabrication of Porous Biopolymers
2.1. Composites with Nanoparticles
2.2. Composites with Polymers
2.3. Composites with Metal Structures
2.4. Gel Formation (Hydrogel and Aerogel)
2.5. Biopolymer Pretreatment
3. Wastewater Treatment Methods
3.1. Membrane Separation
3.2. Adsorption Treatment
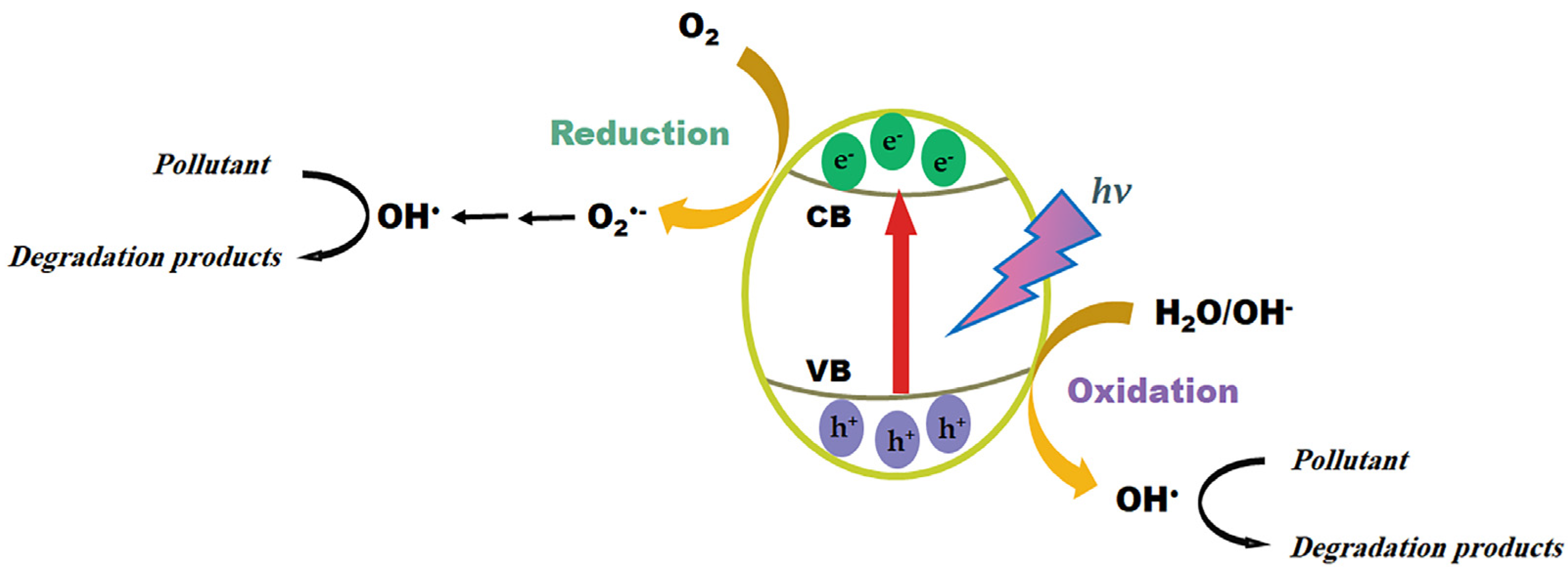
3.3. Degradation and Oxidation
4. Organic Contaminates
4.1. Dyes
| Adsorbent | Biopolymer | Other Components | Dye | qe (mg/g) | Ref. |
|---|---|---|---|---|---|
| Grafted gelatin/MMT nano clay nanocomposites | Gelatin | Montmorillonite, Acrylamide (AAM)—itaconic acid (IA) n, n′-methylene-bis-acrylamide | Malachite green | 950.5 | [67] |
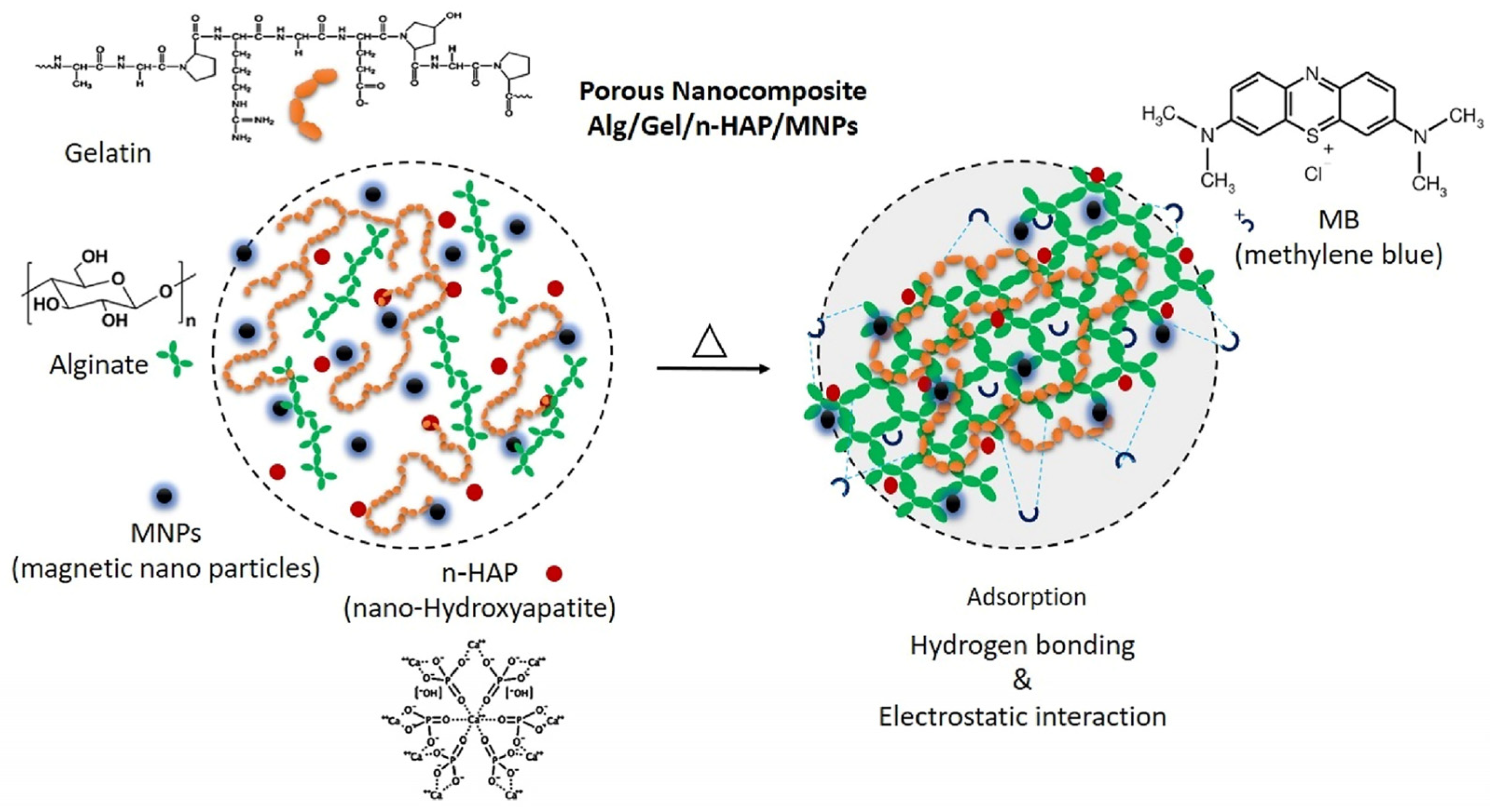
| Nanocomposites Alg/Gel/n-HAP/MNPs | Alginate and gelatin | Nano-hydroxyapatite and magnetic iron oxide nanoparticles (MNPs) | Methylene blue | 561.5 | [28] |
| Ce-UiO-66 MOF @Keratin composites | Keratin | Ce-UiO-66 MOF (1,4-benzene dicarboxylic, dimethylformamide, ammonium ceric nitrate) | Trypan blue | 469.5 | [40] |
| Nanocomposite Zn2+@HAP@CS | Chitosan | Zinc-doped hydroxyapatite | Methyl orange | 453.15 | [68] |
| Composite biopolymer sponge GO-coated PS | Gelatin and chitosan | Poly (vinyl alcohol) and graphene oxide | Congo red | 129.53 | [35] |
| Rudamin B | 99.53 | ||||
| Cs/PEG composite membrane | Chitosan | Polyethylene glycol/TEOS | Methyl orange | 74 mg/g | [32] |
| POP magnetic oak tannin gel (Fe3O4–OT) | Tannin gel | Fe3O4 | Malachite green | 49.00 | [75] |
| Sunset yellow | 53.95 | ||||
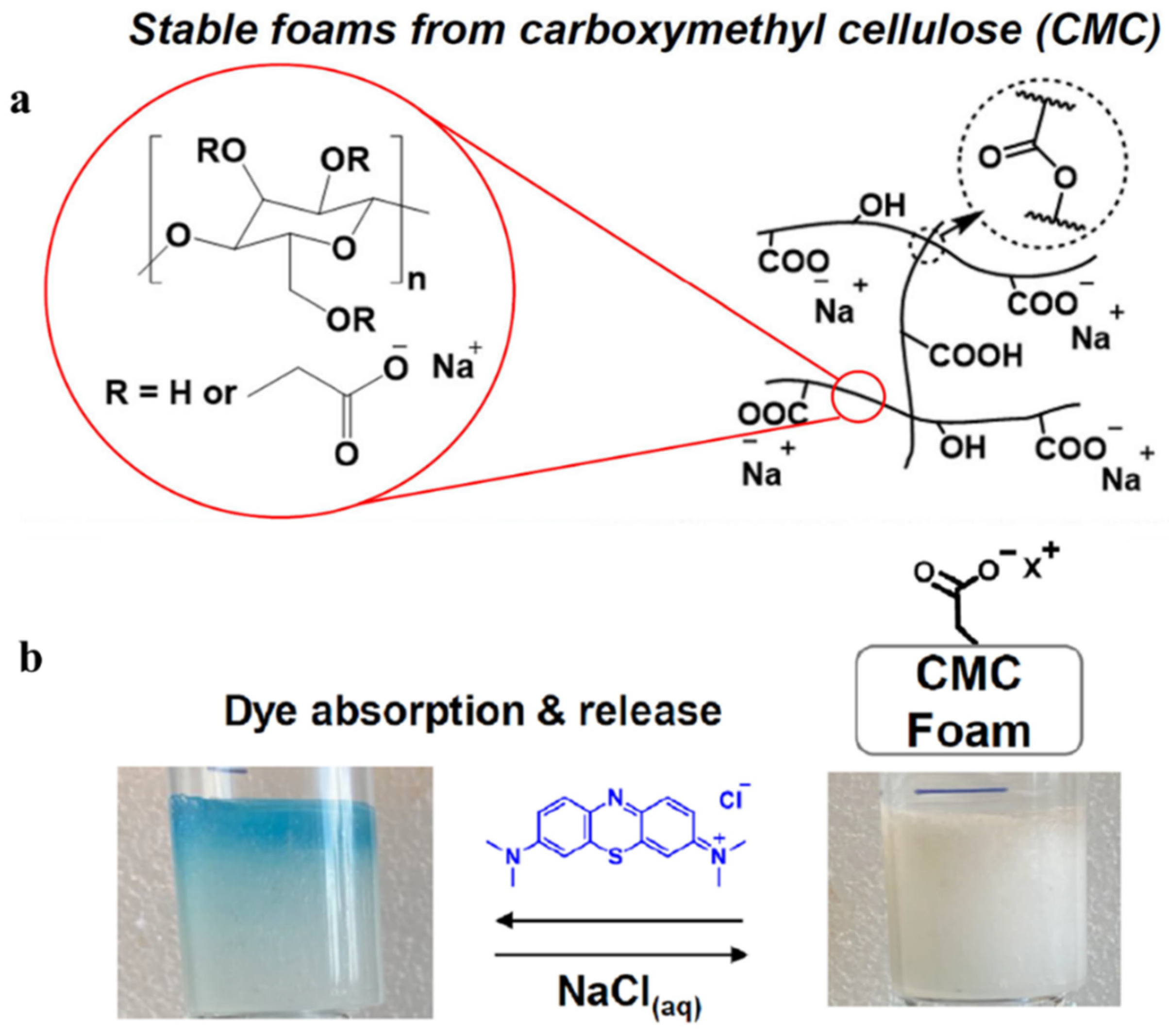
| CMC foams | Carboxymethyl cellulose | Poly (acrylic acid), CaCl2 | Methylene blue | Not mentioned | [34] |
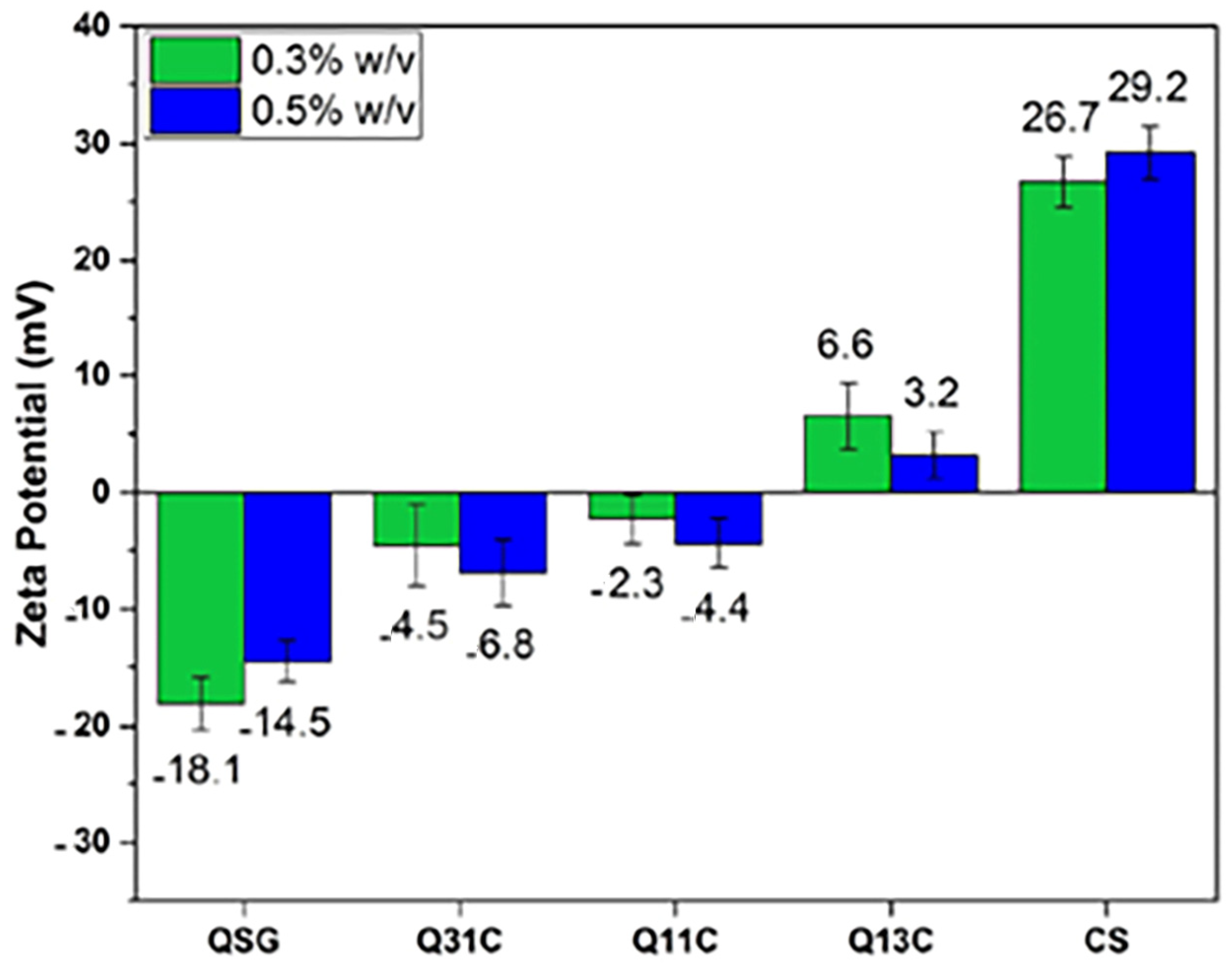
| Polyelectrolyte complexes CS-QSG | Chitosan and Quince seed gum | - | Methylene blue | 30.88 | [31] |
| Rudamin B | 15.16 | ||||
| Methyl orange | 14 |
| Catalyst | Biopolymer | Other Components | Method | Dye | Constant Rate (t−1) | Ref |
|---|---|---|---|---|---|---|
| Porous chitosan-gC3N4 nanosheets | Chitosan | Graphitic carbon nitride | Photocatalytic degradation | Rudamin B | not mentioned (R% = 98) | [53] |
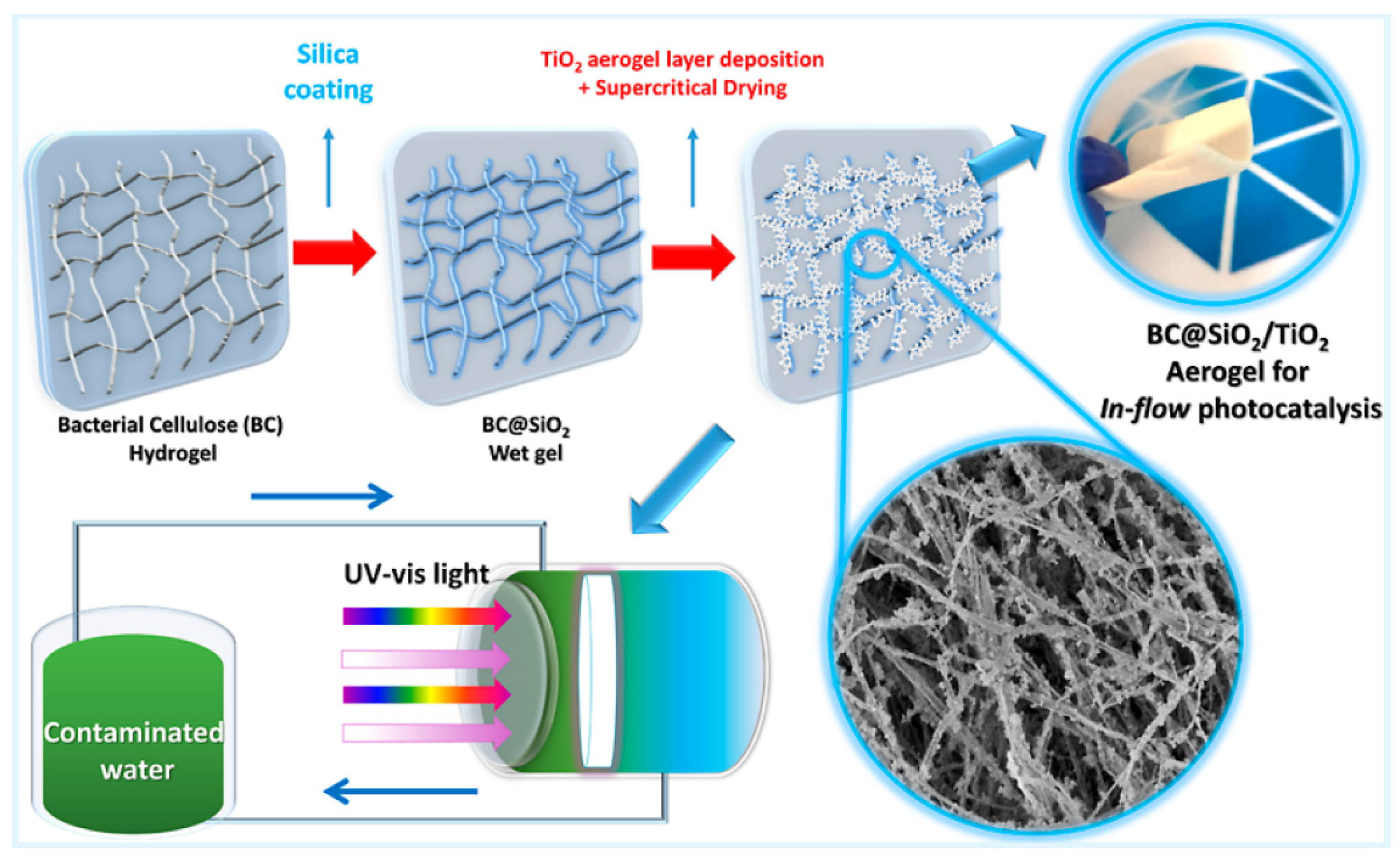
| BC@SiO2/TiO2 Hybrid Aerogel | Bacterial nanocellulose (Komagataeibacter xylinus bacteria) | TEOS and TiO2 | Photocatalytic degradation | Methylene blue | 0.1538 min−1 | [49] |
| Cu-alginate (ZnNPs) hydrogel composite beads | Sodium Alginate | Zn (NO3)2 and Cu (NO3)2 | Catalytic reduction | Methylene blue | 0.798 min−1 | [47] |
| Methyl orange | 0.456 min−1 | |||||
| Congo red | 0.216 min−1 | |||||
| Orange G | 0.252 min−1 | |||||
| AgNPs@Cu@ Alginate aerogel Composite beads | Sodium Alginate | AgNO3 and Cu (NO3)2 | Catalytic reduction | Methylene blue | 0.0061 s−1 | [29] |
| Methyl orange | 0.0131 s−1 | |||||
| Congo red | 0.0016 s−1 | |||||
| Orange G | 0 | |||||
| Alg/XG/AgNPs/Dex/Ca nanocomposite | Alginate/Xanthum Gum/Dextran | AgNPs and CaCl2 | Catalytic reduction | Methylene blue | 0.31907 min−1 | [86] |
| Nanocopper/ Chitosan aerogel biocomposite | Chitosan | CuSO4.5 H2O and extract of durian shell (DS) | Catalytic reduction | Methyl orange | 0.163 min −1 | [87] |
| Metal-oxide catalysts MnOx-PP | Pomelo peels | Manganese oxide (MnOx) | Catalytic oxidation | Methylene blue | not mentioned (qt = 50) | [52] |
4.2. Trace Organic Matter
4.3. Organic Acids Pollution
4.4. Organic Solvent and Oil Contaminate
4.5. Pharmaceutical Contamination
4.6. Natural Organic Matter (NOM)
5. Perspectives and Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, J.F.; Heng, Z.W.; Teoh, H.C.; Chong, W.C.; Pang, Y.L. Recent development of magnetic biochar crosslinked chitosan on heavy metal removal from wastewater—Modification, application and mechanism. Chemosphere 2022, 291, 133035. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Singh, N.B.; Guin, M.; Barthwal, S. Water Purification by Green Synthesized Nanomaterials. Curr. Pharm. Biotechnol. 2022, 24, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, D.; Qiu, Y.; Wang, L.L.; Yang, L.P. Application of macrocycle-crosslinked polymers as adsorbents for the removal of organic micropollutants from water. Curr. Opin. Green Sustain. Chem. 2023, 40, 100755. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A.; Abdi, J.; Hayati, B.; Shekarchi, A.A. Bio-based magnetic metal-organic framework nanocomposite: Ultrasound-assisted synthesis and pollutant (heavy metal and dye) removal from aqueous media. Appl. Surf. Sci. 2019, 480, 288–299. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Dinari, M.; Jamshidian, F. Preparation of MIL-101-NH2 MOF/triazine based covalent organic framework hybrid and its application in acid blue 9 removals. Polymer 2021, 215, 123383. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, T.B.; Huang, C.P.; Chen, C.W.; Bui, X.T.; Dong, C. Di Alkaline modified biochar derived from spent coffee ground for removal of tetracycline from aqueous solutions. J. Water Process Eng. 2021, 40, 101908. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Z.; Wang, S.; Chen, J.; Hu, B.; Shen, C.; Wang, X. Carbon-based nanocomposites for the elimination of inorganic and organic pollutants through sorption and catalysis strategies. Sep. Purif. Technol. 2023, 308, 122862. [Google Scholar] [CrossRef]
- Shabaana, O.A.; Jahina, H.S.; Eldessouky, M.M.I.; Mohamed, G.G. Adsorption of scarlet red dye from industrial wastewater using multiwall carbon nanotubes. Res. J. Environ. Sci. 2021, 8, 32–35. [Google Scholar]
- Song, Y.; Phipps, J.; Zhu, C.; Ma, S. Porous Materials for Water Purification. Angew. Chem.—Int. Ed. 2023, 62, e202216724. [Google Scholar] [CrossRef] [PubMed]
- Chaukura, N.; Mamba, B.B.; Mishra, S.B. Porous materials for the sorption of emerging organic pollutants from aqueous systems: The case for conjugated microporous polymers. J. Water Process Eng. 2017, 16, 223–232. [Google Scholar] [CrossRef]
- Khan, N.A.; López-Maldonado, E.A.; Majumder, A.; Singh, S.; Varshney, R.; López, J.R.; Méndez, P.F.; Ramamurthy, P.C.; Khan, M.A.; Khan, A.H.; et al. A state-of-art-review on emerging contaminants: Environmental chemistry, health effect, and modern treatment methods. Chemosphere 2023, 344, 140264. [Google Scholar] [CrossRef] [PubMed]
- Rekik, S.B.; Gassara, S.; Deratani, A. Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties. Membranes 2023, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Bragança, I.; Homem, V. Personal care products in soil-plant and hydroponic systems: Uptake, translocation, and accumulation. Sci. Total Environ. 2024, 912, 168894. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Devre, P.V.; Patil, A.S.; Sohn, D.; Gore, A.H. Upcycling of spent granular carbon into sustainable and recyclable biopolymeric hybrid hydrogel for highly efficient adsorptive removal of tetracycline pollutant from environmental waters and industrial effluents. J. Environ. Chem. Eng. 2023, 11, 109368. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Yan, D.; Chen, H.; Zhang, M.; Wang, J.; Yang, G. Application of Fe(II)/peroxymonosulfate for efficient alkali lignin wastewater treatment: Insight into the synergistic interactions between redox reactions and coagulation. Sep. Purif. Technol. 2024, 328, 125037. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Du, S.; Liang, W. Synthesis of chitosan-based grafting magnetic flocculants for flocculation of kaolin suspensions. J. Environ. Sci. 2024, 139, 193–205. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, T.; Hao, L.; Guo, Y.; Liu, W.; Guo, L.; Wang, C.; Wang, Z.; Wu, Q. Advances in magnetic porous organic frameworks for analysis and adsorption applications. TrAC—Trends Anal. Chem. 2020, 132, 116048. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Al-Batty, S.; Al-Hamd, R.K.S.; Sims, R.; Hakami, M.W.; Manirul Haque, S.K. Sustainable environment through using porous materials: A review on wastewater treatment. Asia-Pacific J. Chem. Eng. 2023, 18, e2941. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Dinari, M.; Mokhtari, N.; Hatami, M. Covalent triazine based polymer with high nitrogen levels for removal of copper (II) ions from aqueous solutions. J. Polym. Res. 2021, 28, 119. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Ahamad, T.; Ruksana; Naushad, M.; Al-Maswari, B.M.; Alshehri, S.M. Fabrication of highly porous adsorbent derived from bio-based polymer metal complex for the remediation of water pollutants. J. Clean. Prod. 2019, 208, 1317–1326. [Google Scholar] [CrossRef]
- Zhou, W.; Sheng, Y.; Alizadeh, A.; Baghaei, S.; Lv, Q.; Shamsborhan, M.; Nasajpour-Esfahani, N.; Rezaie, R. Synthesis and characterization of Alg/Gel/n-HAP/MNPs porous nanocomposite adsorbent for efficient water conservancy and removal of methylene blue in aqueous environments: Kinetic modeling and artificial neural network predictions. J. Environ. Manag. 2024, 349, 119446. [Google Scholar] [CrossRef]
- Benali, F.; Boukoussa, B.; Issam, I.; Mokhtar, A.; Iqbal, J.; Hachemaoui, M.; Habeche, F.; Cherifi, Z.; Hacini, S.; Patole, S.P.; et al. Assessment of AgNPs@Cu@Alginate Composite for Efficient Water Treatment: Effect of the Content of Cu(II) Crosslinking Agent. J. Polym. Environ. 2023, 31, 4170–4183. [Google Scholar] [CrossRef]
- Das, A.; Kundu, S.; Gupta, M.; Mukherjee, A. Synthesis of porous calcium-guar gum benzoate nano-biohybrids for sorptive removal of congo red and phosphates from water. Int. J. Biol. Macromol. 2023, 253, 126662. [Google Scholar] [CrossRef]
- Kaviani, A.; Pircheraghi, G.; Bagheri, R.; Goharpey, F. Polyelectrolyte Complexes Between Chitosan and Quince Seed Gum: A Rheological, Structural, and Multiple Dye Adsorption Study. J. Polym. Environ. 2023, 31, 852–869. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Hassan, S.; Imran, Z.; Mazhar, D.; Afzal, S.; Ullah, S.A. Green Approach to Water Purification: Investigating Methyl Orange Dye Adsorption Using Chitosan/Polyethylene Glycol Composite Membrane. J. Polym. Environ. 2024, 32, 194–212. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Baklouti, S.; Deratani, A. Fabrication, characterization and permeation studies of ionically cross-linked chitosan/kaolin composite membranes. Period. Polytech. Chem. Eng. 2023, 67, 256–270. [Google Scholar] [CrossRef]
- Cui, J.; Varma, J.; Emrick, T.; Bien, C.; Preda, D.; Gamliel, D. Carboxymethyl cellulose foams: Fabrication, aqueous stability, and water capture. J. Mater. Sci. 2023, 58, 8230–8240. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C. Organic dye removal and recycling performances of graphene oxide-coated biopolymer sponge. Prog. Nat. Sci. Mater. Int. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Dinari, M.; Shirani, M.A.; Maleki, M.H.; Tabatabaeian, R. Green cross-linked bionanocomposite of magnetic layered double hydroxide/guar gum polymer as an efficient adsorbent of Cr(VI) from aqueous solution. Carbohydr. Polym. 2020, 236, 116070. [Google Scholar] [CrossRef]
- Bansal, M.; Pal, B. Starch modified NiFe layered double hydroxide composites for better adsorption and photocatalytic removal of reactive dye and piroxicam-20 drug. Environ. Sci. Pollut. Res. 2023, 30, 73825–73848. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Fiorini, M.; Celli, A.; Coelho, C.; Hennous, M.; Verney, V.; Leroux, F. Poly(butylene succinate)/layered double hydroxide bionanocomposites: Relationships between chemical structure of LDH anion, delamination strategy, and final properties. J. Appl. Polym. Sci. 2013, 130, 1931–1940. [Google Scholar] [CrossRef]
- Esmaeiltarkhani, F.K.; Dinari, M.; Mokhtari, N. Hydrazide-linked perylene-based porous organic polymer: An innovative approach for removing organic dyes from aqueous solution. Results Eng. 2024, 22, 102051. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.T.; Suneetha, M.; Altalhi, T.; Soo Han, S.; Kurkuri, M.D. Functional porous Ce-UiO-66 MOF@Keratin composites for the efficient adsorption of trypan blue dye from wastewater: A step towards practical implementations. Chem. Eng. J. 2023, 461, 142103. [Google Scholar] [CrossRef]
- Su, S.; Zhou, X.; Gong, X.; Idrees, K.B.; Kirlikovali, K.O.; Islamoglu, T.; Farha, O.K.; Gianneschi, N.C. Metal−Organic Frameworks with a Bioinspired Porous Polymer Coating for Sieving Separation. J. Am. Soc. 2023, 145, 13195–13202. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, A.; Zlaoui, K.; Horchani-Naifer, K.; Ennigrou, D.J. Characterization and extraction of sodium alginate from Tunisian algae: Synthesizing a cross-linked ultrafiltration membrane. Iran. Polym. J. 2022, 31, 367–382. [Google Scholar] [CrossRef]
- Rostaminejad, B.; Dinari, M.; Karimi, A.R.; Hadizadeh, M. Oxidative cross-linking of biocompatible chitosan injectable hydrogel by perylene-dopamine to boost phototoxicity of perylene on in vitro melanoma and breast cancer therapy. J. Mol. Liq. 2023, 386, 122553. [Google Scholar] [CrossRef]
- Kumar, N.; Gusain, R.; Pandey, S.; Ray, S.S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Adv. Mater. Interfaces 2023, 10, 2201375. [Google Scholar] [CrossRef]
- de Morais, L.C.; Ferreira, I.A.F.; de Oliveira Meira, A.C.F.; Veríssimo, L.A.A.; de Resende, J.V. Synthesis and characterization of hydrogels from alginate and ora-pro-nóbis (Pereskia aculeata Miller) mucilage. J. Appl. Polym. Sci. 2023, 140, e54568. [Google Scholar] [CrossRef]
- Baigorria, E.; Souza dos Santos, S.; de Moura, M.R.; Fraceto, L.F. Nanocomposite hydrogels 3D printed for application in water remediation. Mater. Today Chem. 2023, 30, 101559. [Google Scholar] [CrossRef]
- Benali, F.; Boukoussa, B.; Issam, I.; Iqbal, J.; Mokhtar, A.; Hachemaoui, M.; Habeche, F.; Hacini, S.; Abboud, M. Zinc nanoparticles encapsulated in porous biopolymer beads for reduction of water pollutants and antimicrobial activity. Int. J. Biol. Macromol. 2023, 248, 125832. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, A.; Lin, L.; El-Sohaimy, S.A.; Li, Y.; Wu, L.; Zhang, H. Schiff base cross-linked dialdehyde cellulose/gelatin composite aerogels as porous structure templates for oleogels preparation. Int. J. Biol. Macromol. 2023, 224, 667–675. [Google Scholar] [CrossRef]
- da Silva, T.C.A.; Marchiori, L.; Mattos, B.O.; Ullah, S.; da Silva Barud, H.; Domeneguetti, R.R.; Rojas-Mantilla, H.D.; Zanoni, M.V.B.; Rodrigues-Filho, U.P.; Ferreira-Neto, E.P.; et al. Designing Highly Photoactive Hybrid Aerogels for In-Flow Photocatalytic Contaminant Removal Using Silica-Coated Bacterial Nanocellulose Supports. ACS Appl. Mater. Interfaces 2023, 15, 23146–23159. [Google Scholar] [CrossRef]
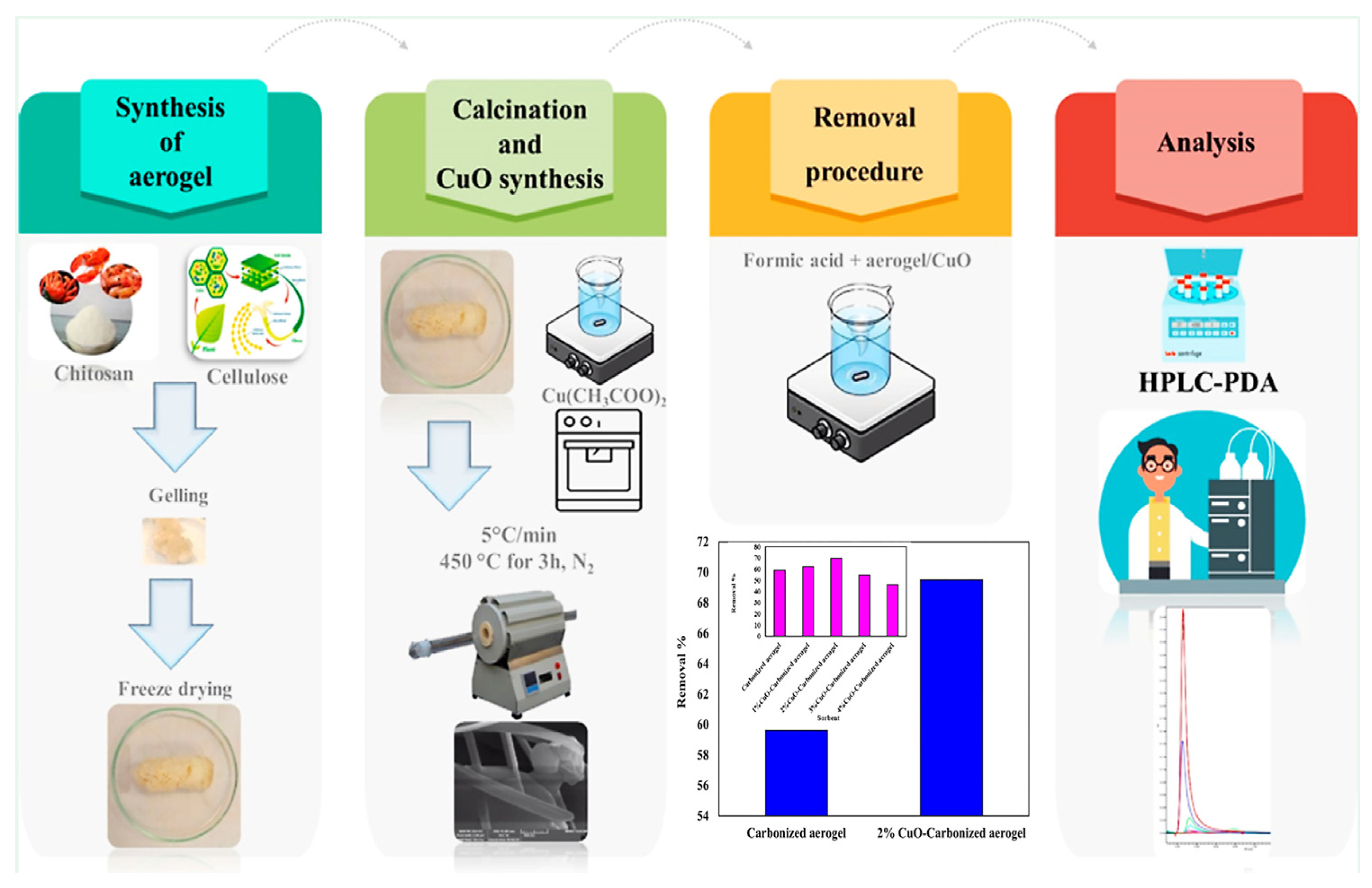
- Amidi, M.; Salehi, E. Calcined Chitosan/Cellulous Aerogel Modified with Copper Oxide Nanoparticles as an Efficient Sorbent for the Optimized Removal of Formic Acid from Water. ACS Appl. Bio Mater. 2023, 6, 4217–4225. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, S.; Strømme, M.; Xu, C. All-cellulose-based freestanding porous carbon nanocomposites and their versatile applications. Compos. Part B Eng. 2022, 232, 109602. [Google Scholar] [CrossRef]
- Zheng, J.Y.; He, J.; Han, C.B.; Huang, G.; Sun, B.C.; Zhao, W.K.; Wang, Y.; Sun, L.; Si, J.; Yan, H. Adsorption-enhanced catalytic oxidation for long-lasting dynamic degradation of organic dyes by porous manganese-based biopolymeric catalyst. Int. J. Biol. Macromol. 2023, 237, 124152. [Google Scholar] [CrossRef] [PubMed]
- Praseetha, P.K.; Godwin, M.A.; AlSalhi, M.S.; Devanesan, S.; Vijayakumar, S.; Sangeetha, R.; Prathipkumar, S.; Kim, W. Porous chitosan-infused graphitic carbon nitride nanosheets for potential microbicidal and photo-catalytic efficacies. Int. J. Biol. Macromol. 2023, 238, 124120. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.O.; Cunha, R.S.; de Sousa Silva, R.; Marangoni, C.; Hotza, D.; Machado, R. Porous chitosan membranes by solvent evaporation technique: Evaluation of the biopolymer/acetone ratio and potentiality for the membrane distillation process. Polym. Technol. Mater. 2023, 62, 2347–2363. [Google Scholar] [CrossRef]
- Rowley, J.; Abu-Zahra, N.H. Synthesis and characterization of polyethersulfone membranes impregnated with (3-aminopropyltriethoxysilane) APTES-Fe3O4 nanoparticles for As(V) removal from water. J. Environ. Chem. Eng. 2019, 7, 102875. [Google Scholar] [CrossRef]
- Kahloul, M.; Ounifi, I.; Agougui, H.; Jabli, M.; Hafiane, A. A novel cellulose acetate-polyoxometalate (PW11Fe(H2O)O39) hybrid membranes: Preparation, characterization and study of their potential for humic acid adsorption. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Pavithra, N.; Méndez-Herrera, P.F.; López-López, J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment; De Gruyter: Berlin, Germany, 2012; Volume 2012. [Google Scholar]
- Tadayoni, N.S.; Dinari, M.; Torbatian, A. Novel flower-like magnetic core-shell covalent triazine polymer as a beneficial Direct Scarlet 4BS adsorbent and comprehensive study of the kinetics and isotherm adsorption. J. Environ. Chem. Eng. 2023, 11, 110647. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Trabolsi, A. Pollutant removal with organic macrocycle-based covalent organic polymers and frameworks. Chem 2021, 7, 882–918. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Narzary, S.; Alamelu, K.; Raja, V.; Jaffar Ali, B.M. Visible light active, magnetically retrievable Fe3O4@SiO2@g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants. J. Environ. Chem. Eng. 2020, 8, 104373. [Google Scholar] [CrossRef]
- Zhong, C.; Jiang, Y.; Liu, Q.; Sun, X.; Yu, J. Natural siderite derivatives activated peroxydisulfate toward oxidation of organic contaminant: A green soil remediation strategy. J. Environ. Sci. 2023, 127, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Sun, F.; Zeng, Y.; Zeng, Q.; Zhang, T.; Tian, W.; Liang, B. Preparation and application of separable magnetic Fe3O4-SiO2-APTES-Ag2O composite particles with high visible light photocatalytic performance. J. Environ. Chem. Eng. 2018, 6, 945–954. [Google Scholar] [CrossRef]
- Habache, N.; Bechiri, O. Thermally activated persulfate oxidation of Basic Fuchsin dye: Effect of different operating parameters, kinetic, and thermodynamic study. Int. J. Chem. Kinet. 2024, 56, 30–42. [Google Scholar] [CrossRef]
- Mahroug, H.; Belkaid, S. Sustainable and Low-cost Hydroxyapatite/Starch for the Removal of Methylene Blue from Aqueous Solutions. Phys. Chem. Res. 2024, 12, 73–84. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Aamer, N.; Abdallah, H.M.; López-Maldonado, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Mohamed, R.R. Novel high-efficient adsorbent based on modified gelatin/montmorillonite nanocomposite for removal of malachite green dye. Sci. Rep. 2024, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- EL Kaim Billah, R.; Zaghloul, A.; Bahsis, L.; Oladoja, N.A.; Azoubi, Z.; Taoufyk, A.; Majdoubi, H.; Algethami, J.S.; Soufiane, A.; López-Maldonado, E.A.; et al. Multifunctional biocomposites based on cross-linked Shrimp waste-derived chitosan modified Zn2+@Calcium apatite for the removal of methyl orange and antibacterial activity. Mater. Today Sustain. 2024, 25, 100660. [Google Scholar] [CrossRef]
- Fan, Y.; Liang, H.; Jian, M.; Liu, R.; Zhang, X.; Hu, C.; Liu, H. Removal of dimethylarsinate from water by robust NU-1000 aerogels: Impact of the aerogel materials. Chem. Eng. J. 2023, 455, 140387. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, Y.; Zhang, B.; Fu, J.; Jiang, X.; Jia, B.; Men, X.; Li, L. Preparation of Chitosan Modified Cu-Metal–Organic Framework Antibacterial Microspheres and Their Application in Adsorption of Cr(VI) from Aqueous Solution. Water Air Soil Pollut. 2023, 234, 97. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Salih, H.G.; Ali, N.S.; Khalbas, A.H.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S.; Albayati, T.M.; Harharah, H.N. Olive stone as an eco-friendly bio-adsorbent for elimination of methylene blue dye from industrial wastewater. Sci. Rep. 2023, 13, 21063. [Google Scholar] [CrossRef]
- Jiang, B.; Shen, F.; Jiang, Y.; Huang, M.; Zhao, L.; Lei, Y.; Hu, J.; Tian, D.; Shen, F. Extraction of super high-yield lignin-carbohydrate complexes from rice straw without compromising cellulose hydrolysis. Carbohydr. Polym. 2024, 323, 121452. [Google Scholar] [CrossRef]
- Karami, N.; Mohammadpour, A.; Samaei, M.R.; Amani, A.M.; Dehghani, M.; Varma, R.S.; Sahu, J.N. Green synthesis of sustainable magnetic nanoparticles Fe3O4 and Fe3O4-chitosan derived from Prosopis farcta biomass extract and their performance in the sorption of lead(II). Int. J. Biol. Macromol. 2024, 254, 127663. [Google Scholar] [CrossRef]
- Asem, M.; Noraini Jimat, D.; Huda Syazwani Jafri, N.; Mohd Fazli Wan Nawawi, W.; Fadhillah Mohamed Azmin, N.; Firdaus Abd Wahab, M. Entangled cellulose nanofibers produced from sugarcane bagasse via alkaline treatment, mild acid hydrolysis assisted with ultrasonication. J. King Saud Univ.—Eng. Sci. 2023, 35, 24–31. [Google Scholar] [CrossRef]
- Sadegh, N.; Haddadi, H.; Asfaram, A. Synthesis of a green magnetic biopolymer derived from oak fruit hull tannin for the efficient and simultaneous adsorption of a mixture of Malachite Green and Sunset Yellow dyes from aqueous solutions. New J. Chem. 2022, 46, 11862–11876. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.; Nam, C. Ultrafast and on-demand oil/water separation with vertically aligned cellulosic smart sponge. J. Hazard. Mater. 2023, 445, 130559. [Google Scholar] [CrossRef]
- Khodayari, J.; Zare, K.; Moradi, O.; Kalaee, M.; Mohammad Mahmoodi, N. Synthesis of eco-friendly carboxymethyl cellulose /metal–organic framework biocomposite and its photocatalytic activity. J. Photochem. Photobiol. A Chem. 2024, 446, 115097. [Google Scholar] [CrossRef]
- Laxmiputra; Nityashree, D.B.; Udayabhanu; Anush, S.M.; Pramoda, K.; Prashantha, K.; Ullala Mata Beena, B.N.; Girish, Y.R.; Nagarajaiah, H. Construction of Z-Scheme MoS2/ZnFe2O4 heterojunction photocatalyst with enhanced photocatalytic activity under visible light. Mater. Res. Bull. 2024, 169, 112489. [Google Scholar] [CrossRef]
- Huang, X.M.; Chen, N.; Ye, D.N.; Zhong, A.G.; Liu, H.; Li, Z.; Liu, S.Y. Structurally Complementary Star-Shaped Unfused Ring Electron Acceptors with Simultaneously Enhanced Device Parameters for Ternary Organic Solar Cells. Sol. RRL 2023, 7, 2300143. [Google Scholar] [CrossRef]
- Zwane, S.; Ingwani, T.; Dlamini, D.S.; Mamba, B.B.; Kuvarega, A.T. Poly(m-phenylene isophthalamide) (PMIA) membranes as a support for WO3/g-C3N4 for the degradation of diclofenac in water. J. Photochem. Photobiol. A Chem. 2024, 446, 115123. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef]
- Ukani, H.; Mehra, S.; Parmar, B.; Kumar, A.; Khan, I.; El Seoud, O.A.; Malek, N. Metal−Organic Framework-Based Aerogel: A Novel Adsorbent for the Efficient Removal of Heavy Metal Ions and Selective Removal of a Cationic Dye from Aqueous Solution. Ind. Eng. Chem. Res. 2023, 62, 5002–50014. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Boukoussa, B.; Cherdouane, K.R.; Zegai, R.; Mokhtar, A.; Hachemaoui, M.; Issam, I.; Iqbal, J.; Patole, S.P.; Zeggai, F.Z.; Hamacha, R.; et al. Preparation of activated carbon-metal nanoparticle composite materials for the catalytic reduction of organic pollutants. Surf. Interfaces 2024, 44, 103622. [Google Scholar] [CrossRef]
- Shrivastava, K.; Dangi, S.S.; Nema, A.; Bano, M.; Rai, M.; Verma, V.; Khan, F. Silver nanoparticle incorporated calcium crosslinked hydrogel composite for reduction of methylene blue dye and nitrite sensing. J. Photochem. Photobiol. A Chem. 2024, 447, 115256. [Google Scholar] [CrossRef]
- Truong, T.B.T.; Nguyen, T.T.T.; Nguyen, P.A.; Do, B.L.; Van Nguyen, T.T.; Huynh, K.P.H.; Phan, H.P.; Dang-Bao, T.; Ho, T.G.T.; Nguyen, T. Green synthesised nanocopper/chitosan aerogel biocomposite as a recyclable and nonprecious catalyst for methyl orange reduction. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 680, 132622. [Google Scholar] [CrossRef]
- Ali, F.; Zahid, S.; Khan, S.; Rehman, S.U.; Ahmad, F. A Comprehensive Review on Adsorption of Dyes from Aqueous Solution by Mxenes. Asian J. Green Chem. 2024, 8, 81–107. [Google Scholar] [CrossRef]
- Daramola, I.O.; Ojemaye, M.O.; Okoh, A.I.; Okoh, O.O. Occurrence of herbicides in the aquatic environment and their removal using advanced oxidation processes: A critical review. Environ. Geochem. Health 2023, 45, 1231–1260. [Google Scholar] [CrossRef]
- Kotnala, S.; Bhushan, B.; Nayak, A. Fabrication of a magnetite hydroxyapatite nanocomposite for the removal of Paraquat dichloride: Adsorption studies. Mater. Today Proc. 2023, 73, 122–127. [Google Scholar] [CrossRef]
- Guo, G.; Li, T.; Liu, Z.; Luo, X.; Zhang, T.; Tang, S.; Wang, X.; Chen, D. Bell pepper derived nitrogen-doped carbon dots as a pH-modulated fluorescence switching sensor with high sensitivity for visual sensing of 4-nitrophenol. Food Chem. 2024, 432, 137232. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, L.; Yin, Z.; Jiang, H. Facile microwave assisted one-pot solid-state construction of Co-Fe spinel oxide/porous biochar for highly efficient 4-nitrophenol degradation: Effect of chemical blowing and surface vulcanization. Sep. Purif. Technol. 2024, 328, 125033. [Google Scholar] [CrossRef]
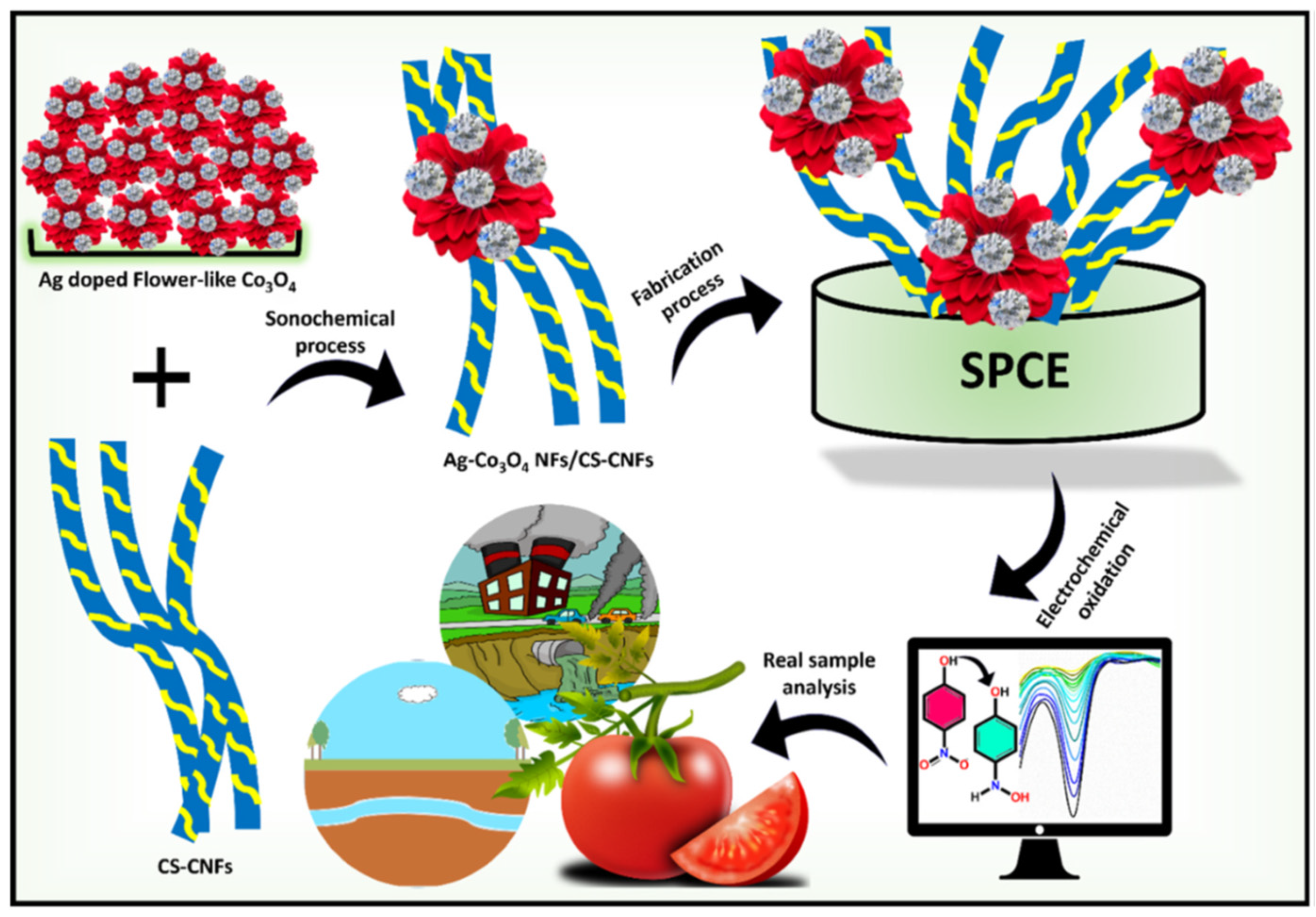
- Balram, D.; Lian, K.Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Bi-functional renewable biopolymer wrapped CNFs/Ag doped spinel cobalt oxide as a sensitive platform for highly toxic nitroaromatic compound detection and degradation. Chemosphere 2022, 291, 132998. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Chapter 26—Analysis of organic acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar]
- Khanashyam, A.C.; Shanker, M.A.; Thomas, P.E.; Babu, K.S.; Nirmal, N.P. Phytochemicals in biofilm inhibition. In Recent Frontiers of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 397–412. [Google Scholar]
- Cheremisinoff, N.P.; Rosenfeld, P.E. Sources of air emissions from pulp and paper mills. In Handbook of Pollution Prevention and Cleaner Production; William Andrew Inc.: Norwich, NY, USA, 2010; pp. 179–259. [Google Scholar]
- Alami, N.H.; Hamzah, A.; Tangahu, B.V.; Warmadewanti, I.; Bachtiar Krishna Putra, A.; Purnomo, A.S.; Danilyan, E.; Putri, H.M.; Aqila, C.N.; Dewi, A.A.N.; et al. Microbiome profile of soil and rhizosphere plants growing in traditional oil mining land in Wonocolo, Bojonegoro, Indonesia. Int. J. Phytoremediation 2023, 25, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, C.; Song, J.; Zhang, X.; Huang, X.; Ma, Y.; Cao, H.; Liu, N. Superwetting Ag/α-Fe2O3 anchored mesh with enhanced photocatalytic and antibacterial activities for efficient water purification. Green Energy Environ. 2024, 9, 89–103. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.H.; Wang, J.J. Biochar-based materials as remediation strategy in petroleum hydrocarbon-contaminated soil and water: Performances, mechanisms, and environmental impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Zhou, J.; Wu, J.; Gu, H.; Lin, W. Hydrophobic sponge derived from natural loofah for efficient oil/water separation. Sep. Purif. Technol. 2024, 330, 125519. [Google Scholar] [CrossRef]
- Maroulas, K.N.; Trikkaliotis, D.G.; Metaxa, Z.S.; AbdelAll, N.; Alodhayb, A.; Khouqeer, G.A.; Kyzas, G.Z. Super-hydrophobic chitosan/graphene-based aerogels for oil absorption. J. Mol. Liq. 2023, 390, 123071. [Google Scholar] [CrossRef]
- Al-Najar, J.A.; Al-Humairi, S.T.; Lutfee, T.; Balakrishnan, D.; Veza, I.; Soudagar, M.E.M.; Fattah, I.M.R. Cost-Effective Natural Adsorbents for Remediation of Oil-Contaminated Water. Water 2023, 15, 1186. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Zhang, Y.Q.; Gao, C.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Superhydrophobic aerogel membrane with integrated functions of biopolymers for efficient oil/water separation. Sep. Purif. Technol. 2022, 282, 120138. [Google Scholar] [CrossRef]
- Afolabi, F.; Mahmood, S.M.; Dzulkarnain, I.; Ewere, D.; Akbari, S. A detailed study on the rheological behavior of a novel cellulose-based hydrophobically-modified polymer. Pet. Sci. Technol. 2023, 41, 1313–1327. [Google Scholar] [CrossRef]
- Parmentier, M.; Gabriel, C.M.; Guo, P.; Isley, N.A.; Zhou, J.; Gallou, F. Switching from organic solvents to water at an industrial scale. Curr. Opin. Green Sustain. Chem. 2017, 7, 13–17. [Google Scholar] [CrossRef]
- Dayan, A.D. Principles of Toxicology: Environmental and Industrial Applications, 2nd ed.; John Wiley: New York, NY, USA, 2001; Volume 58, ISBN 0471293210. [Google Scholar]
- Witkowski, K.M.; Johnson, N.E. Organic-solvent water pollution and low birth weight in Michigan. Soc. Biol. 1992, 39, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Hernández, J.M.; Brillas, E. A critical review over the removal of paracetamol (acetaminophen) from synthetic waters and real wastewaters by direct, hybrid catalytic, and sequential ozonation processes. Chemosphere 2023, 313, 137411. [Google Scholar] [CrossRef] [PubMed]
- Ohale, P.E.; Igwegbe, C.A.; Iwuozor, K.O.; Emenike, E.C.; Obi, C.C.; Białowiec, A. A review of the adsorption method for norfloxacin reduction from aqueous media. MethodsX 2023, 10, 102180. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Xu, J.; Mei, Y.; Fan, S.; Xu, H. Effect of carbonization methods on the properties of tea waste biochars and their application in tetracycline removal from aqueous solutions. Chemosphere 2021, 267, 129283. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Renu; Kaur, M.; Aggarwal, D.; Kumar, V.; Tikoo, K.; Kaushik, A.; Singhal, S. Unveiling the multifaceted applications of magnetically responsive chitosan capped ZnS QDs for sensing and annihilation of pharmaceutical drugs. Talanta 2024, 266, 125084. [Google Scholar] [CrossRef] [PubMed]
- Shahrin, E.W.E.S.; Narudin, N.A.H.; Shahri, N.N.M.; Nur, M.; Lim, J.W.; Bilad, M.R.; Mahadi, A.H.; Hobley, J.; Usman, A. A comparative study of adsorption behavior of rifampicin, streptomycin, and ibuprofen contaminants from aqueous solutions onto chitosan: Dynamic interactions, kinetics, diffusions, and mechanisms. Emerg. Contam. 2023, 9, 100199. [Google Scholar] [CrossRef]
- Kim, M.; Njaramba, L.K.; Yoon, Y.; Jang, M.; Park, C.M. Thermally-activated gelatin–chitosan–MOF hybrid aerogels for efficient removal of ibuprofen and naproxen. Carbohydr. Polym. 2024, 324, 121436. [Google Scholar] [CrossRef]
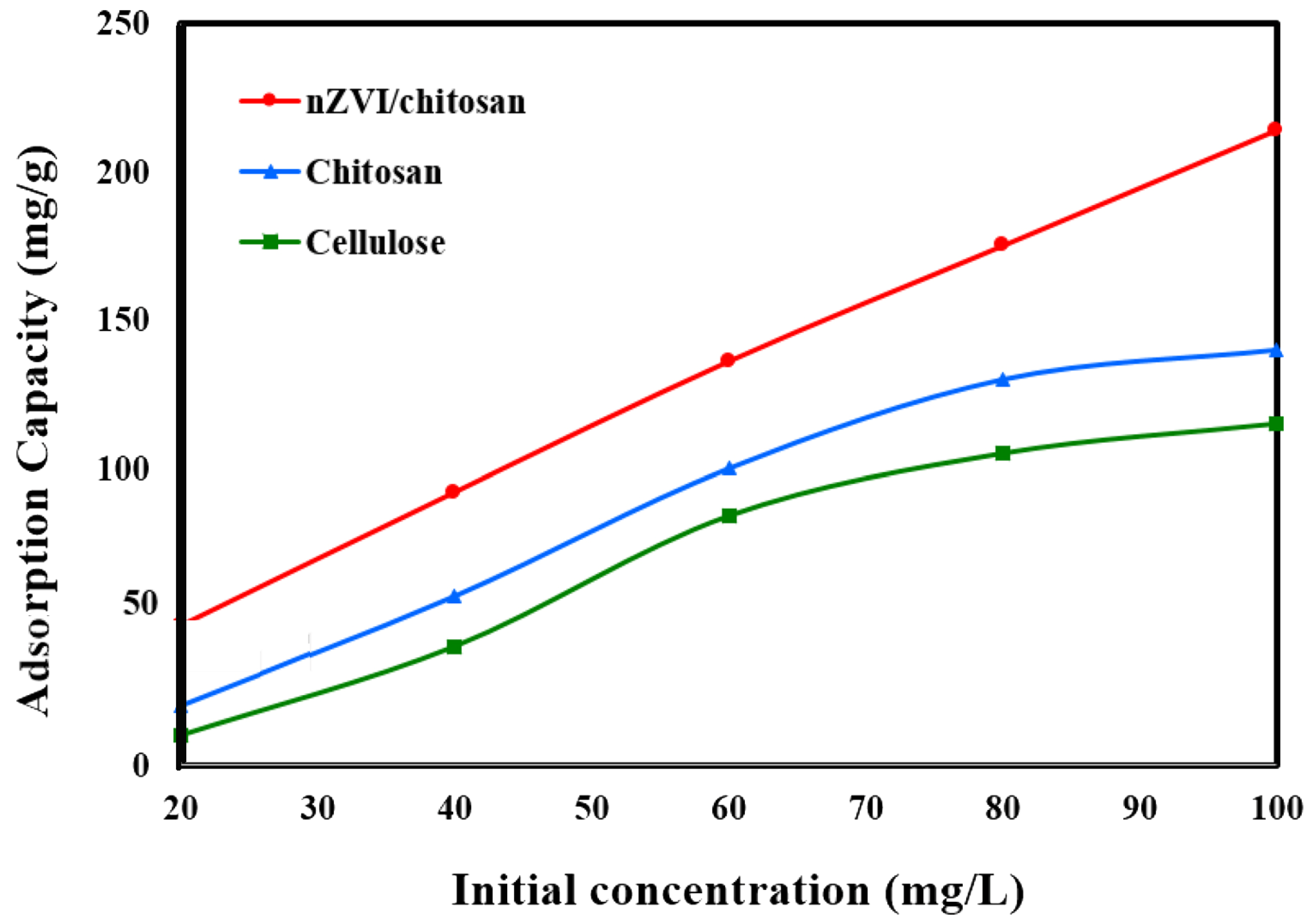
- Pourbaghaei, N.Z.; Anbia, M.; Rahimi, F. Fabrication of Nano Zero valent Iron/Biopolymer Composite with Antibacterial Properties for Simultaneous Removal of Nitrate and Humic Acid: Kinetics and Isotherm Studies. J. Polym. Environ. 2022, 30, 907–924. [Google Scholar] [CrossRef]






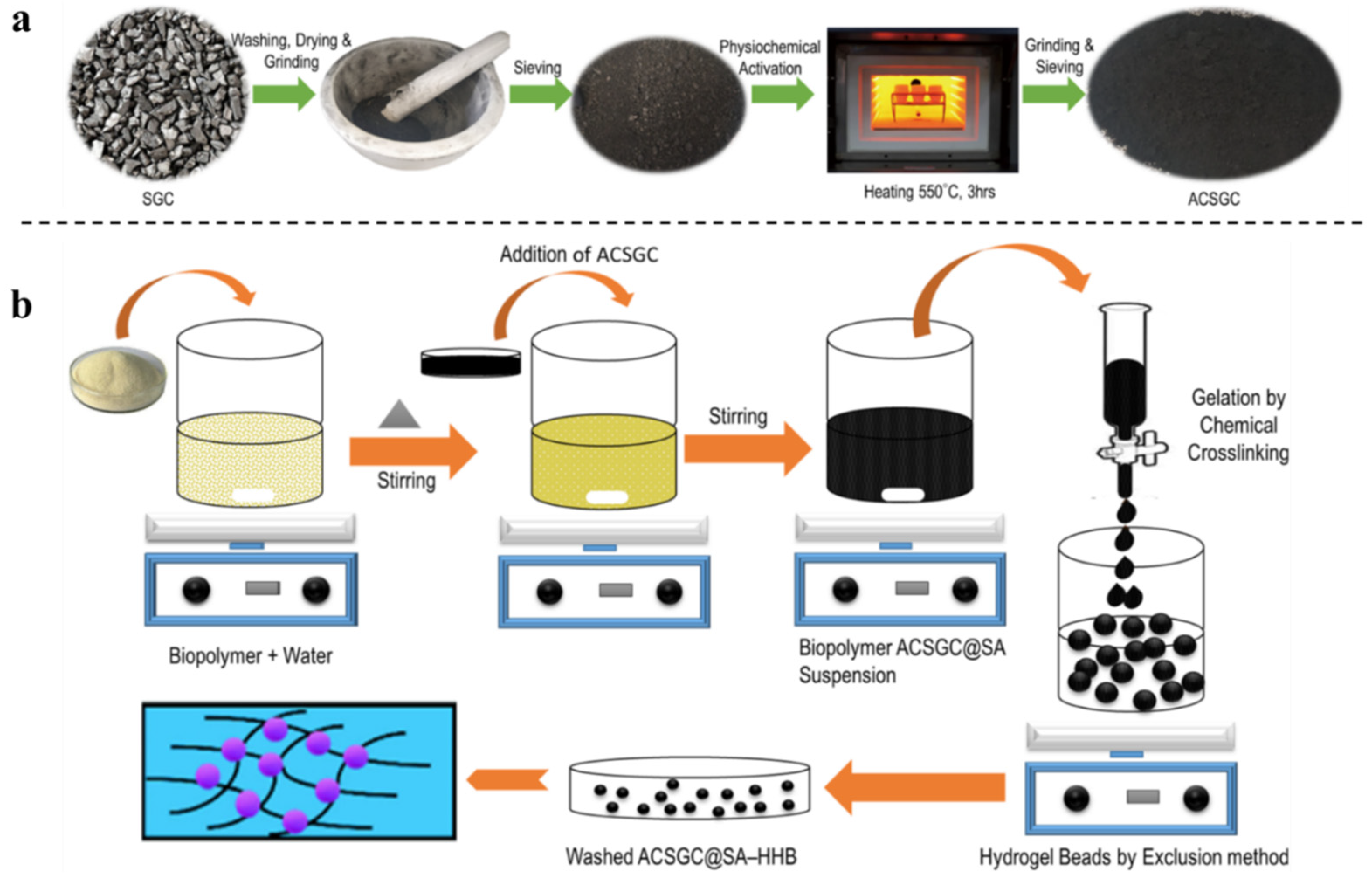
| Adsorbent | Biopolymer | Other Components | Oil and Solvents | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|
| CS/PVA/rGO /Graphene-based aerogels | Chitosan | Reduced graphene oxide, PVA, and methyltrichlorosilane | Diesel oil | 27 g/g | [101] |
| T-SA/lignin/rGO-MTMS aerogel membrane | Lignin and sodium alginate | Reduced graphene oxide and trimethoxymethylsilane | n-hexane | 11.8 g/g | [103] |
| Dichloromethane | 13.9 g/g | ||||
| Chloroform | 14 g/g | ||||
| Isooctane | 11 g/g | ||||
| Pump oil | 8 g/g | ||||
| Xylene | 5.5 g/g | ||||
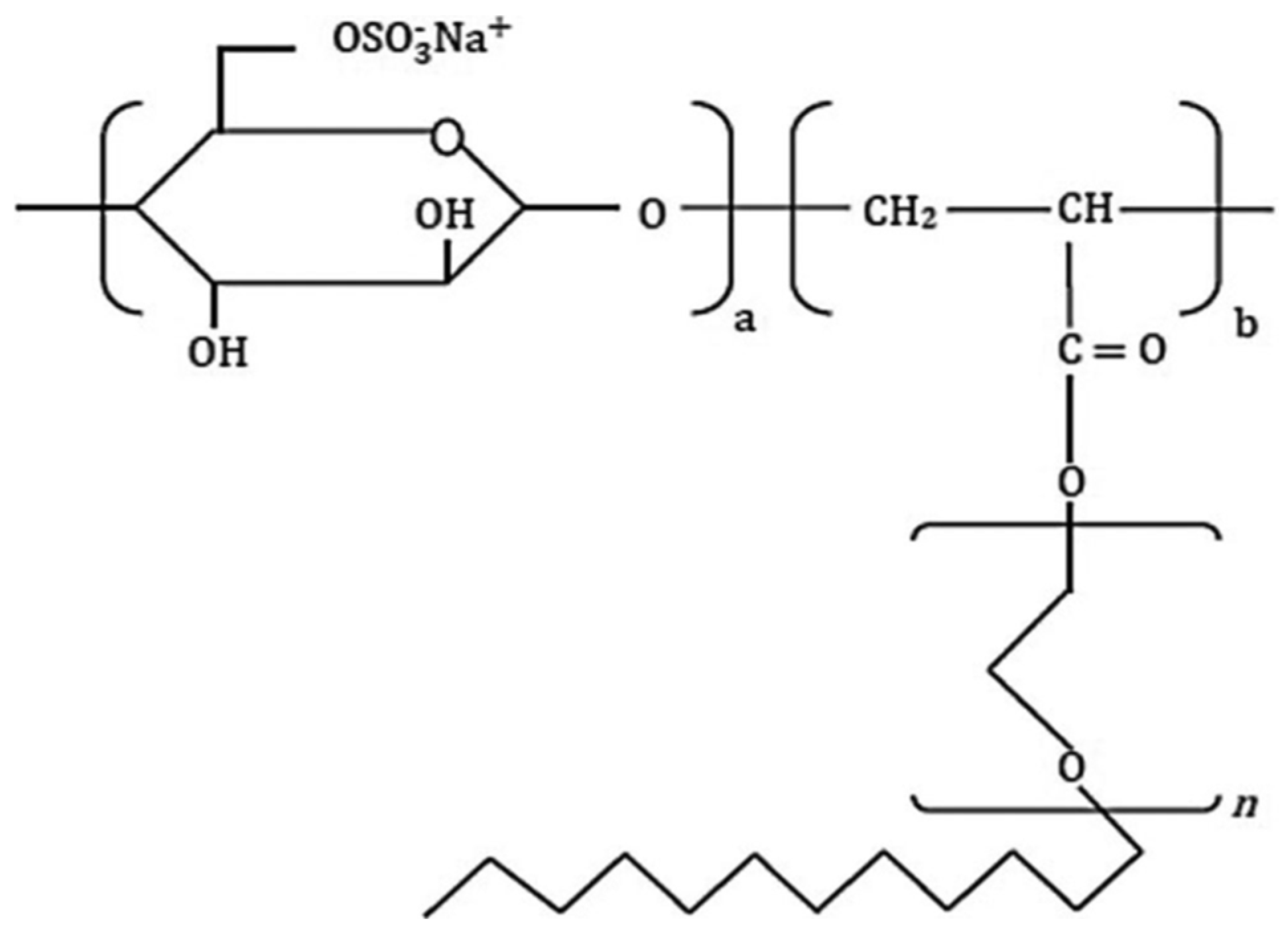
| DPEA-Cell-OSO−3 polymer | Sodium Cellulose Sulfate | Ethylene ixide, acrylic acid, and dodecene | Oil | Not mentioned | [104] |
| Cladophora cellulose-derived porous carbons (CPC) | Cladophora cellulose (CC) | - | Chloroform, acetone, tetrahydrofuran, benzene, and toluene | 100–217 (times own weight of sorbent) | [51] |
| Adsorbent | Biopolymer | Other Components | Drug | qe (mg/g) | Ref. |
|---|---|---|---|---|---|
| Starch-NiFe LDH (S/NiFe-LDH) composite | Corn starch | Fe (NO3)3.9H2O, Ni (NO3)2.6H2O | Piroxicam-20 | 1500.2 | [37] |
| AC-SGC@SA–HHB composite hydrogel adsorbent | Sodium alginate | Activated carbon (AC) from spent granular carbon (SGC), | Tetracycline | 22.6055 | [17] |
| Chitosan derived from mud-crab (Scylla serrata) shell adsorbent | Chitosan (mud crab) | - | Rifampicin, | 10.57 | [112] |
| Streptomycin | 7.512 | ||||
| Ibuprofen | 7.169 | ||||
| gelatin–chitosan–MOF hybrid aerogels (CGC–MOF) | Gelatin Chitosan | UiO-66–NH2 (ZrCl4. 2-aminoterephthalic acid) | Ibuprofen | 1.1 | [113] |
| Naproxen | 1.4 | ||||
| Chitosan-capped ZnS QDs/NiFe2O4 | Chitosan | ZnS QDs (zinc nitrate and sodium sulfide) and NiFe2O4 (nickel nitrate and ferric nitrate) | Tetracycline | Rate constant * (k × 10−3 min −1) = 21.7 | [111] |
| Minocycline | Rate constant * (k × 10−3 min −1) = 28.7 |
| Porous Composite | Method | Mechanism | Ref. |
|---|---|---|---|
| Starch-NiFe LDH (S/NiFe-LDH) composite | Adsorption | Physio-sorption process | [37] |
| Ce-uio-66 MOF @Keratin composites | Hydrogen bonding, π–π interaction, electrostatic interaction, and pore filling | [40] | |
| Sodium alginate–bentonite clay (SA-B) nanocomposite hydrogels | Exothermic adsorption and electrostatic interactions | [46] | |
| Cuo-cellulose and chitosan (CS/CE) aerogel | Exothermic, entropy-reduction, chemisorption, and single layer | [50] | |
| Grafted gelatin/MMT nano clay nanocomposites | Electrostatic forces and coordination bonding (metal ions of MMT nano clay) Hydrogen bonding interactions | [67] | |
| Nanocomposite Zn2+@HAP@CS | Electrostatic attraction (positively charged composite) | [68] | |
| CS/PVA/rGO/graphene-based aerogels | Capillary uptake and then chemical adsorption | [101] | |
| DPEA-Cell-OSO−3 polymer | Hydrogen bonding | [104] | |
| Nanocomposites Alg/Gel/n-HAP/MNPs | Hydrogen bonding Electrostatic interaction | [28] | |
| Cs/PEG composite membrane | [32] | ||
| Composite biopolymer sponge GO-coated PS | [35] | ||
| Pop magnetic oak tannin gel (Fe3O4–OT) | [75] | ||
| Polyelectrolyte complexes CS-QSG | Adsorption (heterogeneous multilayer) | [31] | |
| Chitosan derived from mud crab (Scylla serrata) shells adsorbent | Adsorption (monolayer and multilayer) | [112] | |
| AgNPs@Cu@ alginate aerogel composite beads | 1. Adsorption of electrons and pollutants on the catalyst’s surface 2. Reduction 3. Desorption on the catalyst’s surface | [29] | |
| Cu-alginate (ZnNPs) hydrogel composite beads | 1. Diffuse inside the catalyst’s pore via electrostatic interaction 2. Reduction 3. Desorption on the catalyst’s surface | [47] | |
| Alg/XG/AgNPs/Dex/Ca nanocomposite | 1. Electrostatic adsorption of pollutants on the catalyst’s surface 2. Reduction and weaker electrostatic 3. Desorption on the catalyst’s surface | [86] | |
| Nano copper/chitosan aerogel bio composite | 1. Adsorption of dyes and BH4- ions onto the catalyst’s surface 2. Dye reduction 3. Desorption | [87] | |
| Porous chitosan- gC3N4 nanosheets | Photodegradation (semiconductor photocatalyst) | [53] | |
| Ag–Co3O4 NFs/CS-CNFs/SPCE | Electrochemical redox process | [93] | |
| Chitosan-capped zns qds/NiFe2O4 | Enhancement in the absorption of visible light, robust electrostatic interactions, and charge separation of photogenerated charge carriers | [111] | |
| Metal-oxide catalysts MnOx-PP | Adsorption-enhanced catalytic oxidation | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadayoni, N.S.; Dinari, M.; Roy, A.; Karimi Abdolmaleki, M. Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants. Polymers 2024, 16, 1543. https://doi.org/10.3390/polym16111543
Tadayoni NS, Dinari M, Roy A, Karimi Abdolmaleki M. Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants. Polymers. 2024; 16(11):1543. https://doi.org/10.3390/polym16111543
Chicago/Turabian StyleTadayoni, Nayereh S., Mohammad Dinari, Aleena Roy, and Mahmood Karimi Abdolmaleki. 2024. "Recent Advances in Porous Bio-Polymer Composites for the Remediation of Organic Pollutants" Polymers 16, no. 11: 1543. https://doi.org/10.3390/polym16111543








