Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction
Abstract
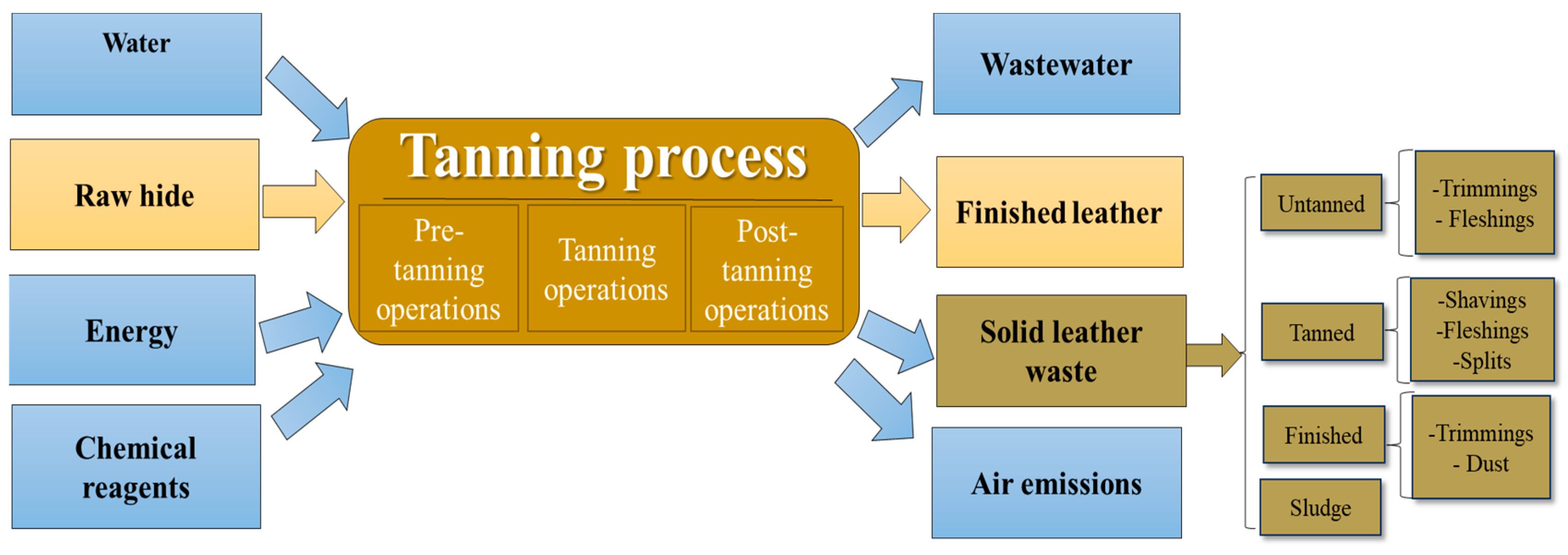
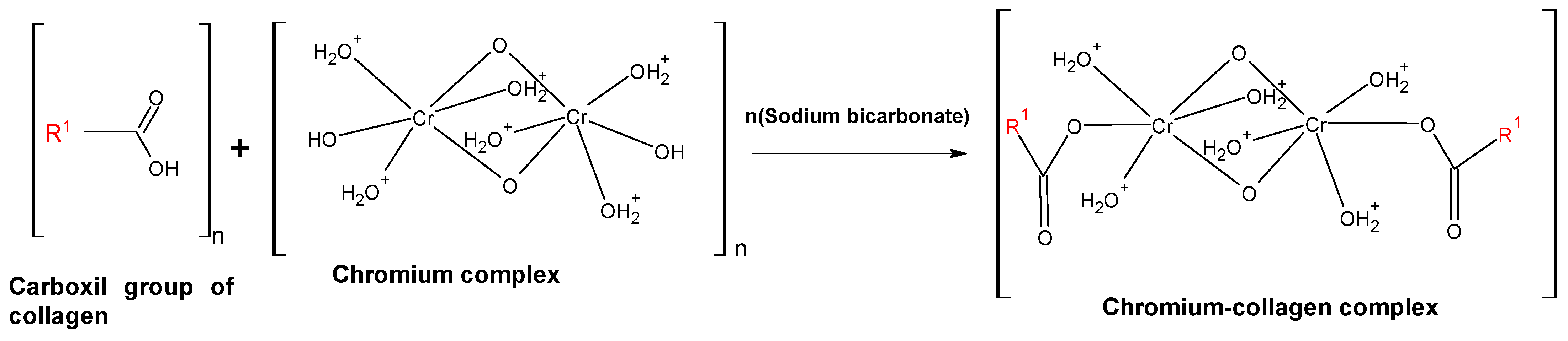
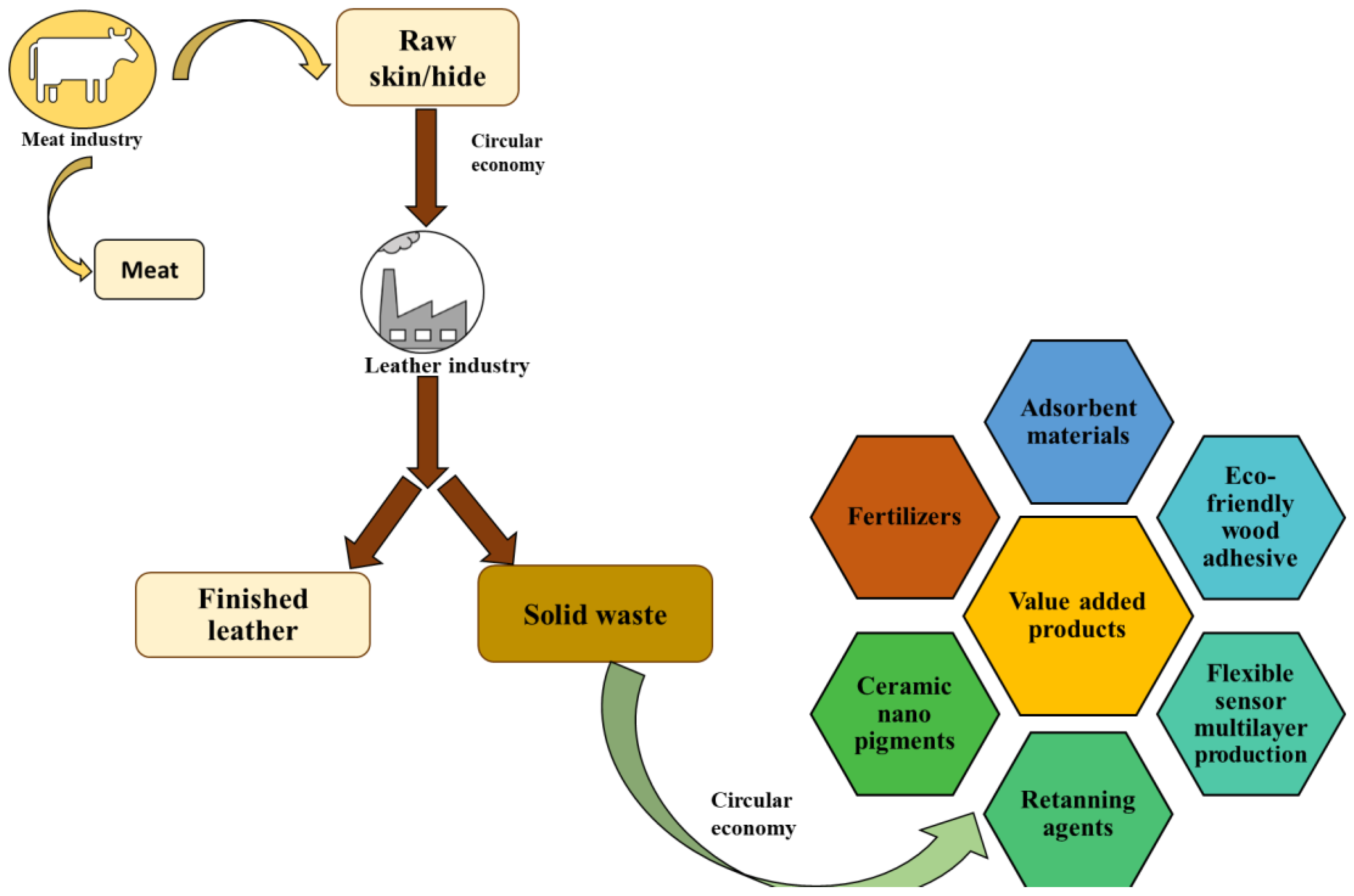
:1. Introduction
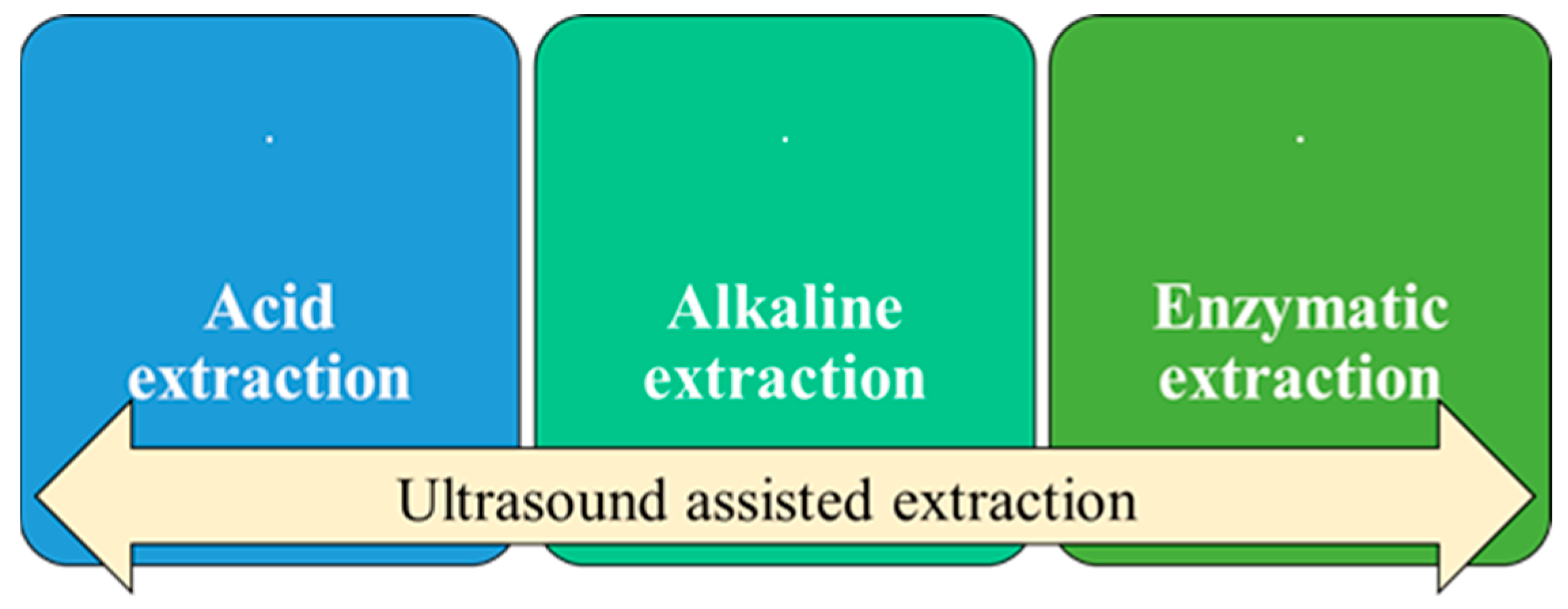
2. Chromium Extraction Methods
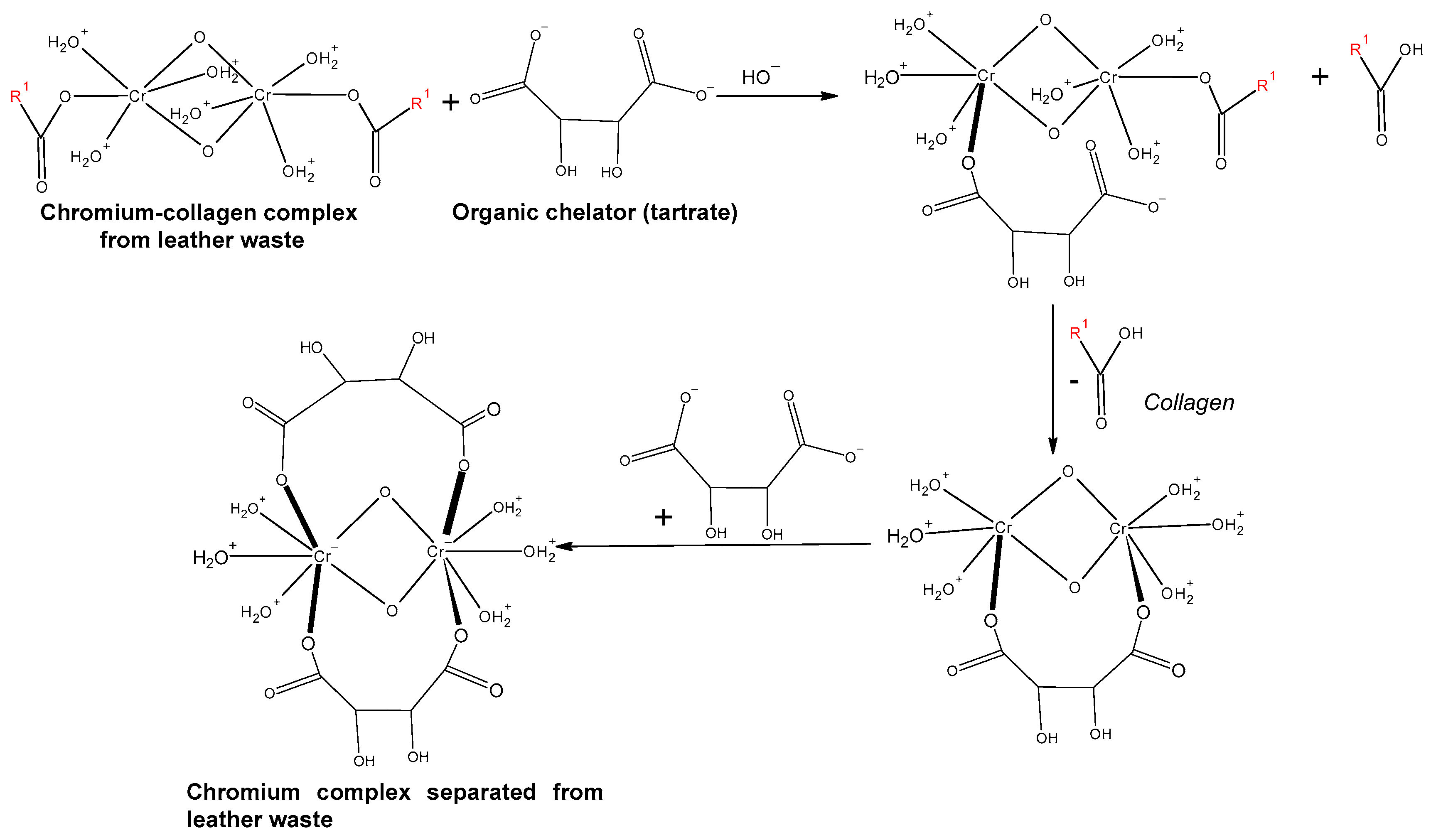
3. Acid Extraction of Chromium
4. Alkaline Extraction of Chromium
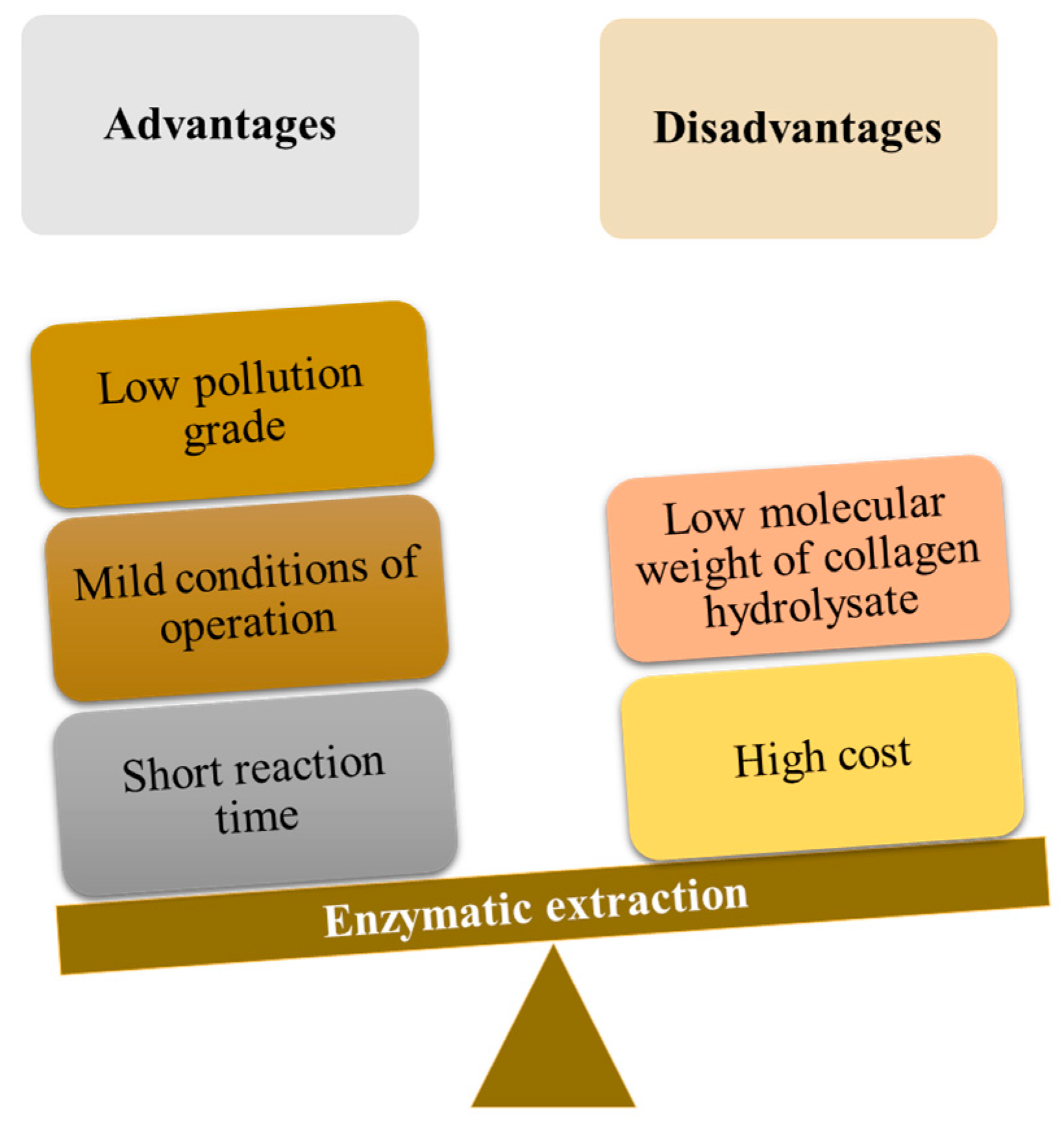
5. Enzymatic Extraction of Chromium
6. Ultrasound-Assisted Extraction of Chromium
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Chen, M.; Li, J.; Jin, Y. A novel substitution-based method for effective leaching of chromium A novel substitution-based method for effective leaching of chromium kinetics and mechanism studies. Waste Manag. 2022, 103, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Satish, M.; Madhan, B.; Sreeram, K.J.; Rao, J.; Nair, B.U. Alternative carrier medium for sustainable leather manufacturing—A review and perspective. J. Clean. Prod. 2016, 112, 49–58. [Google Scholar] [CrossRef]
- Bulijan, J.; Reich, G.; Ludvik, J. Mass Balance in Leather Processing. United Nations Industrial Development Organisation (UNIDO). 9 August 2000. Available online: https://www.unido.org/sites/default/files/2009-05/Mass_balance_in_leather_processing_0.pdf (accessed on 20 December 2023).
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.; Zainescu, G.; Stefa, S.; Meghea, A.; Kallitsis, J. Modification of Collagen Derivatives with Water-Soluble Polymers for the Development of Cross-Linked Hydrogels for Controlled Release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef]
- Rahmawati, D.; Setyadewi, N.M.; Sugihartono. Extraction and characterization of gelatin from skin trimming pickled waste of tannery. Earth Environ. Sci. 2019, 306, 012022. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Massuda, L.A.; Alessandretti, I.A.; Piccin, J.S.; Gettmer, A. Emerging contaminants adsorption by beads from chromium (III) tanned leather waste recovered gelatin. J. Mol. Liq. 2021, 330, 115638. [Google Scholar] [CrossRef]
- Ayele, M.; Limeneh, D.I.; Tesfaye, T.; Mengie, W.; Abuhay, A.; Haile, A.; Gebino, G. A Review on Utilization Routes of the Leather Industry Biomass. Adv. Mater. Sci. Eng. 2021, 2021, 1503524. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Crudu, A.M. Ecofriendly wet-white leather vs. conventional tanned wet-blue. A Photochem. Approach. J. Clean. Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Pre-treatment of tannery sludge for sustainable landfilling. Waste Manag. 2016, 52, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Famielec, S. Chromium Concentrate Recovery from Solid Tannery Waste in a Thermal Process. Materials 2020, 13, 1533. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C.; Kavitha, S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: A comprehensive review. J. Clean. Prod. 2015, 89, 1–17. [Google Scholar] [CrossRef]
- Mella, B.; Glanert, A.C.; Gutterres, M. Removal of chromium from tanning wastewater and its reuse. Process Saf. Environ. Prot. 2015, 95, 195–201. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Chandra Babu, N.K.; Mandal, A.B. Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean. Prod. 2008, 16, 1807–1813. [Google Scholar] [CrossRef]
- Nagueira, F.; Castro, I.; Bastos, A.; Souza, G.; Carvalho, J.; Oliveira, L. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Şaşmaz, S.; Karaağaç, B.; Uyanık, N. Utilization of chrome-tanned leather wastes in natural rubber and styrene-butadiene rubber blends. J. Mater. Cycles Waste Manag. 2019, 21, 166–175. [Google Scholar] [CrossRef]
- Malek, A.; Hachemi, M.; Didier, V. New approach of depollution of solid chromium leather waste by the use of organic chelates. Economical and environmental impacts. J. Hazard. Mater. 2009, 170, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, L.X.; Wang, S.M.; Fang, D. Removal of Cr from tannery sludge by bioleaching method. J. Environ. Sci. 2006, 18, 885–890. [Google Scholar] [CrossRef]
- Leathersmithe. Available online: www.leathersmithe.com/tanning-methods-and-the.html (accessed on 14 May 2024).
- Dwivedi, S.P.; Dixit, A.; Bajaj, R. Development of bio-composite material by utilizing chrome containing leather waste with Al2O3 ceramic particles. Mater. Res. Express 2019, 6, 105105. [Google Scholar] [CrossRef]
- Cardona, N.; Velásquez, S.; Giraldo, D. Characterization of Leather Wastes from Chrome Tanning and its Effect as Filler on the Rheometric Properties of Natural Rubber Compounds. J. Polym. Environ. 2017, 25, 1190–1197. [Google Scholar] [CrossRef]
- Tahiri, S.; DeLaGuardia, M. Treatment and valorization of leather industry solid: A review. J. Am. Leather Chem. Assoc. 2009, 104, 52–67. [Google Scholar]
- The European Commission. EUR-LEX. The European Commission. 18 December 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014D0955 (accessed on 6 November 2023).
- Stefan, D.S.; Bosomoiu, M.; Constantinescu, R.R.; Ignat, M. Composite polymers frim leather waste to produce smart fertilizers. Polymers 2021, 13, 4351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tan, K.; Wang, X.; Chen, Y. A rapid soil Chromium pollution detection method based on hyperspectral remote sensing data. Int. J. Appl. Earth Obs. Geoinf. 2024, 128, 103759. [Google Scholar] [CrossRef]
- Velusamy, M.; Chakali, B.; Ganesan, S.; Tinwala, F. Investigation on pyrolysis and incineration of chrome-tanned solid waste from tanneries for effective treatment and disposal: An experimental study. Environ. Sci. Pollut. Res. 2019, 27, 29778–29790. [Google Scholar] [CrossRef]
- Achmad, R.T.; Budiwan, B.; Auerkari, E.I. Effects of Chromium on Human Body. Annu. Res. Rev. Biology. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Amata, A.I. Chromium in Livestock Nutrition: A Review. Glob. Adv. Res. J. Agric. Sci. 2013, 2, 289–306. [Google Scholar]
- Shanker, A. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S. Surface reactions of chromium in soils and waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Thatoi, H.N.; Dal, B.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar]
- Ocak, B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci. Pollut. Res. 2018, 25, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Pati, A.; Subramani, S. A review on management of chrome-tanned leather shavings: A holistic paradigm to combat the environmental issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. 18 October 2023. Available online: www.atsdr.cdc.gov/spl/index.html#2022sp (accessed on 18 October 2023).
- Chrysochoou, M.; Johnston, C. Reduction of Chromium(VI) in Saturated Zone Sediments by Calcium Polysulfide and Nanoscale Zerovalent Iron Derived From Green Tea Extract. In GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering; ASCE 2012; Geotechnical Special Publication: Oakland, CA, USA, 2012; p. 3959. [Google Scholar]
- Zayed, A.M.; Terry, N. Chromium in the Environment: Fators Affecting Biological Remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Verma, S.K.; Sharma, P.C. Current trends in solid tannery waste management. Crit. Rev. Biotechnol. 2022, 43, 805–822. [Google Scholar] [CrossRef]
- de Matos, E.; Scopel, B.; Dettmer, A. Citronella essential oil microencapsulation by complex coacervation with leather waste gelatin and sodium alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- Sharf, A.; Gasmeleed, A.; Musa, A.E. Extraction of Chromium Six from Chrome Shavings. J. For. Prod. Ind. 2013, 2, 21–26. [Google Scholar]
- Yang, J.; Shan, Z.; Zhang, Y.; Chen, L. Stabilization and cyclic utilization of chrome leather shavings. Environ. Sci. Pollut. Res. 2018, 26, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- da Costa Cunha, G.; Peixoto, J.A.; de Souza, D.R.; Cruz Romao, L.P.; Macedo, A.S. Recycling of chromium wastes from tanning industry to produce ceramic nanopigments. Green Chem. 2016, 18, 5342–5356. [Google Scholar] [CrossRef]
- He, B.; Du, Y.; Xu, H.; Ma, J. Synthesis of Ceramic Pigments with Chromium Content from Leather Waste. Trans. Indian Ceram. Soc. 2021, 2021, 103–109. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, T. Formulating Nitrogen-Phosphorous-Potassium enriched organic manure from solid waste: A novel approach of waste valorization. Process Saf. Environ. Prot. 2019, 132, 160–168. [Google Scholar] [CrossRef]
- Wang, X.; Yue, O.; Liu, X.; Hou, M.; Zheng, M. A novel bio-inspired multi-functional collagen aggregate based flexible sensor with multi-layer and internal 3D network structure. Chem. Eng. J. 2020, 392, 123672. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Dang, X. An eco-friendly wood adhesive based on waterborne polyurethane grafted with gelatin derived from chromium shavings waste. Environ. Res. 2022, 206, 112266. [Google Scholar] [CrossRef] [PubMed]
- Younas, H.; Nazir, A.; Bareen, F. Application of microbe-impregnated tannery solid waste biochar in soil enhances growth performance of sunflower. Environ. Sci. Pollut. Res. 2022, 29, 57669–57687. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Kantarli, I.C.; Yuksel, M.; Saglam, M. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, H.; Yuan, Y.; Peng, J.; Yao, G.; Zhou, P.; Lai, B. Coupling adsorption and in-situ Fenton-like oxidation by waste leather-derived materials in continuous flow mode towards sustainable removal of trace antibiotics. Chem. Eng. J. 2021, 420, 130370. [Google Scholar] [CrossRef]
- Senthil, R.; Kavukcu, S.B.; Cakir, S.; Turkmen, H. Utilization of various solid leather wastes for the production of blended bricks. Clean Technol. Environ. Policy 2022, 24, 1889–1901. [Google Scholar] [CrossRef]
- Zainescu, G.; Deselnicu, V.; Constantinescu, R.; Georgescu, D. Biocomposites from tanned leather fibres with applications in constructions. Leather Footwear J. 2018, 18, 203–206. [Google Scholar] [CrossRef]
- Jeyasingh, J.; Philip, L. Bioremediation of chromium contaminated soil: Optimization of operating parameters under laboratory conditions. J. Hazard. Mater 2005, 118, 113–120. [Google Scholar] [CrossRef]
- Sethunathan, N.; Meghraj, M.; Smith, L.; Kamalideen, S.P.B.; Avudainayagam, S.; Naidu, R. Microbial role in the failure of natural attenuation of chromium (VI) in long-term tannery waste contaminated soil. Agric. Ecosyst. Environ. 2005, 105, 657–661. [Google Scholar] [CrossRef]
- Cavington, A. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Pati, A.; Chaudhary, R. Soybean plant growth study conducted using purified protein hydrolysate-based fertilizer made from chrome-tanned leather ysate-based fertilizer made from chrome-tanned leather. Environ. Sci. Pollut. Res. 2015, 22, 20316–20321. [Google Scholar] [CrossRef]
- Taylor, M. Effect of processing variables on ash content of galable and hydrolyzed portein products isolated from treatment of chromium leather waste. J. Am. Leather Chem. Assoc. 1993, 88, 358–367. [Google Scholar]
- Scopel, B.; Baldasso, C.; Mettmer, A.; Santana, R. Hydrolysis of Chromium Tanned Leather Waste: Turning Waste into Valuable Materials—A Review. J. Am. Leather Chem. Assoc. 2018, 113, 122–129. [Google Scholar]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Sarvanan, P. Extraction of collagen from raw trimming wastes of tannery: A waste to wealth approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Sanlier, S.H. Preparation of magnetic gelatin nanoparticles and investigating the possible use as chemotherapeutic agent. Artif. Cells Nanomed. Biotechnol. 2013, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Jin, Y.; Chen, M. Study on the removal of chromium(III) from leather waste by a two-step method. J. Ind. Eng. Chem. 2019, 79, 172–180. [Google Scholar] [CrossRef]
- Ferreira, M.; Almeida, M.; Pinho, S.; Santos, I. Finished leather waste chromium acid extraction and anaerobic biodegradation of the products. Waste Manag. 2010, 30, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, C.; Zanatta, R.; Santos, D.; Giacobe, K.; Dallago, R.; Mello, P.; Flores, E. Ultrassound assisted extraction of chromium from residual tannd leaher: An inovative strategy for the reuse of waste in tanning industry. Ultrason. Sonochem. 2020, 64, 104682. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Wang, H.; Zhang, K. Regeneration of native collagen from hazardous waste: Chrome tanned leather shavings by acid method. Environ. Sci. Pollut. Res. 2020, 27, 31300–31310. [Google Scholar] [CrossRef]
- Brown, D.A. Investigation of carboxylic acids for the extraction of chromium (III) from the leather waste and the posiible re-use of the extracted chromium tanning industry. Environ. Technol. Lett. 1986, 7, 289–298. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Li, J.; Jin, Y.; Zhang, Y.; Wang, Y. A novel substitution—Based method for effective leaching of chromium (III) from chromium- tanned leather waste: The termodynamics, kinetics and mechanism studies. Waste Manag. 2020, 103, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Popiolski, A.; Dallago, R.; Steffens, J.; Mignoni, M.; Venquiaruto, L.; Santos, D.; Duarte, F. Ultrasound-Assisted Extraction of Cr from Residual Tannery Leather: Feasibility of Ethylenediaminetetraacetic Acid as the Extraction Solution. ACS Omega 2018, 3, 16074–16080. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.; Santos, D.; Cauduro, V.; Bizii, C.; Flores, E. Ultrasound-assisted extraction of chromium from tanned leather shavings: A promising continous flow technology for the treatment of solid waste. Ultrason. Sonochem. 2022, 89, 106124. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W.; Charewicz, W.; Adamski, Z. Recovery of chromium (III) from waste of uncolored chromium leaters. Part I. Kinetic studies on alkaline hydrolytic decomposition of the waste. Sep. Purif. Technol. 2011, 81, 223–236. [Google Scholar] [CrossRef]
- Hinojosa, J.A.B.; Marrufo, L. Optimization of Alkaline Hydrolysis of Chrome Shavings to Recover Collagen Hydrolysate and Chromium Hydroxide. Leather Footwear J. 2020, 20, 15–28. [Google Scholar] [CrossRef]
- Pahlawan, I.F.; Sutyasmi, S.; Griyanitasari, G. Hydrolysis of leather shavings waste for protein binder. In Proceedings of the International Conference on Green Agro-industry and Bioeconomy, Malang, Indonesia, 18–20 September 2019. [Google Scholar]
- Ferreira, M.J.; Almeida, M.F.; Pinho, S.C.; Gomes, J.R.; Rodrigues, J.L. Alkaline hydrolysis of chromium tanned leather scrap fibers and anaerobic biodegration of the products. Waste Biomass Valoriz. 2014, 5, 551–562. [Google Scholar] [CrossRef]
- Poulopoulou, V.; Katakis, D.; Vrachnou, E. A method of removal chromium from tanned leather wastes. J. Air Waste Manag. Assoc. 1998, 48, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xuepin, L.; Zhang, W.; Shi, B. Dechroming of chromium containing leather waste with low hydrolysis degree of colagen. J. Soc. Leather Technol. Chem. 2015, 99, 129–133. [Google Scholar]
- Asava, A.; Sang, P.; Onyuka, A. Recovery of Collagen Hydrolysate from Chrome Leather Shaving Tannery Waste through Two-Step Hydrolysis using Magnesium Oxide and Bating Enzyme. Soc. Leather Technol. Chem. J. 2019, 103, 80–85. [Google Scholar]
- Dettmer, A.; Oliveira dos Santos, R.M. Protein extraction from chromium tanned leather waste by Bacillus subtilis enzymes. J. AQEIC 2014, 65, 93–100. [Google Scholar]
- Qiang, X.H.; Feng, H. Collagen Extracted from Chrome Shavings Using Alkali and Enzyme. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011. [Google Scholar]
- Tahiri, S.; Mohamed, B.; Albizane, A.; Massaoudi, A. Extraction of proteins from chrome shavings with sodium hydroxide and reuse of chromium in the tanning process. J. Am. Leather Chem. Assoc. 2004, 99, 16–25. [Google Scholar]
- Pecha, J.; Barinova, M.; Kolomaznik, K.; Nguyen, T.N. Technological-economic optimization of enzymatic hydrolysis used for the processing of chrome-tanned leather waste. Process Saf. Environ. Prot. 2021, 152, 220–229. [Google Scholar] [CrossRef]
- Rahaman, A.; Raihan, M.; Islam, M.D. Recovery of chromium from chrome shaving dust. Eur. Acad. Res. 2017, 4, 9441–9448. [Google Scholar]
- Pollet, B.; Ashokkumar, M. Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mason, T.J.; Cobley, A.J.; Graves, J.E.; Morgan, D. New evidence for the inverse dependence of mechanical and chemicaleffects on the frequency of ultrasound. Ultrason. Sonochem. 2011, 18, 226–230. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sanchez-Brunete, C.; Albero, B.; Garcia-Valcarcel, A. Application of ultrasound-assisted extraction to the determi-nation of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]


| Chromium Removal Method | Chromium Removal Method | Chromium Extraction Yield | The Degree of Collagen Hydrolysis | Reference |
|---|---|---|---|---|
| Acid extraction | - Concentration of extraction solution = 8% H2SO4 - H2SO4:sample ratio = 11:1 - Time = 2.5 h - Temperature = 343 K | >95% | - | [63] |
| Acid extraction | - Concentration of extraction solution = 25 mL/L H2SO4 | 30–60% ± 5% | 3–6 ± 1% | [64] |
| - Time = 3 or 6 days | ||||
| Acid extraction | - Sample amount = 150 mg | 92% | - | [65] |
| - Concentration of extraction solution = 3 mol/L HNO3 | ||||
| - Temperature = 30 °C | ||||
| - Time = 30 min - Amplitude = 90% US bath = 37 kHz | ||||
| Acid extraction | - H2C2O4/H2SO4/sample ratio = 2:1:1 - Time = 12 h - Processing times = 1 h - Stirring speed = 250 r/min - Temperature = 40 °C | 95.6% | 90.6% | [66] |
| Acid extraction | - Extraction agent: oxalic acid - Time = 36 h - Room temperature - pH = 5.5 - Cr–oxalic acid ratio = 1:3 | 71% | - | [67] |
| Acid extraction | - Concentration of potassium tartrate = 0.5 M - NaOH solution concentration = 0.25 M - Room temperature - Time = 72 h | 95% | - | [19] |
| Acid extraction | - Concentration of sodium oxalate = 2% - Sodium oxalate/sample ratio = 200 mL/g - Thickness of sample = 0.5 mm - Temperature = 333 K - Time = 5 h - Stirring speed = 150 rpm | 98% | >95% | [68] |
| Acid extraction | - Sample amount = 3 g - Cr3+/EDTA ratio = 1:3 - Temperature = 80 °C - Time = 30 de minutes - Ultrasonic bath = 25 kHz Amplitude = 100% - 5 washing cycles with water (V = 50 mL), at a temperature of 50 °C, for 3 min each | 98% | - | [69] |
| Acid extraction | - EDTA/Cr 3+ ratio = 3:1 - US power = 150 W - Frequency = 20 KHz - Residence time = 60 min - Temperature = 70 °C | 71.7% | - | [70] |
| Alkaline extraction | - Concentration of extraction solution = 0.2 M NaOH - NaOH/sample ratio = 80 cm3/g - Time = 1 h - Temperature = 60 °C | 90% | ~100% | [71] |
| Alkaline extraction | - Concentration of extraction solution = 0.47 M NaOH - Time = 90 min - Temperature = 70 °C | 750.8 g | 87.165% | [72] |
| Alkaline extraction | - Concentration of extraction solution = 3% NaOH - Time = 180 min - Temperature = 90 °C - NaOH/sample ratio = 5:1 | ~100% | - | [73] |
| Alkaline extraction | - Concentration of extraction solution = 4 M NaOH - NaOH/sample ratio = 0.15 - Time = 90 min - Temperature = 423 K | 85% | 98% | [74] |
| Alkaline extraction | - H2SO4 concentration = 0.1 N H2SO4 - Dose of Gamma radiation 60Co = 60 Krad - Concentration of extraction solution = 1 N NaOH | ~100% | 25–40% | [75] |
| Alkaline extraction | - Concentration of extraction solution 1 = 2 g/L NaOH, stirring for 30 min at 30 °C, urea concentration = 40 g/L - Concentration of extraction solution 2 = 50 g/L H2SO4, stirring for 1 h at 30 °C - Concentration of extraction solution 3 = 40 g/L CaOH, stirring for 2 ore at 30 °C - Concentration of extraction solution 4 = 50 g/L H2SO4, stirring for 1 h at 30 °C | 97% | 10% | [76] |
| Enzymatic extraction | - Extraction solution concentration = 6% MgO - Bating enzyme concentration = 0.75% - Time = 30 h - Temperature = 33–37 °C - pH = 8.3–8.5 | 99.99% | - | [77] |
| Enzymatic extraction | - Extraction solution MgO - Stirring speed = 60 rpm - Temperature = 70 °C - Time = 6 h - Bacillus subtilis enzyme A proteolytic activity = 130.5 U/mL - pH = 9 - Time = 15 h - Temperature = 45 °C - Stirring speed = 60 rpm | ~100% | - | [78] |
| Enzymatic extraction | - Extraction solution concentration = 3% MgO - Extraction solution concentration = 3% CaO - Temperature = 80 °C - Time = 4 h - 1398 neutral protease concentration = 0.125% - Temperature = 46 °C | ~100% | >60% | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codreanu, A.-M.N.; Stefan, D.S.; Kim, L.; Stefan, M. Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction. Polymers 2024, 16, 1546. https://doi.org/10.3390/polym16111546
Codreanu A-MN, Stefan DS, Kim L, Stefan M. Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction. Polymers. 2024; 16(11):1546. https://doi.org/10.3390/polym16111546
Chicago/Turabian StyleCodreanu (Manea), Ana-Maria Nicoleta, Daniela Simina Stefan, Lidia Kim, and Mircea Stefan. 2024. "Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction" Polymers 16, no. 11: 1546. https://doi.org/10.3390/polym16111546







