A Cellulose-Based Dual-Crosslinked Framework with Sensitive Shape and Color Changes in Acid/Alkaline Vapors
Abstract
1. Introduction
2. Experimental Section
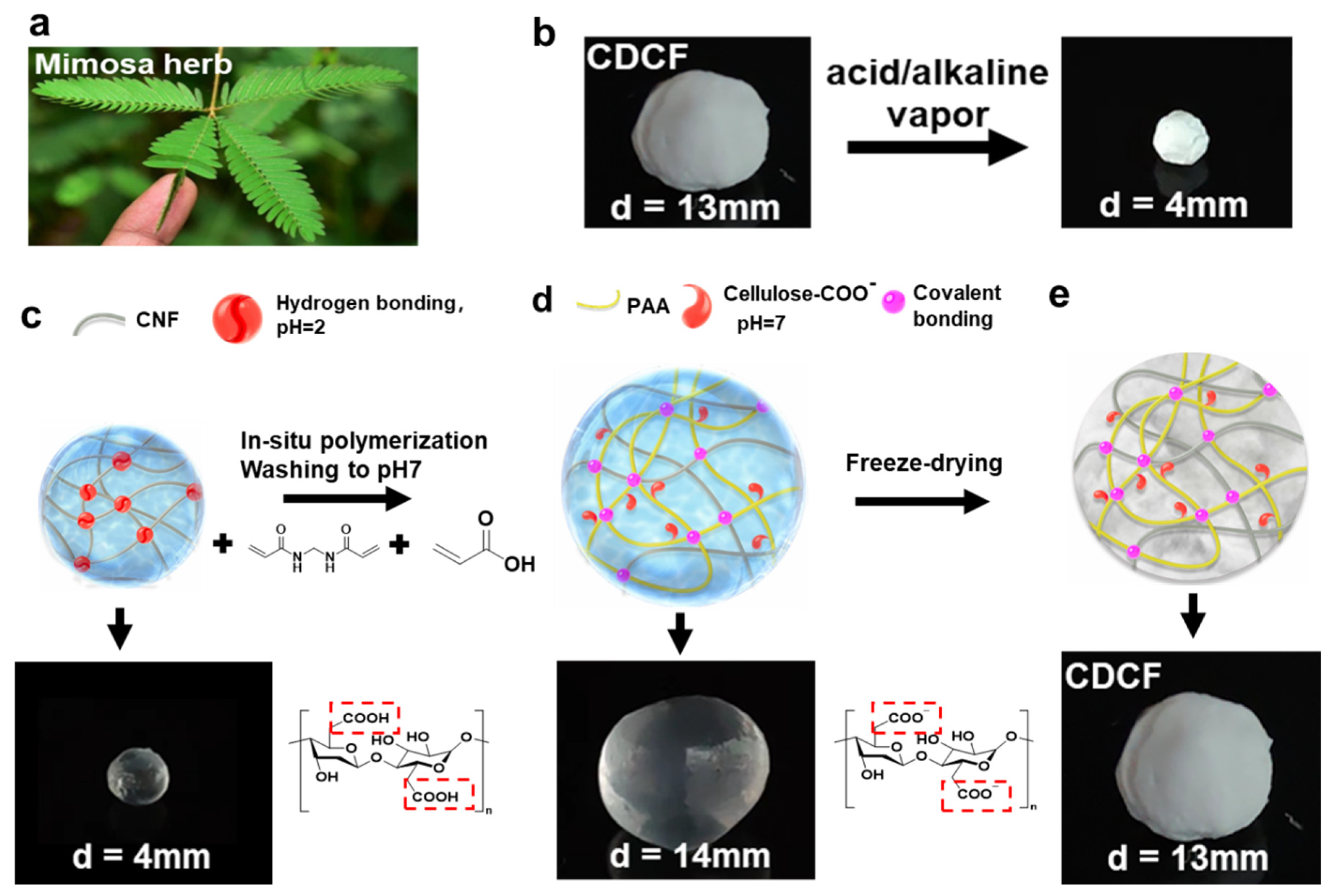
2.1. Fabrication of Nanocellulose Aerogels
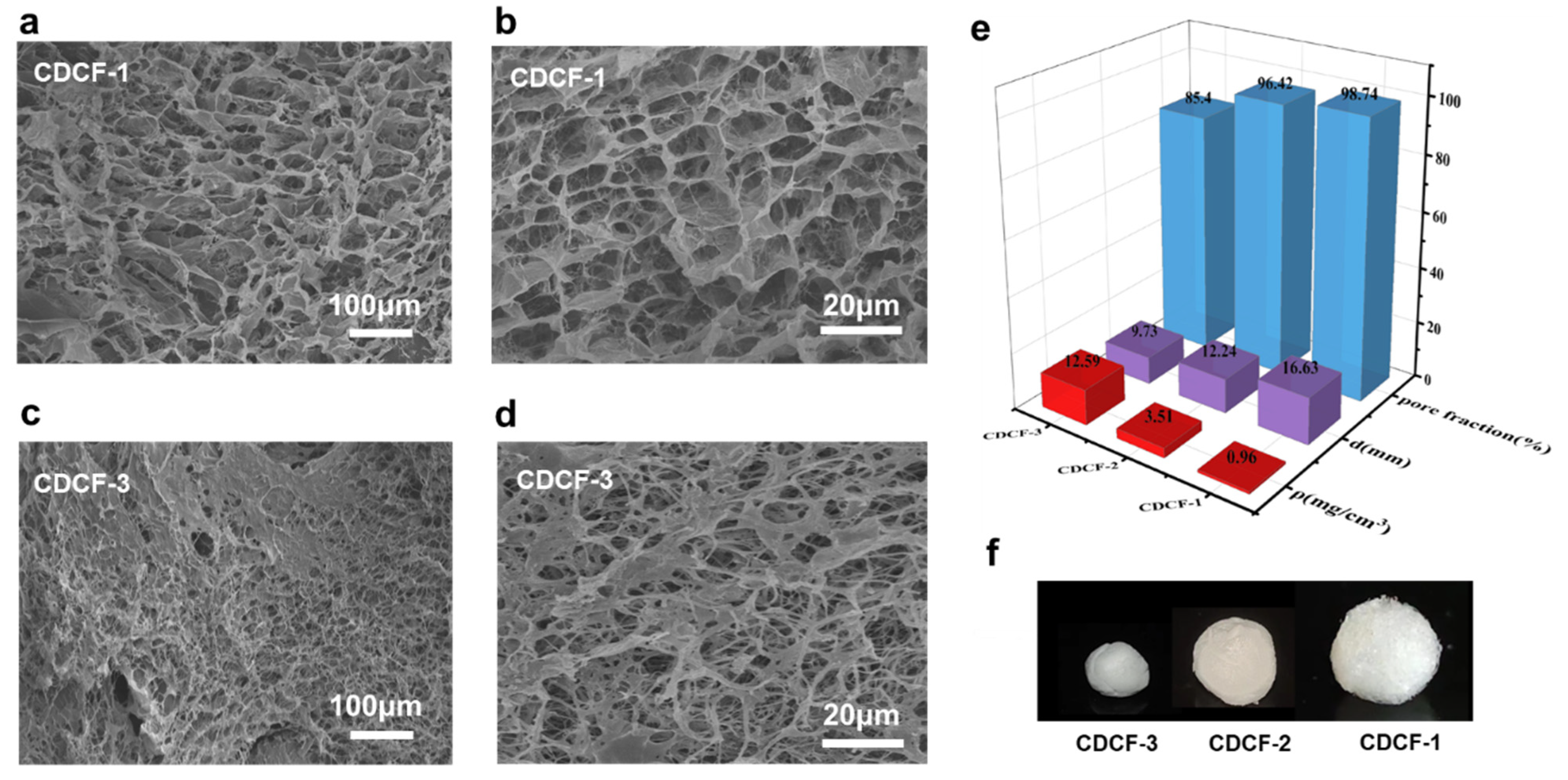
2.2. Density and Porosity
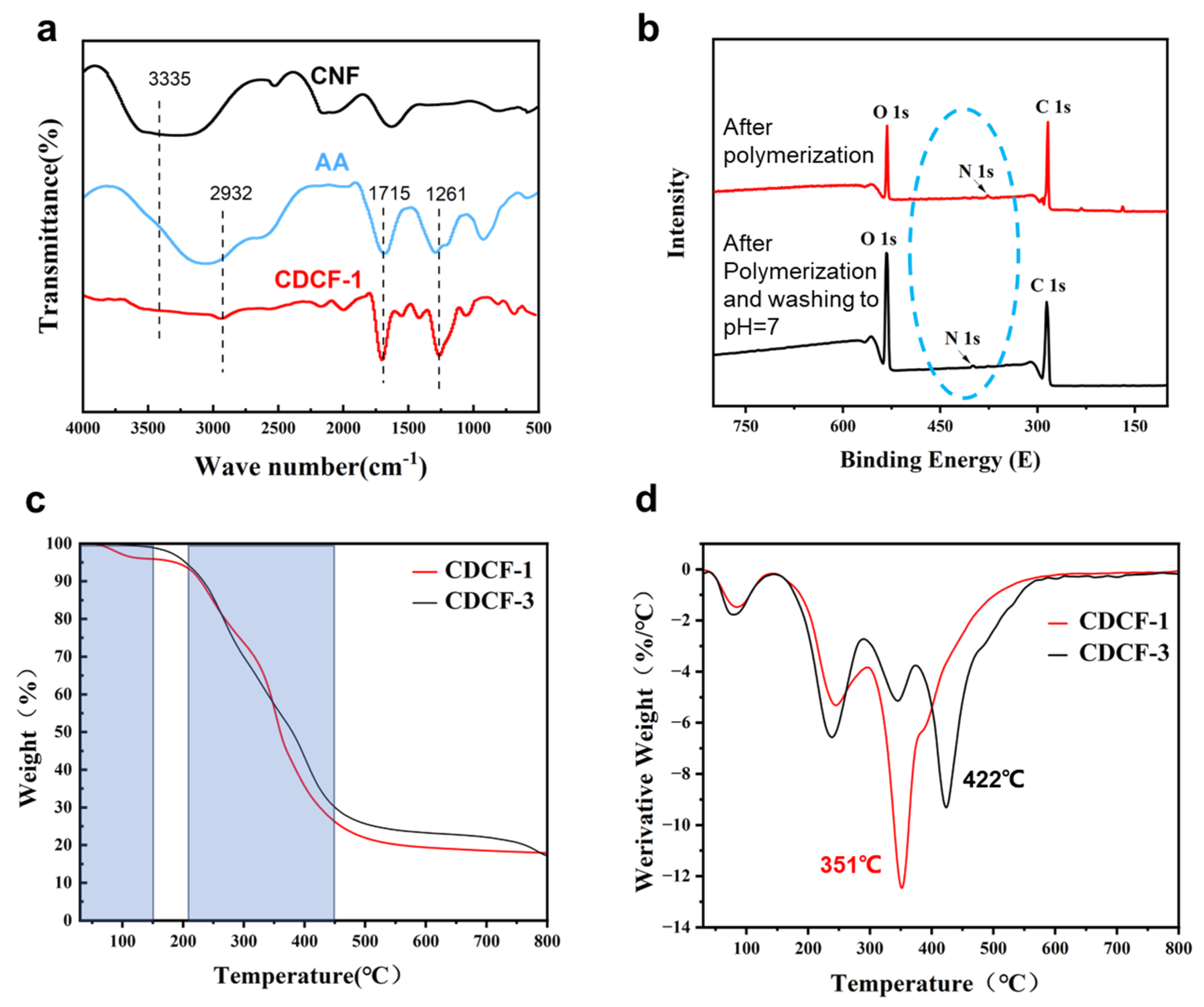
2.3. The Characterization of Aerogels
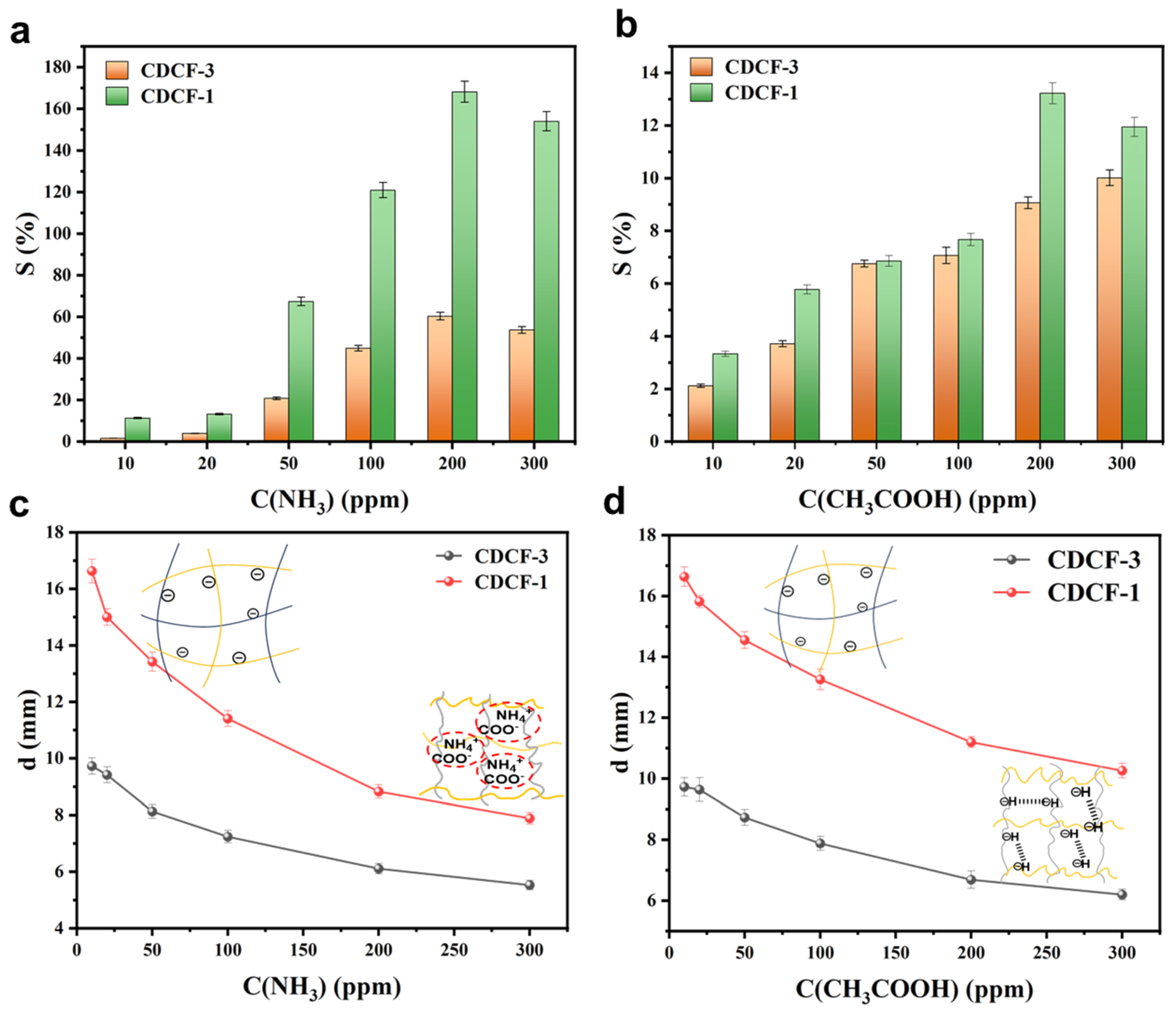
2.4. Vapor Sensitivity Testing
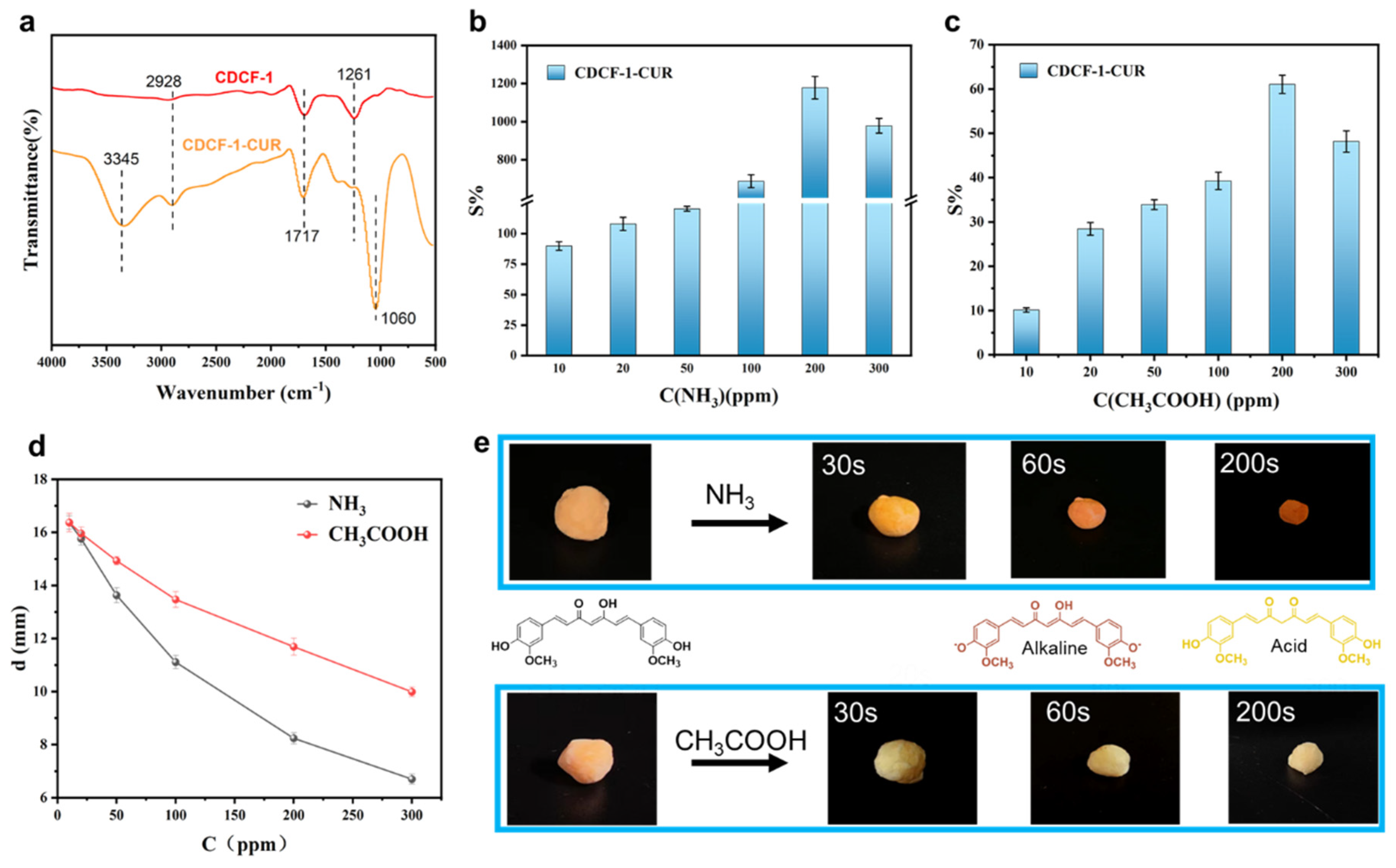
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Cai, X.; Zhang, X.; Zhang, J.; Fan, J.; Ma, F.; Zhu, W.; Jia, X.; Wang, S.; Meng, Z. Hazardous Gases-Responsive Photonic Crystals Cryogenic Sensors Based on Antifreezing and Water Retention Hydrogels. ACS Appl. Mater. Interfaces 2023, 15, 42046–42055. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, S.; Moghadam, T.T.; Sajedi, R.H. Facile and Rapid Detection of Microalbuminuria by Antibody-Functionalized Gold Nanorods. Plasmonics 2022, 17, 1269–1277. [Google Scholar] [CrossRef]
- Yu, Q.; Li, M.; Gao, J.; Xu, P.; Chen, Q.; Xing, D.; Yan, J.; Zaworotko, M.J.; Xu, J.; Chen, Y.; et al. Fabrication of Large Single Crystals for Platinum-Based Linear Polymers with Controlled-Release and Photoactuator Performance. Angew. Chem. Int. Ed. 2019, 58, 18634–18640. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, S.Q.; Liu, Z.; Chen, Y.; Zaworotko, M.J.; Cheng, P.; Ma, J.G.; Zhang, Z. Fabrication of Moisture-Responsive Crystalline Smart Materials for Water Harvesting and Electricity Transduction. J. Am. Chem. Soc. 2021, 143, 7732–7739. [Google Scholar] [CrossRef] [PubMed]
- Odent, J.; Vanderstappen, S.; Toncheva, A.; Pichon, E.; Wallin, T.J.; Wang, K.; Shepherd, R.F.; Dubois, P.; Raquez, J.-M. Hierarchical chemomechanical encoding of multi-responsive hydrogel actuators via 3D printing. J. Mater. Chem. A 2019, 7, 15395–15403. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, J.; Huang, L.L.; Chen, L.H.; Gao, H.L.; Ni, Y.H.; Zheng, Q.H. High-Sensitivity Multiresponses Cellulose-Based Actuators with Configurable Amplitude. ACS Sustain. Chem. Eng. 2022, 10, 6414–6425. [Google Scholar] [CrossRef]
- Mizutani, M.; Kanaoka, M.M. Environmental sensing and morphological plasticity in plants. Semin. Cell Dev. Biol. 2018, 83, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.F.; Lou, Y.L.; Jiang, S.; Dai, H.Q.; Yang, P.; Zhou, X.Y.; Fang, G.G.; Wu, W.B. High flux composite membranes based on glass/cellulose fibers for efficient oil-water emulsion separation. Colloids Surf. A 2022, 647, 6414–6425. [Google Scholar] [CrossRef]
- Karakus, S.; Insel, M.A.; Kahyaoglu, I.M.; Albayrak, I.; Ustun-Alkan, F. Characterization; optimization, and evaluation of preservative efficacy of carboxymethyl cellulose/hydromagnesite stromatolite bio-nanocomposite. Cellulose 2022, 29, 3871–3887. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Lalsangi, S.; Santra, S.; Banerjee, R. Nanocellulose as a carrier for improved drug delivery: Progresses and innovation. J. Drug Deliv. Sci. Technol. 2024, 97, 105743. [Google Scholar] [CrossRef]
- Mendes, G.; Faria, M.; Carvalho, A.; Gonçalves, M.C.; de Pinho, M.N. Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties. Carbohydr. Polym. 2018, 189, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, M.; Fujikawa, S.; Kunitake, T. Robust, Hyper-Permeable Nanomembrane Composites of Poly(dimethylsiloxane) and Cellulose Nanofibers. ACS Appl. Mater. Inter. 2021, 13, 61189–61195. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.T.; Du, J.J.; Kou, J.F.; Zhang, Z.Y.; Liu, F.Y. Hierarchical porous cellulose/lanthanide hybrid materials as luminescent sensor. J. Rare Earth 2018, 36, 1036–1043. [Google Scholar] [CrossRef]
- Shahi, N.; Lee, E.; Min, B.; Kim, D.J. Rice Husk-Derived Cellulose Nanofibers: A Potential Sensor for Water-Soluble Gases. Sensors 2021, 21, 4415. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Manuspiya, H. Fabrication of hybrid thin film based on bacterial cellulose nanocrystals and metal nanoparticles with hydrogen sulfide gas sensor ability. Carbohydr. Polym. 2020, 230, 115566. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Effect of freeze-drying parameters on the microstructure and thermal insulating properties of nanofibrillated cellulose aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 475–485. [Google Scholar] [CrossRef]
- Tüzün, E. Synthesis of novel antioxidant carboxymethylcellulose nanocomposites for Cu–Ni–Mo-based steel foams. Cellulose 2023, 30, 8753–8768. [Google Scholar] [CrossRef]
- Hossain, L.; Raghuwanshi, V.S.; Tanner, J.; Wu, C.M.; Kleinerman, O.; Cohen, Y.; Garnier, G. Structure and swelling of cross-linked nanocellulose foams. J. Colloid Interface Sci. 2020, 568, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Camargos, C.H.M.; Poggi, G.; Chelazzi, D.; Baglioni, P.; Rezende, C.A. Strategies to mitigate the synergistic effects of moist-heat aging on TEMPO-oxidized nanocellulose. Polym. Degrad. Stab. 2022, 200, 109943. [Google Scholar] [CrossRef]
- Li, P.P.; Li, M.H.; Sun, B.; Li, X.R.; Xiao, Q.Y.; Yue, D.M.; Gao, S.; Wang, B.; Jiang, X.B.; Jiang, J.W.; et al. Integrated Three-Dimensional Microdevice with a Modified Surface for Enhanced DNA Separation from Biological Samples. ACS Appl. Mater. Inter. 2023, 15, 55297–55307. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shao, C.; Zhou, S.; Yang, J.; Xu, F. Preparation of carbon aerogels from TEMPO-oxidized cellulose nanofibers for organic solvents absorption. RSC Adv. 2017, 7, 38220–38230. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Cai, Z.Y.; Gong, S.Q. Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Li, M.; Liu, L.; Liu, L.; Yu, J. Cellulose nanofibril (CNF) based aerogels prepared by a facile process and the investigation of thermal insulation performance. Cellulose 2020, 27, 6217–6233. [Google Scholar] [CrossRef]
- Zhao, J.; Xi, X.T.; Ouyang, H.Y.; Yang, J.Y.; Wang, Y.; Yi, L.F.; Song, D.Y.; Song, Y.J.; Zhao, L.J. Acidic and alkaline gas sensitive and self-healing chitosan aerogel based on electrostatic interaction. Carbohydr. Polym. 2021, 272, 118445. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.S.A.; Greish, Y.E.; Mahmoud, S.T.; Qamhieh, N.N.; El-Maghraby, H.F.; Zeze, D. Fabrication and characterization of cellulose acetate-based nanofibers and nanofilms for H2S gas sensing application. Carbohydr. Polym. 2021, 258, 117643. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldaña, M.D. Lupin hull cellulose nanofiber aerogel preparation by supercritical CO2 and freeze drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Amirjani, A.; Salehi, K.; Sadrnezhaad, S.K. Simple SPR-based colorimetric sensor to differentiate Mg2+ and Ca2+ in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120692. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Kamani, P.; Hosseini, H.R.M.; Sadrnezhaad, S.K. SPR-based assay kit for rapid determination of Pb(2). Anal. Chim. Acta 2022, 1220, 340030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhan, Z.; Zhu, M.; Lai, H.; He, X.; Deng, W.; Zhang, Q.; Wang, Y. An efficient electrocatalytic system composed of nickel oxide and nitroxyl radical for the oxidation of bio-platform molecules to dicarboxylic acids. J. Energy Chem. 2023, 80, 58–67. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Wiens, R.A.; Gough, K.M.; Aluko, R.E. Influence of acetylation on physicochemical and morphological characteristics of pigeon pea starch. Food Hydrocolloid 2020, 100, 105424. [Google Scholar] [CrossRef]
- Wei, N.; Lv, Z.; Meng, X.; Liang, Q.; Jiang, T.; Sun, S.; Li, Y.; Feng, J. Sodium alginate-carboxymethyl chitosan hydrogels loaded with difenoconazole for pH-responsive release to control wheat crown rot. Int. J. Biol. Macromol. 2023, 252, 126396. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Bai, W.; He, J.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Superwetting Monolithic Hollow-Carbon-Nanotubes Aerogels with Hierarchically Nanoporous Structure for Efficient Solar Steam Generation. Adv. Energy Mater. 2018, 9, 1802158. [Google Scholar] [CrossRef]
- Li, F.; Tang, Q. Understanding the role of functional groups of thiolate ligands in electrochemical CO2 reduction over Au(Ⅲ) from first-principles. J. Mater. Chem. A 2019, 7, 19872–19880. [Google Scholar] [CrossRef]
- He, H.; Song, Y.; Li, M.; Zhang, H.; Li, J.; Huang, H.; Li, Y. A novel anthocyanin electrospun film by caffeic acid co-pigmentation for real-time fish freshness monitoring. Anal. Methods 2023, 15, 228–239. [Google Scholar] [CrossRef]
- Pourreza, N.; Golmohammadi, H. Application of curcumin nanoparticles in a lab-on-paper device as a simple and green pH probe. Talanta 2015, 131, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocolloid 2020, 100, 105438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Qian, X.; Gou, Y.; Zheng, C.; Zhang, F. A Cellulose-Based Dual-Crosslinked Framework with Sensitive Shape and Color Changes in Acid/Alkaline Vapors. Polymers 2024, 16, 1547. https://doi.org/10.3390/polym16111547
Sun Y, Qian X, Gou Y, Zheng C, Zhang F. A Cellulose-Based Dual-Crosslinked Framework with Sensitive Shape and Color Changes in Acid/Alkaline Vapors. Polymers. 2024; 16(11):1547. https://doi.org/10.3390/polym16111547
Chicago/Turabian StyleSun, Yuxin, Xinye Qian, Yan Gou, Chunling Zheng, and Fang Zhang. 2024. "A Cellulose-Based Dual-Crosslinked Framework with Sensitive Shape and Color Changes in Acid/Alkaline Vapors" Polymers 16, no. 11: 1547. https://doi.org/10.3390/polym16111547
APA StyleSun, Y., Qian, X., Gou, Y., Zheng, C., & Zhang, F. (2024). A Cellulose-Based Dual-Crosslinked Framework with Sensitive Shape and Color Changes in Acid/Alkaline Vapors. Polymers, 16(11), 1547. https://doi.org/10.3390/polym16111547




