Lunar Regolith Geopolymer Concrete for In-Situ Construction of Lunar Bases: A Review
Abstract
:1. Introduction
2. Geopolymer Materials
2.1. Reaction Mechanism of Geopolymer
2.2. Factors Influencing Mechanical Properties
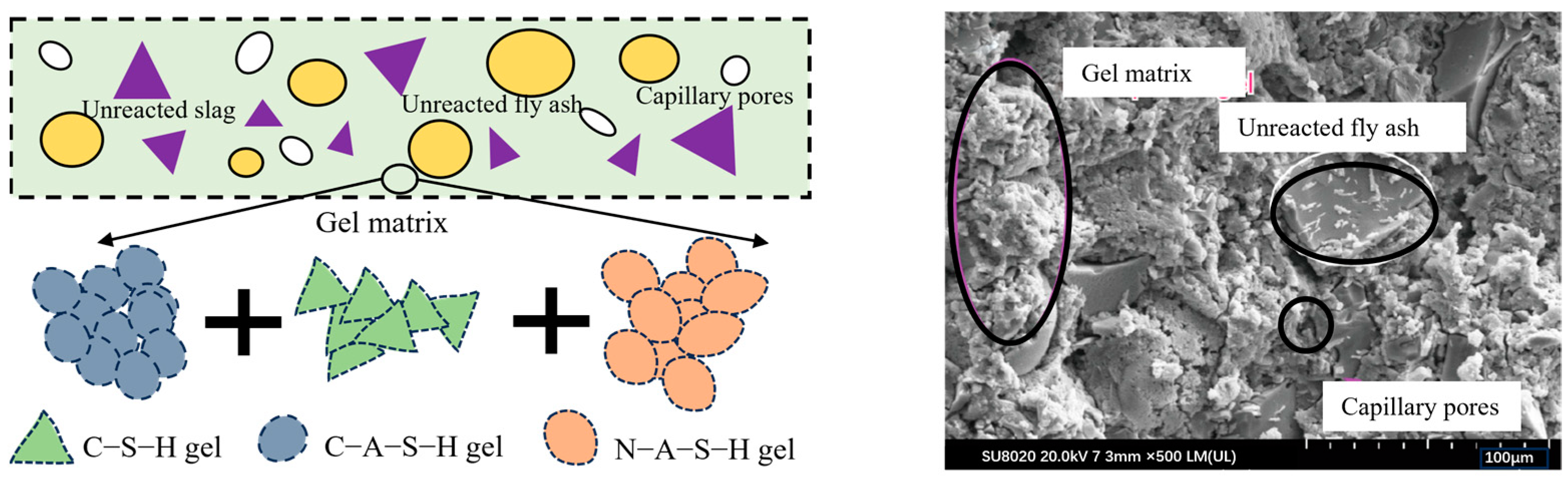
2.2.1. Content of Amorphous Substances
2.2.2. Chemical Composition
| System | Precursors | Activator | n(SiO2/Na2O) | Na2O% | Compressive Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| High calcium | GGBS | Na2SiO3 | 2.6 | 15% | 44.9~78.4 | [53] |
| GGBS | Na2SiO3 | 2.6 | 12% | 41.0~75.4 | ||
| GGBS | Na2SiO3 | 1.5~2.4 | 14% | 47.3~76.2 | [29] | |
| Low calcium | FA | Na2SiO3 | 1.2 | 8% | 27.4~41.7 | [28] |
| FA | Na2SiO3 | 0.5~1.0 | 4% | 19.6~42.3 | [59] | |
| FA | Na2SiO3 | 1.0 | 8% | 23.0~64.0 | ||
| FA | Na2SiO3 | 0.6~1.5 | 7% | 24.9~43.6 | [48] | |
| FA | Na2SiO3 | 2.0 | 9% | 24.3~32.0 | [60] | |
| FA | KOH+ Na2SiO3 | 2.2 | 9% | 22.0~26.0 | ||
| Mixed | GGBS+ FA | Na2SiO3 | 0.5~2.3 | 12% | 30.0~56.0 | [61] |
| VA | Na2SiO3 | 1.5 | 14% | 33.2 | [62] | |
| VA | NaOH | 2.5 | 6% | 22.0 | [50] |
2.2.3. Activators
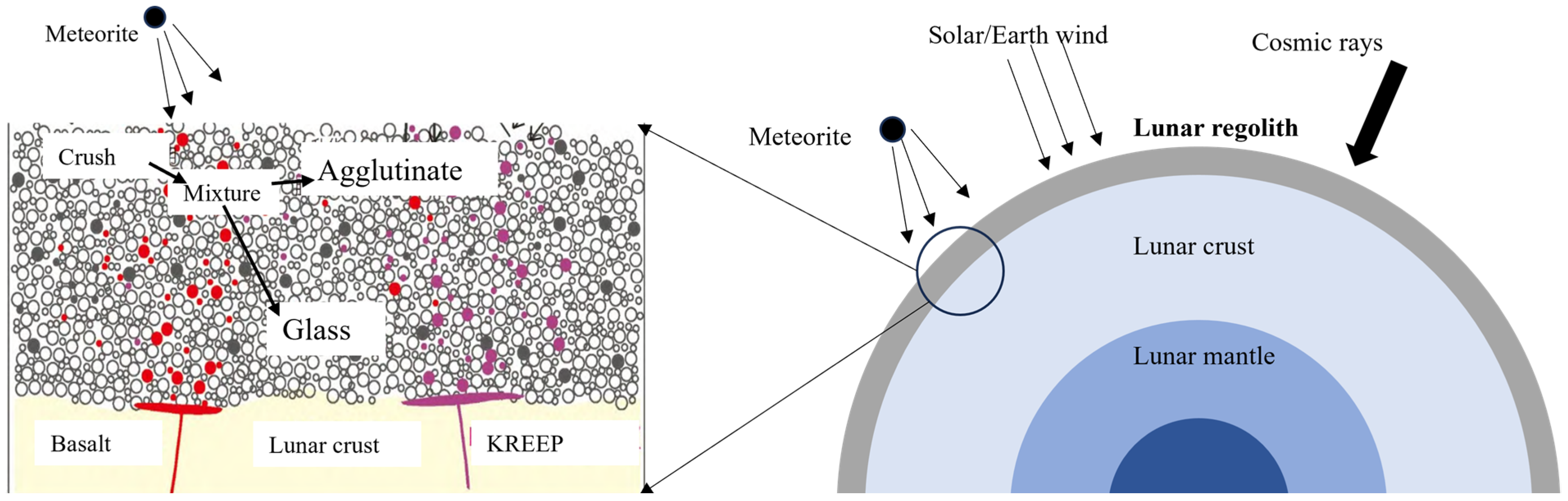
2.3. Applicability of Geopolymers in the Lunar Environment
2.3.1. Temperature
2.3.2. Moonquake
2.3.3. Vacuum
2.3.4. Radiation
3. Lunar Regolith as a Precursor
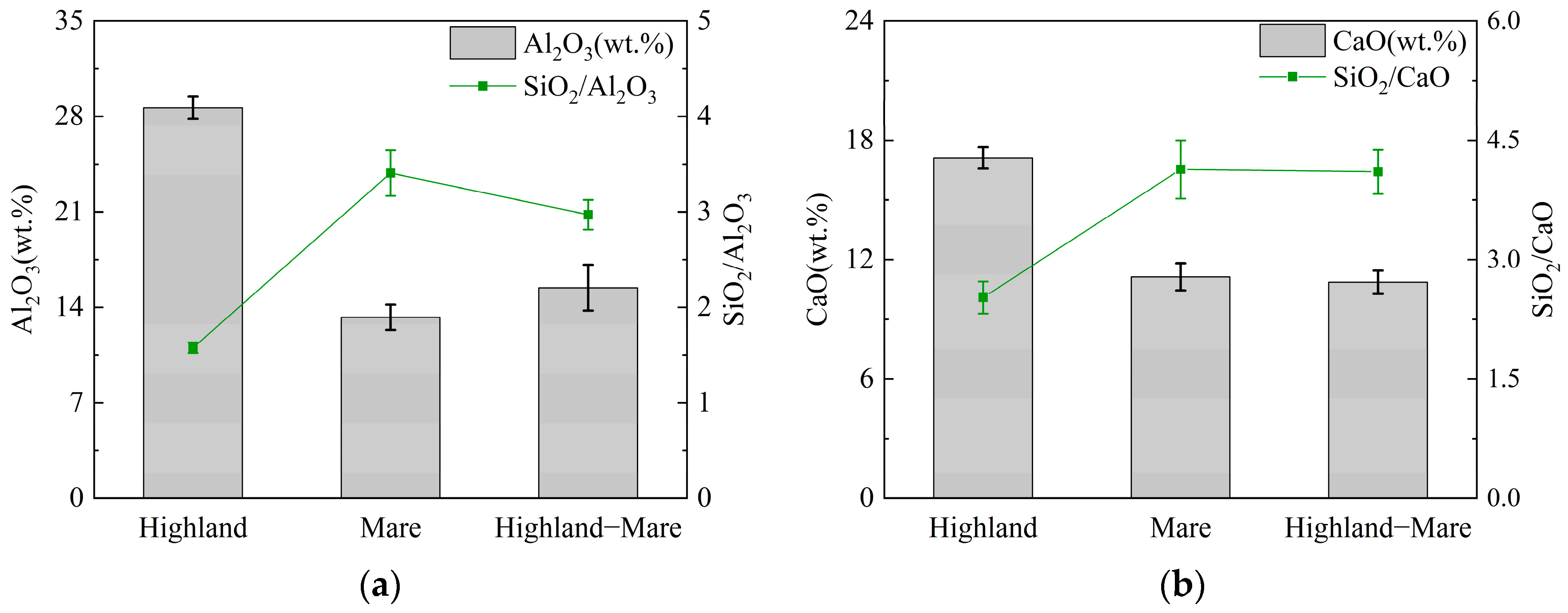
3.1. Chemical Composition of the Lunar Regolith
3.2. Mineral Composition of the Lunar Regolith
| Specimens | Amorphous Substances (wt.%) | Crystalline Minerals (wt.%) | Ref. | |||
|---|---|---|---|---|---|---|
| Glass | Agglutinate | Plagioclase | Pyroxene | Olivine | ||
| CE-5 | 15.5 | 30.4 | 44.5 | 3.6 | [2] | |
| Apollo (67461) | 15.4 | 72.2 | 8.7 | 4.8 | [104] | |
| Apollo (64501) | 32.0 | 45.2 | 5.2 | 14.1 | ||
| Apollo (69961) | 35.3 | 51.8 | 5.3 | 5.6 | ||
| Apollo (12001) | 32.4 | 19.2 | 34.9 | 7.5 | ||
| Apollo (12044) | 31.9 | 17.7 | 16.9 | 5.7 | ||
| Apollo (15531) | 27.6 | 16.0 | 42.7 | 4.1 | ||
| Apollo (15558) | 14.5 | 7.3 | 14.2 | 42.2 | 1.0 | |
| Apollo (10018) | 7.7 | 13.5 | — | — | — | |
| Apollo (10094) | 4.8 | 5.5 | — | — | — | |
| Luna16 | 15.1 | 32.3 | 16.1 | 15.8 | 5.7 | [105] |
| Luna20 | 8.2 | 26.6 | 25.3 | 13.2 | 3.2 | [106] |
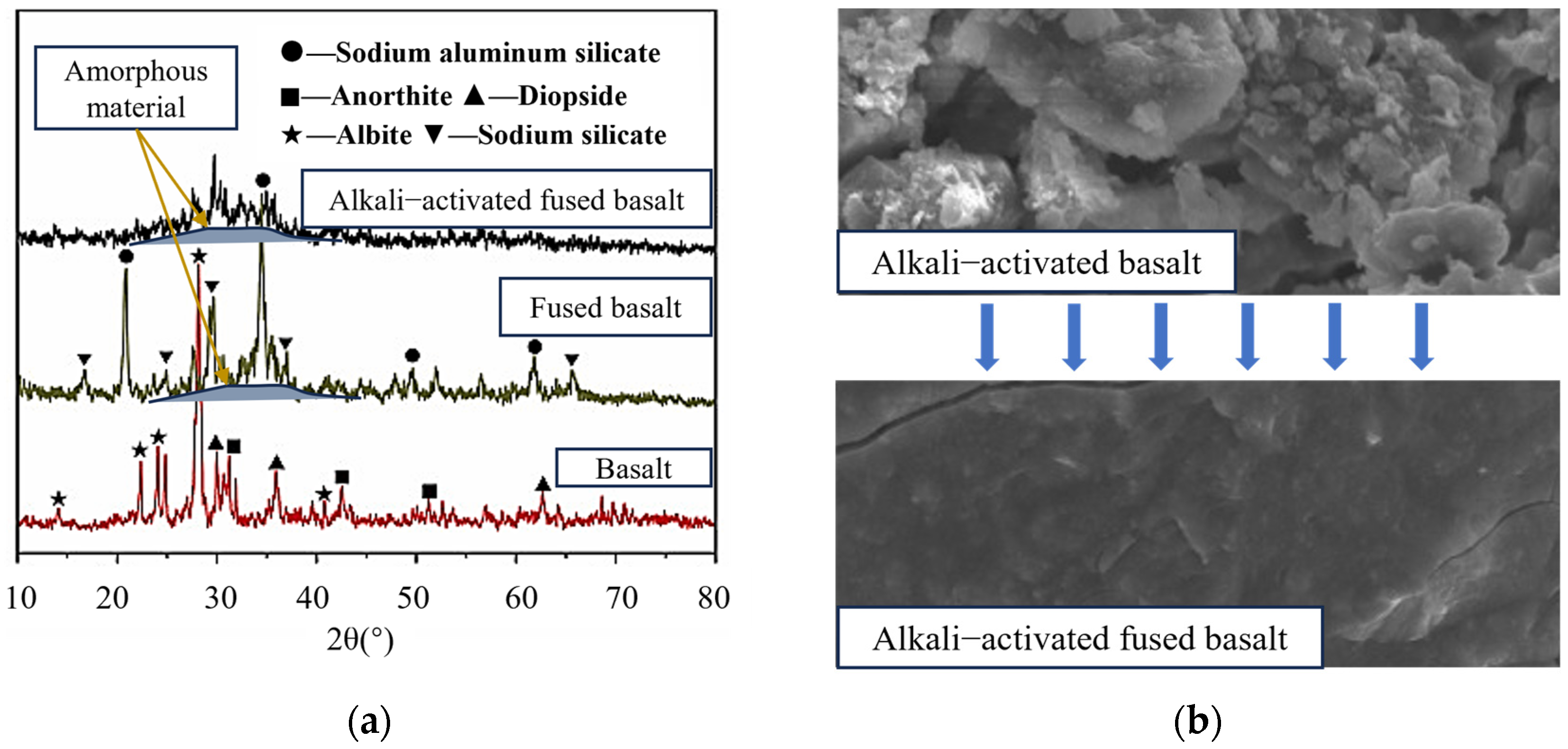
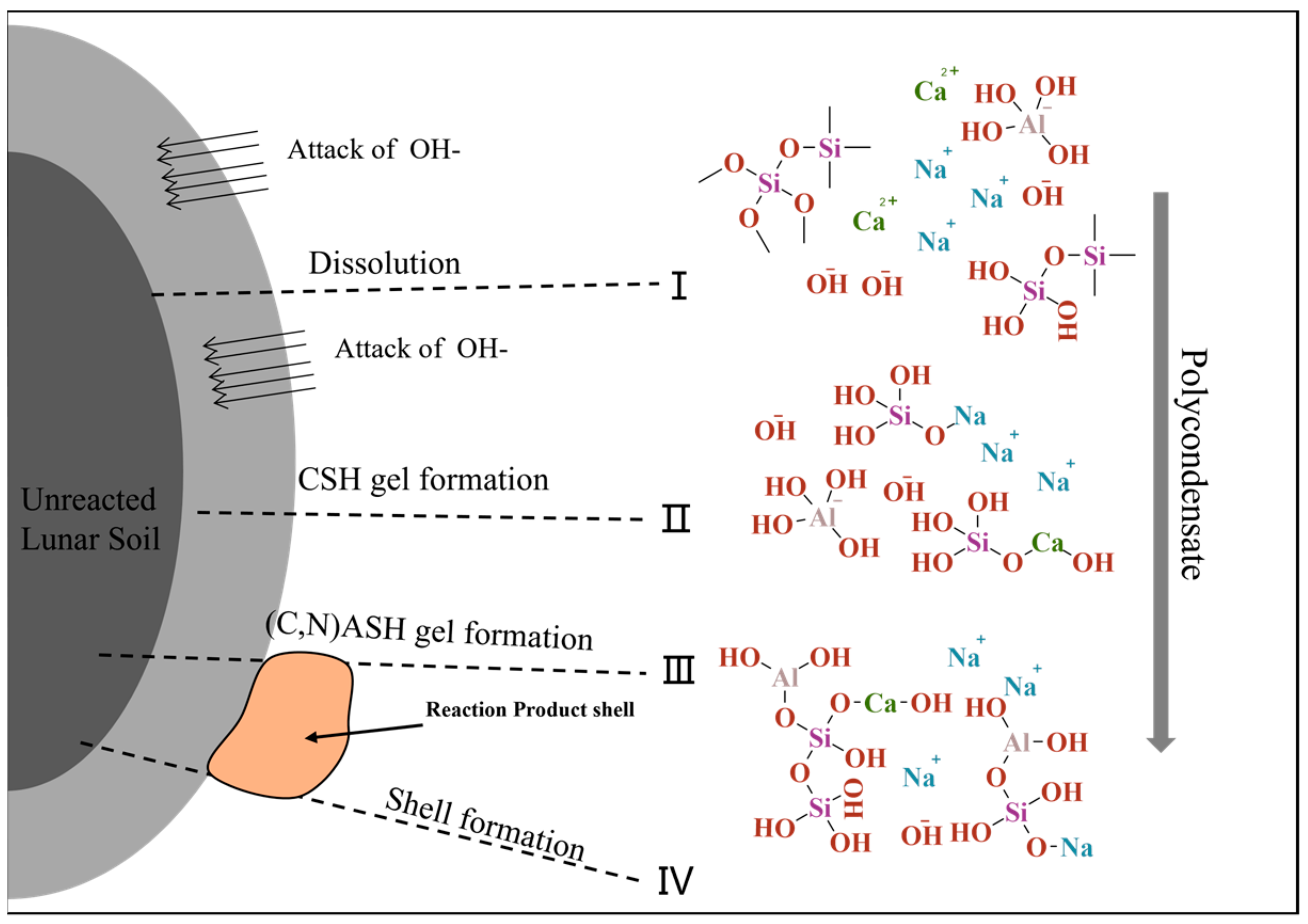
3.3. Reaction Mechanism of Alkali-Activated Lunar Regolith Geopolymer
- Depolymerization: Induced by the action of OH−, it results in the dissolution of amorphous lunar regolith constituents, including CaO, into a free ionic state. This process involves the disruption of Si-O-Si, Si-O-Al, and Al-O-Al bonds, which hydroxyl groups subsequently replace. This process results in the formation of various free radicals.
- 2.
- Condensation: As the degree of depolymerization increases, the number of groups in the system rises, and the contact between the groups intensifies, leading to an increase in the polycondensation degree, with Na+ and Ca2+ in the system replacing some of the hydroxyl groups. This results in the formation of a condensation gel, which occurs concurrently with the formation of a gel in the system containing C-S-H.
- 3.
- Al3⁺ addition: Al3⁺ dissolution is slower, and in the third stage, Al(OH)₄− groups are replaced, forming Si-O-Al bonds once again. The product at this stage is (N,C)-A-S-H.
- 4.
- Shell formation: The gel product continues to be generated and precipitates into a shell until the precipitation hardens.
4. Research on Alkali-Activated Lunar Regolith Geopolymer
4.1. Lunar Regolith Simulants
| Category | Name | Year | Agency | Source | Feature | Ref. |
|---|---|---|---|---|---|---|
| I | JLU-H | 2022 | Jilin University | Damiao Mine, Chengde City, Hebei Province | Focus on the simulation of the lunar regolith crystalline phase | [108] |
| ISRM-1 | 2023 | Chinese Academy of Sciences | VA, titanium magnetite | Focus on the simulation of the Apollo 17 sample’s titanium | [109] | |
| TJ-1 | 2011 | Tongji University | Volcanic ash from Jingyu County, Jilin Province | Focus on geotechnical properties. | [110] | |
| MLS-1 | 1990 | University of Minnesota | Basalt | Does not contain amorphous substances, with a smaller average particle size | [111] | |
| II | JSC-1 | 1993 | Johnson Space Center | Volcanic ash from Merriam Crater, Arizona | Non-residual | [112] |
| CAS-1 | 2009 | Chinese Academy of Sciences | Volcanic ash from Jingyu County, Jilin Province | The median grain size is chemically similar to that of the Apollo 14 lunar regolith sample | [113] | |
| JSC-1A | 2010 | Johnson Space Center | Volcanic ash from Merriam Crater, Arizona | A substitute for JSC-1 | [114] | |
| BP-1 | 2013 | Geological Survey Denver | Black Point lava flow, Arizona. | The grain size is more akin to the lunar regolith than JSC-1 | [115] | |
| BH-1 | 2020 | Beijing University of Aeronautics and Astronautics | Volcanic ash from Huinan County, Jilin Province | Particle size distribution close to the Apollo 17 lunar regolith sample | [83] | |
| DNA-1 | - | ESA | Italy | Amorphous substance simulations were considered | [82] | |
| LHS-1 | 2021 | University of Central Florida | Mineral and rock debris | Similar to the highland sample | [116] | |
| LMS-1 | 2021 | University of Central Florida | Mineral and rock debris | Similar to the mare sample |
4.2. Mechanical Properties of Alkali-Activated Lunar Regolith Simulants
| Precursor | w/b | Activator | Na2O%/ K2O% | n(SiO2/Na2O) | 28-Day Compressive Strength (MPa) | Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| BH-1 | 0.28 | NaOH | 9% | — | 13.00~32.00 | 40~80 °C | [80] |
| VA | 0.27 | NaOH | ~8% | — | 32.46~50.36 | −196~25 °C | [22] |
| LHS-1 | 0.25 | K2SiO3 | ~7% | 0.8 | 12.25 | 76~105 °C | [118] |
| LHS-1 | 0.25 | K2SiO3 | ~8% | 1.0 | 16.73 | ||
| LMS-1 | 0.25 | K2SiO3 | ~7% | 0.8 | 13.87 | ||
| LMS-1 | 0.25 | K2SiO3 | ~8% | 1.0 | 18.09 | ||
| LHS-1 | 0.23 | Na2SiO3 | ~7% | 0.6 | 27.95 | ||
| LHS-1 | 0.23 | Na2SiO3 | ~8% | 0.8 | 41.23 | ||
| LMS-1 | 0.23 | Na2SiO3 | ~7% | 0.6 | 24.59 | ||
| LMS-1 | 0.23 | Na2SiO3 | ~8% | 0.8 | 31.86 | ||
| JSC-1A | 0.21 | Na2SiO3 | ~8% | 2.0 | 24.35 | 60 °C | [117] |
| JSC-2A | 54.85 | ||||||
| OPRL2N | 22.47 | ||||||
| OPRH2N | 22.96 | ||||||
| EAC-1A | 19.20 | ||||||
| LN | 0.26 | Na2SiO3 | 8% | 1.4 | 59.60 | 60 °C | [21] |
4.3. Advantages of Lunar Regolith Geopolymer
5. Future Perspectives
- At the current stage, retrieving lunar regolith samples on a large scale for testing purposes is not feasible. Consequently, lunar regolith simulants remain the most available material for testing purposes. There is still a lack of a unified standard for preparing lunar regolith simulants. The simulated materials are designed to replicate the chemical properties of lunar regolith and primarily utilize volcanic ash, with variations in amorphous substances, chemical compositions, and particle size distributions derived from different volcanic sources.
- The type and quantity of alkaline activators exert a considerable influence on the mechanical properties of the final lunar regolith geopolymer. Given that activators for in-situ construction of lunar bases must be transported by rocket, selecting the most appropriate type of activator is essential to achieving optimal activation efficiency.
- Vacuum and temperature variations have adverse effects on geopolymers. Therefore, future research needs to consider the degeneration of geopolymer solidification under the high vacuum and extreme temperature conditions of the lunar surface environment. The accurate and quantitative performance evaluation of geopolymer is essential to designing safe and reliable architectural structures fabricated with lunar regolith polymer.
6. Conclusions
- The lunar regolith geopolymer reaction mechanisms belong to the mixed system, and the types of gels generated are mainly N-A-S-H and C-A-S-H. The chemical and mineralogical compositions of the highland and the mare lunar regolith exhibit notable differences. Highland lunar regolith samples contain approximately 35% amorphous substances; the ratio of Si/Al is approximately 1.5; the content of aluminum is approximately 28%; the ratio of Si/Ca is approximately 2.6; and the content of calcium is approximately 17%. Compared with the mare lunar regolith, the highland lunar regolith is more suitable as a geopolymer precursor material.
- Previous studies on lunar regolith simulants can be divided into two types. The first type focuses on the simulation of physical properties, while the second type focuses on the simulation of physical and chemical properties. The lunar regolith simulants for alkali-activated geopolymers belong to the second type. The mechanical properties of alkali-activated lunar regolith simulants are mainly concentrated in the range of 18 MPa to 30 MPa, except for test data exhibiting significant differences in amorphous content compared to the lunar regolith samples. Sodium silicate is the most commonly used activator for lunar regolith geopolymers, and alkalinity in the range of 7% to 10% and modulus in the range of 0.8 to 2.0 are suitable.
- Geopolymers can be adaptable for lunar surface construction environments, and the residual compressive strength after multiple temperature cycles within the temperature range of −40 °C to 120 °C, simulated in the lunar surface environment of the mid-latitude region, is above 70%. The vacuum environment on the lunar surface will prematurely evaporate the water inside the reaction system, increasing the porosity of the geopolymers. A vacuum degree of approximately 2 × 10−6 can decrease the mechanical properties of the geopolymer by 8~40%.
- The interval between deep-source moonquakes is approximately 16.8 h. The final setting time of the geopolymer can be adjusted to less than 400 min. Therefore, the curing process can be completed within the interval between moonquakes. The duration of the deep-source moonquake is approximately 30 to 120 min, which may influence the rheological properties of the freshly mixed geopolymer. Therefore, vibration disturbance must be considered when designing materials and structures for in-situ lunar construction.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nickless, E. Resourcing future generations: A global effort to meet the world’s future needs head-on. Eur. Geol. 2016, 42, 46–50. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.; Yang, M.; Pei, Z.; Zhou, Q.; Ren, X.; Liu, B.; Liu, D.; Zeng, X.; Zhang, G.; et al. Characteristics of the lunar samples returned by the Chang’E-5 mission. Natl. Sci. Rev. Sci. Rev. 2021, 9, nwab188. [Google Scholar] [CrossRef] [PubMed]
- Toklu, Y.C.; Akpinar, P. Lunar soils, simulants and lunar construction materials: An overview. Adv. Space Res. 2022, 70, 762–779. [Google Scholar] [CrossRef]
- Smith, M.; Craig, D.; Herrmann, N.; Mahoney, E.; Krezel, J.; McIntyre, N.; Goodliff, K. The Artemis Program: An Overview of NASA’s Activities to Return Humans to the Moon. In Proceedings of the 2020 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Palos, M.F.; Serra, P.; Fereres, S.; Stephenson, K.; González-Cinca, R. Lunar ISRU energy storage and electricity generation. Acta Astronaut. 2020, 170, 412–420. [Google Scholar] [CrossRef]
- Benaroya, H.; Bernold, L.; Chua, K.M. Engineering, Design and Construction of Lunar Bases. J. Aerosp. Eng. 2002, 15, 33–45. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, D.; Wang, Q.; Cui, Y.; Feng, P. Lunar In Situ Large-Scale Construction: Quantitative Evaluation of Regolith Solidification Techniques. Engineering, 2024; in press. [Google Scholar] [CrossRef]
- Meurisse, A.; Beltzung, J.C.; Kolbe, M.; Cowley, A.; Sperl, M. Influence of mineral composition on sintering lunar regolith. J. Aerosp. Eng. 2017, 30, 4017014. [Google Scholar] [CrossRef]
- Crockett, R.S.; Fabes, B.D.; Nakamura, T.; Senior, C.L. Construction of large lunar structures by fusion welding of sintered regolith. In Proceedings of the 4th International Conference on Engineering, Construction and Operations in Space, Albuquerque, New Mexico, 26 February–3 March 1994; pp. 1116–1127. [Google Scholar]
- Lin, T.D.; Skaar, S.B.; O’Gallagher, J.J. Proposed remote-control, solar-powered concrete production experiment on the moon. J. Aerosp. Eng. 1997, 10, 104–109. [Google Scholar] [CrossRef]
- Roedel, H.; Lepech, M.D.; Loftus, D.J. Protein-regolith composites for space construction. In Earth and Space 2014; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 291–300. ISBN 9780784479179. [Google Scholar]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Z.; Zhang, R.; Zhu, X.; Li, F. Preparation and evaluation of geopolymer based on BH-2 lunar regolith simulant under lunar surface temperature and vacuum condition. Acta Astronaut. 2021, 189, 90–98. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Palomo, A.; López Dela Fuente, J.I. Alkali-activated cementitous materials: Alternative matrices for the immobilisation of hazardous wastes: Part I. Stabilisation of boron. Cem. Concr. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Parathi, S.; Nagarajan, P.; Pallikkara, S.A. Ecofriendly geopolymer concrete: A comprehensive review. Clean Technol. Environ. Policy 2021, 23, 1701–1713. [Google Scholar] [CrossRef]
- Apollo, P.E.T. The Apollo 16 lunar samples: Petrographic and chemical description. Science 1973, 179, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, N.J.; Meyer, C.; Gast, P.W.; Wiesmann, H. The composition and derivation of Apollo 12 soils. Earth Planet. Sci. Lett. 1971, 10, 341–350. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Liu, Y.; Xiao, Z.; Lin, Y.; Li, J.; Ma, H.; Tang, G.; Guo, S.; Tang, X.; et al. Two-billion-year-old volcanism on the Moon from Chang’e-5 basalts. Nature 2021, 600, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Guo, X.; Yuan, S.; Xia, M.; Wang, Z. The mechanical and structural properties of lunar regolith simulant based geopolymer under extreme temperature environment on the moon through experimental and simulation methods. Constr. Build. Mater. 2022, 325, 126679. [Google Scholar] [CrossRef]
- Wang, K.; Lemougna, P.N.; Tang, Q.; Li, W.; Cui, X. Lunar regolith can allow the synthesis of cement materials with near-zero water consumption. Gondwana Res. 2017, 44, 1–6. [Google Scholar] [CrossRef]
- Gharzouni, A.; Bourbon, X.; Michau, N.; Rossignol, S. Curing temperature’s effect on Argillite-metakaolin based geopolymer grouts. Ceram. Int. 2024, 50, 23356–23366. [Google Scholar] [CrossRef]
- Catenacci, M.J.; Luckarift, H.R.; Friedman, R.J.; Male, A.; Owens, J.R. Effect of fly ash composition and component quantities on the gamma radiation shielding properties of geopolymer. Prog. Nucl. Energy 2021, 140, 103889. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, S.; Li, F. Preparation of geopolymer based on lunar regolith simulant at in-situ lunar temperature and its durability under lunar high and cryogenic temperature. Constr. Build. Mater. 2022, 318, 126033. [Google Scholar] [CrossRef]
- Pilehvar, S.; Arnhof, M.; Pamies, R.; Valentini, L.; Kjøniksen, A. Utilization of urea as an accessible superplasticizer on the moon for lunar geopolymer mixtures. J. Clean Prod. 2020, 247, 119177. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride diffusion resistance and chloride binding capacity of fly ash-based geopolymer concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. De 2014, 64, 1. [Google Scholar] [CrossRef]
- Pandey, A.; Dalal, S.; Dutta, S.; Dixit, A. Structural characterization of polycrystalline thin films by X-ray diffraction techniques. J. Mater. Sci. Mater. Electron. 2021, 32, 1341–1368. [Google Scholar] [CrossRef]
- Library, U.D. Crystalline and Amorphous Solids. Available online: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/12%3A_Solids/12.01%3A_Crystalline_and_Amorphous_Solids (accessed on 1 March 2024).
- Takayama, S. Amorphous structures and their formation and stability. J. Mater. Sci. 1976, 11, 164–185. [Google Scholar] [CrossRef]
- Khedmati, M.; Alanazi, H.; Kim, Y.; Nsengiyumva, G.; Moussavi, S. Effects of Na2O/SiO2 molar ratio on properties of aggregate-paste interphase in fly ash-based geopolymer mixtures through multiscale measurements. Constr. Build. Mater. 2018, 191, 564–574. [Google Scholar] [CrossRef]
- Němeček, J.; Amilauer, V.; Kopecký, L. Nanoindentation characteristics of alkali-activated aluminosilicate materials. Cem. Concr. Compos. 2011, 33, 163–170. [Google Scholar] [CrossRef]
- Palomo, Á.; Alonso, S.; Fernandez Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mat. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M.; Bucher, R. Influence of the initial water content in flash calcined metakaolin-based geopolymer. Constr. Build. Mater. 2019, 201, 421–429. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- El-Hassan, H.; Ismail, N. Effect of process parameters on the performance of fly ash/GGBS blended geopolymer composites. J. Sustain. Cen.-Based Mater. 2018, 7, 122–140. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the CASH gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Lee, W.; Wang, J.; Ding, Y.; Cheng, T. A study on the characteristics and microstructures of GGBS/FA based geopolymer paste and concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. Mix design development of fly ash and ground granulated blast furnace slag based geopolymer concrete. J. Build. Eng. 2018, 20, 712–722. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Rodríguez-González, E. Recycling Waste Materials to Fabricate Solar-Driven Self-Cleaning Geopolymers. Waste Biomass Valorization 2024, 15, 2833–2843. [Google Scholar] [CrossRef]
- Yilmazoglu, A.; Yildirim, S.T.; Behçet, Ö.F.; Yıldız, S. Performance evaluation of fly ash and ground granulated blast furnace slag-based geopolymer concrete: A comparative study. Struct. Concr. 2022, 23, 3898–3915. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S. Strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Nofalah, M.; Ghadir, P.; Hasanzadehshooiili, H.; Aminpour, M.; Javadi, A.A.; Nazem, M. Effects of binder proportion and curing condition on the mechanical characteristics of volcanic ash-and slag-based geopolymer mortars; machine learning integrated experimental study. Constr. Build. Mater. 2023, 395, 132330. [Google Scholar] [CrossRef]
- Mao, Q.; Li, Y.; Liu, K.; Peng, H.; Shi, X. Mechanism, characterization and factors of reaction between basalt and alkali: Exploratory investigation for potential application in geopolymer concrete. Cem. Concr. Compos. 2022, 130, 104526. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Yang, K.; Yu, L.; Yang, C. Setting behaviours and early-age microstructures of alkali-activated ground granulated blast furnace slag (GGBS) from different regions in China. Cem. Concr. Compos. 2020, 114, 103782. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. Final setting time and compressive strength of fly ash and GGBS-based geopolymer paste and mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074. [Google Scholar] [CrossRef]
- Huang, Z.; Ou Yang, Y.; Li, C. Mechanical Property and Microstructrue of Geopolymers with Quartz Powder by Autoclaving Curing. Build. Chin. Ceram. Soc. 2015, 34, 2925–2929. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Yao, P.; Wang, C.; Ma, Z. Microstructure and macro properties of sustainable alkali-activated fly ash mortar with various construction waste fines as binder replacement up to 100%. Cem. Concr. Compos. 2022, 134, 104733. [Google Scholar] [CrossRef]
- Lou, Y.; Huang, M.; Kang, S.; Hu, M.; Wu, W.; Chen, S. Study on Basic performance and Drying Shrinkage of Binary Solid Waste Geopolymer Prepared with Recycled Powders and Slag. Case Stud. Constr. Mater. 2024, 20, e03195. [Google Scholar] [CrossRef]
- Dietel, J.; Warr, L.N.; Bertmer, M.; Steudel, A.; Grathoff, G.H.; Emmerich, K. The importance of specific surface area in the geopolymerization of heated illitic clay. Appl. Clay Sci. 2017, 139, 99–107. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of particle fineness on engineering properties and microstructure of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef]
- Cheng, Y.; Cong, P.; Zhao, Q.; Hao, H.; Mei, L.; Zhang, A.; Han, Z.; Hu, M. Study on the effectiveness of silica fume-derived activator as a substitute for water glass in fly ash-based geopolymer. J. Build. Eng. 2022, 51, 104228. [Google Scholar] [CrossRef]
- Sontia Metekong, J.V.; Kaze, C.R.; Deutou, J.G.; Venyite, P.; Nana, A.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Evaluation of performances of volcanic-ash-laterite based blended geopolymer concretes: Mechanical properties and durability. J. Build. Eng. 2021, 34, 101935. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Effect of silica fume on efflorescence formation and alkali leaching of alkali-activated slag. J. Clean Prod. 2021, 315, 128210. [Google Scholar] [CrossRef]
- Taghvayi, H.; Behfarnia, K.; Khalili, M. The effect of alkali concentration and sodium silicate modulus on the properties of alkali-activated slag concrete. J. Adv. Concr. Technol. 2018, 16, 293–305. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Sajan, P.; Jiang, T.; Lau, C.; Tan, G.; Ng, K. Combined effect of curing temperature, curing period and alkaline concentration on the mechanical properties of fly ash-based geopolymer. Clean. Mater. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Fernández Jiménez, A.; Pastor, J.Y.; Martín, A.; Palomo, A. High-temperature resistance in alkali-activated cement. J. Am. Ceram. Soc. 2010, 93, 3411–3417. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fang, Y.; Wang, X.; Yang, H.; Xing, F. Compressive properties of sustainable geopolymers at elevated temperatures: Strength and elastic modulus evolution. Constr. Build. Mater. 2024, 411, 134515. [Google Scholar] [CrossRef]
- Nakamura, Y.; Latham, G.V.; Dorman, H.J. Apollo lunar seismic experiment—Final summary. J. Geophys. Res. Solid Earth 1982, 87, A117–A123. [Google Scholar] [CrossRef]
- Latham, G.; Ewing, M.; Dorman, J.; Nakamura, Y.; Press, F.; Toksőz, N.; Sutton, G.; Duennebier, F.; Lammlein, D. Lunar structure and dynamics—Results from the apollo passive seismic experiment. Moon 1973, 7, 396–421. [Google Scholar] [CrossRef]
- Goins, N.R.; Dainty, A.M.; Toksöz, M.N. Seismic energy release of the Moon. J. Geophys. Res. Solid Earth 1981, 86, 378–388. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, A.E.M.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Shi, H.; Song, L.; Chen, W.; Zhang, H.; Wang, G.; Yuan, G.; Zhang, W.; Chen, G.; Wang, Y.; Lin, G. New non-destructive method for testing the strength of cement mortar material based on vibration frequency of steel bar: Theory and experiment. Constr. Build. Mater. 2020, 262, 120931. [Google Scholar] [CrossRef]
- Swamy, N.; Rigby, G. Dynamic properties of hardened paste, mortar and concrete. Matér. Constr. 1971, 4, 13–40. [Google Scholar] [CrossRef]
- Safawi, M.I.; Iwaki, I.; Miura, T. A study on the applicability of vibration in fresh high fluidity concrete. Cem. Concr. Res. 2005, 35, 1834–1845. [Google Scholar] [CrossRef]
- Popovics, S. A review of the concrete consolidation by vibration. Matér. Constr. 1973, 6, 453–463. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, R.; Yang, Z.; Li, F.; Zhu, X. Preparation and Performance Evaluation on 3D Printed Road Material Based on Lunar Soil Simulant. China J. Highw. Transp. 2022, 35, 105. [Google Scholar] [CrossRef]
- Johnson, F.S.; Carroll, J.M.; Evans, D.E. Vacuum measurements on the lunar surface. J. Vac. Sci. Technol. 1972, 9, 450–456. [Google Scholar] [CrossRef]
- Pilehvar, S.; Arnhof, M.; Erichsen, A.; Valentini, L.; Kjøniksen, A.L. Investigation of severe lunar environmental conditions on the physical and mechanical properties of lunar regolith geopolymers. J. Mater. Res. Technol. 2021, 11, 1506–1516. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, C.; Zhu, X.; Li, F. Preparation and characterization of high-strength geopolymer based on BH-1 lunar soil simulant with low alkali content. Engineering 2021, 7, 1631–1645. [Google Scholar] [CrossRef]
- Lucey, P.; Korotev, R.L.; Gillis, J.J.; Taylor, L.A.; Lawrence, D.; Campbell, B.A.; Elphic, R.; Feldman, B.; Hood, L.L.; Hunten, D. Understanding the lunar surface and space-Moon interactions. Rev. Mineral. Geochem. 2006, 60, 83–219. [Google Scholar] [CrossRef]
- Vaniman, D.; Reedy, R.; Heiken, G.; Olhoeft, G.; Mendell, W. The Lunar Environment. Lunar Sourceb. 1991, 1, 27–60. [Google Scholar]
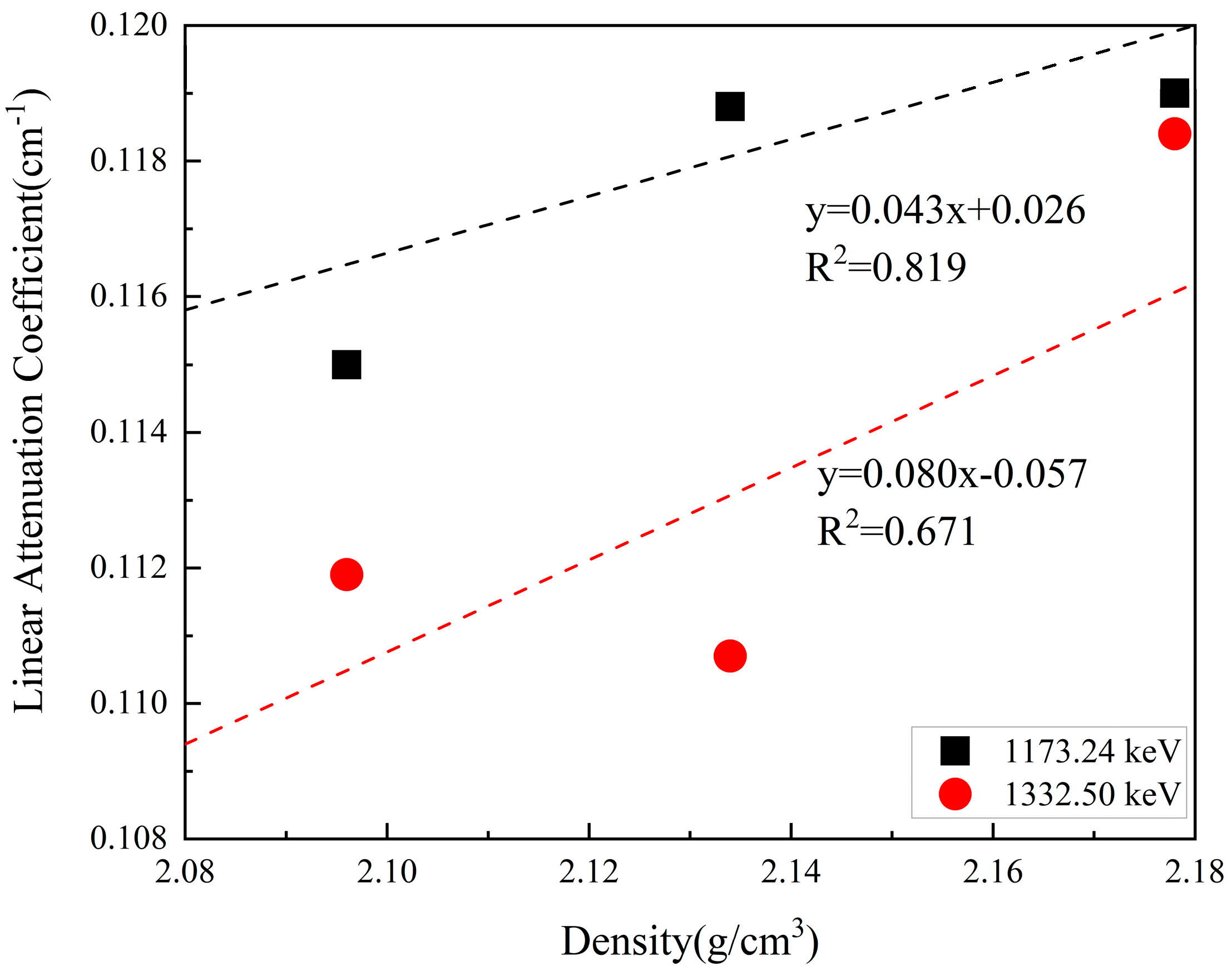
- Singh, K.; Singh, S.; Dhaliwal, A.S.; Singh, G. Gamma radiation shielding analysis of lead-flyash concretes. Appl. Radiat. Isot. 2015, 95, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, K. On the use of green concrete composite as a nuclear radiation shielding material. Prog. Nucl. Energy 2021, 136, 103730. [Google Scholar] [CrossRef]
- Isachenkov, M.; Chugunov, S.; Landsman, Z.; Akhatov, I.; Metke, A.; Tikhonov, A.; Shishkovsky, I. Characterization of novel lunar highland and mare simulants for ISRU research applications. Icarus 2022, 376, 114873. [Google Scholar] [CrossRef]
- Fang, M.; Park, D.; Singuranayo, J.L.; Chen, H.; Li, Y. Aggregate gradation theory, design and its impact on asphalt pavement performance: A review. Int. J. Pavement Eng. 2019, 20, 1408–1424. [Google Scholar] [CrossRef]
- Ferrone, K.L.; Taylor, A.B.; Helvajian, H. In situ resource utilization of structural material from planetary regolith. Adv. Space Res. 2022, 69, 2268–2282. [Google Scholar] [CrossRef]
- Langevin, Y.; Arnold, J.R. The evolution of the lunar regolith. Annu. Rev. Earth Planet. Sci. 1977, 5, 449–489. [Google Scholar] [CrossRef]
- Stern, S.A. The lunar atmosphere: History, status, current problems, and context. Rev. Geophys. 1999, 37, 453–491. [Google Scholar] [CrossRef]
- Zhang, S.; Wimmer-Schweingruber, R.F.; Yu, J.; Wang, C.; Fu, Q.; Zou, Y.; Sun, Y.; Wang, C.; Hou, D.; Böttcher, S.I.; et al. First measurements of the radiation dose on the lunar surface. Sci. Adv. 2020, 6, eaaz1334. [Google Scholar] [CrossRef] [PubMed]
- Plescia, J.B.; Cahill, J.; Greenhagen, B.; Hayne, P.; Mahanti, P.; Robinson, M.S.; Spudis, P.D.; Siegler, M.; Stickle, A.; Williams, J.P. Lunar surface processes. Rev. Mineral. Geochem. 2023, 89, 651–690. [Google Scholar] [CrossRef]
- Tian, H.; Wang, H.; Chen, Y.; Yang, W.; Zhou, Q.; Zhang, C.; Lin, H.; Huang, C.; Wu, S.; Jia, L.; et al. Non-KREEP origin for Chang’e-5 basalts in the Procellarum KREEP Terrane. Nature 2021, 600, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.N.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Istuque, D.B.; Soriano, L.; Akasaki, J.L.; Melges, J.; Borrachero, M.V.; Monzó, J.; Payá, J.; Tashima, M.M. Effect of sewage sludge ash on mechanical and microstructural properties of geopolymers based on metakaolin. Constr. Build. Mater. 2019, 203, 95–103. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Liao, S.; Yin, Z.; Hsu, W. Mineral chemistry and 3D tomography of a Chang’E 5 high-Ti basalt: Implication for the lunar thermal evolution history. Sci. Bull. 2022, 67, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Ganju, E.; Torbatisarraf, H.; Thompson, M.S.; Chawla, N. Advanced microstructural and compositional analysis of a lunar agglutinate from the Apollo 11 mission. Meteorit. Planet. Sci. 2024, 2024, 1–18. [Google Scholar] [CrossRef]
- Rui, Z.; Wei-Hua, W. Lunar Glasses. Physics 2022, 10, 1–47. [Google Scholar] [CrossRef]
- Zhao, R.; Shen, L.; Xiao, D.; Chang, C.; Huang, Y.; Yu, J.; Zhang, H.; Liu, M.; Zhao, S.; Yao, W.; et al. Diverse glasses revealed from Chang’E-5 lunar regolith. Natl. Sci. Rev. 2023, 10, nwad079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, G.; Dong, K.; Deng, X.; Gao, X.; Yang, Y.; Xiao, Y.; Bai, X.; Liang, K.; et al. Size, morphology, and composition of lunar samples returned by Chang’E-5 mission. Sci. China Phys. Mech. Astron. 2022, 65, 229511. [Google Scholar] [CrossRef]
- Taylor, G.J.; Martel, L.M.V.; Lucey, P.G.; Gillis-Davis, J.J.; Blake, D.F.; Sarrazin, P. Modal analyses of lunar soils by quantitative X-ray diffraction analysis. Geochim. Cosmochim. Acta 2019, 266, 17–28. [Google Scholar] [CrossRef]
- Lpis. Lunar Sample Atlas. Available online: https://www.lpi.usra.edu/lunar/samples/atlas/ (accessed on 1 January 2024).
- Hubbard, N.J.; Nyquist, L.E.; Rhodes, J.M.; Bansal, B.M.; Wiesmann, H.; Church, S.E. Chemical features of the Luna 16 regolith sample. Earth Planet. Sci. Lett. 1972, 13, 423–428. [Google Scholar] [CrossRef]
- Taylor, G.J.; Drake, M.J.; Wood, J.A.; Marvin, U.B. The Luna 20 lithic fragments, and the composition and origin of the lunar highlands. Geochim. Cosmochim. Acta 1973, 37, 1087–1106. [Google Scholar] [CrossRef]
- Cai, L.; Ding, L.; Luo, H.; Yi, X. Preparation of autoclave concrete from basaltic lunar regolith simulant: Effect of mixture and manufacture process. Constr. Build. Mater. 2019, 207, 373–386. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, R.; Li, X.; Zou, M.; Wang, C.; Chen, L. JLU-H: A novel lunar highland regolith simulant for use in large-scale engineering experiments. Planet Space Sci. 2022, 221, 105562. [Google Scholar] [CrossRef]
- Ningxi, Z.; Jian, C.; Juehao, H. Development and properties of a magnetic high-titanium lunar regolith simulant IRSM-1. Chin. J. Geotech. Eng. 2023, 45, 110–113. [Google Scholar] [CrossRef]
- Jiang, M.; Li, L. Development of TJ-1 lunar soil simulant. Chin. J. Geotech. Eng. 2011, 33, 209. [Google Scholar]
- Taylor, L.A.; Liu, Y. Important considerations for lunar soil simulants. In Proceedings of the Earth and Space 2010: Engineering, Science, Construction, and Operations in Challenging Environments, Honolulu, HI, USA, 14–17 March 2010; pp. 106–118. [Google Scholar]
- Willman, B.M.; Boles, W.W.; Mckay, D.S.; Allen, C.C. Properties of lunar soil simulant JSC-1. J. Aerosp. Eng. 1995, 8, 77–87. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Feng, J.; Ou Yang, Z.; Liu, J.; Liu, C. CAS-1 Lunar soil simulant. Acta Mineral. Sin. 2007, 27, 571–578. [Google Scholar] [CrossRef]
- Zeng, X.; He, C.; Oravec, H.; Wilkinson, A.; Agui, J.; Asnani, V. Geotechnical Properties of JSC-1A Lunar Soil Simulant. J. Aerosp. Eng. 2010, 23, 111–116. [Google Scholar] [CrossRef]
- Stoeser, D.B.; Rickman, D.L.; Wilson, S. Preliminary Geological Findings on the BP-1 Simulant; NASA: Washington, DC, USA, 2010; Volume TM-2010. [Google Scholar]
- Long-Fox, J.M.; Landsman, Z.A.; Easter, P.B.; Millwater, C.A.; Britt, D.T. Geomechanical properties of lunar regolith simulants LHS-1 and LMS-1. Adv. Space Res. 2023, 71, 5400–5412. [Google Scholar] [CrossRef]
- Collins, P.J.; Edmunson, J.; Fiske, M.; Radlińska, A. Materials characterization of various lunar regolith simulants for use in geopolymer lunar concrete. Adv. Space Res. 2022, 69, 3941–3951. [Google Scholar] [CrossRef]
- Javed, U.; Ahmed Shaikh, F.U.; Rahman, A.K.M.S. Synthesizing lunar regolith-geopolymer emulating lunar positive temperature regime. Planet Space Sci. 2024, 244, 105890. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Chin, S.; Ilavsky, J.; Andrews, R.N.; Bumajdad, A.; Büyüköztürk, O. Hydration kinetics and morphology of cement pastes with pozzolanic volcanic ash studied via synchrotron-based techniques. J. Mater. Sci. 2018, 53, 1743–1757. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, R.; Chen, F.; Jia, X.; Cong, P. Effects of reactive MgO on the reaction process of geopolymer. Materials 2019, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Hintze, P.E.; Quintana, S. Building a lunar or martian launch pad with in situ materials: Recent laboratory and field studies. J. Aerosp. Eng. 2013, 26, 134–142. [Google Scholar] [CrossRef]
- Roberts, A.D.; Whittall, D.R.; Breitling, R.; Takano, E.; Blaker, J.J.; Hay, S.; Scrutton, N.S. Blood, sweat, and tears: Extraterrestrial regolith biocomposites with in vivo binders. Mater. Today Bio 2021, 12, 100136. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhu, J.; Chang, S.; Xue, G.; Pang, J.; Zhu, H. Lunar regolith-AlSi10Mg composite fabricated by selective laser melting. Vacuum 2021, 187, 110122. [Google Scholar] [CrossRef]
- Farries, K.W.; Visintin, P.; Smith, S.T.; van Eyk, P. Sintered or melted regolith for lunar construction: State-of-the-art review and future research directions. Constr. Build. Mater. 2021, 296, 123627. [Google Scholar] [CrossRef]
- Engelschiøn, V.S.; Eriksson, S.R.; Cowley, A.; Fateri, M.; Meurisse, A.; Kueppers, U.; Sperl, M. EAC-1A: A novel large-volume lunar regolith simulant. Sci. Rep. 2020, 10, 5473. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Xi, X.; Guo, Z.; Yue, X.; Hao, B.; Liang, C. Study on the feasibility of preparing a continuous fibre using lunar soil simulant. Sci. Sin. Tech. 2020, 12, 1625–1633. (In Chinese) [Google Scholar] [CrossRef]
- Hoshino, T.; Wakabayashi, S.; Yoshihara, S.; Hatanaka, N. Key Technology Development for Future Lunar Utilization—Block Production Using Lunar Regolith. Trans. Jpn. Soc. Aeronaut. Space Sci. Aerosp. Technol. Jpn. 2016, 14, 35–40. [Google Scholar] [CrossRef]
- Goulas, A.; Binner, J.G.; Engstrøm, D.S.; Harris, R.A.; Friel, R.J. Mechanical behaviour of additively manufactured lunar regolith simulant components. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 1629–1644. [Google Scholar] [CrossRef]
- Faierson, E.J.; Logan, K.V.; Stewart, B.K.; Hunt, M.P. Demonstration of concept for fabrication of lunar physical assets utilizing lunar regolith simulant and a geothermite reaction. Acta Astronaut. 2010, 67, 38–45. [Google Scholar] [CrossRef]
- Delgado, A.; Shafirovich, E. Towards better combustion of lunar regolith with magnesium. Combust. Flame 2013, 160, 1876–1882. [Google Scholar] [CrossRef]
- Toutanji, H.A.; Evans, S.; Grugel, R.N. Performance of lunar sulfur concrete in lunar environments. Constr. Build. Mater. 2012, 29, 444–448. [Google Scholar] [CrossRef]
- Li, S.; Lucey, P.G.; Milliken, R.E.; Hayne, P.O.; Fisher, E.; Williams, J.; Hurley, D.M.; Elphic, R.C. Direct evidence of surface exposed water ice in the lunar polar regions. Proc. Natl. Acad. Sci. USA 2018, 115, 8907–8912. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ji, G.; Zhang, Y.; Ma, G.; Mechtcherine, V.; Pan, J.; Wang, L.; Ding, T.; Duan, Z.; Du, S. Large-scale 3D printing concrete technology: Current status and future opportunities. Cem. Concr. Compos. 2021, 122, 104115. [Google Scholar] [CrossRef]
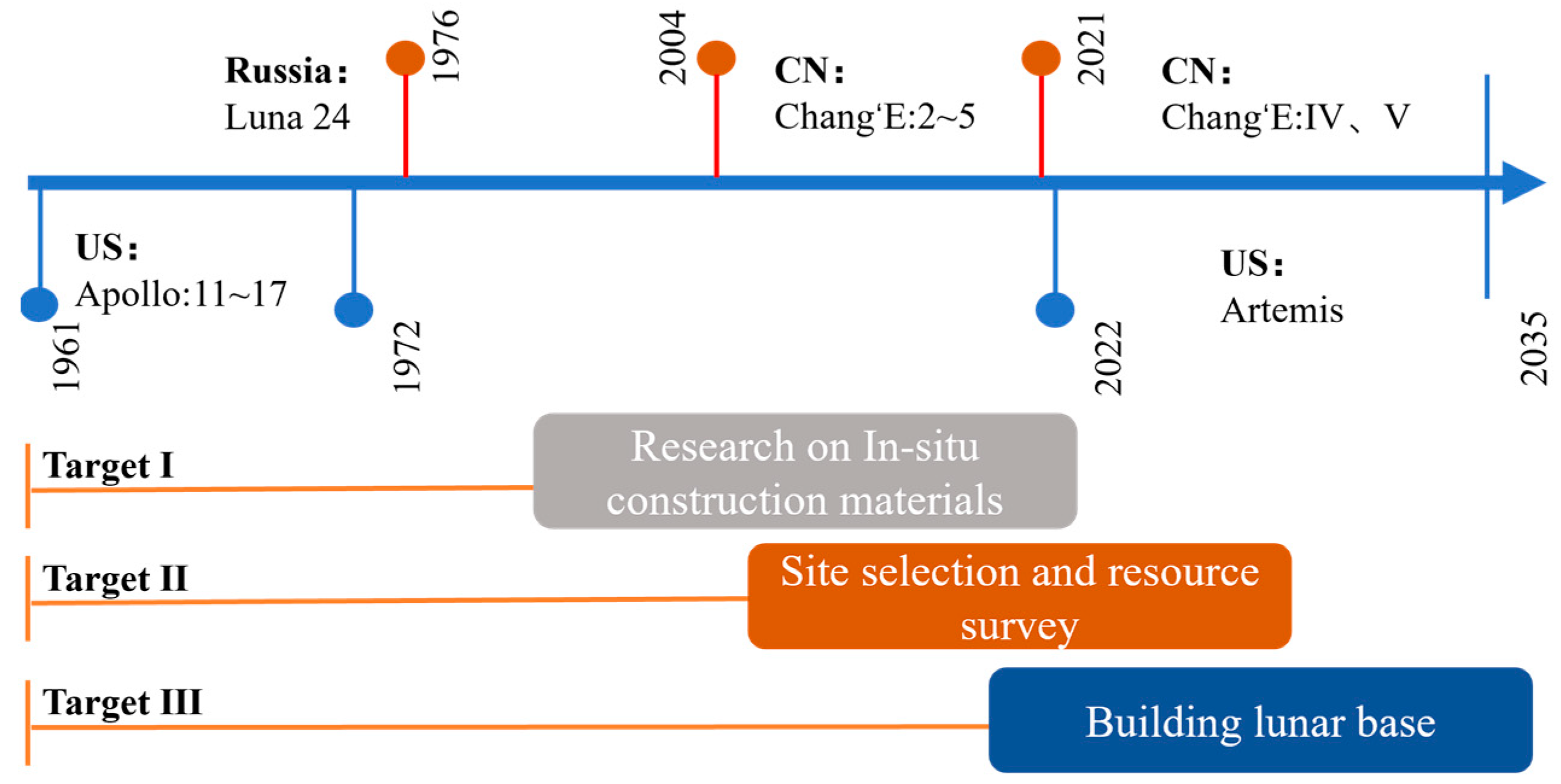



| Polar Shadow Crater | Remaining Polar Regions | Equator | Mid-Latitudes | |
|---|---|---|---|---|
| Average temperature | −233 | −53 | −19 | −53~−19 |
| Range | — | 10 | 140 | 110 |
| Factors | Features | Challenges |
|---|---|---|
| Temperature | Range: −230~140 °C | Low temperatures impede the chemical reaction. Temperature-induced cyclic shock has a detrimental effect on the mechanical properties of geopolymers. |
| Moonquake | Frequency: 500 occurrences per year, equivalent to an earthquake of magnitude 2 on the Richter scale Duration: 30~120 min | Frequent seismic activities affect the rheological properties of the fresh-state geopolymers and the required setting time. |
| Vacuum | Vacuum level: 10−11~10−14 Pa | A vacuum can prematurely evaporate the water in the reaction system, increasing the geopolymer’s internal porosity and weakening its mechanical properties. |
| Radiation | Radiation dose: 300 mSv per year | Geopolymers need to be as dense as possible, and sufficient weathering should be applied to cover the building surface so that it absorbs the radiation. |
| Precursor | Earth material | Technique | Temperature (°C) | Compressive Strength (MPa) | Ref. |
|---|---|---|---|---|---|
| JSC-1A | — | Melting | 1070~1125 | 152 | [125] |
| DNA | — | 213 | |||
| CAS-1 | AlSi10Mg powder | Melting (Laser) | 1700 | 264 | [123] |
| CAS-1 | — | Melting | 1200~1500 | 1012~1955 | [126] |
| FJS-1 | — | Sintering | 200~600 | 33 | [127] |
| JSC-1A | — | Sintering (Laser) | 50~200 | 4 | [128] |
| JSC-1A | Al | Sintering (Reduction-oxidation reaction) | 660 | 10~18 | [129] |
| JSC-1 | Mg | 100 | 10.2 | [130] | |
| —— | Portland cement | Hot pressing (Steam autoclave) | 175~203 | 75 | [10] |
| JSC-1 | Sulfur | 193 | 31~33 | [131] | |
| CAS-1 | Portland cement | Hot pressing (Steam autoclave) | 190 | 27 | [107] |
| JSC, OPR | Alkali activator | Reaction solidification (Geopolymer) | 80 | 25~54 | [117] |
| BH-1 | Alkali activator | 37~90 | 4~21 | [80] | |
| LN | Alkali activator | 25~80 | 29~46 | [22] | |
| JSC-1A | Protein matrix | Reaction solidification (Biopolymer) | 25 | 4~13 | [11] |
| LHS-1 | Protein matrix | 40 | 20~40 | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhao, C.; Sun, X.; Dong, W. Lunar Regolith Geopolymer Concrete for In-Situ Construction of Lunar Bases: A Review. Polymers 2024, 16, 1582. https://doi.org/10.3390/polym16111582
Zheng X, Zhao C, Sun X, Dong W. Lunar Regolith Geopolymer Concrete for In-Situ Construction of Lunar Bases: A Review. Polymers. 2024; 16(11):1582. https://doi.org/10.3390/polym16111582
Chicago/Turabian StyleZheng, Xiaowei, Cong Zhao, Xiaoyan Sun, and Weiwei Dong. 2024. "Lunar Regolith Geopolymer Concrete for In-Situ Construction of Lunar Bases: A Review" Polymers 16, no. 11: 1582. https://doi.org/10.3390/polym16111582
APA StyleZheng, X., Zhao, C., Sun, X., & Dong, W. (2024). Lunar Regolith Geopolymer Concrete for In-Situ Construction of Lunar Bases: A Review. Polymers, 16(11), 1582. https://doi.org/10.3390/polym16111582









