Freeze-Dried β-Glucan and Poly-γ-glutamic Acid: An Efficient Stabilizer to Strengthen Subgrades of Low Compressible Fine-Grained Soils with Varying Curing Periods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Atterberg’s Limits
3.2. Compaction Characteristics
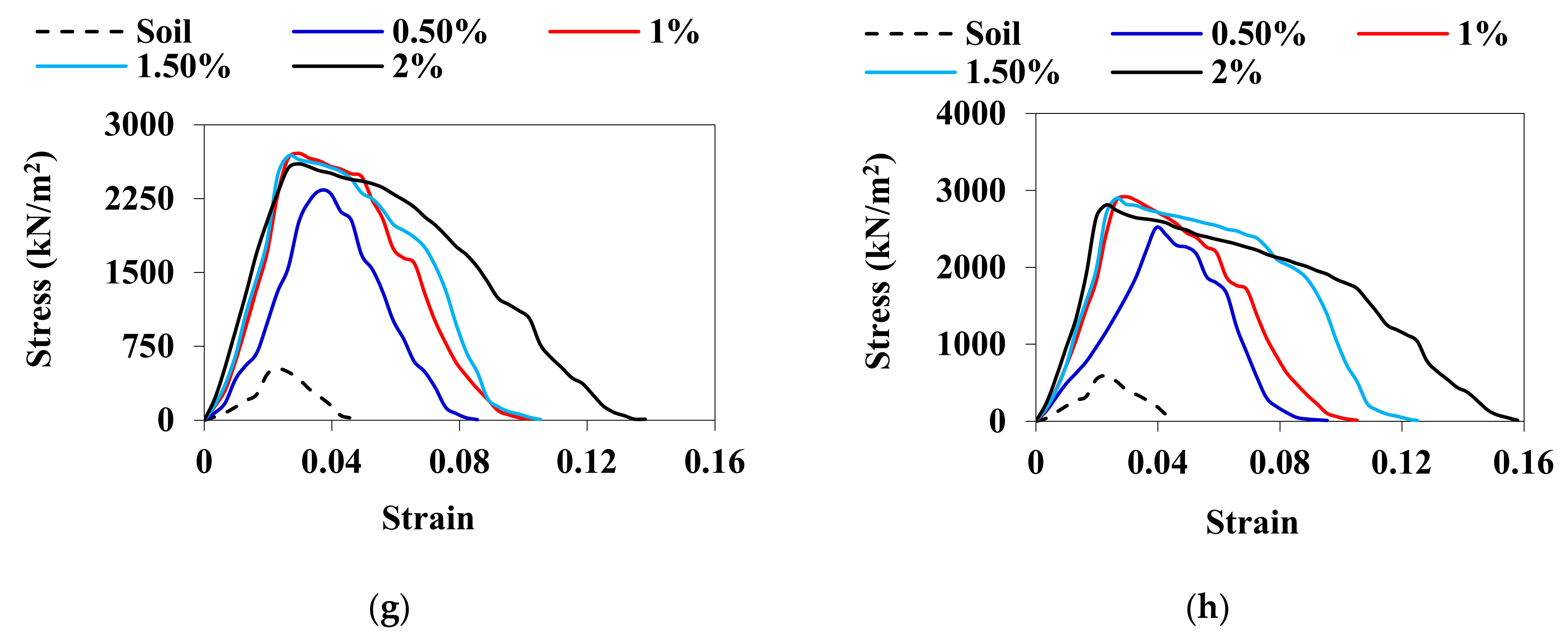
3.3. UCS
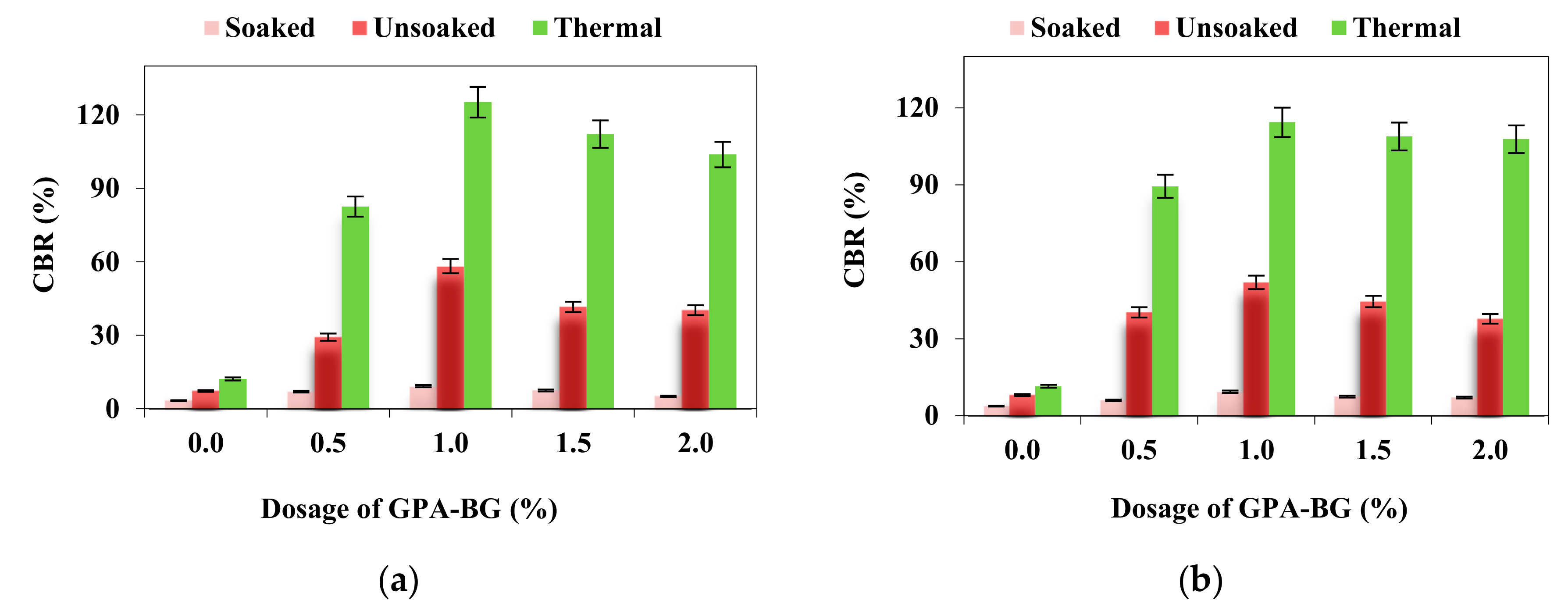
3.4. CBR
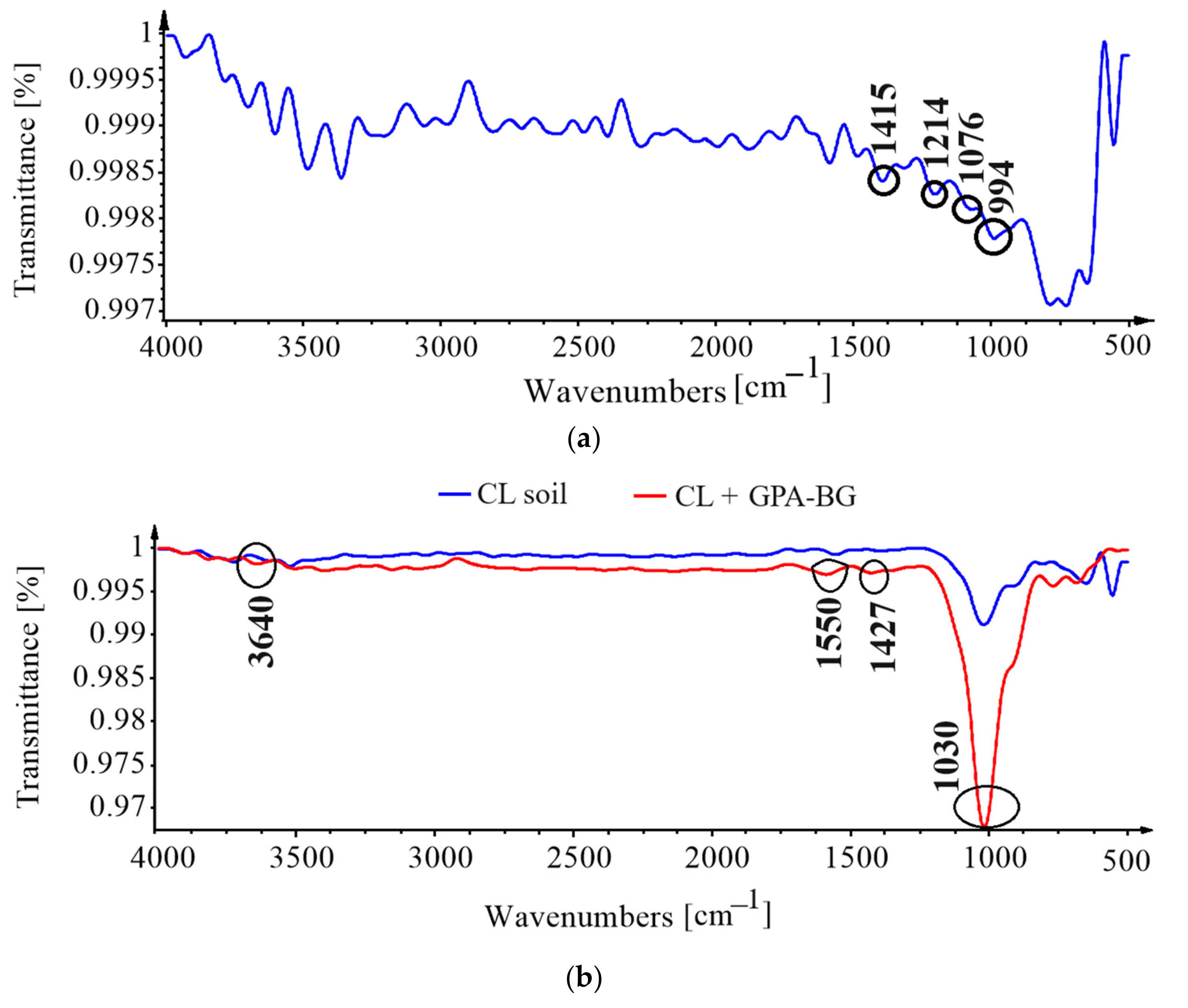
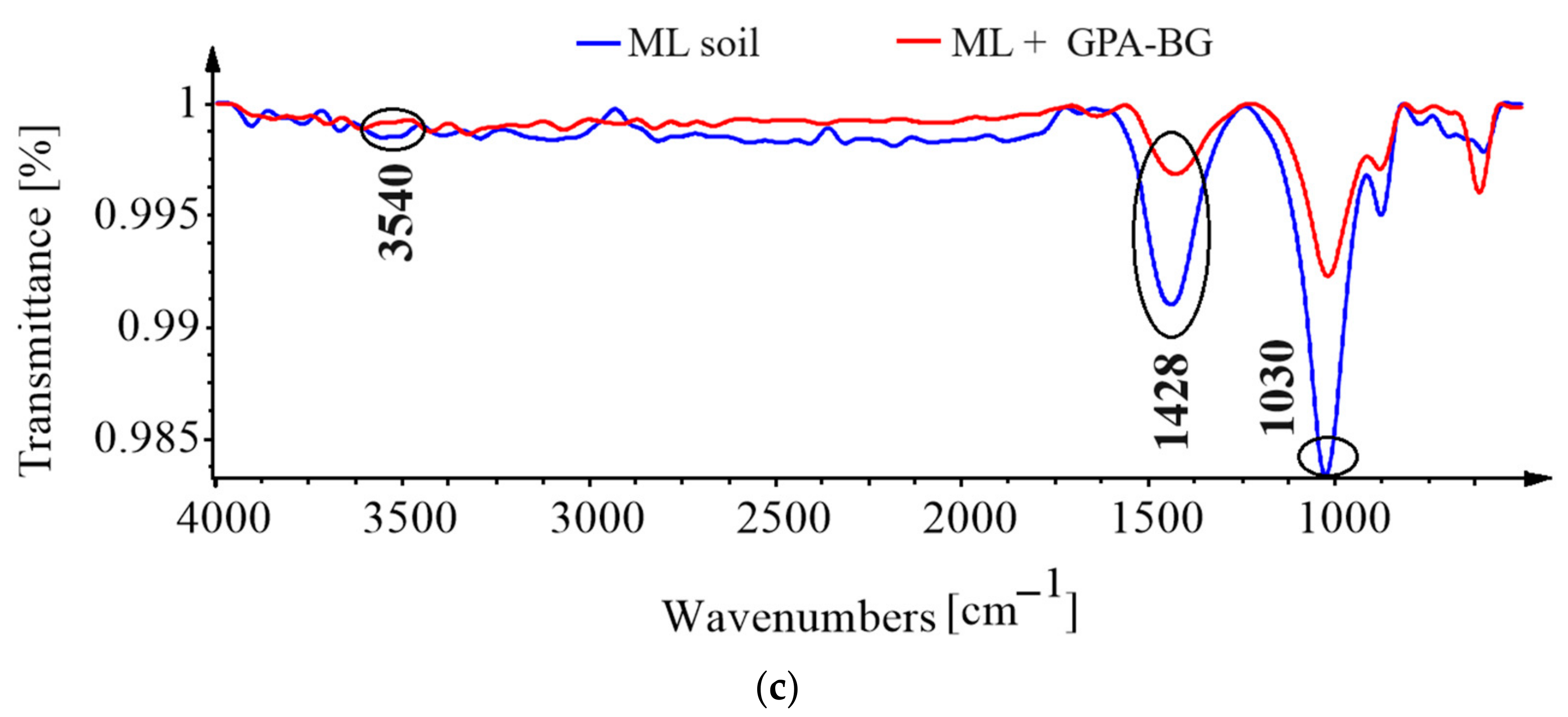
3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.6. SEM Analysis
3.7. Zetasizer and BET Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedarla, A.; Puppala, A.J.; Savigamin, C.; Yu, X. Performance of Sand-Treated Clay Subgrade Supporting a Low-Volume Flexible Pavement. Transp. Res. Rec. 2015, 2473, 91–97. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, M.; Wang, Q.; Wang, Y.; Liu, J.; Cao, C.; Zheng, W.; Bao, Y.; Rocchi, I. Use of Sulfur-Free Lignin as a Novel Soil Additive: A Multi-Scale Experimental Investigation. Eng. Geol. 2020, 269, 105551. [Google Scholar] [CrossRef]
- Basu, D.; Lee, M. A Combined Sustainability-Reliability Approach in Geotechnical Engineering. In Risk, Reliability and Sustainable Remediation in the Field of Civil and Environmental Engineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 379–413. [Google Scholar]
- Shekh, M.I.; Ahmed, S. Biopolymers: An Overview. Adv. Appl. Biobased Mater. 2023, 2023, 3–21. [Google Scholar]
- Biswas, S.; Pal, A. Application of Biopolymers as a New Age Sustainable Material for Surfactant Adsorption: A Brief Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100145. [Google Scholar] [CrossRef]
- Bagheri, P.; Gratchev, I.; Son, S.; Rybachuk, M. Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil. Polymers 2023, 15, 1556. [Google Scholar] [CrossRef]
- Lemboye, K.; Almajed, A. Effect of Varying Curing Conditions on the Strength of Biopolymer Modified Sand. Polymers 2023, 15, 1678. [Google Scholar] [CrossRef]
- Hamza, M.; Nie, Z.; Aziz, M.; Ijaz, N.; Fang, C.; Ghani, M.U.; Ijaz, Z.; Noshin, S.; Salman, M. Geotechnical Properties of Problematic Expansive Subgrade Stabilized with Guar Gum Biopolymer. Clean. Technol. Environ. Policy 2023, 25, 1699–1719. [Google Scholar] [CrossRef]
- Babatunde, Q.O.; Byun, Y.-H. Soil Stabilization Using Zein Biopolymer. Sustainability 2023, 15, 2075. [Google Scholar] [CrossRef]
- Martin, K.K.; Khodadadi Tirkolaei, H.; Kavazanjian, E. Mid-Scale Biocemented Soil Columns via Enzyme-Induced Carbonate Precipitation (EICP). Soils Found. 2021, 61, 1529–1542. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Li, X. Geotechnical Engineering Properties of Soils Solidified by Microbially Induced CaCO3 Precipitation (MICP). Adv. Civ. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Reddy, K.R.; Yaghoubi, P.; Yukselen-Aksoy, Y. Effects of Biochar Amendment on Geotechnical Properties of Landfill Cover Soil. Waste Manag. Res. 2015, 33, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in Polysaccharide and Protein Films and Coatings for Food Contact—A Review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.; Li, C.; Liu, H.; Cheng, Y.; Lu, J.; Zhang, K.; Wang, H. Super-Swelling Lignin-Based Biopolymer Hydrogels for Soil Water Retention from Paper Industry Waste. Int. J. Biol. Macromol. 2019, 135, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Khatami, H.; O’Kelly, B.C. Prevention of Bleeding of Particulate Grouts Using Biopolymers. Constr. Build. Mater. 2018, 192, 202–209. [Google Scholar] [CrossRef]
- Ren, F.; Ding, H.; Dong, B.; Qian, X.; Liu, J.; Tan, J. Study on the Improvement of Soil Properties Using Hydrophilic-Hydrophobic Biopolymer Crosslinking. Constr. Build. Mater. 2024, 415, 135101. [Google Scholar] [CrossRef]
- Ni, J.; Li, S.S.; Geng, X.Y. Mechanical and Biodeterioration Behaviours of a Clayey Soil Strengthened with Combined Carrageenan and Casein. Acta Geotech. 2022, 17, 5411–5427. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.L.; Dubey, A.A.; Dhami, N.K.; Mukherjee, A. Multiscale Study of Soil Stabilization Using Bacterial Biopolymers. J. Geotech. Geoenviron. Eng. 2021, 147, 04021074. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, J.; Bai, Y.; Xiao, H. Effect of Xanthan Gum on the Mechanical Properties of Fiber-Reinforced Sandy Soil. Bull. Eng. Geol. Environ. 2024, 83, 177. [Google Scholar] [CrossRef]
- Chen, C.; Wei, K.; Gu, J.; Huang, X.; Dai, X.; Liu, Q. Combined Effect of Biopolymer and Fiber Inclusions on Unconfined Compressive Strength of Soft Soil. Polymers 2022, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liang, B.; Sun, W.; He, X.; Yi, F.; Wan, Y. Mechanical Properties of Solidified Dredged Soils Considering the Effects of Compaction Degree and Residual Moisture Content. Dev. Built Environ. 2023, 16, 100235. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Heinonen, J.; Sainio, T. Acid Hydrolysis of Glycosidic Bonds in Oat Β-glucan and Development of a Structured Kinetic Model. AIChE J. 2018, 64, 2570–2580. [Google Scholar] [CrossRef]
- Shima, F.; Schulte, B.; Keul, H.; Moeller, M.; Akashi, M. Preparation of Microparticles Composed of Amphiphilic Poly (γ-Glutamic Acid) through Hydrophobic Interactions. Polym. J. 2014, 46, 184–188. [Google Scholar] [CrossRef]
- Moradi, G.; Shafaghatian, S.; Katebi, H. Effect of Chemical and Biological Stabilization on the Resilient Modulus of Clay Subgrade Soil. Int. J. Pavement Res. Technol. 2022, 15, 415–432. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a Sustainable Solution for the Enhancement of Soil Mechanical Properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wu, J.; Niu, S.; Gu, Z.; Zheng, J.-J.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. An Anionic Biopolymer γ-Polyglutamate Enhanced the Microbially Induced Carbonate Precipitation for Soil Improvement: Mechanical Behaviors and Underlying Mechanism. Acta Geotech. 2022, 17, 4485–4496. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Petrisor, I.G.; Kwan, C.C.; Yen, T.F. Biopolymer Plugging Effect: Laboratory-Pressurized Pumping Flow Studies. J. Pet. Sci. Eng. 2003, 38, 13–21. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Sibakov, J.; Nappa, M.; Hytönen, E.; Koksel, H.; Poutanen, K. Extraction of Soluble β-Glucan from Oat and Barley Fractions: Process Efficiency and Dispersion Stability. J. Cereal Sci. 2018, 81, 60–68. [Google Scholar] [CrossRef]
- Chowhan, Z. pH-solubility Profiles of Organic Carboxylic Acids and Their Salts. J. Pharm. Sci. 1978, 67, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Kropp, M.; Morawa, K.-M.; Mihov, G.; Salz, A.K.; Harmening, N.; Franken, A.; Kemp, A.; Dias, A.A.; Thies, J.; Johnen, S. Biocompatibility of Poly (Ester Amide)(PEA) Microfibrils in Ocular Tissues. Polymers 2014, 6, 243–260. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C. IPNs Derived from Biopolymers. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 211–233. [Google Scholar]
- Zhang, H.; Zhang, F.; Wu, J. Physically Crosslinked Hydrogels from Polysaccharides Prepared by Freeze–Thaw Technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Rezaeimalek, S.; Nasouri, N.; Huang, J.; Shafique, S.B.; Gilazghi, S.T. Comparison of Short-Term and Long-Term Performances for Polymer-Stabilized Sand and Clay. J. Traffic Transp. Eng. 2017, 4, 145–155. [Google Scholar] [CrossRef]
- IS 2720-5 (1985); Methods of Test for Soils, Part 5: Determination of Liquid and Plastic Limit. Bureau of Indian Standards: New Delhi, India, 1985.
- IS 2720-7 (1980); Methods of Test for Soils, Part 7: Determination of Water Content-Dry Density Relation Using Light Compaction. Bureau of Indian Standards: New Delhi, India, 1980.
- IS 2720-10 (1991); Methods of Test for Soils, Part 10: Determination of Unconfined Compressive Strength. Bureau of Indian Standards: New Delhi, India, 1991.
- IS 2720-16 (1987); Methods of Test for Soils, Part 16: Laboratory Determination of CBR. Bureau of Indian Standards: New Delhi, India, 1987.
- IS 2720-17 (1986); Methods of Test for Soils, Part 17: Laboratory Determination of Permeability. Bureau of Indian Standards: New Delhi, India, 1986.
- IRC:37:2018; Guidelines for the Design of Flexible Pavements. IRC: New Delhi, India, 2018.
- Nugent, R.; Zhang, G.; Gambrell, R. Effect of Exopolymers on the Liquid Limit of Clays and Its Engineering Implications. Transp. Res. Rec. 2010, 2101, 34–43. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Cho, G.-C. Soil Strengthening Using Thermo-Gelation Biopolymers. Constr. Build. Mater. 2015, 77, 430–438. [Google Scholar] [CrossRef]
- Khaleghi, M.; Heidarvand, M. A Novel Study on Hydro-Mechanical Characteristics of Biopolymer-Stabilized Dune Sand. J. Clean. Prod. 2023, 398, 136518. [Google Scholar] [CrossRef]
- Pavan Sai Gopal, K.; Shyam Chamberlin, K. Experimental Study on Black Cotton Soil Treated with Xanthan Gum and Palm Oil Fuel Ash. E3S Web Conf. 2023, 391, 01017. [Google Scholar] [CrossRef]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR Spectroscopic Analysis of Saccharomyces Cerevisiae Cell Walls: Study of an Anomalous Strain Exhibiting a Pink-Colored Cell Phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, Z.; Liu, J.; Song, C.; Zhu, F.; Wang, S. The Evaluation of Bacillus-Secreted Polyglutamic Acid as Anti-Scaling Treatment for Circulating Cooling Water. Environ. Sci. Pollut. Res. 2022, 29, 82762–82771. [Google Scholar] [CrossRef]
- Nikolic, G. Fourier Transforms: New Analytical Approaches and FTIR Strategies; BoD–Books on Demand; InTech: Rijeka, Croatia, 2011; ISBN 953-307-232-6. [Google Scholar]
- Ozaki, Y.; Baranska, M.; Lednev, I.K.; Wood, B.R. Vibrational Spectroscopy in Protein Research: From Purified Proteins to Aggregates and Assemblies; Academic Press: Cambridge, MA, USA, 2020; ISBN 0-12-818611-9. [Google Scholar]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Abd Majid, M.Z.; Tahir, M.M.; Mohamad, E.T. Improvement of Problematic Soils with Biopolymer—An Environmentally Friendly Soil Stabilizer. J. Mater. Civ. Eng. 2017, 29, 04016204. [Google Scholar] [CrossRef]
- Bonini, M.; Gabbani, A.; Del Buffa, S.; Ridi, F.; Baglioni, P.; Bordes, R.; Holmberg, K. Adsorption of Amino Acids and Glutamic Acid-Based Surfactants on Imogolite Clays. Langmuir 2017, 33, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as Green Binders for Soil Improvement in Geotechnical Applications: A Review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- López-Perea, P.; Schwarz, P.; Figueroa, J.; Hernández-Estrada, Z. Effect of β-Glucans on Viscoelastic Properties of Barley Kernels and Their Relationship to Structure and Soluble Dietary Fibre. J. Cereal Sci. 2012, 56, 595–602. [Google Scholar] [CrossRef]
- Ayeldeen, M.K.; Negm, A.M.; El Sawwaf, M.A. Evaluating the Physical Characteristics of Biopolymer/Soil Mixtures. Arab. J. Geosci. 2016, 9, 371. [Google Scholar] [CrossRef]
- Vetrik, M.; Pradny, M.; Kobera, L.; Slouf, M.; Rabyk, M.; Pospisilova, A.; Stepanek, P.; Hruby, M. Biopolymer-Based Degradable Nanofibres from Renewable Resources Produced by Freeze-Drying. RSC Adv. 2013, 3, 15282. [Google Scholar] [CrossRef]
- Silvestro, I.; Sergi, R.; d’Abusco, A.S.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan Scaffolds with Enhanced Mechanical Strength and Elastic Response by Combination of Freeze Gelation, Photo-Crosslinking and Freeze-Drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef]
- Vishweshwaran, M.; Sujatha, E.R.; Harshith, N.; Umesh, C. Geotechnical Properties of β-Glucan-Treated Clayey Sand; Springer: Berlin/Heidelberg, Germany, 2021; pp. 63–73. [Google Scholar]
- Taniguchi, M.; Kato, K.; Shimauchi, A.; Xu, P.; Fujita, K.-I.; Tanaka, T.; Tarui, Y.; Hirasawa, E. Physicochemical Properties of Cross-Linked Poly-γ-Glutamic Acid and Its Flocculating Activity against Kaolin Suspension. J. Biosci. Bioeng. 2005, 99, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Skujins, J.J.; McLaren, A.D. Persistence of Enzymatic Activities in Stored and Geologically Preserved Soils. Enzymologia 1968, 34, 213–225. [Google Scholar] [PubMed]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 04018170. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef]
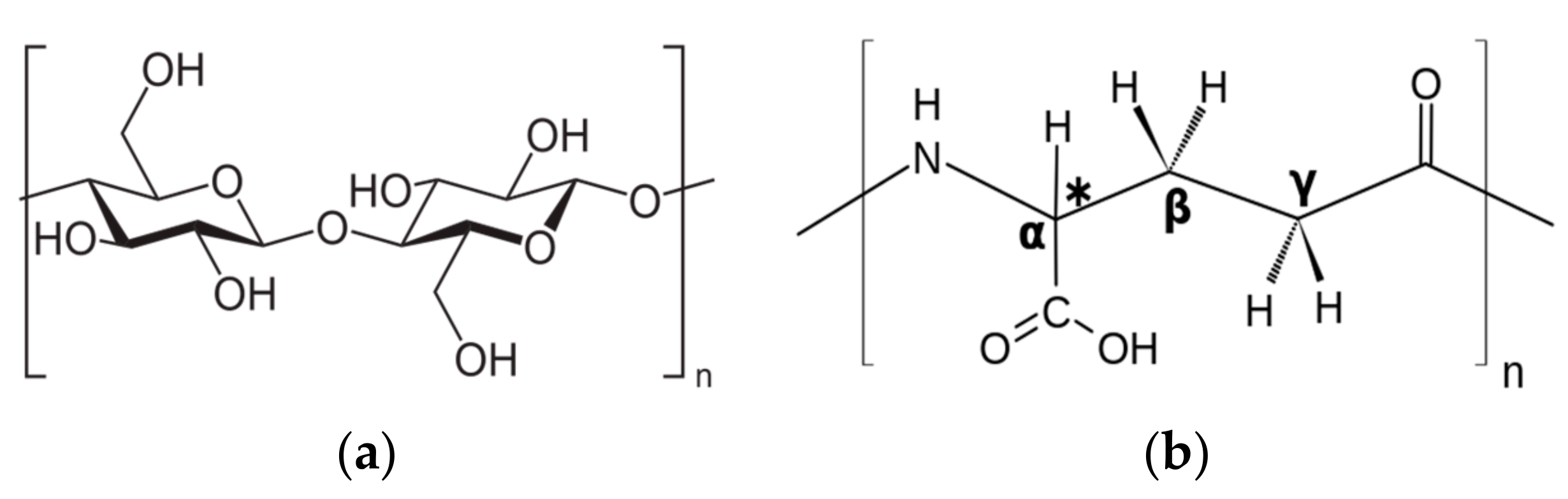
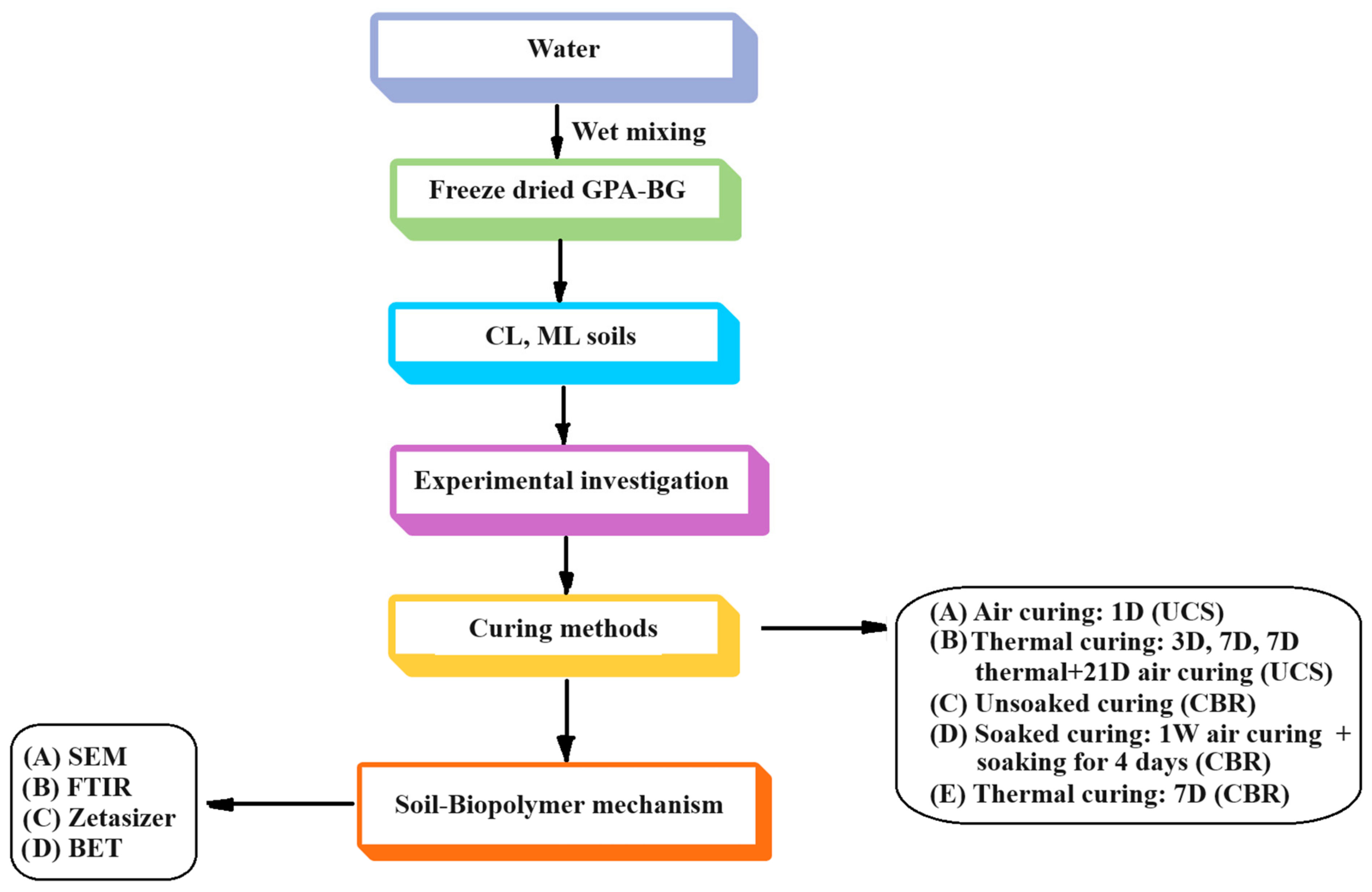
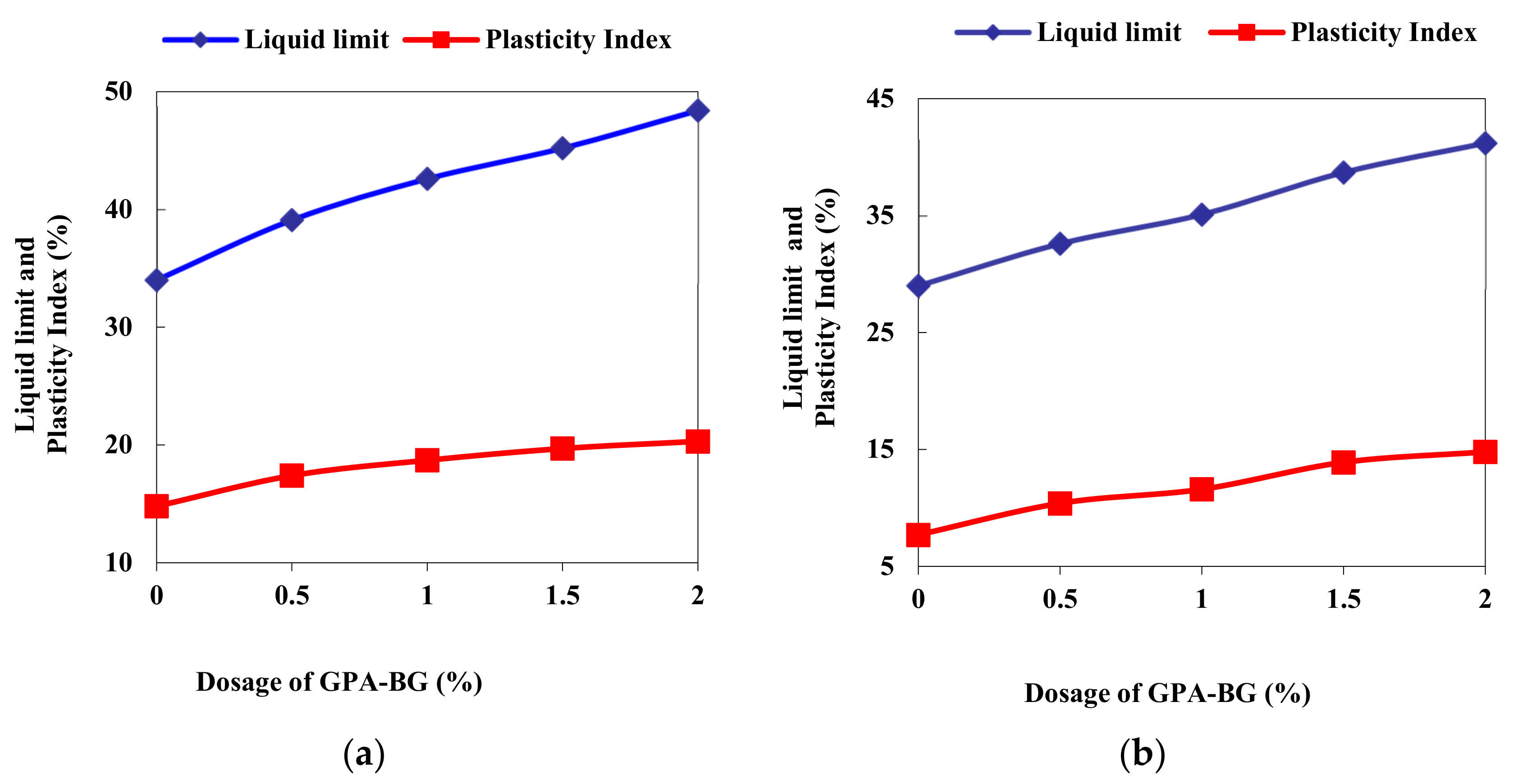

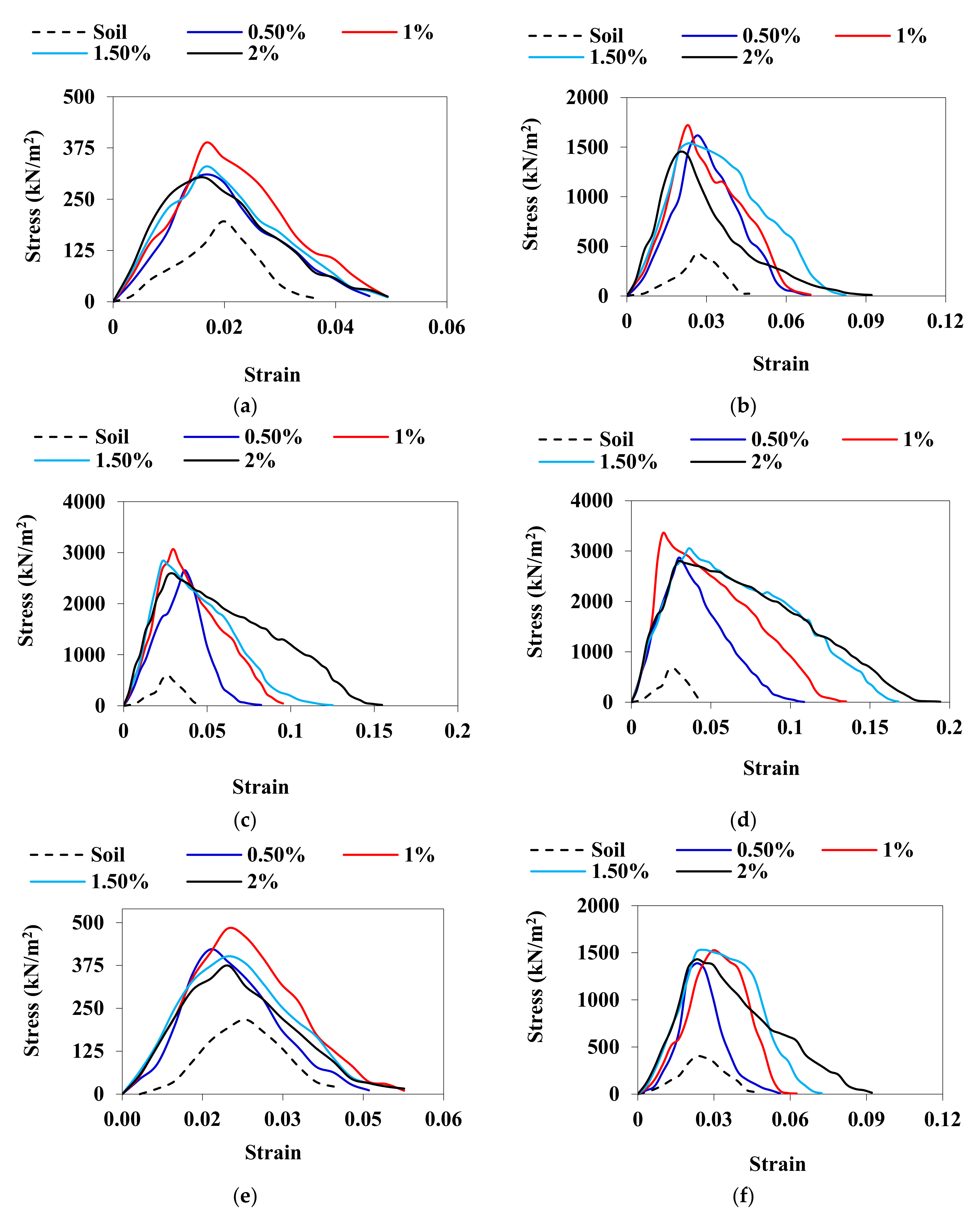

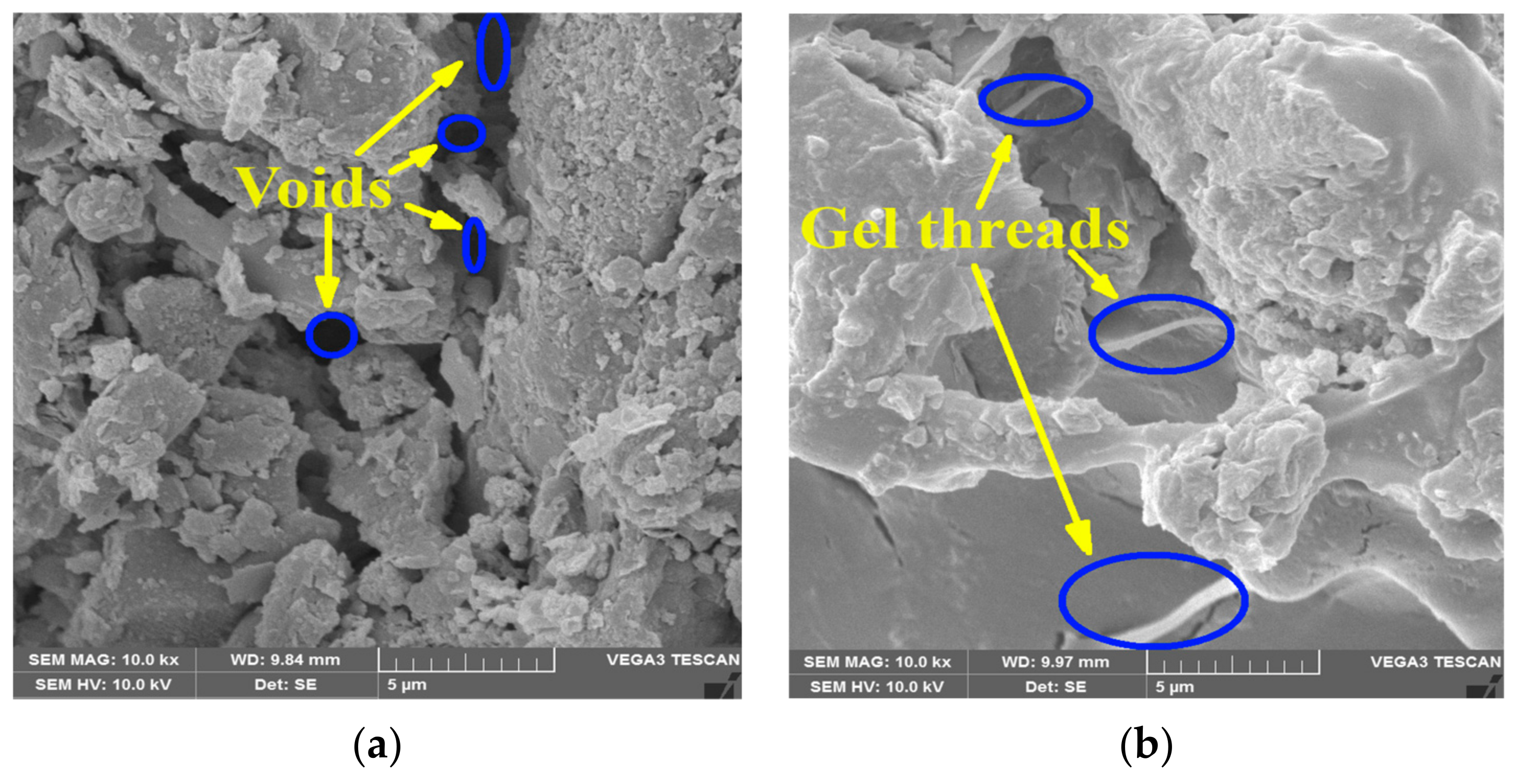
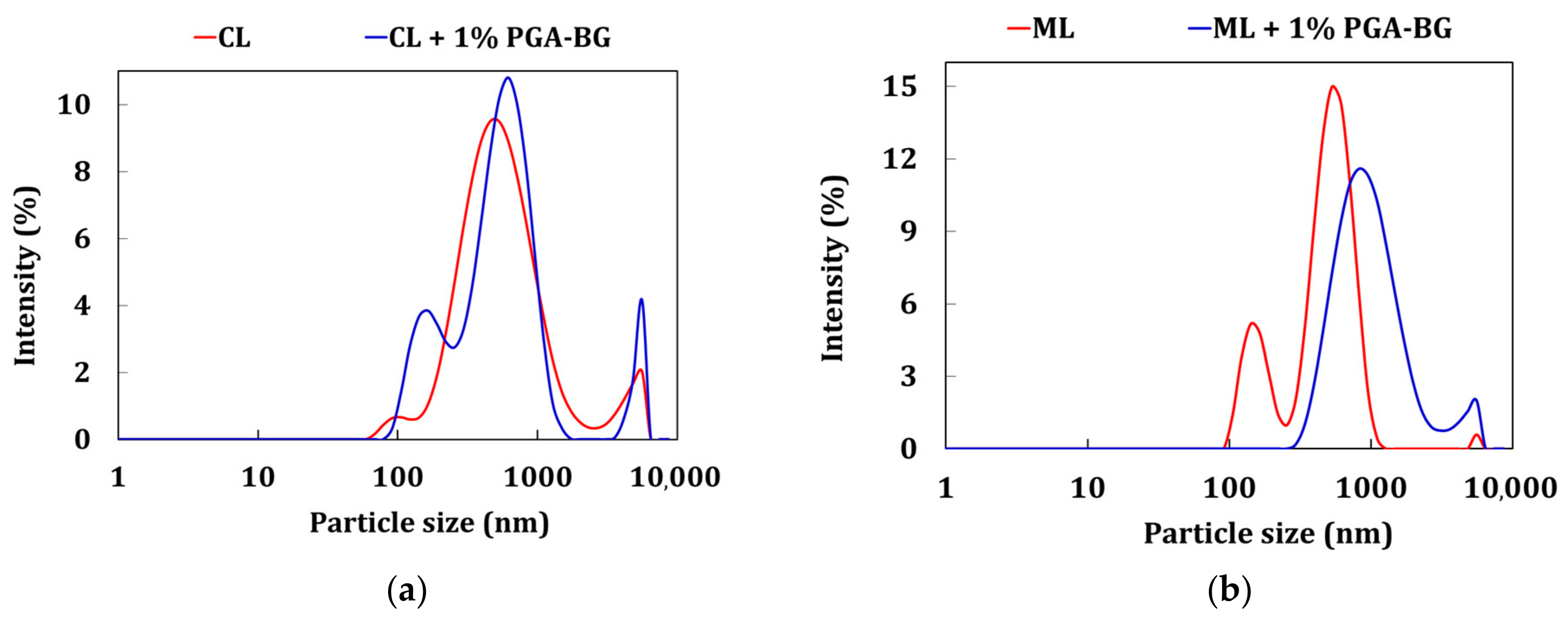
| Properties | Low Compressible Clay | Low Compressible Silt |
|---|---|---|
| Specific gravity | 2.74 | 2.71 |
| Sand (%) | 13 | 17 |
| Clay (%) | 54 | 36 |
| Silt (%) | 33 | 47 |
| Liquid limit (%) | 34 | 29 |
| Plasticity index (%) | 14.8 | 7.7 |
| USCS | CL | ML |
| Coefficient of permeability (cm/s) | 7.31 × 10−5 | 2.6 × 10−4 |
| Optimum moisture content (OMC) (%) | 8 | 10 |
| Maximum dry unit (MDU) weight (kN/m3) | 21.8 | 19.7 |
| UCS (kPa) | 196 | 217 |
| Soaked CBR (%) | 3.4 | 3.8 |
| Unsoaked CBR (%) | 7.3 | 8.1 |
| pH | 7.27 | 7.46 |
| MSA | Modulus of Granular Base + Sub-Base (MPa) | Modulus of Subgrade (MPa) | Thickness of Dense Bituminous Macadam (mm) | Thickness of Granular Layer (mm) |
|---|---|---|---|---|
| 5 | 135.44 | 62.41 | 140 | 200 |
| 10 | 138.45 | 62.41 | 160 | 210 |
| 15 | 135.44 | 62.41 | 180 | 200 |
| 20 | 141.38 | 62.41 | 190 | 220 |
| 25 | 141.38 | 62.41 | 195 | 220 |
| 30 | 142.81 | 62.41 | 205 | 225 |
| 35 | 139.92 | 62.41 | 210 | 215 |
| 40 | 135.44 | 62.41 | 220 | 200 |
| 45 | 127.54 | 62.41 | 225 | 175 |
| 50 | 125.89 | 62.41 | 230 | 170 |
| S. No. | CBR of Soil | Cost of Bituminous Surfacing (INR) | Cost of the Granular Layer (INR) | Transportation Cost of Granular Layer (INR 50/m3) | Cost of GPA-BG in the Subgrade (INR) | Total Cost (INR) |
|---|---|---|---|---|---|---|
| 1 | 3.77% | 2,353,754 | 969,373 | 45,938 | NA | 3,369,065 |
| 2 | 9.38% | 2,032,788 | 870,458 | 42,281 | 688 | 2,945,870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishweshwaran, M.; Sujatha, E.R.; Baldovino, J.A. Freeze-Dried β-Glucan and Poly-γ-glutamic Acid: An Efficient Stabilizer to Strengthen Subgrades of Low Compressible Fine-Grained Soils with Varying Curing Periods. Polymers 2024, 16, 1586. https://doi.org/10.3390/polym16111586
Vishweshwaran M, Sujatha ER, Baldovino JA. Freeze-Dried β-Glucan and Poly-γ-glutamic Acid: An Efficient Stabilizer to Strengthen Subgrades of Low Compressible Fine-Grained Soils with Varying Curing Periods. Polymers. 2024; 16(11):1586. https://doi.org/10.3390/polym16111586
Chicago/Turabian StyleVishweshwaran, Muralidaran, Evangelin Ramani Sujatha, and Jair Arrieta Baldovino. 2024. "Freeze-Dried β-Glucan and Poly-γ-glutamic Acid: An Efficient Stabilizer to Strengthen Subgrades of Low Compressible Fine-Grained Soils with Varying Curing Periods" Polymers 16, no. 11: 1586. https://doi.org/10.3390/polym16111586
APA StyleVishweshwaran, M., Sujatha, E. R., & Baldovino, J. A. (2024). Freeze-Dried β-Glucan and Poly-γ-glutamic Acid: An Efficient Stabilizer to Strengthen Subgrades of Low Compressible Fine-Grained Soils with Varying Curing Periods. Polymers, 16(11), 1586. https://doi.org/10.3390/polym16111586







