Magnesium Transfer between Atomic Force Microscopy Probes and Metal Electrodes in Aqueous Alginate Electrolytes
Abstract
:1. Introduction
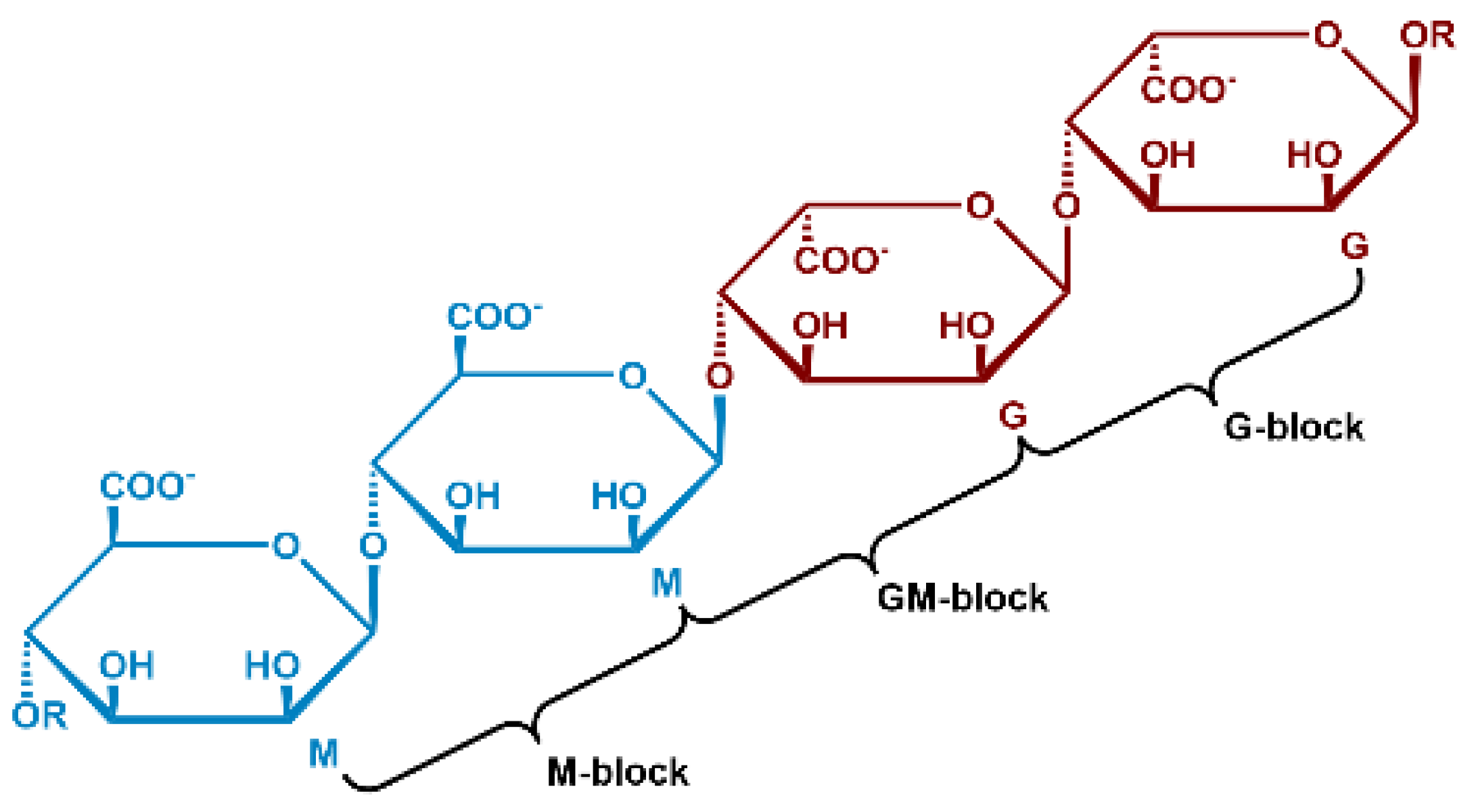
1.1. Alginate Structure
1.2. Magnesium Surface Reactions
2. Experimental Details
2.1. Mg-Alginate Electrolyte Synthesis
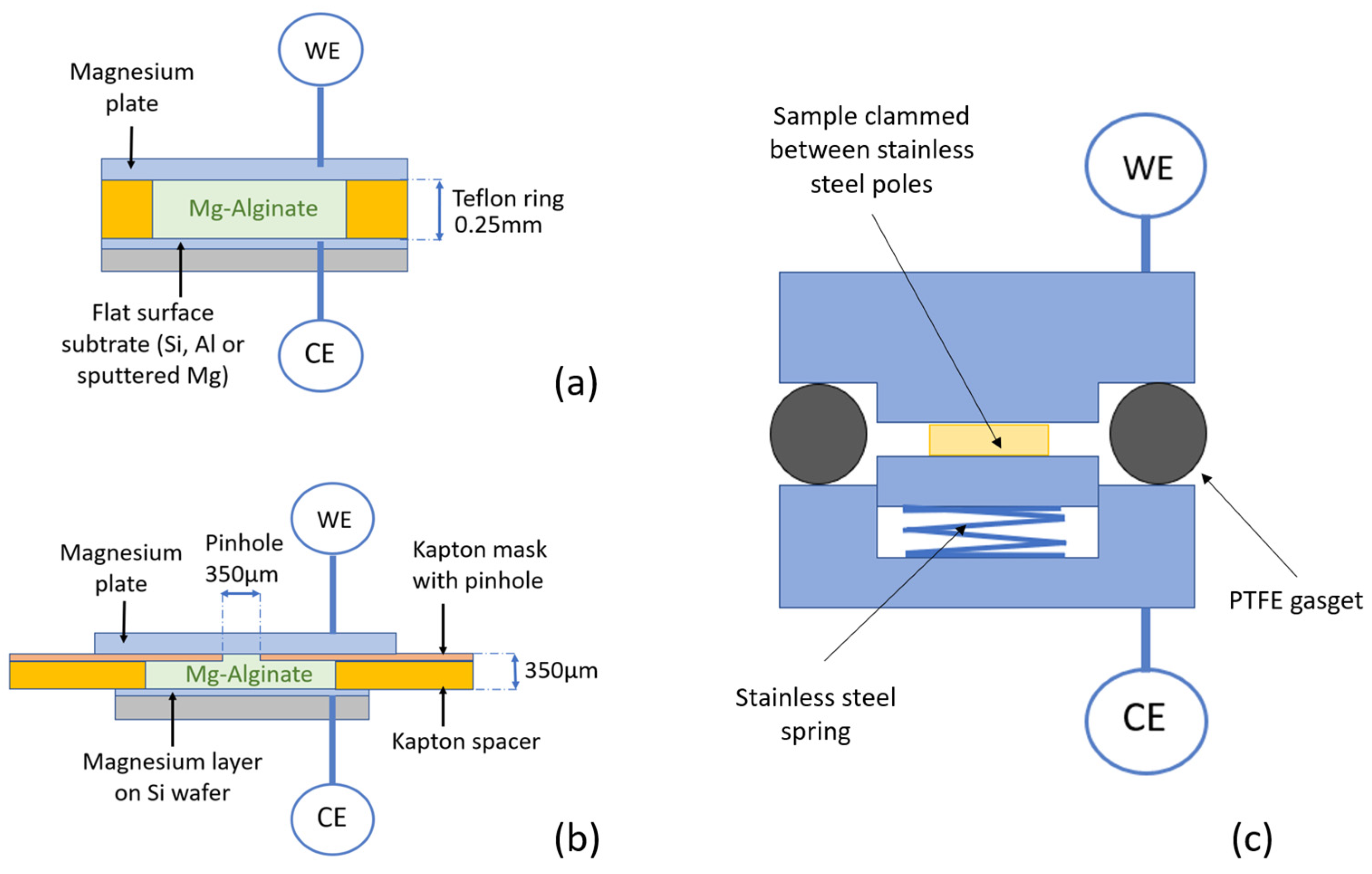
2.2. Sample Preparation for Deposition and Stripping Experiments
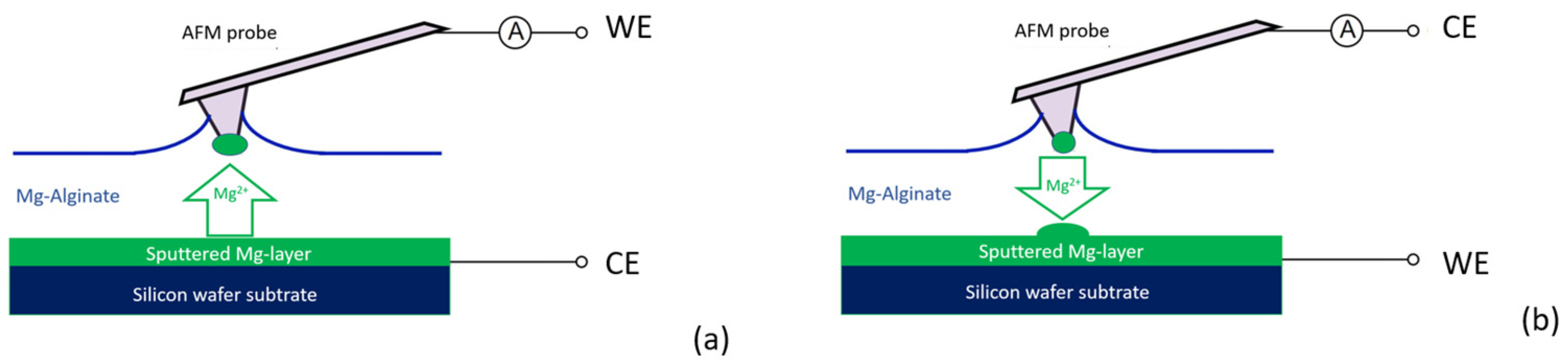
2.3. Electro-Chemical Experiments Using AFM
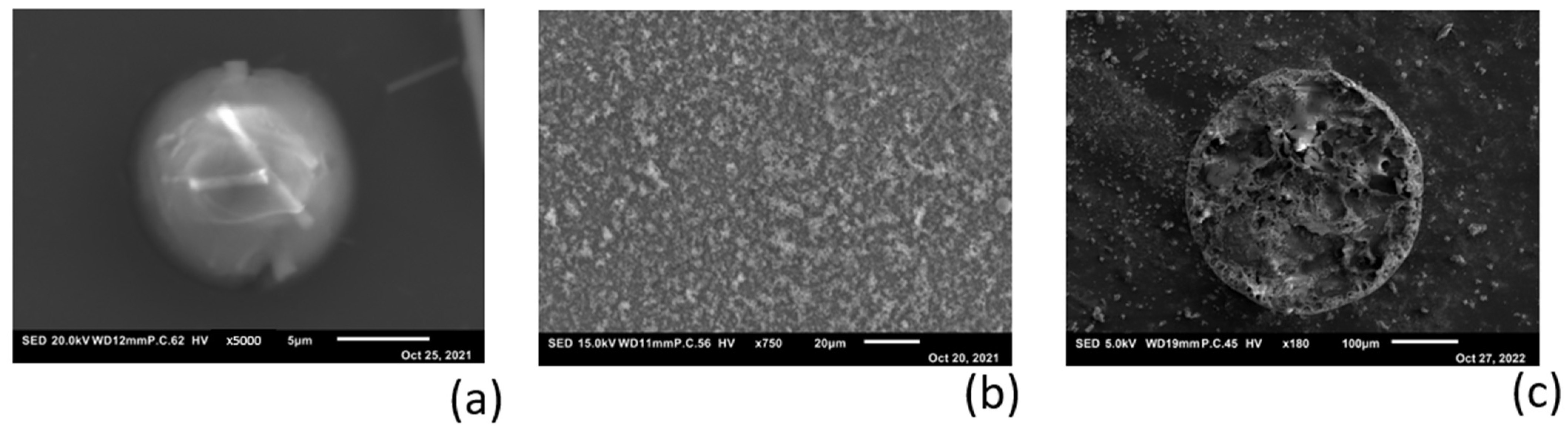
2.4. Bulk Stripping and Deposition Measurements
3. Results and Discussion
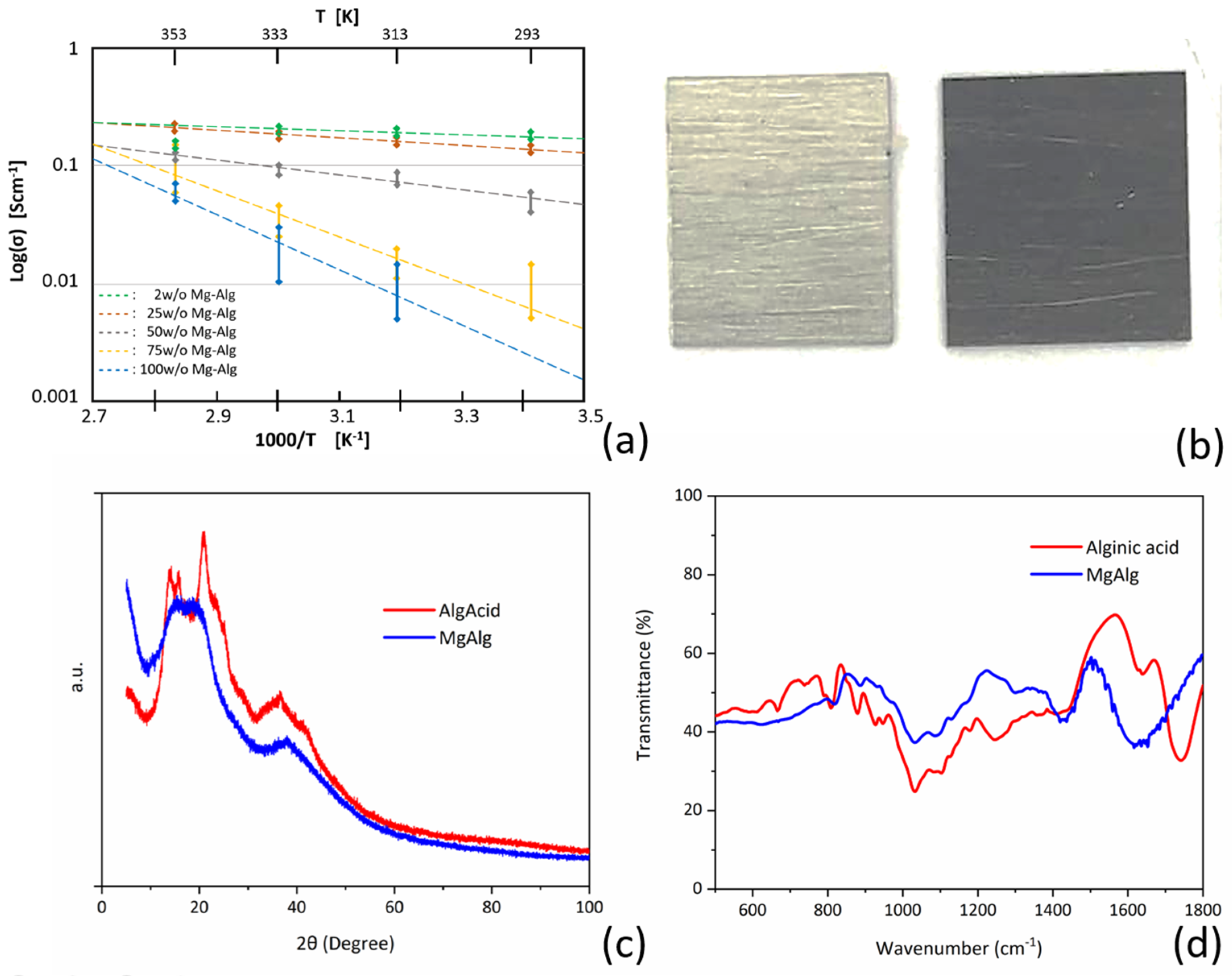
3.1. Characterization of Mg-Alginate Solutions
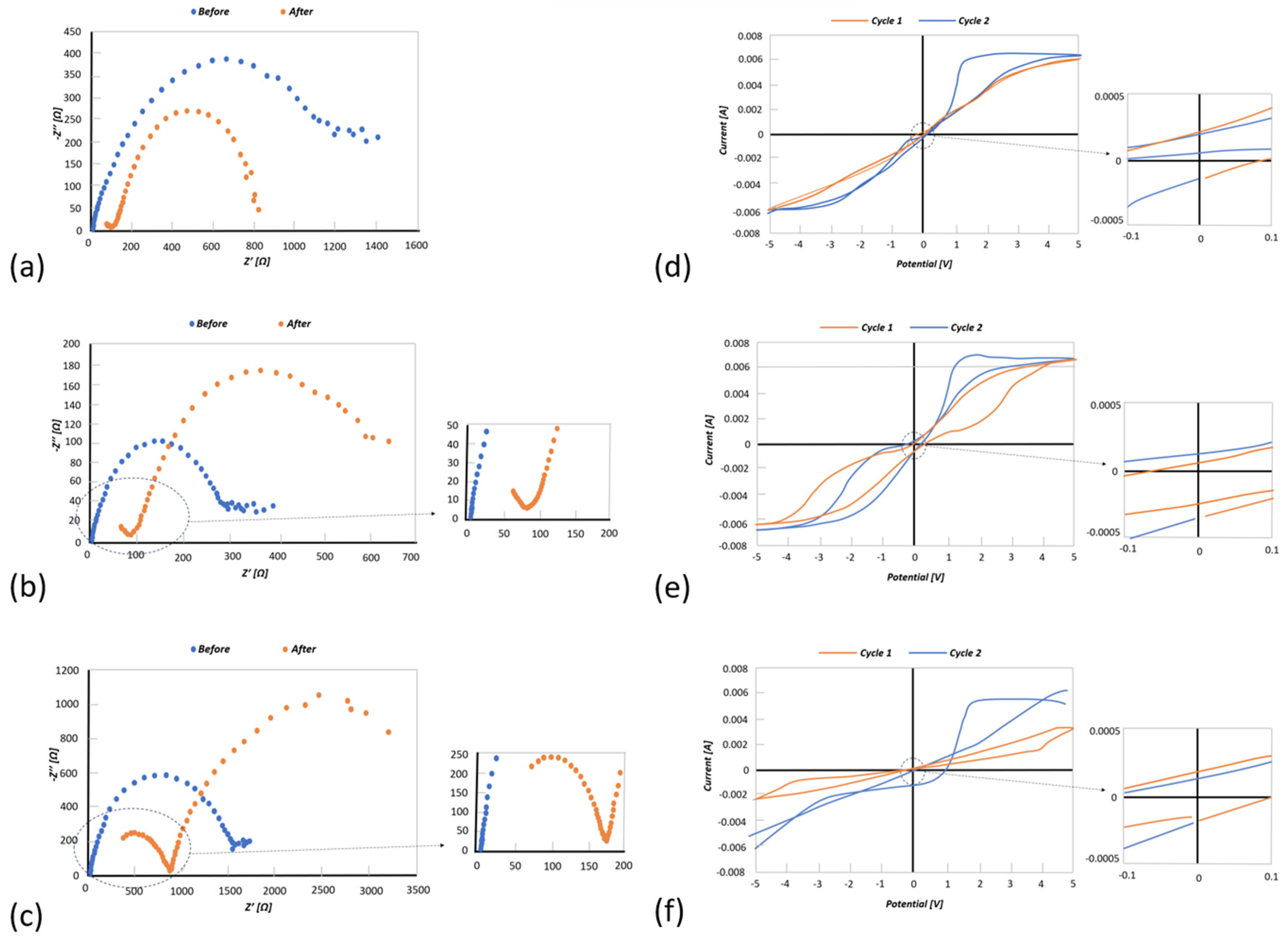
3.2. Electrochemical Characterization
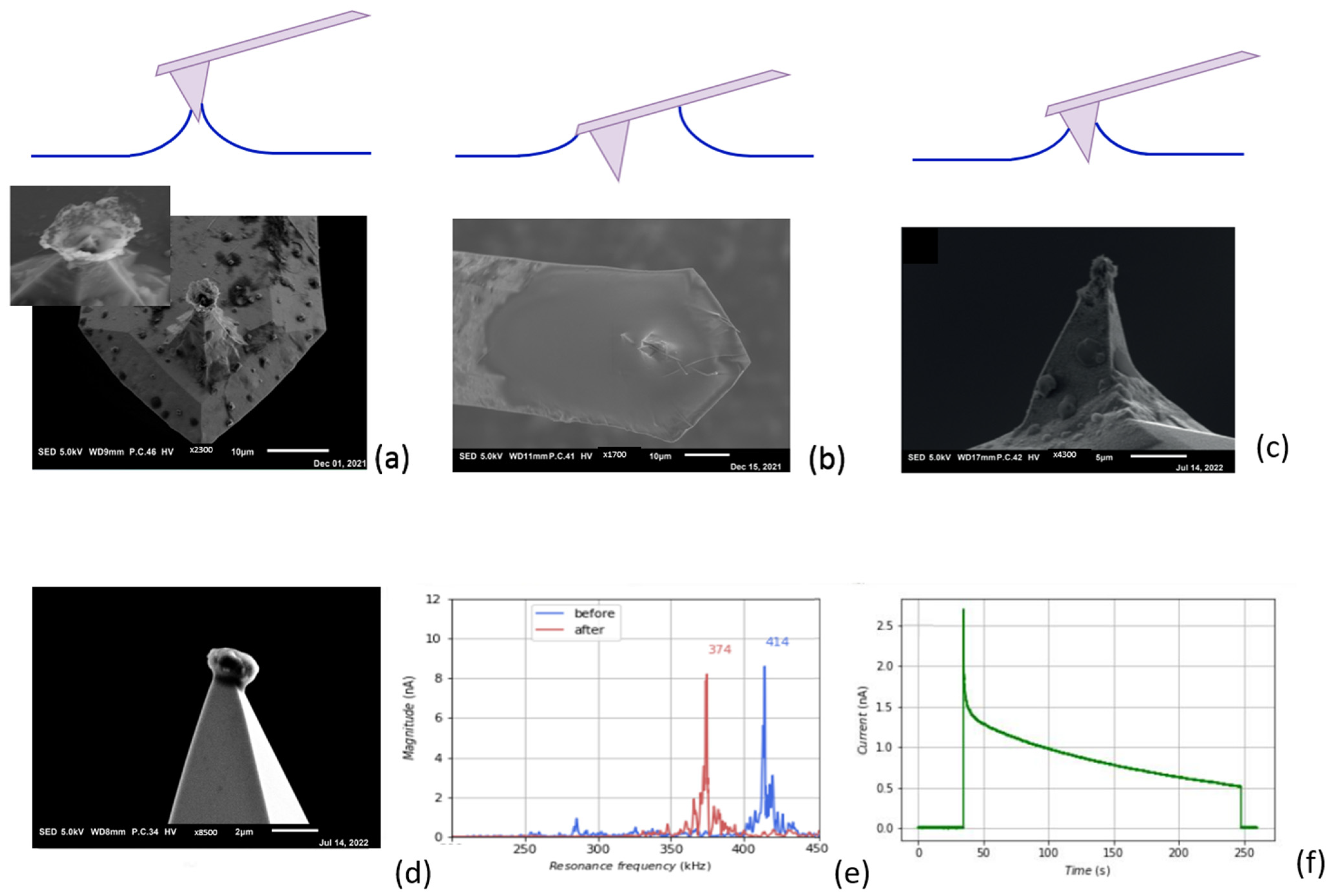
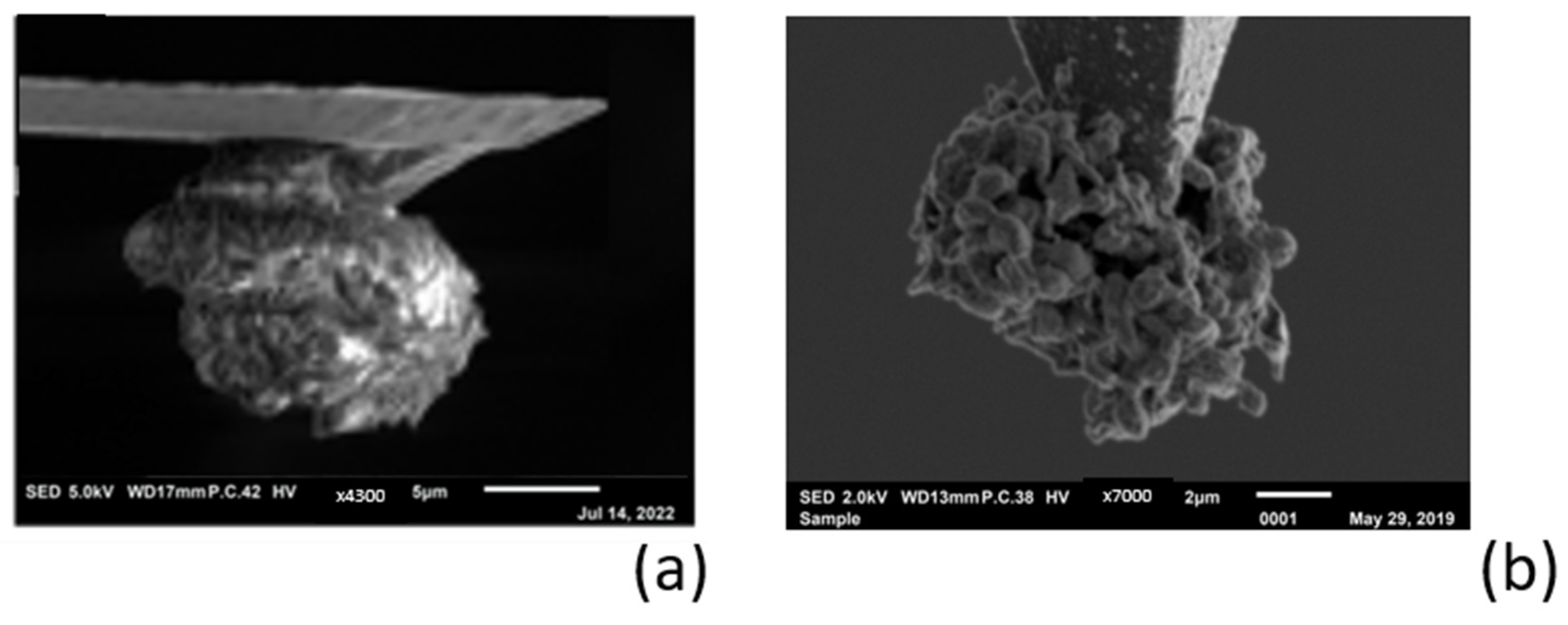
3.3. Electrochemical Deposition of Magnesium Using AFM Probe as an Electrode
3.4. Reversable Magnesium Deposition between Probe Tip and Substrate
3.5. Electro-Chemical Magnesium Deposition at Bulk Level
4. General Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, X.; Liu, H.; Cheng, X.B.; Yan, C.; Huang, J.Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Biemolt, J.; Jungbacker, P.; van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond lithium-based batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Matsui, M. Study on electrochemically deposited Mg metal. J. Power Sources 2011, 196, 7048–7055. [Google Scholar] [CrossRef]
- Aurbach, D.; Gizbar, H.; Schechter, A.; Chusid, O.; Gottlieb, H.E.; Gofer, Y.; Goldberg, I. Electrolyte solutions for rechargeable magnesium batteries based on organomagnesium chloroaluminate complexes. J. Electrochem. Soc. 2001, 149, A115. [Google Scholar] [CrossRef]
- Salama, M.; Attias, R.; Hirsch, B.; Yemini, R.; Gofer, Y.; Noked, M.; Aurbach, D. On the feasibility of practical Mg–S batteries: Practical limitations associated with metallic magnesium anodes. ACS Appl. Mater. Interfaces 2018, 10, 36910–36917. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-voltage aqueous magnesium ion batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Legerstee, W.J.; Boekel, M.; Boonstra, S.; Kelder, E.M. Scanning Probe Microscopy Facility for Operando Study of Redox Processes on Lithium ion Battery Electrodes. Front. Chem. 2021, 9, 505876. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The Egg−Box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrod, O.A.; Larsen, B.; Gronowitz, S.; Hoffman, R.A.; Westerdahl, A. The Effect of Divalent Metals on the Properties of Alginate Solutions. II. Comparison of Different Metal Ions. Acta Chem. Scand. 1965, 19, 341–351. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross−Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Gore, P.; Raja, V.S.; Birbilis, N. Use of sodium bicarbonate as a chloride-free aqueous electrolyte to explore film formation and the negative difference effect on pure magnesium. J. Electrochem. Soc. 2018, 165, C849. [Google Scholar] [CrossRef]
- Salleh, S.H.; Thomas, S.; Yuwono, J.A.; Venkatesan, K.; Birbilis, N. Enhanced hydrogen evolution on MgOH2 covered Mg surfaces. Electrochim. Acta 2015, 161, 144–152. [Google Scholar] [CrossRef]
- Jäckle, M.; Helmbrecht, K.; Smits, M.; Stottmeister, D.; Groß, A. Self-diffusion barriers: Possible descriptors for dendrite growth in batteries? Energy Environ. Sci. 2018, 11, 3400–3407. [Google Scholar] [CrossRef]
- Jäckle, M.; Groß, A. Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 2014, 141, 174710. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legerstee, W.J.; Kiriinya, L.; Kwakernaak, M.; Kelder, E.M. Magnesium Transfer between Atomic Force Microscopy Probes and Metal Electrodes in Aqueous Alginate Electrolytes. Polymers 2024, 16, 1615. https://doi.org/10.3390/polym16121615
Legerstee WJ, Kiriinya L, Kwakernaak M, Kelder EM. Magnesium Transfer between Atomic Force Microscopy Probes and Metal Electrodes in Aqueous Alginate Electrolytes. Polymers. 2024; 16(12):1615. https://doi.org/10.3390/polym16121615
Chicago/Turabian StyleLegerstee, Walter J., Lindah Kiriinya, Mark Kwakernaak, and Erik M. Kelder. 2024. "Magnesium Transfer between Atomic Force Microscopy Probes and Metal Electrodes in Aqueous Alginate Electrolytes" Polymers 16, no. 12: 1615. https://doi.org/10.3390/polym16121615
APA StyleLegerstee, W. J., Kiriinya, L., Kwakernaak, M., & Kelder, E. M. (2024). Magnesium Transfer between Atomic Force Microscopy Probes and Metal Electrodes in Aqueous Alginate Electrolytes. Polymers, 16(12), 1615. https://doi.org/10.3390/polym16121615





