Development of Halloysite Nanotube-Infused Thermoset Soybean Bio-Resin for Advanced Medical Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Halloysite
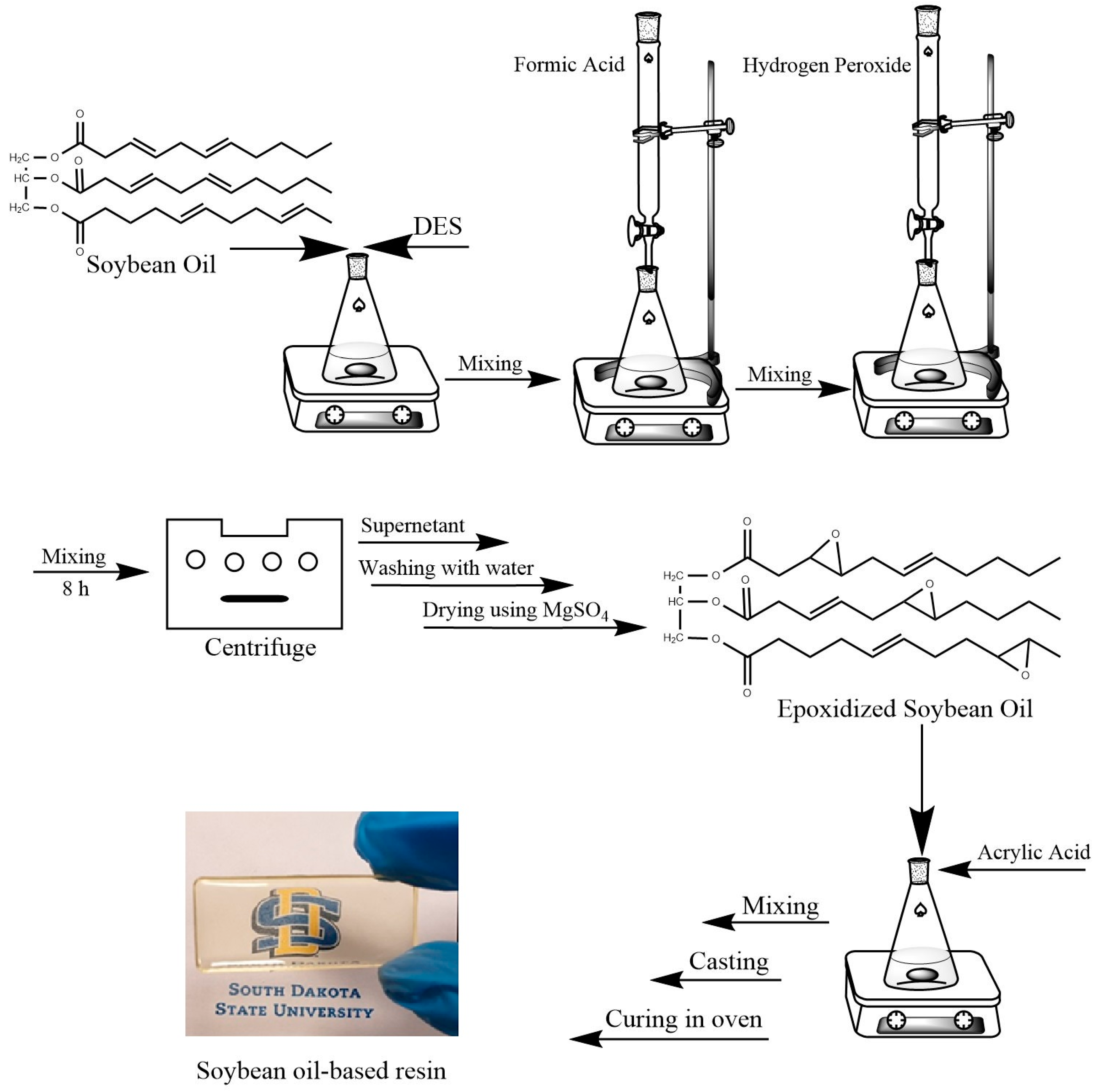
2.3. Epoxidation of Soybean Oil
2.4. Thermoset Resin Preparation
2.5. UV–Vis Spectroscopy
2.6. FT-IR
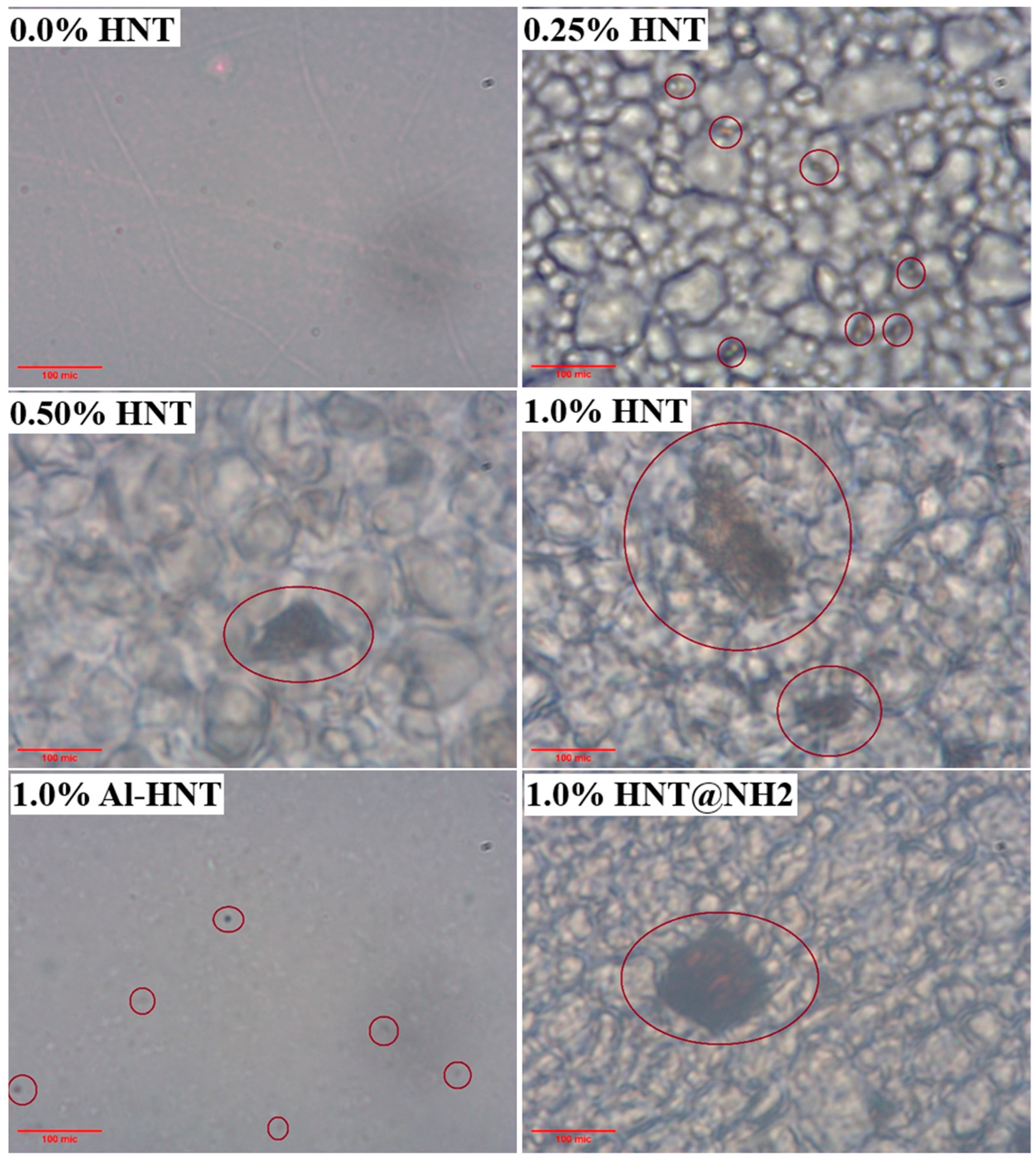
2.7. Optical Microscopy
2.8. Mechanical Test
2.9. Thermal Stability
2.10. Water Absorption Tests
- WA = Water absorption (%),
- Mf = Sample weight after water absorption (g),
- Moi = Oven dry weight after impregnation (g).
3. Results and Discussion
3.1. FT-IR of HNTs and Prepared Resins
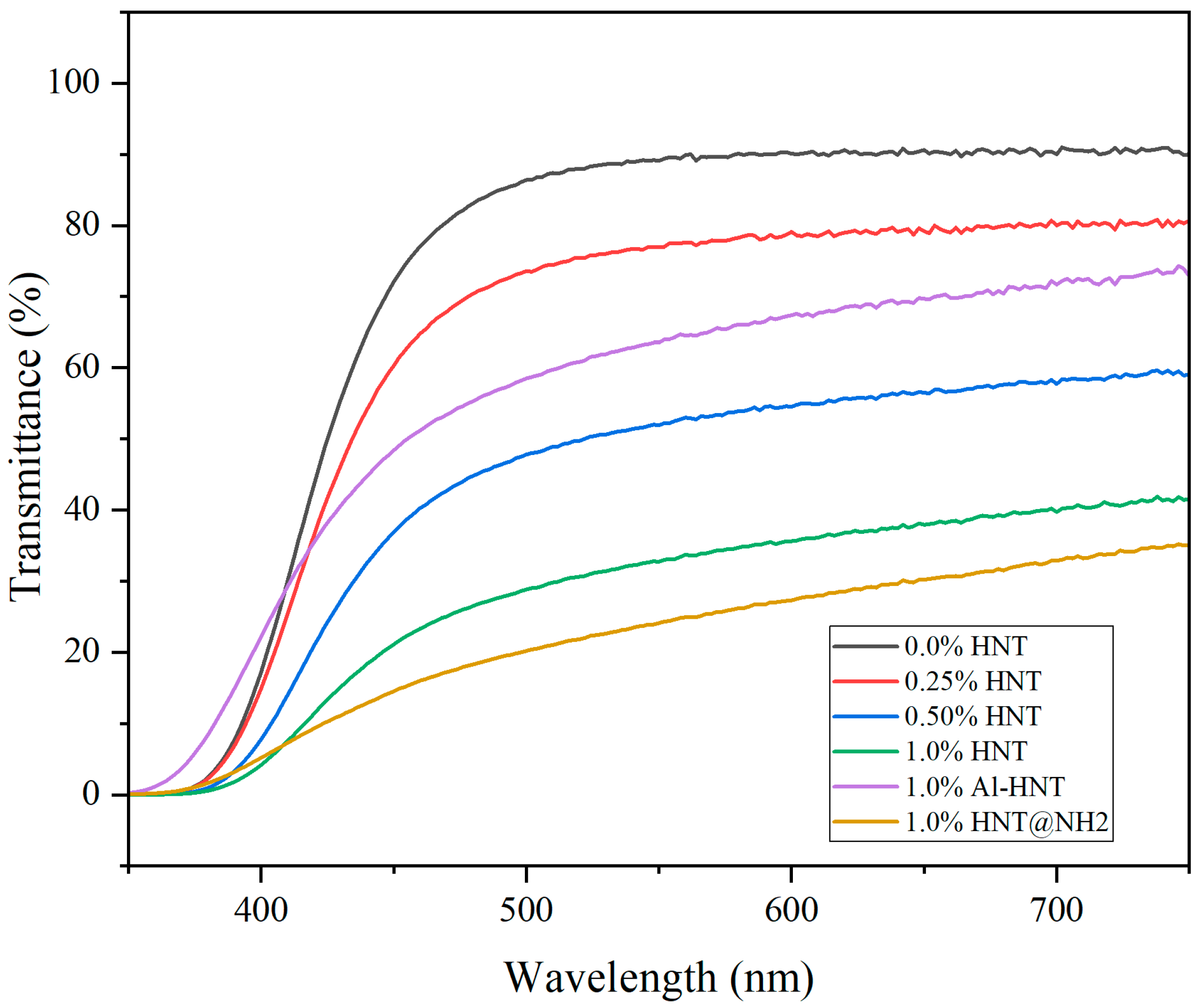
3.2. Transparency and Light Transmission
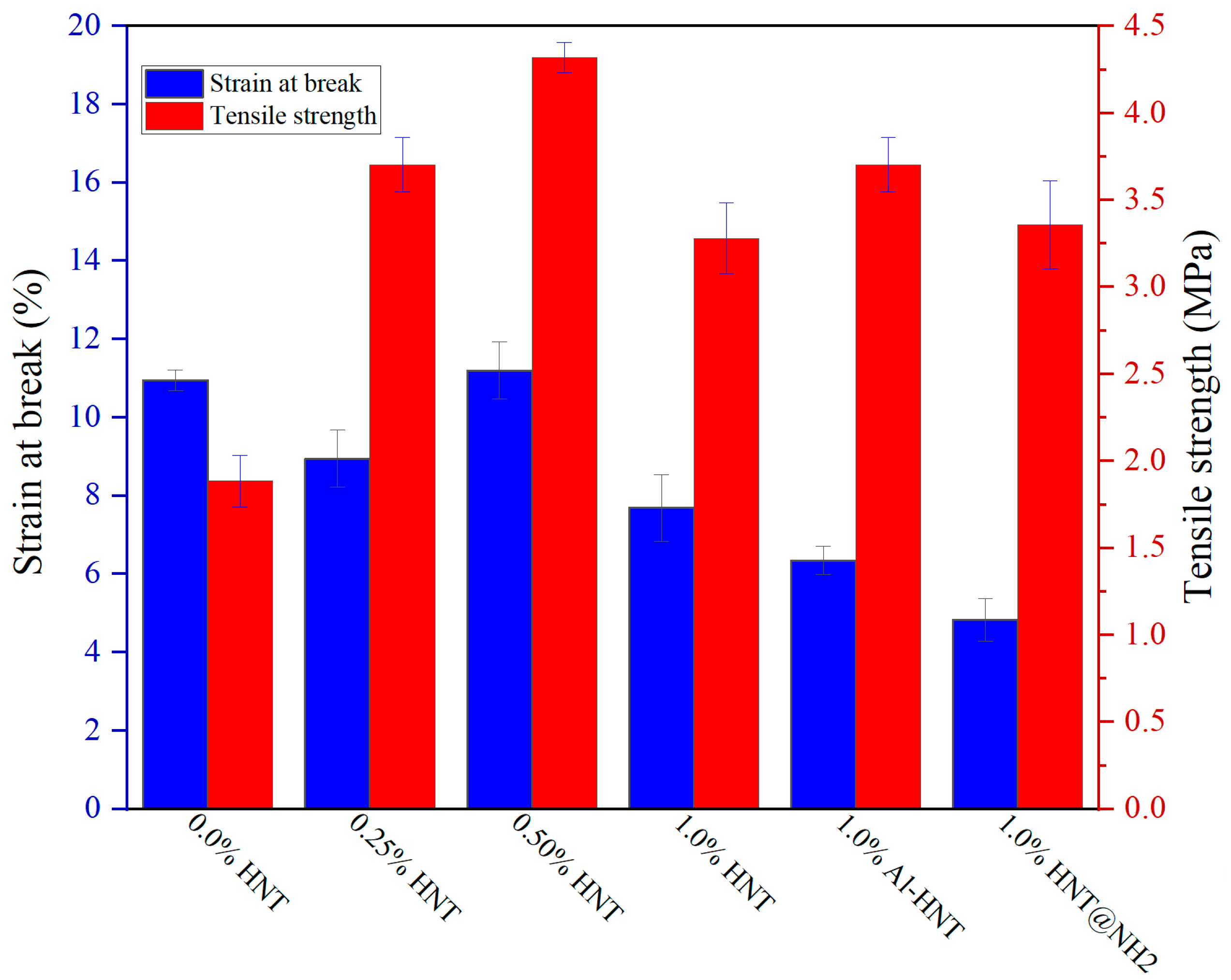
3.3. Mechanical Strength
3.4. Thermal Gravimetric Analysis
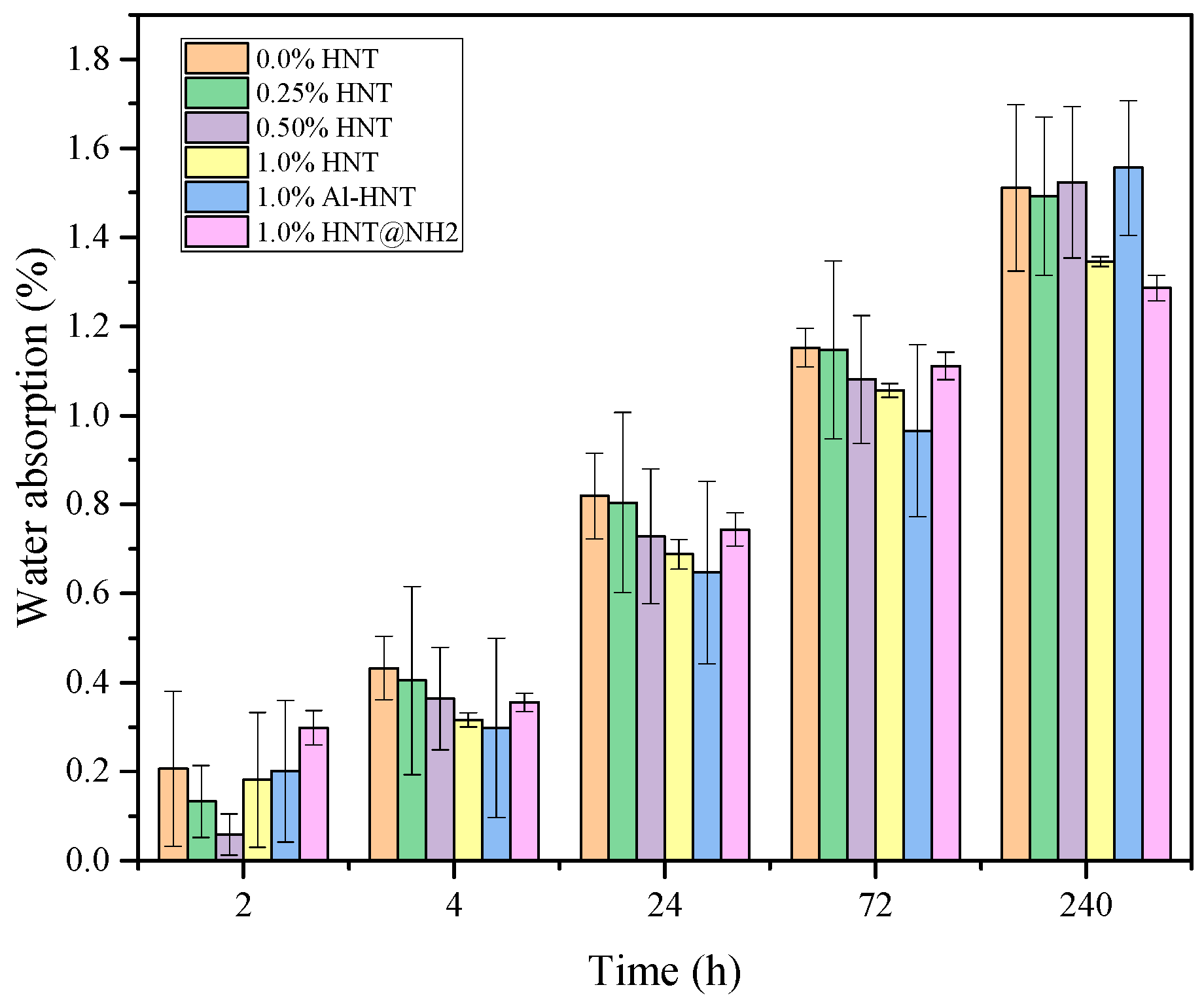
3.5. Water Absorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al-HNT | Alkali-modified halloysite nanotubes |
| APTES | (3-Aminopropyl) triethoxysilane |
| DES | Deep eutectic solvent |
| ESO | Epoxidized soybean oil |
| ESOR | Epoxidized soybean oil resin |
| FT-IR | Fourier transform infrared |
| HNTs | Halloysite nanotubes |
| HNT@NH2 | (3-Aminopropyl) triethoxysilane-modified halloysite nanotubes |
| PLA | Polylactic acid |
| PVC | Polyvinyl chloride |
| SO | Soybean oil |
| WA | Water absorption |
References
- Pal, P.; Sambhakar, S.; Dave, V.; Paliwal, S.K.; Paliwal, S.; Sharma, M.; Kumar, A.; Dhama, N. A review on emerging smart technological innovations in healthcare sector for increasing patient’s medication adherence. Glob. Health J. 2021, 5, 183–189. [Google Scholar] [CrossRef]
- Stasevych, M.; Zvarych, V. Innovative robotic technologies and artificial intelligence in pharmacy and medicine: Paving the way for the future of health care—A review. BDCC 2023, 7, 147. [Google Scholar] [CrossRef]
- Sah, S.K.; Kaity, S. Pharmaceutical packaging: Recent trends and challenges. In Dosage Forms, Formulation Developments and Regulations; Elsevier: Amsterdam, The Netherlands, 2024; pp. 663–684. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Albuquerque, A.K.; Almeida, D.E.; Barreto, J.V.; Silva, I.D.; Jaques, N.G.; Nepomuceno, N.C.; Medeiros, E.S.; Wellen, R.M. Effect of hardener and catalyst contents on curing and degradation of epoxidized soybean oil. J. Appl. Polym. Sci. 2023, 140, e53343. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Y.; Zhou, X.; Wen, Q.; Ye, C.; Ye, Z.; Li, P.; Yang, S.; Yang, Z. A solvent-free and scalable method to prepare alkali soluble soybean oil-based epoxy acrylic resin for photoresist application. Ind. Crops Prod. 2023, 191, 115877. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Santos, M.; Cernadas, T.; Ferreira, P.; Alves, P. Advances in the development of biobased epoxy resins: Insight into more sustainable materials and future applications. Int. Mater. Rev. 2022, 67, 119–149. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Bio-Based Epoxy Resins: A Review. Polym. Plast. Technol. Eng. 2016, 57, 133–155. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, e44103. [Google Scholar] [CrossRef]
- Nepomuceno, N.C.; Fook, M.V.L.; Ries, A.; Mija, A.; Wellen, R.M.R. Bio-Based Epoxy Resins of Epoxidized Soybean oil Cured with Salicylic acid Loaded with Chitosan: Evaluation of Physical–Chemical Properties. J. Polym. Environ. 2023, 31, 2566–2575. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Chong, K.L.; Lai, J.C.; Rahman, R.A.; Adrus, N.; Al-Saffar, Z.H.; Hassan, A.; Lim, T.H.; Wahit, M.U. A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils. Ind. Crops Prod. 2022, 189, 115857. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Sobhan, A.; Saedi, S.; Hoff, M.; Liang, Y.; Muthukumarappan, K. Evaluation and Improvement of Bio-Based Sustainable Resin Derived from Formic-Acid-Modified Epoxidized Soybean Oil for Packaging Applications. Polymers 2023, 15, 4255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, S.; Zhan, G.; Tang, X.; Yu, Y. Biobased nanocomposites from clay modified blend of epoxidized soybean oil and cyanate ester resin. Prog. Org. Coat. 2013, 76, 1683–1690. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Roy, S.; Rhim, J.-W. Silver loaded aminosilane modified halloysite for the preparation of carrageenan-based functional films. Appl. Clay Sci. 2021, 211, 106170. [Google Scholar] [CrossRef]
- Brantseva, T.V.; Ilyin, S.O.; Gorbunova, I.Y.; Antonov, S.V.; Korolev, Y.M.; Kerber, M.L. Epoxy reinforcement with silicate particles: Rheological and adhesive properties—Part II: Characterization of composites with halloysite. Int. J. Adhes. Adhes. 2016, 68, 248–255. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and shear resistance. J. Adhes. Sci. Technol. 2015, 29, 1831–1848. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible Bionanocomposites from Epoxidized Hemp Seed Oil Thermosetting Resin Reinforced with Halloysite Nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kasapis, S.; Rhim, J.-W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef]
- Bischoff, E.; Daitx, T.; Simon, D.A.; Schrekker, H.S.; Liberman, S.A.; Mauler, R.S. Organosilane-functionalized halloysite for high performance halloysite/heterophasic ethylene–propylene copolymer nanocomposites. Appl. Clay Sci. 2015, 112–113, 68–74. [Google Scholar] [CrossRef]
- Uner, G.; Karakus, G.; Kaplan Can, H. Design, fabrication and characterization of silane tailored surface of halloysite based polymer nanocomposites. Polym. Compos. 2023, 44, 1305–1330. [Google Scholar] [CrossRef]
- Maurya, A.; Sinha, S.; Kumar, P.; Singh, V. A review: Impact of surface treatment of nanofillers for improvement in thermo mechanical properties of the epoxy based nanocomposites. Mater. Today Proc. 2023, 78, 164–172. [Google Scholar] [CrossRef]
- Lee, N.; Kim, U.; Shon, M. Development of corrosion-resistant epoxy coating by hydrophobically surface-modified halloysite nanotubes using trimethylchlorosilane and 1,1,1,3,3,3-hexamethyldisilazane. J. Coat. Technol. Res. 2023, 20, 1007–1108. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, J.; Ye, L. Halloysite–epoxy nanocomposites with improved particle dispersion through ball mill homogenisation and chemical treatments. Compos. Sci. Technol. 2009, 69, 2497–2505. [Google Scholar] [CrossRef]
- Albdiry, M.T.; Yousif, B.F. Role of silanized halloysite nanotubes on structural, mechanical properties and fracture toughness of thermoset nanocomposites. Mater. Des. 2014, 57, 279–288. [Google Scholar] [CrossRef]
- Jelić, A.; Marinković, A.; Sekulić, M.; Dikić, S.; Ugrinović, V.; Pavlović, V.; Putić, S. Design of halloysite modification for improvement of mechanical properties of the epoxy based nanocomposites. Polym. Compos. 2021, 42, 2180–2192. [Google Scholar] [CrossRef]
- Sobhan, A.; Ahirekar, V.; Hoff, M.; Muthukumarappan, K. Derivation and characterization of epoxidized soybean oil and epoxy resin film produced using a three step-washing neutralization process. Ind. Crops Prod. 2023, 198, 116675. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhou, Z.; Fu, Y.; Chang, J. Epoxidation of soybean oil catalyzed by deep eutectic solvents based on the choline chloride–carboxylic acid bifunctional catalytic system. Ind. Eng. Chem. Res. 2017, 56, 8224–8234. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting (D882-88). American Society for Testing and Materials: Philadelphia, PA, USA, 1989.
- Kabasakal, Y.; Baysal, E.; Babahan-Bircan, İ.; Altay, Ç.; Toker, H. Investigation of some physical and mechanical properties of wood coated with plant-oil based epoxide nanocomposite materials. Prog. Org. Coat. 2023, 176, 107383. [Google Scholar] [CrossRef]
- Zou, M.; Du, M.; Zhu, H.; Xu, C.; Fu, Y. Green synthesis of halloysite nanotubes supported Ag nanoparticles for photocatalytic decomposition of methylene blue. J. Phys. D Appl. Phys. 2012, 45, 325302. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Fernandes, A.C.; Pereira, C.; Pires, J.; Freire, C. Physicochemical characterization of organosilylated halloysite clay nanotubes. Micropor. Mesopor. Mat. 2016, 219, 145–154. [Google Scholar] [CrossRef]
- Wamba, A.G.; Kofa, G.P.; Koungou, S.N.; Thue, P.S.; Lima, E.C.; dos Reis, G.S.; Kayem, J.G. Grafting of Amine functional group on silicate based material as adsorbent for water purification: A short review. J. Environ. Chem. Eng. 2018, 6, 3192–3203. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Alkali activation of halloysite for adsorption and release of ofloxacin. Appl. Surf. Sci. 2013, 287, 54–61. [Google Scholar] [CrossRef]
- He, T.; Jin, B. Alkali-modified halloysite for enhanced cadmium/lead vapor capture: Focus on silicic surface via experimental and computational investigations. Chem. Eng. J. 2023, 475, 145726. [Google Scholar] [CrossRef]
- Ferrante, F.; Bertini, M.; Ferlito, C.; Lisuzzo, L.; Lazzara, G.; Duca, D. A computational and experimental investigation of halloysite silicic surface modifications after alkaline treatment. Appl. Clay Sci. 2023, 232, 106813. [Google Scholar] [CrossRef]
- Gao, B.; Huang, S.; Liu, Y.; Shi, X.; Jiang, R.; Shu, Z. A novel biocomposite based on lignocellulose modified with epoxidized soybean oil resin. J. Appl. Polym. Sci. 2023, 140, e54600. [Google Scholar] [CrossRef]
- Ge, X.; Yu, L.; Liu, Z.; Liu, H.; Chen, Y.; Chen, L. Developing acrylated epoxidized soybean oil coating for improving moisture sensitivity and permeability of starch-based film. Int. J. Biol. Macromol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Silver ion loaded 3-aminopropyl trimethoxysilane-modified Fe3O4 nanoparticles for the fabrication of carrageenan-based active packaging films. Colloids Surf. B Biointerfaces 2021, 208, 112085. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, M.; Stetsyshyn, Y.; Harhay, K.; Melnyk, Y.; Donchak, V.; Gubriy, Z.; Kracalik, M. Impact of the functionalized clay nanofillers on the properties of the recycled polyethylene terephthalate nanocomposites. J. Appl. Polym. Sci. 2024, 141, e55543. [Google Scholar] [CrossRef]
- Donchak, V.; Stetsyshyn, Y.; Bratychak, M.; Broza, G.; Harhay, K.; Stepina, N.; Kostenko, M.; Voronov, S. Nanoarchitectonics at surfaces using multifunctional initiators of surface-initiated radical polymerization for fabrication of the nanocomposites. Appl. Surf. Sci. Adv. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.-W. Preparation of carrageenan-based nanocomposite films incorporated with functionalized halloysite using AgNP and sodium dodecyl sulfate. Food Hydrocoll 2020, 106, 105934. [Google Scholar] [CrossRef]
- Babu, J.S.; SMurthy, V.S.; Kumar, U.P.; Sathiyamoorthy, V.; Saravanakumar, A. Novel Spinifex littoreus fibre and sugarcane biosilica on mechanical, wear, time dependent and water absorption behaviour of epoxy structural composite. Biomass Conv. Bioref. 2024, 14, 4017–4028. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Zhang, Y.; Cao, B.; Wu, K.; Wang, L. Understanding Water Diffusion Behaviors in Epoxy Resin through Molecular Simulations and Experiments. Langmuir 2024, 40, 4871–4880. [Google Scholar] [CrossRef]
- Lavoratti, A.; Bianchi, O.; Cruz, J.A.; Al-Maqdasi, Z.; Varna, J.; Amico, S.C.; Joffe, R. Impact of water absorption on the creep performance of epoxy/microcrystalline cellulose composites. J. Appl. Polym. Sci. 2024, 141, e55365. [Google Scholar] [CrossRef]


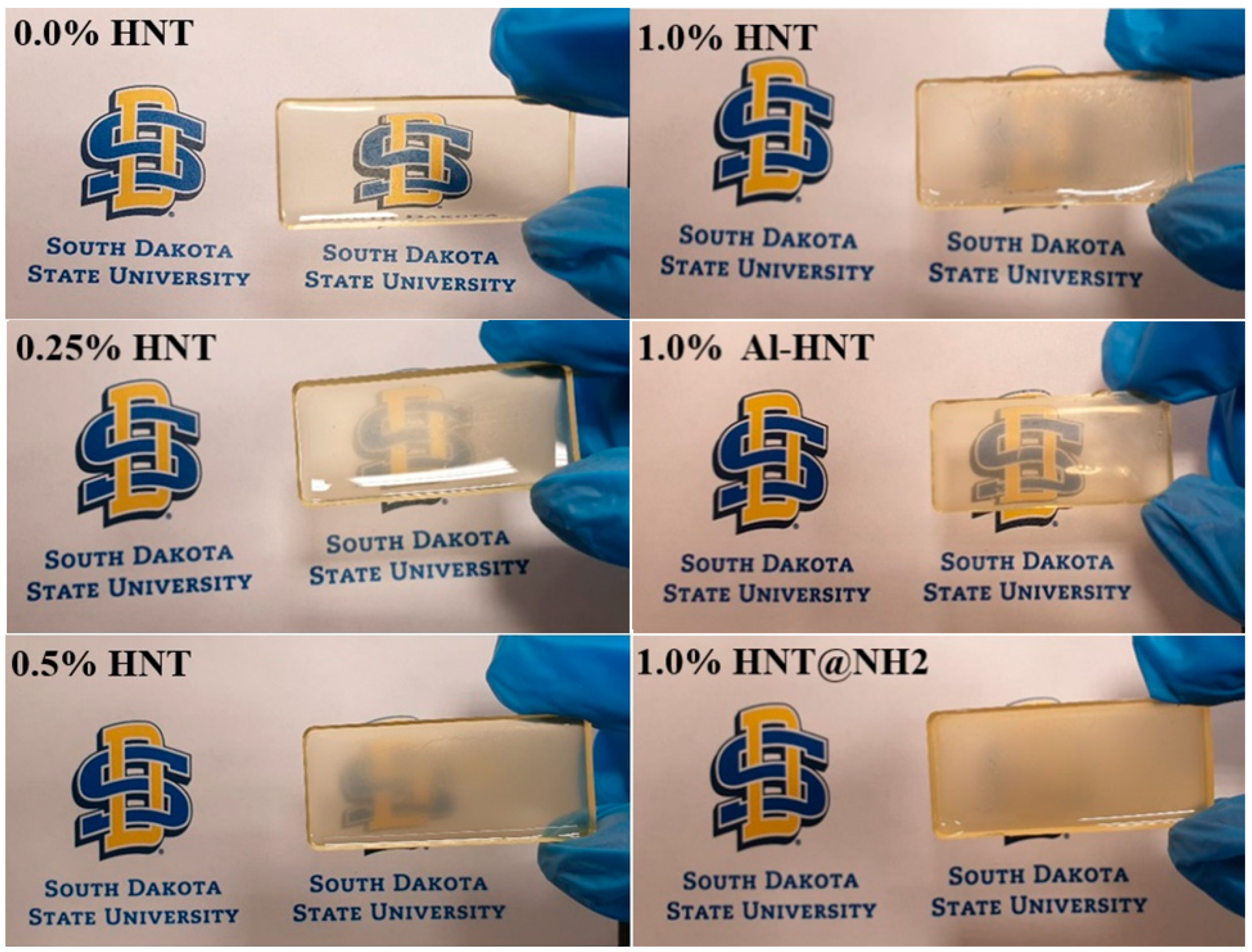

| Sample | Filler (g) | Matrix | |
|---|---|---|---|
| - | - | ESO (g) | Acrylic Acid (g) |
| 0.0% HNT | 0.0 HNTs | 50 | 50 |
| 0.25% HNT | 0.125 HNTs | 49.875 | 50 |
| 0.50% HNT | 0.250 HNTs | 49.75 | 50 |
| 1.0% HNT | 0.50 NTs | 49.5 | 50 |
| 1.0% Al-HNT | 0.5 Al-HNT | 49.5 | 50 |
| 1.0% HNT@NH2 | 0.5 HNT@NH2 | 49.5 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saedi, S.; Sobhan, A.; Hoff, M.; Wang, S.; Muthukumarappan, K. Development of Halloysite Nanotube-Infused Thermoset Soybean Bio-Resin for Advanced Medical Packaging. Polymers 2024, 16, 1616. https://doi.org/10.3390/polym16121616
Saedi S, Sobhan A, Hoff M, Wang S, Muthukumarappan K. Development of Halloysite Nanotube-Infused Thermoset Soybean Bio-Resin for Advanced Medical Packaging. Polymers. 2024; 16(12):1616. https://doi.org/10.3390/polym16121616
Chicago/Turabian StyleSaedi, Shahab, Abdus Sobhan, Magdalene Hoff, Siqun Wang, and Kasiviswanathan Muthukumarappan. 2024. "Development of Halloysite Nanotube-Infused Thermoset Soybean Bio-Resin for Advanced Medical Packaging" Polymers 16, no. 12: 1616. https://doi.org/10.3390/polym16121616





