Performance Evaluation of Out-of-Spec Carbon Prepregs for Upcycling Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cure Kinetics Modeling
2.2. Gel Time and Vitrification Time Evaluation and Modeling
2.3. DiBenedetto Model
3. Results and Discussion
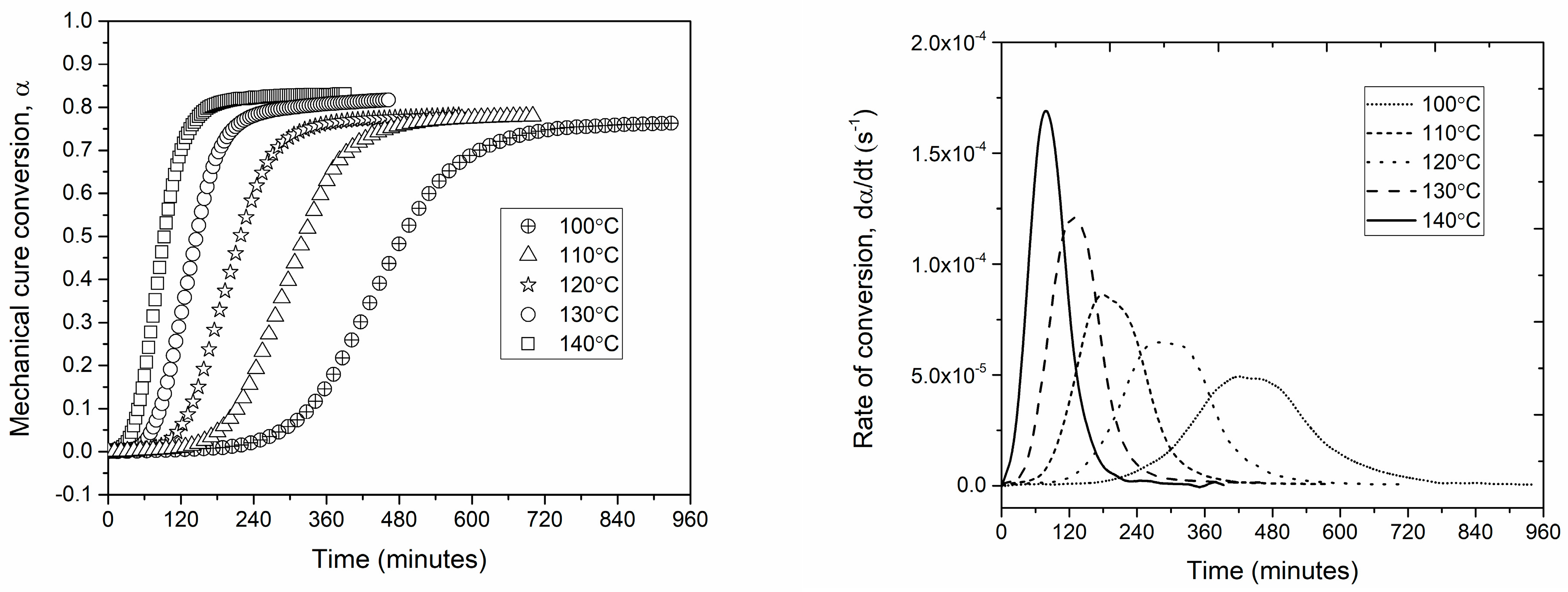
3.1. Cure Kinetics
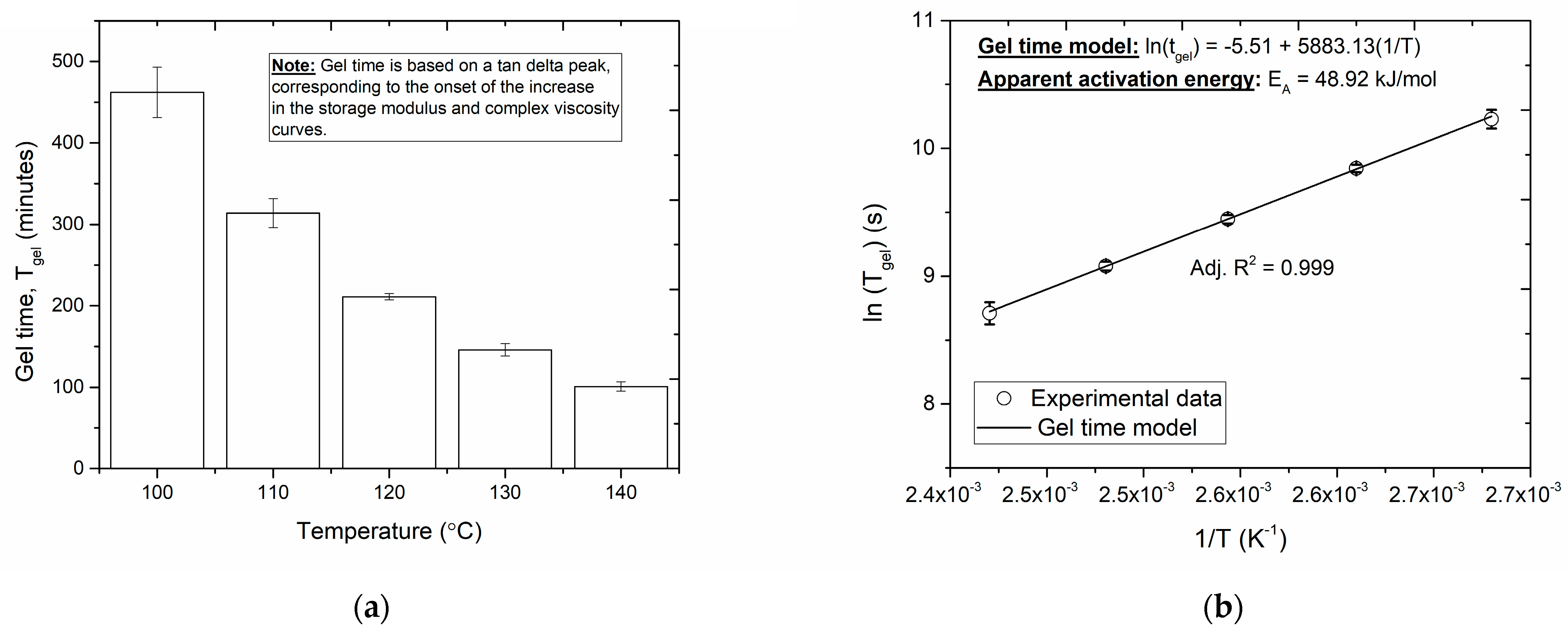
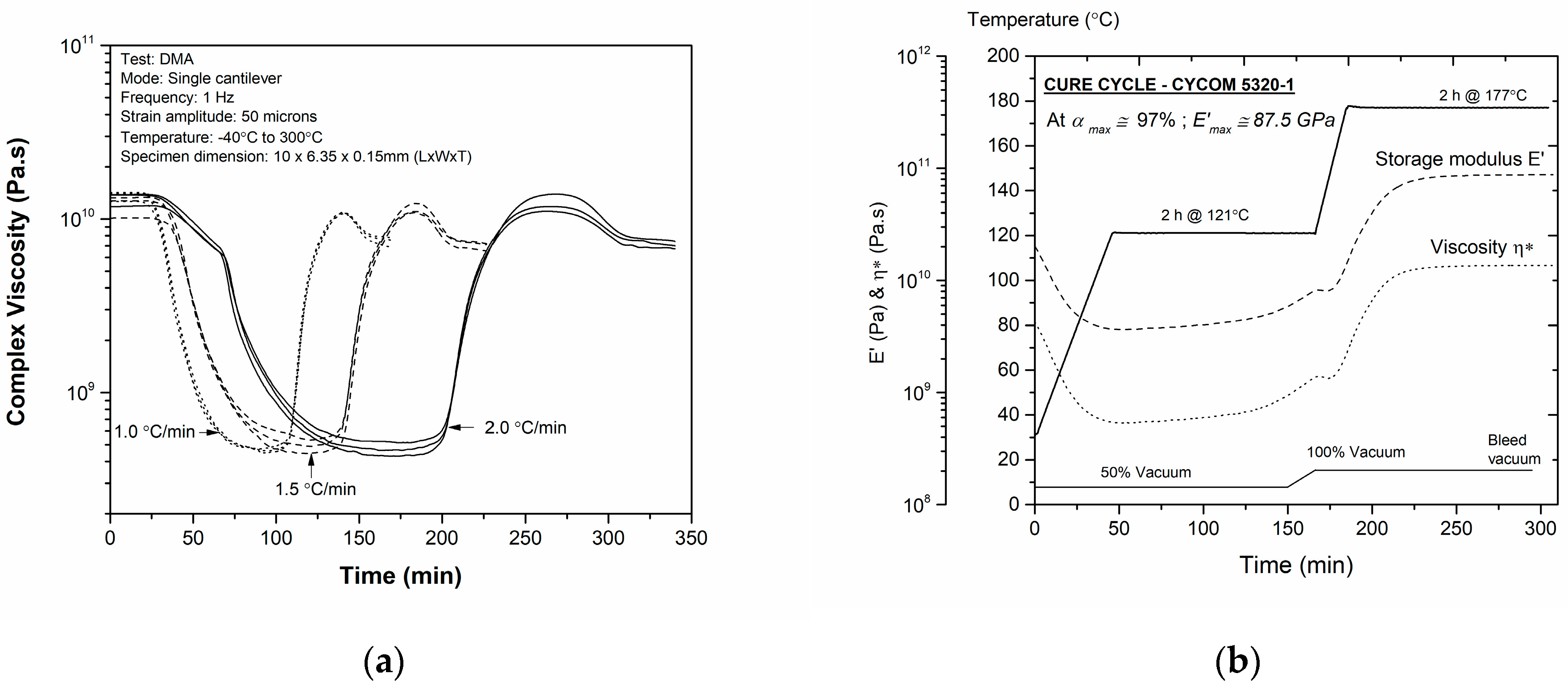
3.2. Gel Time and Vitrification Time Modeling
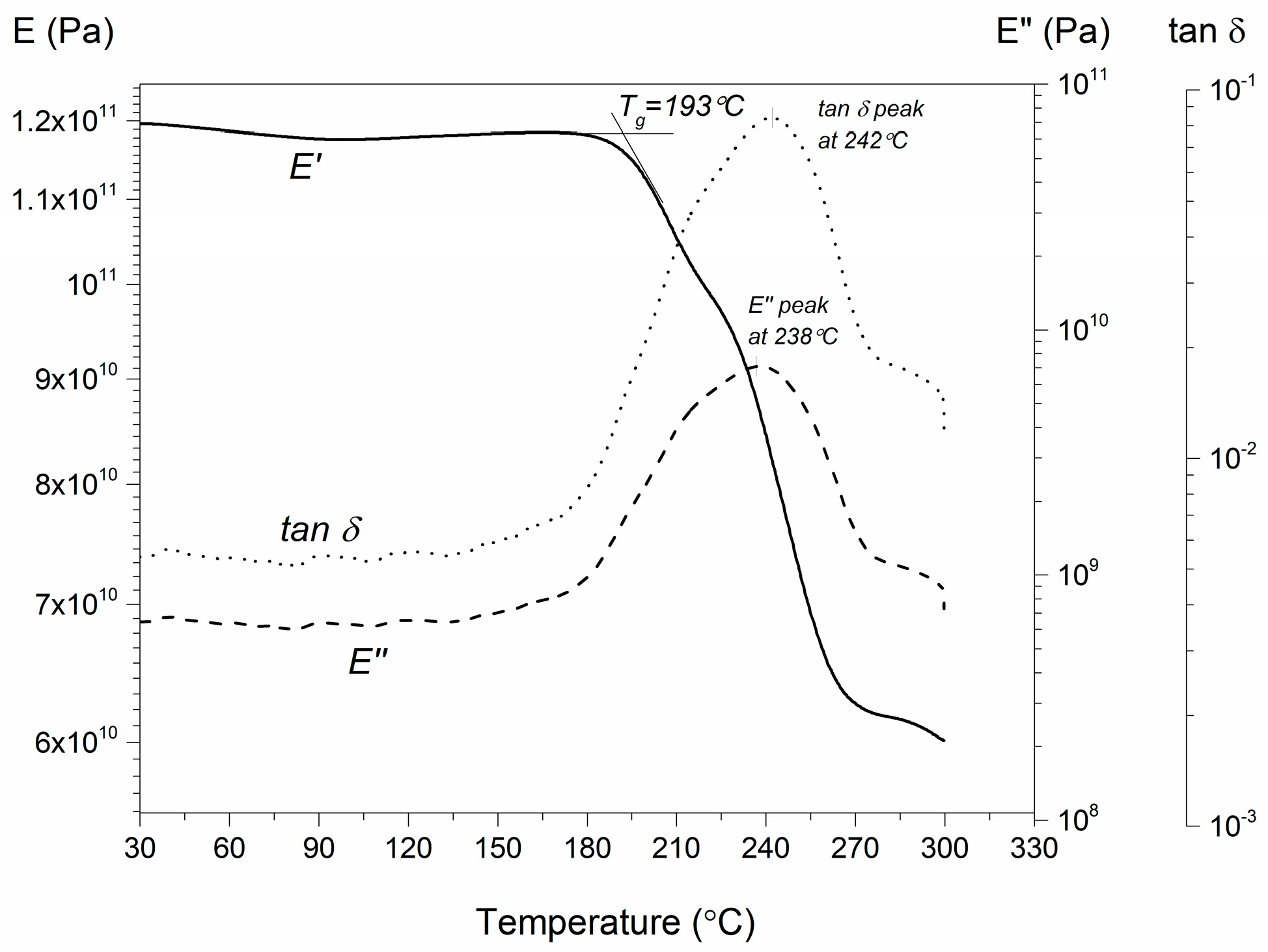
3.3. Tg-α Conversion
3.4. Manufacturing Cure Cycle
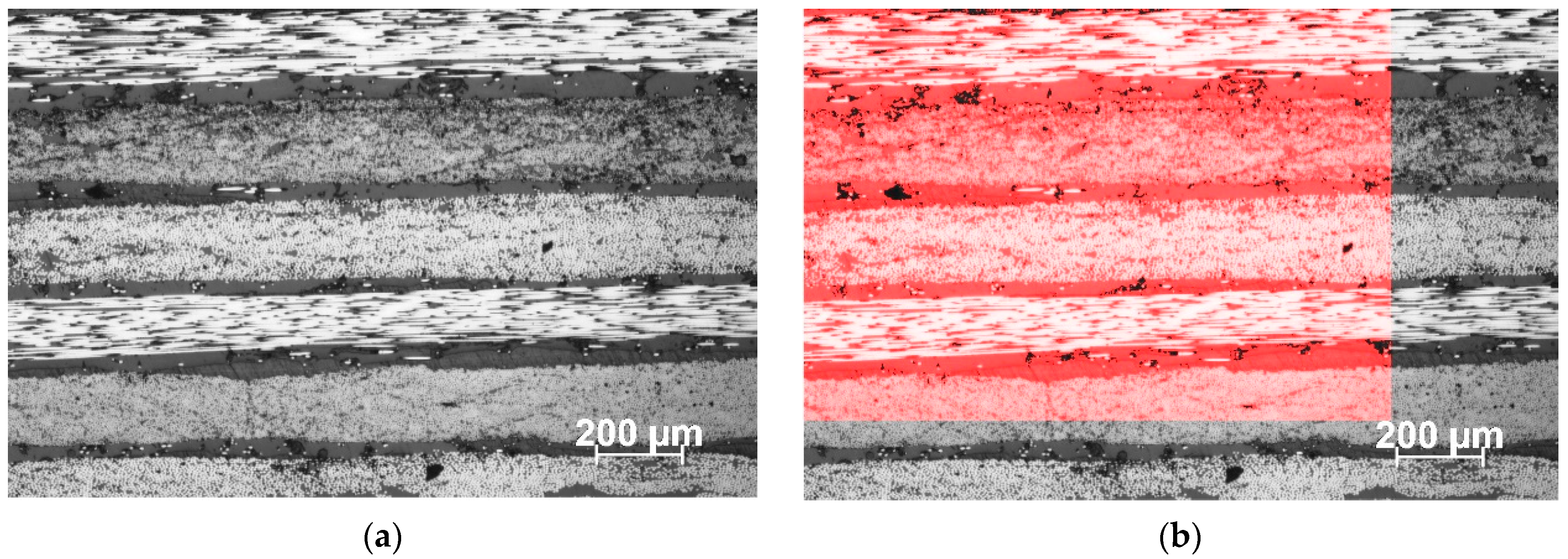
3.5. Mechanical Tests, Extent of Cure, and Glass Transition Tg
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and Council of the European Union. Directive 2000/53/EC of The European Parliament and of The Council of 18 September 2000 on End-of Life Vehicles; European Parliament and Council of the European Union: Brussels, Belgium, 2000; p. 34. [Google Scholar]
- Genna, S.; Papa, I.; Lopresto, V.; Tagliaferri, V. Mechanical Characterisation of CFRP Laminates with Recycled Carbon Fiber Obtained by Resin Infusion under Flexible Tooling (RIFT) Technology. Compos. Sci. Technol. 2020, 199, 108328. [Google Scholar] [CrossRef]
- Liu, Z.; Turner, T.A.; Wong, K.H.; Pickering, S.J. Development of High Performance Recycled Carbon Fibre Composites with an Advanced Hydrodynamic Fibre Alignment Process. J. Clean. Prod. 2021, 278, 123785. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.A.; Dierkes, W.; Akkerman, R.; Brem, G. A Critical Review on Recycling of End-of-Life Carbon Fibre/Glass Fibre Reinforced Composites Waste Using Pyrolysis towards a Circular Economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Abdou, T.R.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of Polymeric Composites from Industrial Waste by Pyrolysis: Deep Evaluation for Carbon Fibers Reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of Carbon Fibre Reinforced Epoxy Resin Composites under Various Oxygen Concentrations in Nitrogen-Oxygen Atmosphere. J. Anal. Appl. Pyrolysis 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.M.; An, J.H.; Chung, D.C.; An, K.H.; Kim, B.J. Recycling and Characterization of Carbon Fibers from Carbon Fiber Reinforced Epoxy Matrix Composites by a Novel Super-Heated-Steam Method. J. Environ. Manag. 2017, 203, 872–879. [Google Scholar] [CrossRef]
- Jiang, L.; Ulven, C.A.; Gutschmidt, D.; Anderson, M.; Balo, S.; Lee, M.; Vigness, J. Recycling Carbon Fiber Composites Using Microwave Irradiation: Reinforcement Study of the Recycled Fiber in New Composites. J. Appl. Polym. Sci. 2015, 132, 42658. [Google Scholar] [CrossRef]
- Wei, Y.; Hadigheh, S.A. Development of an Innovative Hybrid Thermo-Chemical Recycling Method for CFRP Waste Recovery. Compos. Part B Eng. 2023, 260, 110786. [Google Scholar] [CrossRef]
- Termine, S.; Naxaki, V.; Semitekolos, D.; Trompeta, A.-F.; Rovere, M.; Tagliaferro, A.; Charitidis, C. Investigation of Carbon Fibres Reclamation by Pyrolysis Process for Their Reuse Potential. Polymers 2023, 15, 768. [Google Scholar] [CrossRef]
- Nicolai, G.; Bôas, A.P.; Méndez, M.O.; Taranto, O.P. Thermochemical Recycling of Epoxy Resin Composites Reinforced with Carbon Fiber. J. Compos. Mater. 2023, 57, 3201–3212. [Google Scholar] [CrossRef]
- Wong, K.; Rudd, C.; Pickering, S.; Liu, X.L. Composites Recycling Solutions for the Aviation Industry. Sci. China Technol. Sci. 2017, 60, 1291–1300. [Google Scholar] [CrossRef]
- El Gersifi, K.; Durand, G.; Tersac, G. Solvolysis of Bisphenol A Diglycidyl Ether/Anhydride Model Networks. Polym. Degrad. Stab. 2006, 91, 690–702. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Huang, Y.; Du, J. Recycling of Carbon/Epoxy Composites. J. Appl. Polym. Sci. 2004, 94, 1912–1916. [Google Scholar] [CrossRef]
- Braun, D.; Von Gentzkow, W.; Rudolf, A.P. Hydrogenolytic Degradation of Thermosets. Polym. Degrad. Stab. 2001, 74, 25–32. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Degradation of a Model Epoxy Resin by Solvolysis Routes. Polym. Degrad. Stab. 2015, 118, 96–103. [Google Scholar] [CrossRef]
- Pegoretti, A. Towards Sustainable Structural Composites: Recycling of Continuous-Fiber-Reinforced Thermoplastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 105–115. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Karuppannan Gopalraj, S.; Kärki, T. A Review on the Recycling of Waste Carbon Fibre/Glass Fibre-Reinforced Composites: Fibre Recovery, Properties and Life-Cycle Analysis. SN Appl. Sci. 2020, 2, 433. [Google Scholar] [CrossRef]
- Nilakantan, G.; Nutt, S. Reuse and Upcycling of Thermoset Prepreg Scrap: Case Study with out-of-Autoclave Carbon Fiber/Epoxy Prepreg. J. Compos. Mater. 2018, 52, 341–360. [Google Scholar] [CrossRef]
- Kiss, P.; Stadlbauer, W.; Burgstaller, C.; Stadler, H.; Fehringer, S.; Haeuserer, F.; Archodoulaki, V.M. In-House Recycling of Carbon- and Glass Fibre-Reinforced Thermoplastic Composite Laminate Waste into High-Performance Sheet Materials. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106110. [Google Scholar] [CrossRef]
- Cestari, S.P.; Mendes, L.C.; Altstädt, V.; Lopes, L.M.A. Upcycling Polymers and Natural Fibers Waste—Properties of a Potential Building Material. Recycling 2016, 1, 205–218. [Google Scholar] [CrossRef]
- Nilakantan, G.; Nutt, S. Reuse and Upcycling of Aerospace Prepreg Scrap and Waste. Reinf. Plast. 2015, 59, 44–51. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, Z.; Gu, Y.; Li, M.; Su, Y. A New Method to Characterize the Cure State of Epoxy Prepreg by Dynamic Mechanical Analysis. Thermochim. Acta 2009, 487, 8–17. [Google Scholar] [CrossRef]
- Stark, W. Investigation of the Curing Behaviour of Carbon Fibre Epoxy Prepreg by Dynamic Mechanical Analysis DMA. Polym. Test. 2013, 32, 231–239. [Google Scholar] [CrossRef]
- AITM 1-0003; Airbus Test Method, Determination of the Glass Transition Temperatures. Airbus: Applus Illescas, Spain, 2010.
- DIN EN 2565; Aerospace Series—Preparation of Carbon Fibre Reinforced Resin Panels for Test Purposes. Deutsches Institut für Normung: Berlin, Germany, 2013.
- Rao, S.; Umer, R.; Thomas, J.; Cantwell, W.J. Investigation of Peel Resistance during the Fibre Placement Process. J. Reinf. Plast. Compos. 2016, 35, 275–286. [Google Scholar] [CrossRef]
- BS EN 2743:2002; Aerospace Series. Fibre Reinforced Plastics. Standard Procedures for Conditioning Prior to Testing Unaged Materials. British-Adopted European Standard. BSI Group: London, UK, 2002.
- BS EN 2559:2022; Aerospace Series. Carbon, Glass and Aramid Fibre Preimpregnates. Determination of the Resin and Fibre Content and the Mass of Fibre per Unit Area. British-Adopted European Standard. BSI Group: London, UK, January 2023.
- Kamal, M.; Sourour, S. Kinetics and Thermal Characterization of Epoxy-Amine Systems. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Sun, L. Thermal Rheological Analysis of Cure Process of Epoxy Prepreg. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2002. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- ASTM D7028-07(2015); Standard Test Method for Glass Transition Temperature (DMA Tg) of Polymer Matrix Composites by Dynamic Mechanical Analysis (DMA). ASTM: West Conshohocken, PA, USA, 2008.
- Dibenedetto, A.T. Prediction of the Glass Transition Temperature of Polymers: A Model Based on the Principle of Corresponding States. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1949–1969. [Google Scholar] [CrossRef]
- Rahmani, N.; Willard, B.; Lease, K.; Legesse, E.T.; Soltani, S.A.; Keshavanarayana, S. The Effect of Post Cure Temperature on Fiber/Matrix Adhesion of T650/Cycom 5320-1 Using the Micro-Droplet Technique. Polym. Test. 2015, 46, 14–20. [Google Scholar] [CrossRef]
- Kratz, J.; Hsiao, K.; Fernlund, G.; Hubert, P. Thermal Models for MTM45-1 and Cycom 5320 out-of-Autoclave Prepreg Resins. J. Compos. Mater. 2013, 47, 341–352. [Google Scholar] [CrossRef]
- Moroni, A.; Mijovic, J.; Pearce, E.M.; Foun, C.C. Cure Kinetics of Epoxy Resins and Aromatic Diamines. J. Appl. Polym. Sci. 1986, 32, 3761–3773. [Google Scholar] [CrossRef]
- Du, W.; Tan, L.; Zhang, Y.; Yang, H.; Chen, H. Rheological and Kinetic Investigation into Isothermal Curing of a Thermoset Polythiourethane System. Polym.-Plast. Technol. Mater. 2020, 59, 63–71. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Nazockdast, H.; Behzad, T.; Salimijazi, H.R. Investigation of the Cure Kinetics of an Epoxy Resin by Advanced Isoconversional and Model-Fitting Methods. AIP Conf. Proc. 2016, 1713, 110004. [Google Scholar] [CrossRef]
- Francucci, G.; Cardona, F.; Manthey, N.W. Cure Kinetics of an Acrylated Epoxidized Hemp Oil-Based Bioresin System. J. Appl. Polym. Sci. 2013, 128, 2030–2037. [Google Scholar] [CrossRef]
- Bilyeu, B.; Brostow, W.; Menard, K.P. Epoxy Thermosets and Their Applications. II. Thermal Analysis. J. Mater. Educ. 2000, 22, 107–109. [Google Scholar]
- Soltani, S.; Keshavanarayana, S. Development of Time-Temperature Viscosity Diagram for Effective Cure Monitoring of Thermosetting Composite Materials. In Proceedings of the International SAMPE Technical Conference, Paris, France, 10–11 March 2014. [Google Scholar]
- Reddy, B.R.; Eoff, L.; Dalrymple, E.D.; Black, K.; Brown, D.; Rietjens, M. A Natural Polymer-Based Cross-Linker System for Conformance Gel Systems. SPE J. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- ASTM D7028-07e1; Glass Transition Temperature (DMA Tg) of Polymer Matrix Composites by Dynamic Mechanical Analysis (DMA). ASTM: West Conshohocken, PA, USA, 2007.
- Hardis, R.; Jessop, J.L.P.; Peters, F.E.; Kessler, M.R. Cure Kinetics Characterization and Monitoring of an Epoxy Resin Using DSC, Raman Spectroscopy, and DEA. Compos. Part A Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef]
- Cytec Industries Inc. CYCOM® 5320-1 Epoxy Resin System; Cytec Industries Inc.: Smyrna, GA, USA, 2015. [Google Scholar]
- Centea, T.; Nutt, S.R. Manufacturing Cost Relationships for Vacuum Bag-Only Prepreg Processing. J. Compos. Mater. 2016, 50, 2305–2321. [Google Scholar] [CrossRef]
- Ma, Y.; Centea, T.; Nutt, S.R. Defect Reduction Strategies for the Manufacture of Contoured Laminates Using Vacuum BAG-Only Prepregs. Polym. Compos. 2017, 38, 2016–2025. [Google Scholar] [CrossRef]
- Hwang, S.S.; Park, S.Y.; Kwon, G.C.; Choi, W.J. Cure Kinetics and Viscosity Modeling for the Optimization of Cure Cycles in a Vacuum-Bag-Only Prepreg Process. Int. J. Adv. Manuf. Technol. 2018, 99, 2743–2753. [Google Scholar] [CrossRef]

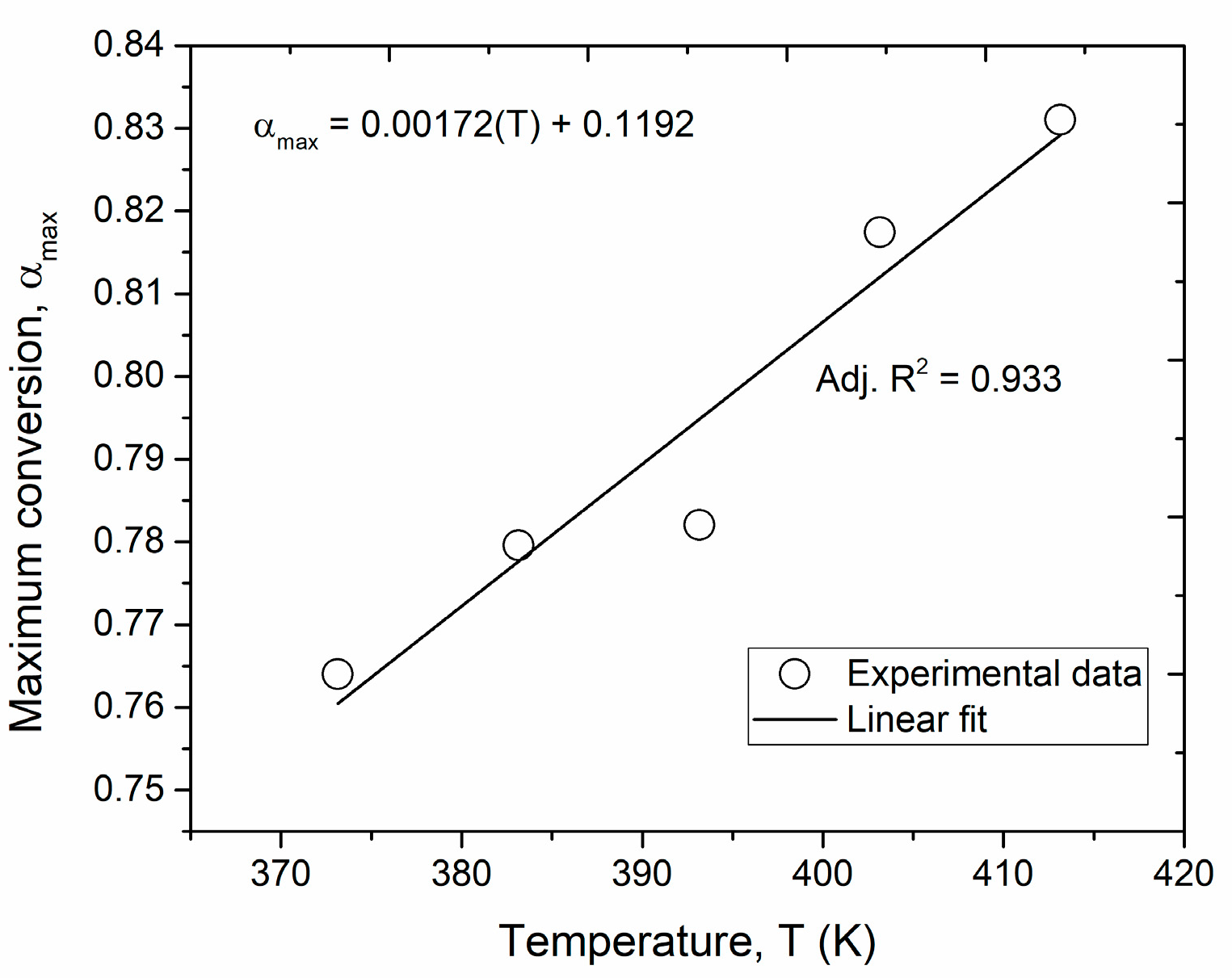
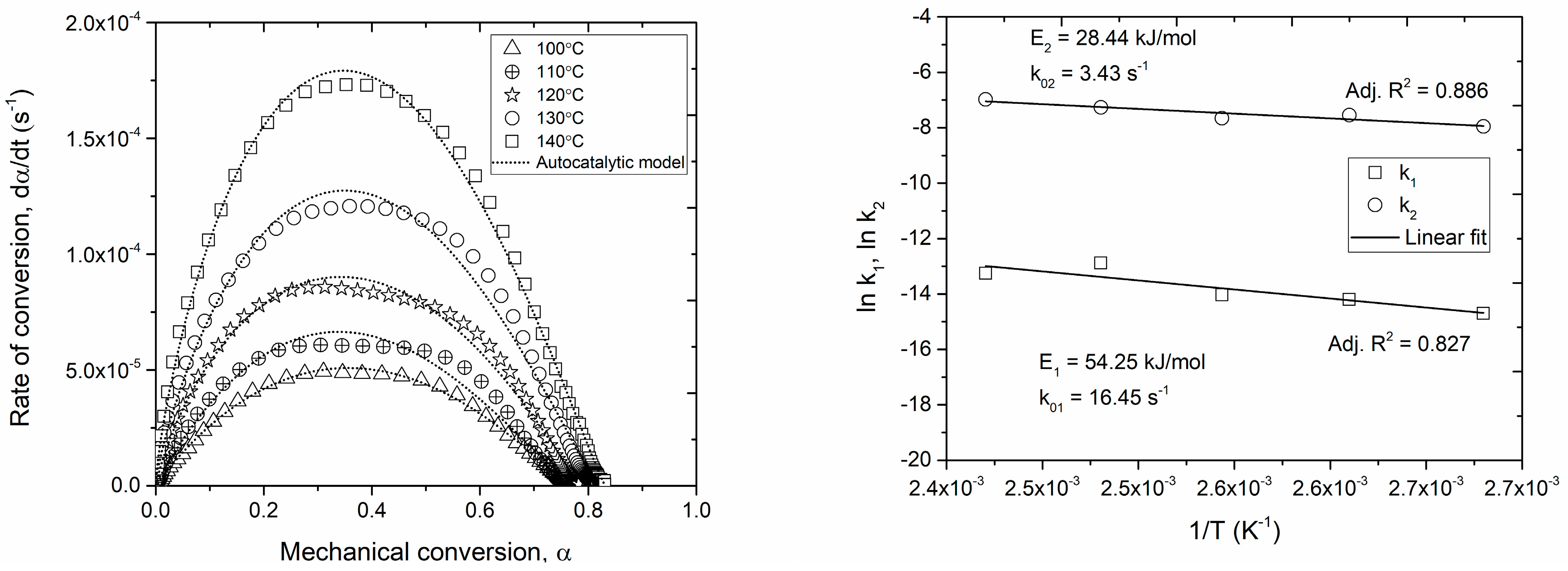

| Pre-Exponential Factors | Apparent Activation Energy | Reaction Order | Maximum Conversion | ||||
|---|---|---|---|---|---|---|---|
| (s−1) | 16.45 | (kJ/mol) | 54.25 | 0.86 | 0.00172(T) + 0.119 | ||
| (s−1) | 3.43 | (kJ/mol) | 28.44 | 1.11 | |||
| Tensile Strength (MPa) | Tensile Modulus (GPa) | |
|---|---|---|
| Manufacturer’s data | 917 | 59 |
| Test coupons | 800 (±20) | 50 (±5) |
| Sample | ∆H100 (J/g) | Tonset (°C) | Tpeak (°C) | ∆H (J/g) | α′ (%) |
|---|---|---|---|---|---|
| 1 | 443.1 | 190.7 | 250.5 | 25.84 | 94.2 |
| 2 | 443.1 | 185.9 | 249.0 | 20.60 | 95.3 |
| 3 | 443.1 | 183.0 | 250.3 | 13.66 | 96.9 |
| Resolution | |||
|---|---|---|---|
| 10× | 20× | ||
| Sample 1 | ROI 1 (%) | 2.5 | 1.9 |
| ROI 2 (%) | 2.6 | 2.5 | |
| ROI 3 (%) | 2.3 | 2.8 | |
| Sample 2 | ROI 1 (%) | 2.8 | 2.4 |
| ROI 2 (%) | 2.1 | 2.2 | |
| ROI 3 (%) | 2.2 | 1.8 | |
| Sample 3 | ROI 1 (%) | 2.5 | 2.6 |
| ROI 2 (%) | 2.8 | 2.6 | |
| ROI 3 (%) | 1.8 | 2.8 | |
| Average | 2.4 | 2.4 | |
| Standard deviation | 0.3 | 0.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Bastienne, D. Performance Evaluation of Out-of-Spec Carbon Prepregs for Upcycling Purposes. Polymers 2024, 16, 1625. https://doi.org/10.3390/polym16121625
Rao S, Bastienne D. Performance Evaluation of Out-of-Spec Carbon Prepregs for Upcycling Purposes. Polymers. 2024; 16(12):1625. https://doi.org/10.3390/polym16121625
Chicago/Turabian StyleRao, Sanjeev, and Dereck Bastienne. 2024. "Performance Evaluation of Out-of-Spec Carbon Prepregs for Upcycling Purposes" Polymers 16, no. 12: 1625. https://doi.org/10.3390/polym16121625
APA StyleRao, S., & Bastienne, D. (2024). Performance Evaluation of Out-of-Spec Carbon Prepregs for Upcycling Purposes. Polymers, 16(12), 1625. https://doi.org/10.3390/polym16121625







