Albumin-Based Hydrogel Films Covalently Cross-Linked with Oxidized Gellan with Encapsulated Curcumin for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Methods for Obtaining Albumin-Based Hydrogel Precursors and Films with or without Encapsulated Curcumin–β-Cyclodextrin Inclusion Complexes
- Obtaining Oxidized Gellan
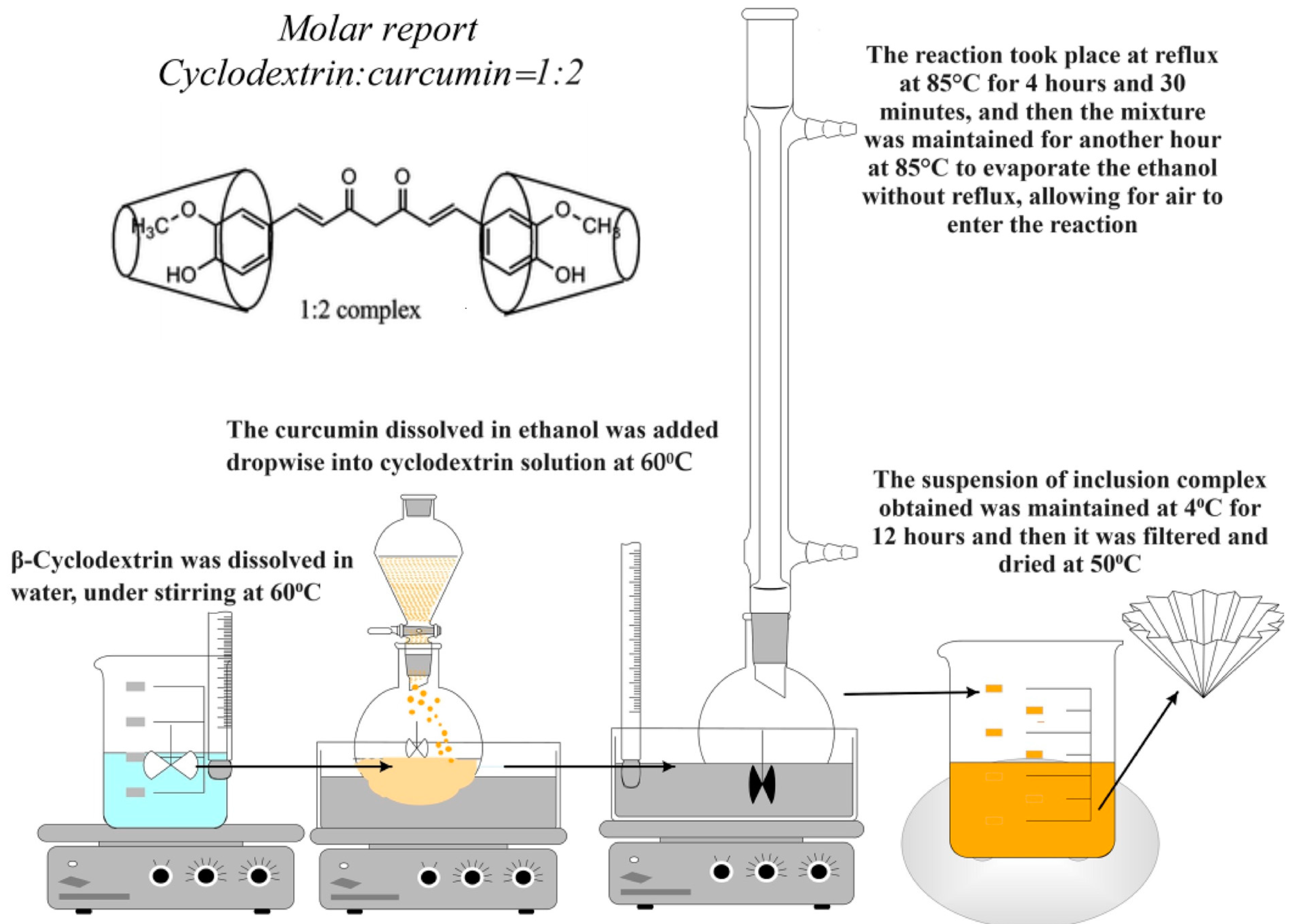
- Obtaining the Inclusion Complexes of β-Cyclodextrin with Curcumin
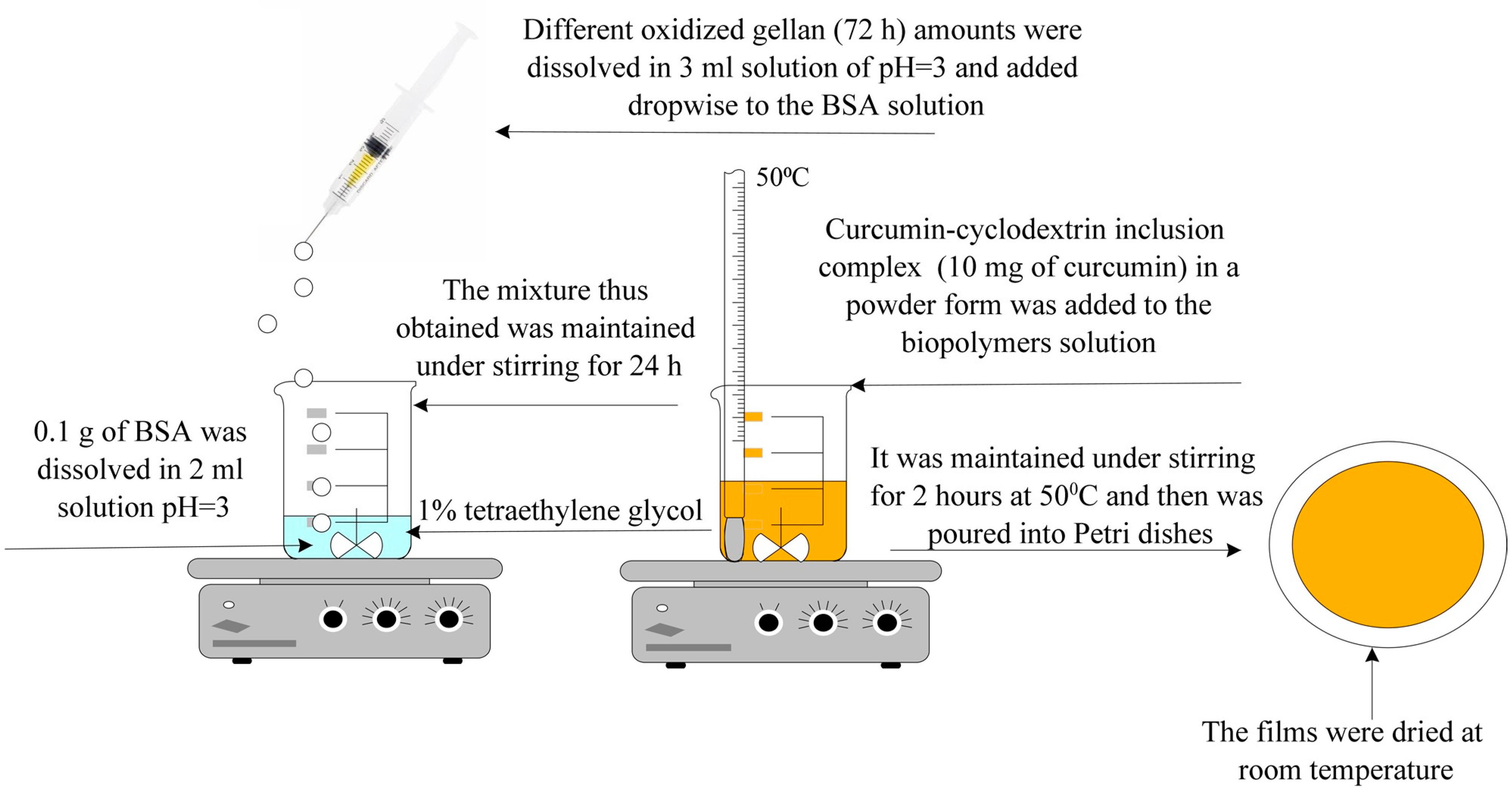
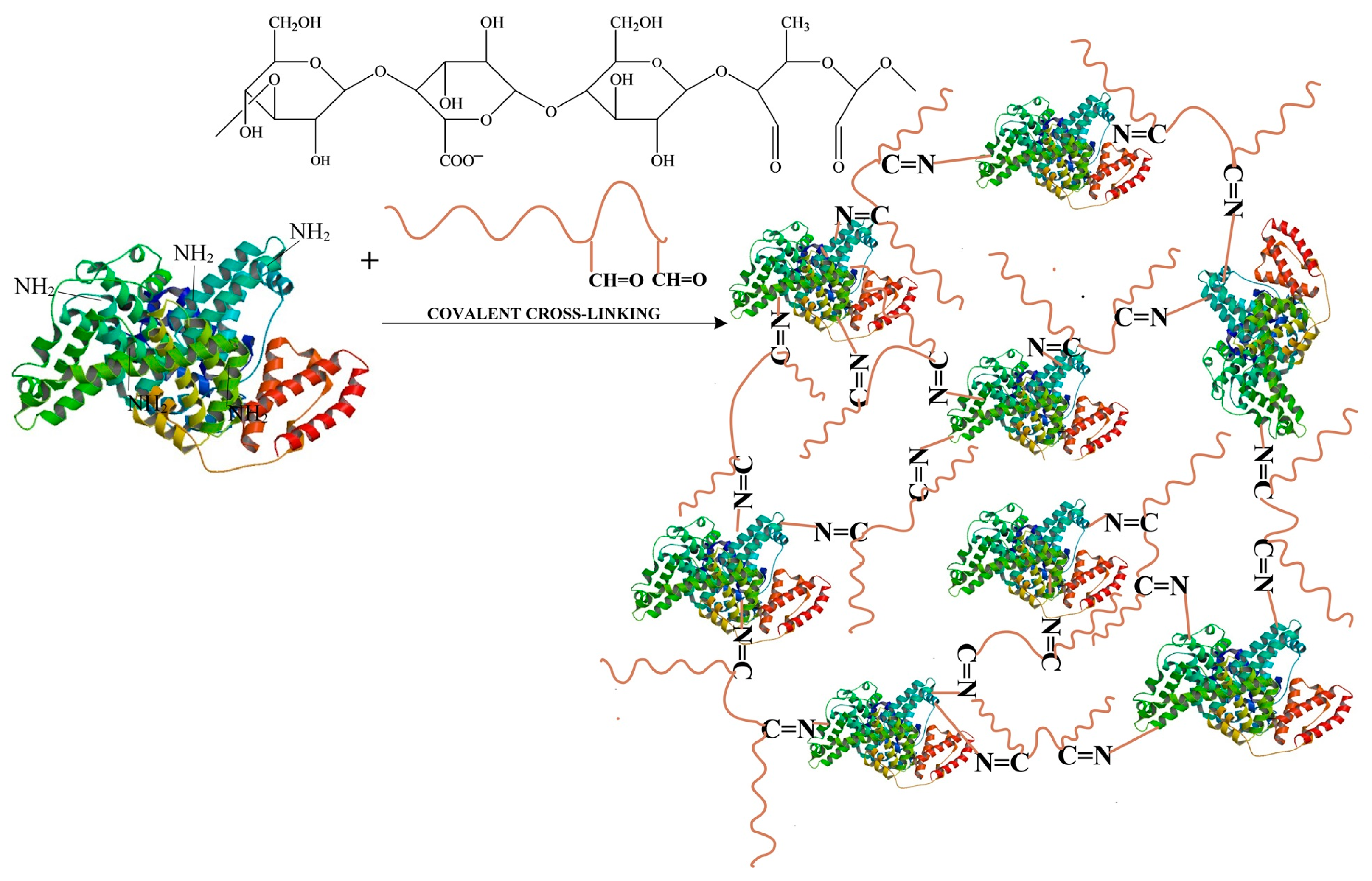
- Obtaining Hydrogel Films Based on Bovine Serum Albumin and Oxidized Gellan with Immobilized Inclusion Complexes
2.2.2. Methods for Oxidized Gellan Characterization
- Kinetics of the Oxidation Reaction and Quantitative Determination of Aldehyde Groups Obtained from the Oxidation of Gellan with NaIO4
- KIO4 + 7KI + 8HCl → 8KCl + 4I2 + 4H2O
- I2 + 2Na2S2O3 × 5H2O → 2NaI + Na2S4O6
- Determination of the Molecular Weight of Oxidized Gellan
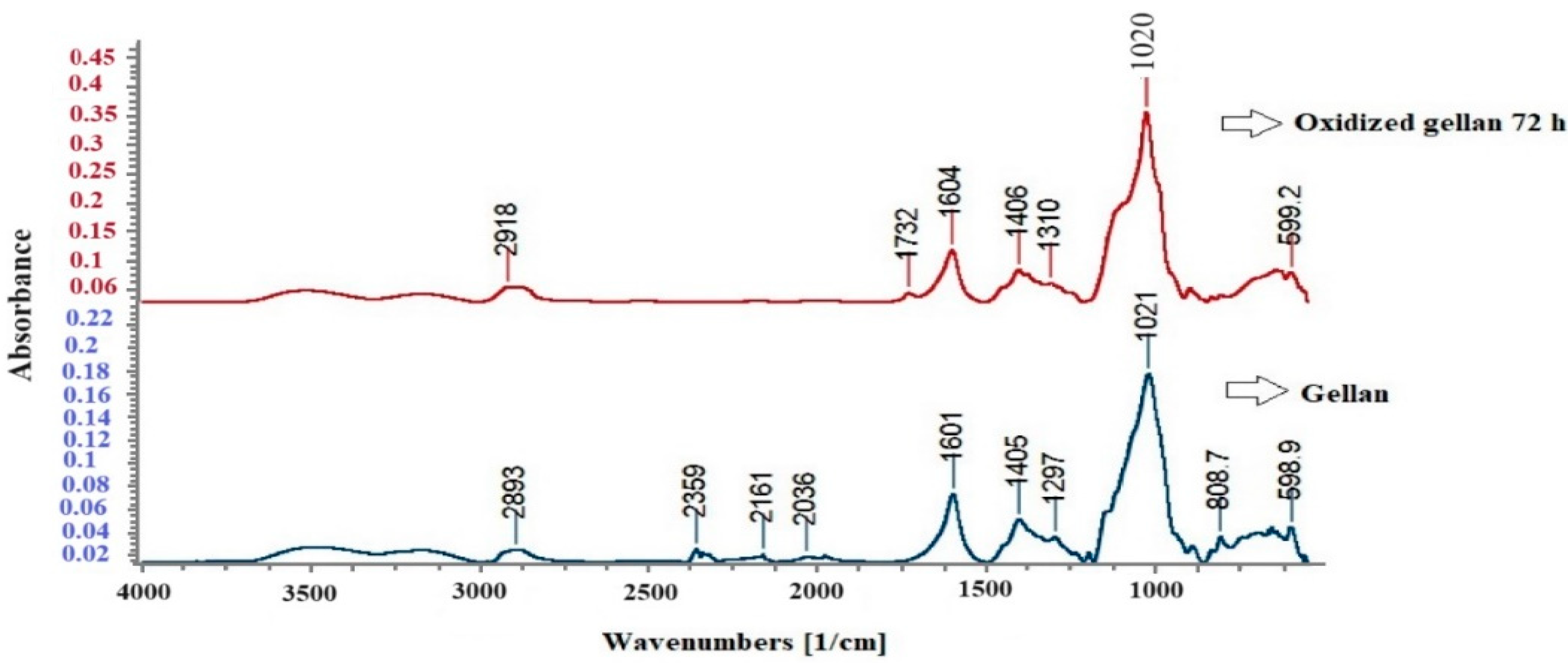
- FT-IR Spectroscopy for Gellan and Oxidized Gellan
- Nuclear Magnetic Resonance Spectroscopy
2.2.3. Hydrogel Film Characterization Methods
- Determination of Albumin’s Free Amine Groups Using Ninhydrin Assay and Evaluation of Conversion Index of Hydrogel Films
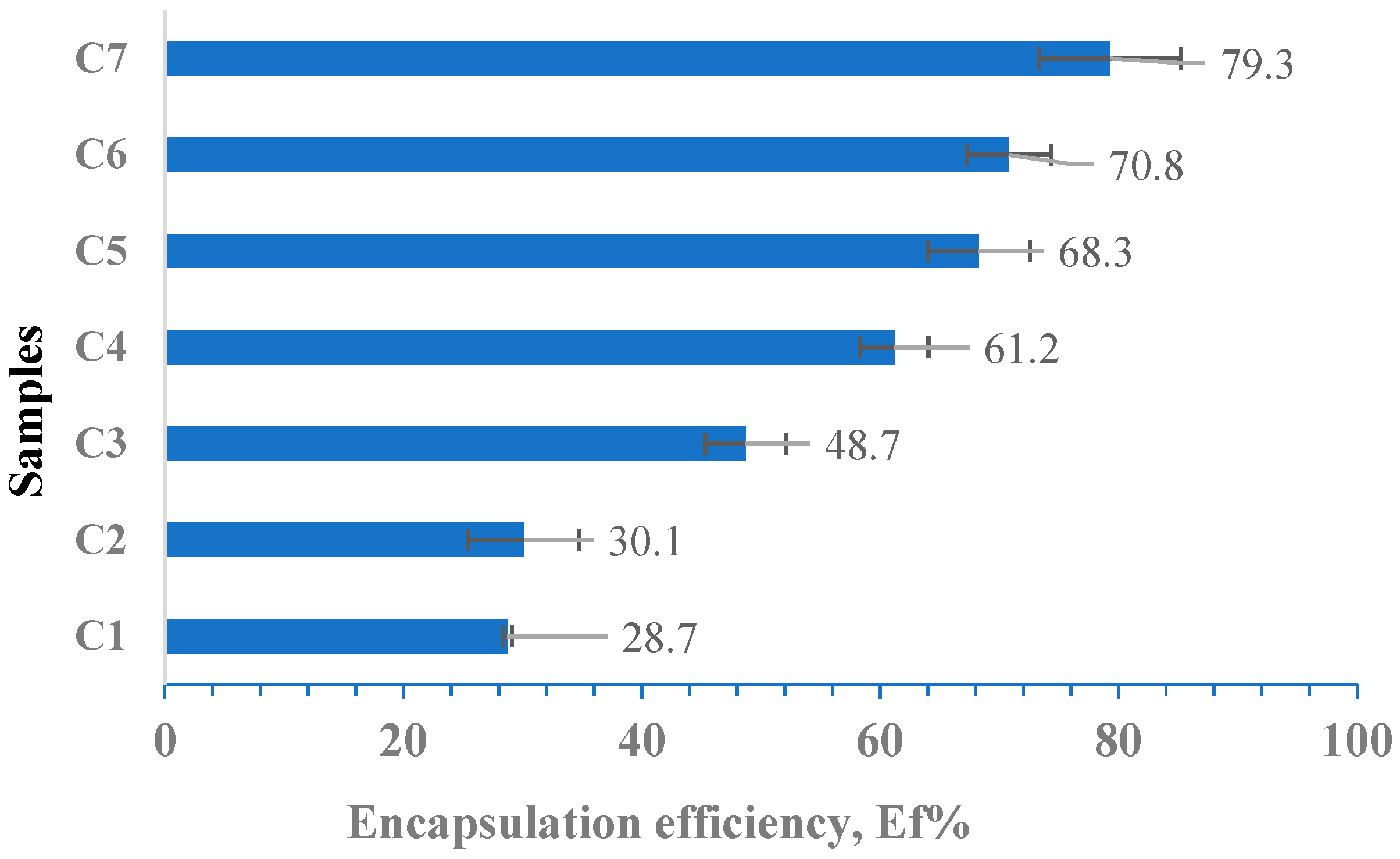
- Determination of Encapsulation Efficiency
- FT-IR Spectroscopy of Hydrogel Films with Immobilized Curcumin
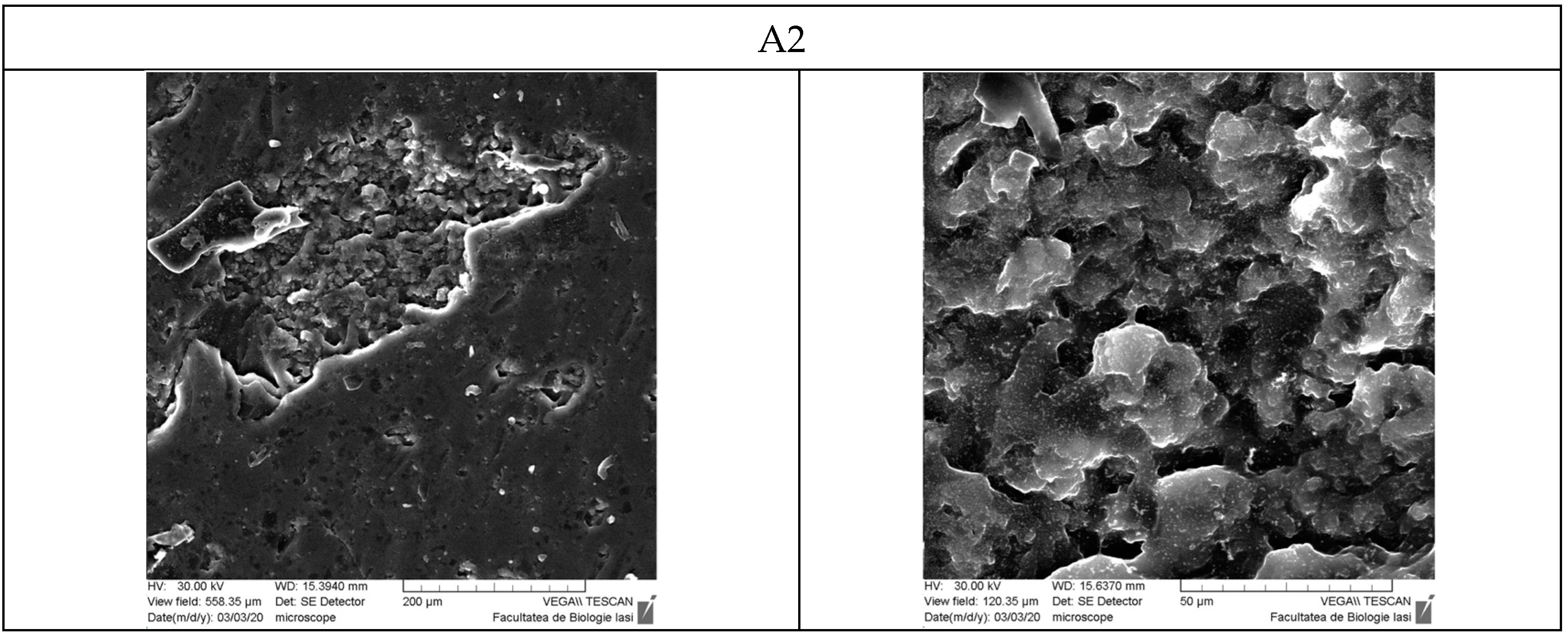
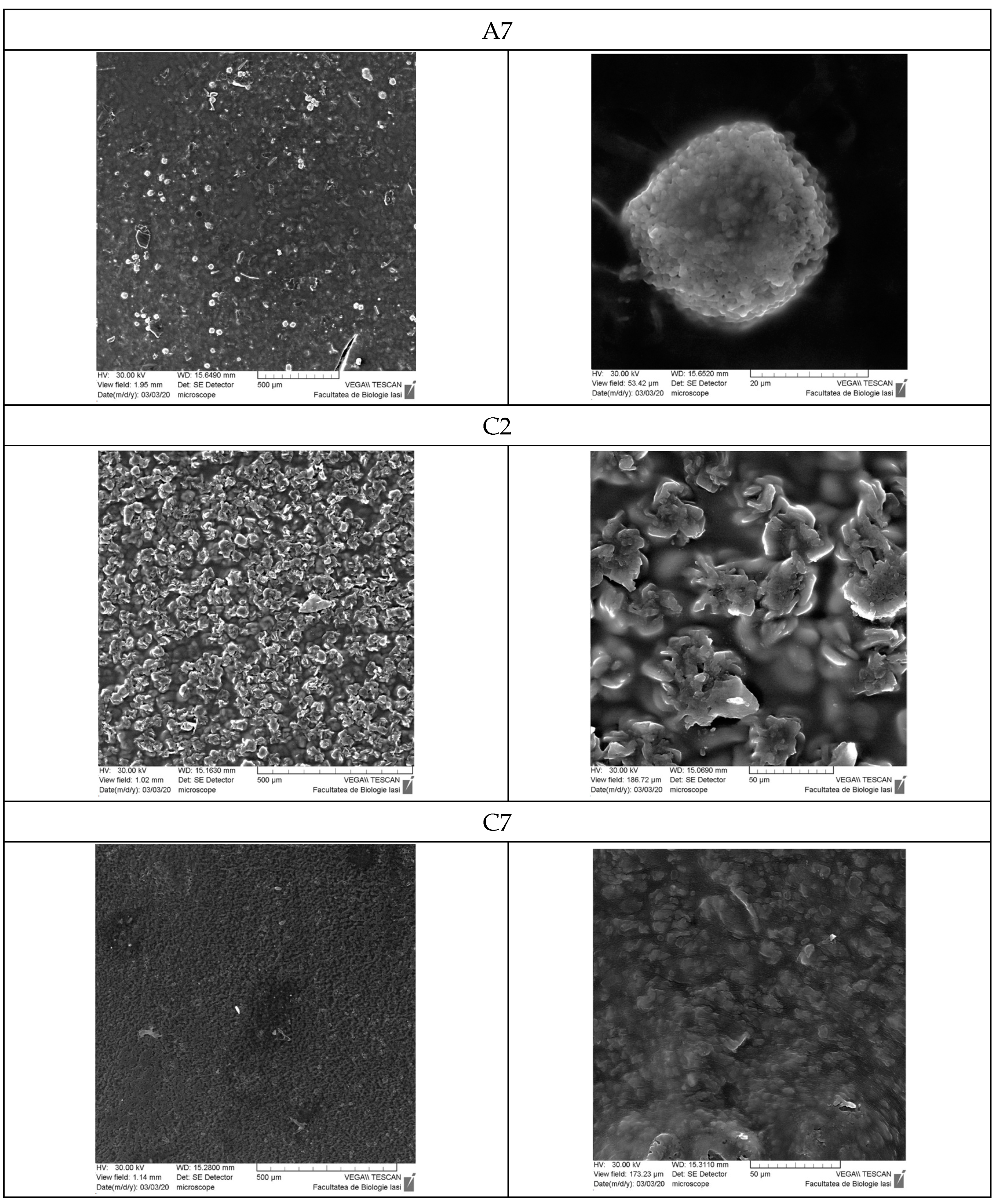
- Scanning Electron Microscopy (SEM)
- Determination of the Ability of Hydrogel Films to Retain Water
- Determination of Antioxidant Activity
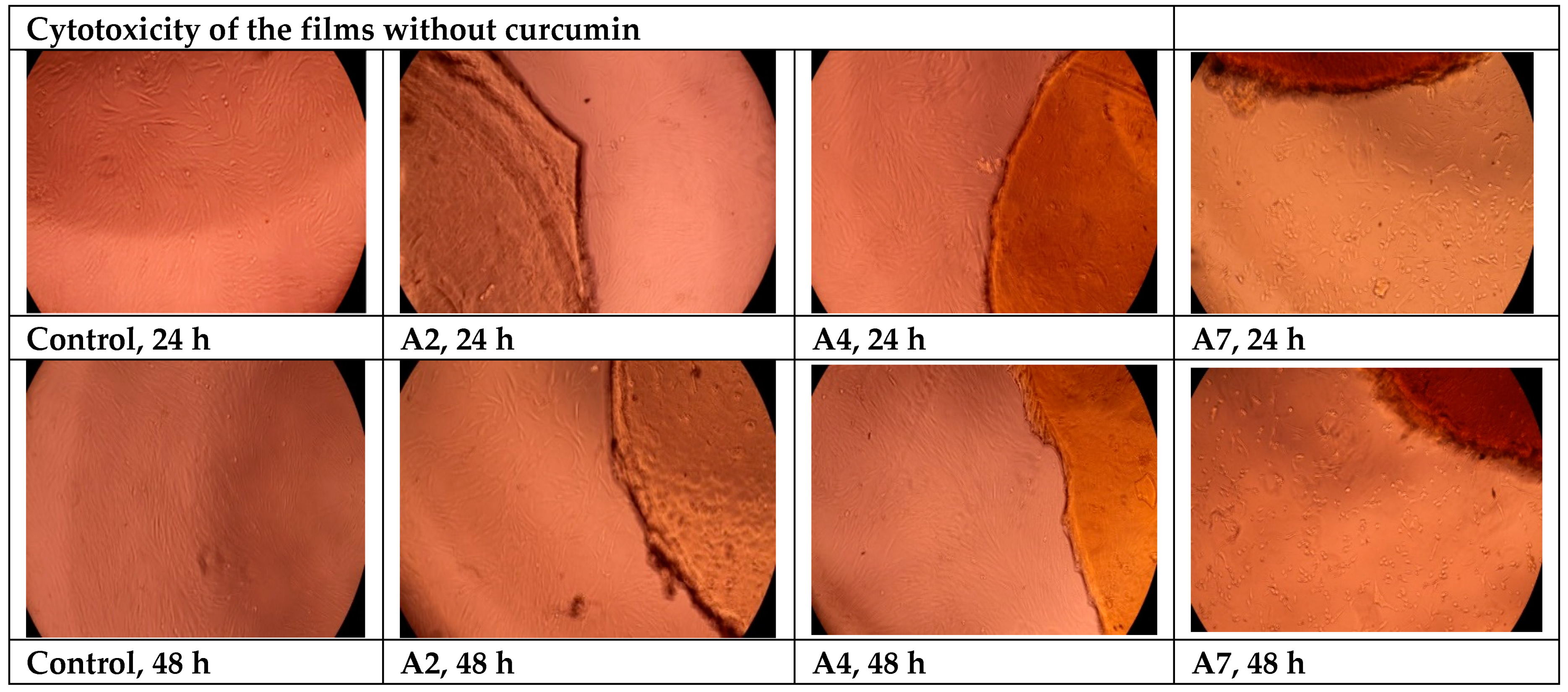
- Cytotoxicity Evaluation of Hydrogel Films without Curcumin
- Release Kinetics of Curcumin from Hydrogel Films
- -
- For calibration curve of curcumin in 0.1 M PBS at pH = 7.4: y = 0.0079x (R2 = 0.9983).
- -
- For calibration curve of curcumin in 0.1 M in ABS at pH = 5.5: y = 0.0104x (R2 = 0.9991).
3. Results and Discussions
3.1. Obtaining Oxidized Gellan and Covalently Cross-Linked Albumin-Based Hydrogel Films Containing Cyclodextrin Inclusion Complex with Curcumin
3.2. Kinetics of the Oxidation Reaction and Quantitative Determination of Aldehyde Groups Resulting from Gellan Oxidation
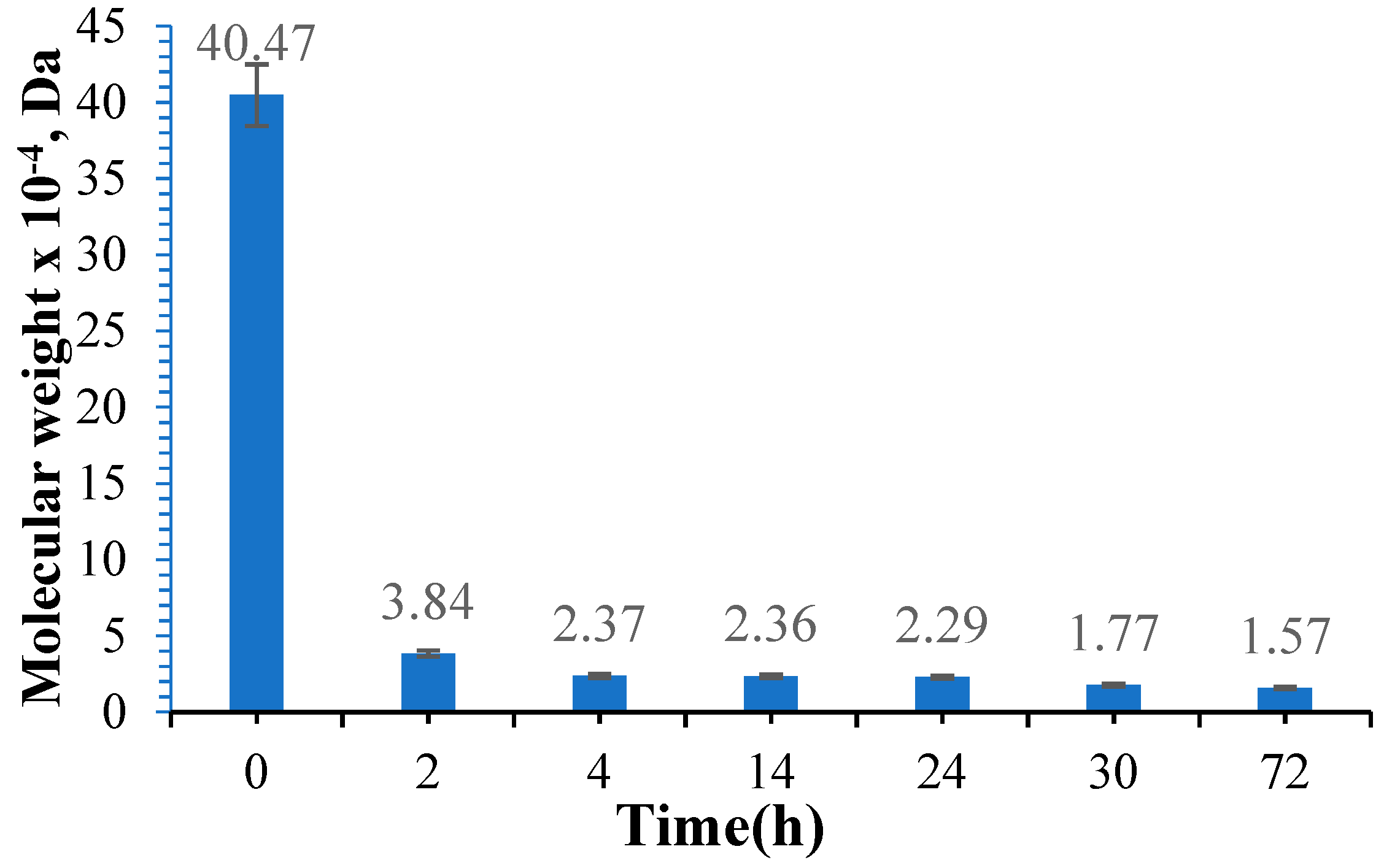
3.3. Determination of the Molecular Weight of Oxidized Gellan
3.4. FT-IR Spectroscopy for Standard Gellan and Oxidized Gellan
3.5. 1H-NMR Spectroscopy
3.6. Determination of Free Amine Groups in Albumin Using the Ninhydrin Assay and Determination of Degree of Conversion in Hydrogel Films
3.7. Determination of the Encapsulation Efficiency (Ef %)
3.8. FT-IR Spectroscopy for Curcumin–β-Cyclodextrin Inclusion Complex and Hydrogel Films without Immobilized Curcumin
3.9. Scanning Electron Microscopy
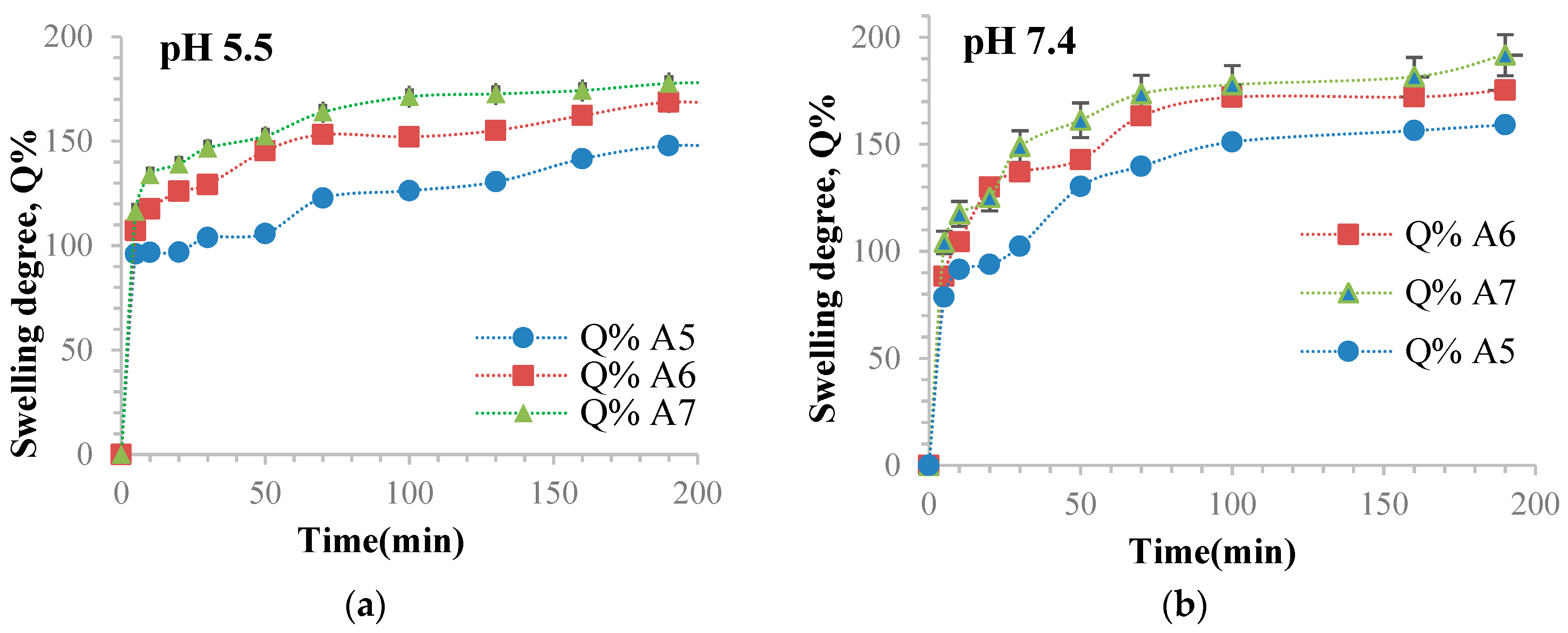
3.10. Determination of the Ability of Hydrogel Films to Retain Water
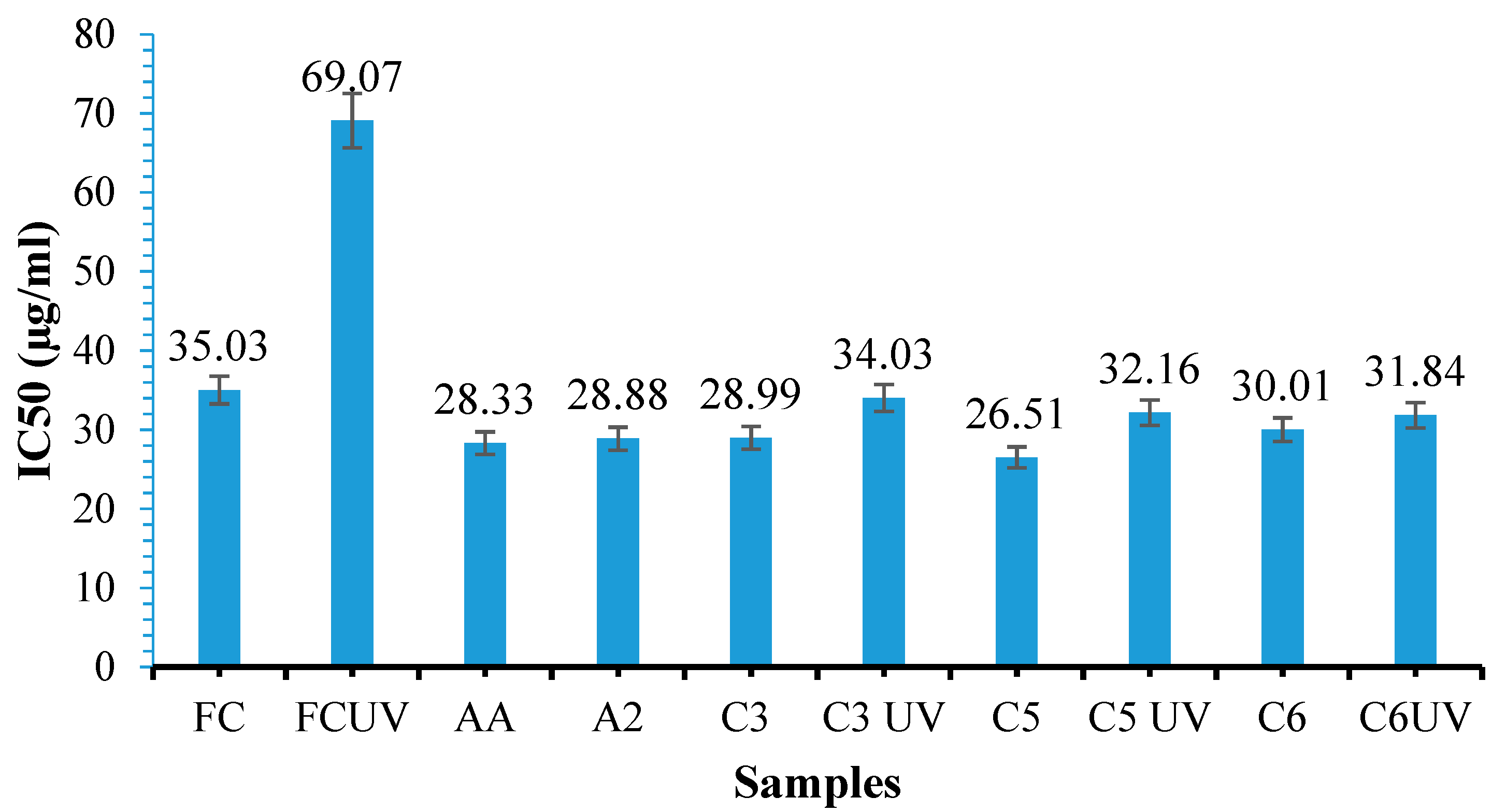
3.11. Antioxidant Activity Determination
3.12. Cytotoxicity Evaluation of the Films Using the MTT Assay
3.13. Curcumin Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Haque, S.T.; Saha, S.K.; Haque, M.E.; Biswas, N. Nanotechnology-based therapeutic applications: In vitro and in vivo clinical studies for diabetic wound healing. Biomater. Sci. 2021, 9, 7705–7747. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Yang, D.; Chen, H.; Wei, H.; Liu, A.; Wei, D.; Chen, J. Hydrogel wound dressings containing bioactive compounds originated from traditional Chinese herbs: A review. Smart Mater. Med. 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, 2100475. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Ren, S.; Guo, S.; Yang, L.; Wang, C. Effect of composite biodegradable biomaterials on wound healing in diabetes. Front. Bioeng. Biotechnol. 2022, 10, 1060026. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Lee, K.Z.; Jeon, J.; Jiang, B.; Subramani, S.V.; Li, J.; Zhang, F. Protein-Based Hydrogels and Their Biomedical Applications. Molecules 2023, 28, 4988. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-based hydrogels for regenerative engineering and cell transplantation. Biotechnol. Bioeng. 2019, 116, 3457–3468. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.; Wang, C.; Jiang, X.; Qu, M.; Zhu, J.; Ren, L.; Vajhadin, F.; et al. Biodegradable β-cyclodextrin conjugated gelatin methacryloyl microneedle for delivery of water-insoluble drug. Adv. Healthc. Mater. 2020, 9, e2000527. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Nourbakhsh, N.; Kenari, M.A.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Raja, W.K.; MacCorkle, S.; Diwan, I.M.; Abdurrob, A.; Lu, J.; Omenetto, F.G.; Kaplan, D.L. Transdermal Delivery Devices: Fabrication, Mechanics and Drug Release from Silk. Small 2013, 9, 3704–3713. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Vulpe, R.; Popa, M.; Picton, L.; Balan, V.; Dulong, V.M.; Butnaru, M.; Verestiuc, L. Crosslinked hydrogels based on biological macromolecules with potential use in skin tissue engineering. Int. J. Biol. Macromol. 2016, 84, 174–181. [Google Scholar] [CrossRef]
- Vulpe, R.; Picton, L.; Le Cerf, D.; Dulong, V.; Popa, M.; Peptu, C.; Verestiuc, L. Rheological study of in-situ crosslinkable hydrogels based on hyaluronic acid, collagen and sericin. Mater. Sci. Eng. C 2016, 69, 388–397. [Google Scholar] [CrossRef]
- Vulpe, R.; Popa, M.; Picton, L.; Peptu, C.A.; Tudorachi, N.; Verestiuc, L. Scaffolds based on collagen, hyaluronan and sericin with potential applications as controlled drug delivery systems. J. Nanosci. Nanotechnol. 2018, 18, 1528–1533. [Google Scholar] [CrossRef]
- Yue, K.; Li, X.; Schrobback, K.; Sheikhi, A.; Annabi, N.; Leijten, J.; Zhang, W.; Zhang, Y.S.; Hutmacher, D.W.; Klein, T.J.; et al. Structural analysis of photocrosslinkable methacryloyl-modified protein derivatives. Biomaterials 2017, 139, 163–171. [Google Scholar] [CrossRef]
- Kong, F.; Mehwish, N.; Lee, B.H. Emerging albumin hydrogels as personalized biomaterials. Acta Biomater. 2023, 157, 67–90. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Tincu, C.-E.; Andrițoiu, C.V.; Popa, M.; Ochiuz, L. Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma. Polymers 2023, 15, 3969. [Google Scholar] [CrossRef]
- Xia, T.; Jiang, X.; Deng, L.; Yang, M.; Chen, X. Albumin-based dynamic double cross-linked hydrogel with self-healing property for antimicrobial application. Colloids Surf. B Biointerfaces 2021, 208, 112042. [Google Scholar] [CrossRef]
- An, F.; Zhang, H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef]
- Arabi, S.H.; Aghelnejad, B.; Schwieger, C.; Meister, A.; Kerth, A.; Hinderberger, D. Serum albumin hydrogels in broad pH and temperature ranges: Characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomater. Sci. 2018, 6, 478–492. [Google Scholar] [CrossRef]
- Baler, K.; Michael, R.; Szleifer, I.; Ameer, G.A. Albumin hydrogels formed by electrostatically triggered self-assembly and their drug delivery capability. Biomacromolecules 2014, 15, 3625–3633. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, R.; Shi, H.; Wang, Y.; Ji, L.; Zhang, P.; Zhang, Q. Design of ruthenium-albumin hydrogel for cancer therapeutics and luminescent imaging. J. Inorg. Biochem. 2019, 194, 19–25. [Google Scholar] [CrossRef]
- Ouyang, J.; Bu, Q.; Tao, N.; Chen, M.; Liu, H.; Zhou, J.; Liu, J.; Deng, B.; Kong, N.; Zhang, X.; et al. A facile and general method for synthesis of antibiotic-free protein-based hydrogel: Wound dressing for the eradication of drug-resistant bacteria and biofilms. Bioact. Mater. 2022, 18, 446–458. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; Gomes da Silva, R.L.C.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. B 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Iurciuc, C.E.; Savin, A.; Lungu, C.; Martin, P.; Popa, M. Gellan. Food applications. Cellul. Chem. Technol. 2016, 50, 1–13. [Google Scholar]
- Iurciuc, C.E.; Lungu, C.; Martin, P.; Popa, M. Gellan. Pharmaceutical, medical and cosmetic applications. Cellul. Chem. Technol. 2017, 51, 187–202. [Google Scholar]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Razi, B.; Aslani, S.; Abbasifard, M.; Imani, D.; Sathyapalan, T.; Sahebkar, A. Effect of curcumin on proinflammatory cytokines: A meta-analysis of randomized controlled trials. Cytokine 2021, 143, 155541. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin Modulates Nuclear Factor κB (NF-κB)-mediated Inflammation in Human Tenocytes in Vitro: Role Of The Phosphatidylinositol 3-Kinase/Akt Pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Patel, P.; Wang, J.Y.; Mineroff, J.; Jagdeo, J. Evaluation of curcumin for dermatologic conditions: A systematic review. Arch. Dermatol. Res. 2024, 316, 37. [Google Scholar] [CrossRef]
- Tang, B.; Ma, L.; Wang, H.; Zhang, G. Study on the Supramolecular Interaction of Curcumin and β-cyclodextrin by Spectrophotometry and Its Analytical Application. J. Agric. Food Chem. 2002, 50, 1355–1361. [Google Scholar] [CrossRef]
- Dellali, M.; Iurciuc, C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules 2021, 26, 2185. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1999; p. 1292. Available online: http://fptl.ru/biblioteka/spravo4niki/dean.pdf (accessed on 6 February 2023).
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Abdel-Akher, M.; Hamilton, J.K.; Montgomery, R.; Smith, F. A new procedure for the determination of the fine structure of polysaccharides. J. Am. Chem. Soc. 1952, 74, 4970–4971. [Google Scholar] [CrossRef]
- Simas-Tosin, F.F.; de Souza, L.M.; Wagner, R.; Pereira, G.C.Z.; Barraza, R.R.; Wendel, C.F.; Sassaki, G.L.; Iacomini, M.; Gorin, P.A.J. Structural characterization of a glucuronoarabinoxylan from pineapple (Ananas comosus (L.) Merrill) gum exudate. Carbohydr. Polym. 2013, 94, 704–711. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef]
- da Silva, L.M.; Araújo, L.F.S.; Alvez, R.C.; Ono, L.; Sá, D.A.T.; da Cunha, P.L.R.; Monteiro de Paula, R.C.; Maciel, J.S. Promising alternative gum: Extraction, characterization, and oxidation of the galactomannan of Cassia fistula. Int. J. Biol. Macromol. 2020, 165, 436–444. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Achterweust, M.; Janssen, H.; Westphal, Y.; Schols, H.A. Periodate oxidation of plant polysaccharides provides polysaccharide-specific oligosaccharides. Carbohydr. Polym. 2022, 291, 119540. [Google Scholar] [CrossRef]
- Bobbitt, J. Periodate Oxidation of Carbohydrates. Adv. Carbohydr. Chem. 1955, 11, 1–41. [Google Scholar] [CrossRef]
- Lindstedt, G. Periodate Oxidation of Sugars in Neutral Phosphate Buffer. Nature 1945, 156, 448–449. [Google Scholar] [CrossRef]
- Cassanelli, M.; Prosapio, V.; Norton, I.; Mills, T. Acidified/basified gellan gum gels: The role of the structure in drying/rehydration mechanisms. Food Hydrocoll. 2018, 82, 346–354. [Google Scholar] [CrossRef]
- Picone, C.S.F.; Cunha, R.L. Influence of pH on formation and properties of gellan gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of Cyclodextrin Complexation of Curcumin on its Solubility and Antiangiogenic and Anti-inflammatory Activity in Rat Colitis Model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef]
- Hutapea, T.P.H.; Madurani, K.A.; Syahputra, M.Y.; Hudha, M.N.; Asriana, A.N.; Kurniawan, F. Albumin: Source, preparation, determination, applications, and prospects. J. Sci. Adv. Mater. Devices 2023, 8, 100549. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-Nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Amer, H.; Nypelö, T.; Sulaeva, I.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Synthesis and Characterization of Periodate-Oxidized Polysaccharides: Dialdehyde Xylan (DAX). Biomacromolecules 2016, 17, 2972–2980. [Google Scholar] [CrossRef]
- Dentini, M.; Coviello, T.; Burchard, W.; Crescenzi, V. Solution properties of exocellular microbial polysaccharides. 3. Light scattering from gellan and from the exocellular polysaccharide of Rhizobium trifolii (strain TA-1) in the ordered state. Macromolecules 1988, 21, 3312–3320. [Google Scholar] [CrossRef]
- Dreveton, E.; Monot, F.; Lecourtier, J.; Ballerini, D.; Choplin, L. Influence of fermentation hydrodynamics on gellan gum physico-chemical characteristics. J. Ferment. Bioeng. 1996, 82, 272–276. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Fang, S. Characterization, Antimicrobial Properties and Coatings Application of Gellan Gum Oxidized with Hydrogen Peroxide. Foods 2019, 8, 31. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.; Mi, X.; Xu, L.; Yang, Y. Potent and regularizable cross-linking of ultrafine fibrous protein scaffolds for tissue engineering using a cytocompatible disaccharide derivative. J. Mater. Chem. B 2015, 3, 3609–3616. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.D.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. Systematic FTIR Spectroscopy Study of the Secondary Structure Changes in Human Serum Albumin under Various Denaturation Conditions. Biomolecules 2019, 9, 359. [Google Scholar] [CrossRef]
- Grdadolnik, J.; Maréchal, Y. Bovine serum albumin observed by infrared spectrometry. I. Methodology, structural investigation, and water uptake. Biopolymers 2000, 62, 40–53. [Google Scholar] [CrossRef]
- Ma, X.; Sun, X.; Hargrove, D.; Chen, J.; Song, D.; Dong, Q.; Lu, X.; Fan, H.; Fu, Y.; Lei, Y. A Biocompatible and Biodegradable Protein Hydrogel with Green and Red Autofluorescence: Preparation, Characterization and In Vivo Biodegradation Tracking and Modeling. Sci. Rep. 2016, 6, 19370. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Biol. Eng. Comput. 2007, 45, 1237–1241. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, H.A.; Kim, M.R.; Hong, J. Changes in chemical stability and bioactivities of curcumin by ultraviolet radiation. Food Sci. Biotechnol. 2013, 22, 279–282. [Google Scholar] [CrossRef]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical Studies on Binding of Curcumin to Bovine Serum Albumin. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; de Dios Figueroa, J.; Marinho Mano, C.; Bechara, J.E.H.; Godınez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate. J. Agric. Food Chem. 2017, 65, 10812–10819. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodríguez-Fernández, J.; Gallego-Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef]
- Elahipanah, S.; O’Brien, P.J.; Rogozhnikov, D.; Yousaf, M.N. General dialdehyde click chemistry for amine bioconjugation. Bioconjug. Chem. 2017, 28, 1422–1433. [Google Scholar] [CrossRef]
- Morra, M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules 2005, 6, 1205–1223. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Boni, F.I.; Ferreira, N.N.; Campana-Filho, S.P.; Almeida, A.; Vasconcelos, T.; Daflon Gremião, M.P.; Ferreira Cury, B.S.; Sarmento, B. Gellan Gum/pectin beads are safe and efficient for the targeted colonic delivery of resveratrol. Polymers 2018, 10, 50. [Google Scholar] [CrossRef]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, H.; Lim, E.; Loh, Y.; Lai, S. Contribution of Aldehydes and Their Derivatives to Antimicrobial and Immunomodulatory Activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef]
- Hassan, S.F.; Asghar, S.; Khan, I.U.; Munir, R.; Khalid, S.H. Curcumin Encapsulation in Geranium Oil Microemulsion Elevates Its Antibacterial, Antioxidant, Anti-Inflammatory, and Anticancer Activities. ACS Omega 2024, 9, 5624–5636. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Gao, P.; Wang, L.; Qi, Y.; Chen, J.; Bai, K.; Fan, Y.; Liu, X. Microemulsion-Based Keratin–Chitosan Gel for Improvement of Skin Permeation/Retention and Activity of Curcumin. Gels 2023, 9, 587. [Google Scholar] [CrossRef]
- Ravikumar, R.; Ganesh, M.; Ubaidulla, U.; Young Choi, E.; Tae Jang, H. Preparation, characterization, and in vitro diffusion study of nonwoven electrospun nanofiber of curcumin-loaded cellulose acetate phthalate polymer. Saudi Pharm. J. 2017, 25, 921–926. [Google Scholar] [CrossRef]
- Mekhloufi, G.; Vilamosa, N.; Agnely, F. Nanoemulsion stabilized by β-lactoglobulin: A promising strategy to encapsulate curcumin for topical delivery. Mater. Today Proc. 2021, 53, 168–173. [Google Scholar] [CrossRef]
- Ferrara, F.; Bondi, A.; Pula, W.; Contado, C.; Baldisserotto, A.; Manfredini, S.; Boldrini, P.; Sguizzato, M.; Montesi, L.; Benedusi, M.; et al. Ethosomes for Curcumin and Piperine Cutaneous Delivery to Prevent Environmental-Stressor-Induced Skin Damage. Antioxidants 2024, 13, 91. [Google Scholar] [CrossRef]
- Miao, X.; Zhao, J.; Xiang, H.; Shi, X. Hyaluronidase Promote Transdermal Diffusion of Small Sized Curcumin Nanocrystal by Dissolving Microneedles Delivery. Pharmaceutics 2023, 15, 788. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602. [Google Scholar] [CrossRef]







| Sample Code * | Molar Report, -NH2/-CHO | Number of -NH2 Moles from BSA | Number of -CHO Moles from Oxidized Gellan | Amount of Curcumin within the Inclusion Complex (mg) |
|---|---|---|---|---|
| A1 | 1:1 | 4.59 × 10−5 | 4.59 × 10−5 | - |
| A2 | 1:4 | 1.84 × 10−4 | ||
| A3 | 1:6 | 2.75 × 10−4 | ||
| A4 | 1:8 | 3.67 × 10−4 | ||
| A5 | 1:10 | 4.59 × 10−4 | ||
| A6 | 1:12 | 5.51 × 10−4 | ||
| A7 | 1:16 | 7.34 × 10−4 | ||
| C1 | 1:1 | 4.59 × 10−4 | 10 | |
| C2 | 1:4 | 1.84 × 10−4 | ||
| C3 | 1:6 | 2.75 × 10−4 | ||
| C4 | 1:8 | 3.67 × 10−4 | ||
| C5 | 1:10 | 4.59 × 10−4 | ||
| C6 | 1:12 | 5.51 × 10−4 | ||
| C7 | 1:16 | 7.34 × 10−4 |
| Sample Code | The Maximum Value of the Swelling Degree (Q%) in ABS, at pH = 5.5 | The Maximum Value of the Swelling Degree (Q%) in PBS, at pH = 7.4 |
|---|---|---|
| A1 | 23.81 | 73.52 |
| A2 | 57.73 | 77.23 |
| A3 | 100.7 | 128.53 |
| A4 | 105.54 | 134.04 |
| A5 | 147.78 | 159.11 |
| A6 | 166.56 | 175.28 |
| A7 | 178.28 | 191.64 |
| Sample Code | Release Efficiency after 24 h, % | The Permeability Coefficient of CURC Found in the Receptor Compartment after 24 h, μg/cm2/h | Curcumin Permeability Coefficient in the Skin Membrane, μg/cm2/h | Total Permeability Coefficient, μg/cm2/h | Exponential Factor, n | R2 |
|---|---|---|---|---|---|---|
| C2-7.4 | 54.03 ± 0.14 | 1.9 ± 0.005 | 0.09 | 1.99 | 0.5 | 0.9151 |
| C3-7.4 | 59.52 ± 1.95 | 1.9 ± 0.08 | 0.1 | 2 | 0.48 | 0.9557 |
| C4-7.4 | 66.9 ± 1.1 | 2 ± 0.05 | 0.13 | 2.13 | 0.47 | 0.9949 |
| C5-7.4 | 73 ± 0.64 | 2.1 ± 0.03 | 0.13 | 2.26 | 0.3 | 0.9893 |
| C2-5.5 | 79.9 ± 0.3 | 5.5 ± 0.01 | 0.27 | 5.77 | 0.6 | 0.8547 |
| C4-5.5 | 88.13 ± 0.4 | 5.83 ± 0.016 | 0.52 | 6.35 | 0.4 | 0.9607 |
| Nr. Crt | Curcumin Release System Type | Medium Used in the Receptor Compartment | Dimension | Membrane Type | Permeability μg/cm2/h | Ref. |
|---|---|---|---|---|---|---|
| 1 | Stable microemulsions of curcumin using different oils and surfactants | PBS and ethanol 1:1 | Droplet diameter 199.39 ± 0.017 | Mouse skin | 130.91 ± 0.02 | [95] |
| 2 | Microemulsion-based keratin–chitosan gel with encapsulated curcumin | PBS with 1% Tween | The particle size of the CME-KCS gel was 186.45 ± 0.75 nm | Rat skin | 0.16 ± 0.01 | [96] |
| 3 | Curcumin-loaded cellulose acetate phthalate nonwoven electrospun nanofiber | Phosphate buffer at pH = 7.4 with 20% ethanol | 300 nm | Pig abdominal skin | 12.87 | [97] |
| 4 | Nanoemulsion based on β-lactoglobulin with curcumin-encapsulated | 90/10 (v/v) PB/ethanol, 0.1 wt% Tween 20, and 0.04 wt% ascorbic acid | 220 nm | Synthetic membrane | 0.47 | [98] |
| 5 | Chitosan nanoparticles with curcumin encapsulated | PBS. pH = 7.4 with 1% Tween 80 | 167.3 ± 3.8 nm–251.5 ± 5.8 nm | Strat-M membrane made of polyester sulfone | 0.54 ± 0.03 and 0.44 ± 0.03 | [92] |
| 6 | Ethosomes (ETs) with encapsulated curcumin | Ethanol:water mixture (50:50, v/v) | 200 nm | STRAT-M® membranes | 0.70 ± 0.21 | [99] |
| 7 | Microneedles containing hyaluronidase with nano curcumin encapsulated | PEG 400 aqueous solution was used as a receptor medium | 55 nm | Pig skin | From 0.68 ± 0.16 to 2.79 ± 0.20 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tincu, C.E.; Daraba, O.M.; Jérôme, C.; Popa, M.; Ochiuz, L. Albumin-Based Hydrogel Films Covalently Cross-Linked with Oxidized Gellan with Encapsulated Curcumin for Biomedical Applications. Polymers 2024, 16, 1631. https://doi.org/10.3390/polym16121631
Tincu CE, Daraba OM, Jérôme C, Popa M, Ochiuz L. Albumin-Based Hydrogel Films Covalently Cross-Linked with Oxidized Gellan with Encapsulated Curcumin for Biomedical Applications. Polymers. 2024; 16(12):1631. https://doi.org/10.3390/polym16121631
Chicago/Turabian StyleTincu (Iurciuc), Camelia Elena, Oana Maria Daraba, Christine Jérôme, Marcel Popa, and Lăcrămioara Ochiuz. 2024. "Albumin-Based Hydrogel Films Covalently Cross-Linked with Oxidized Gellan with Encapsulated Curcumin for Biomedical Applications" Polymers 16, no. 12: 1631. https://doi.org/10.3390/polym16121631






