Bioinspired Dopamine and N-Oxide-Based Zwitterionic Polymer Brushes for Fouling Resistance Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
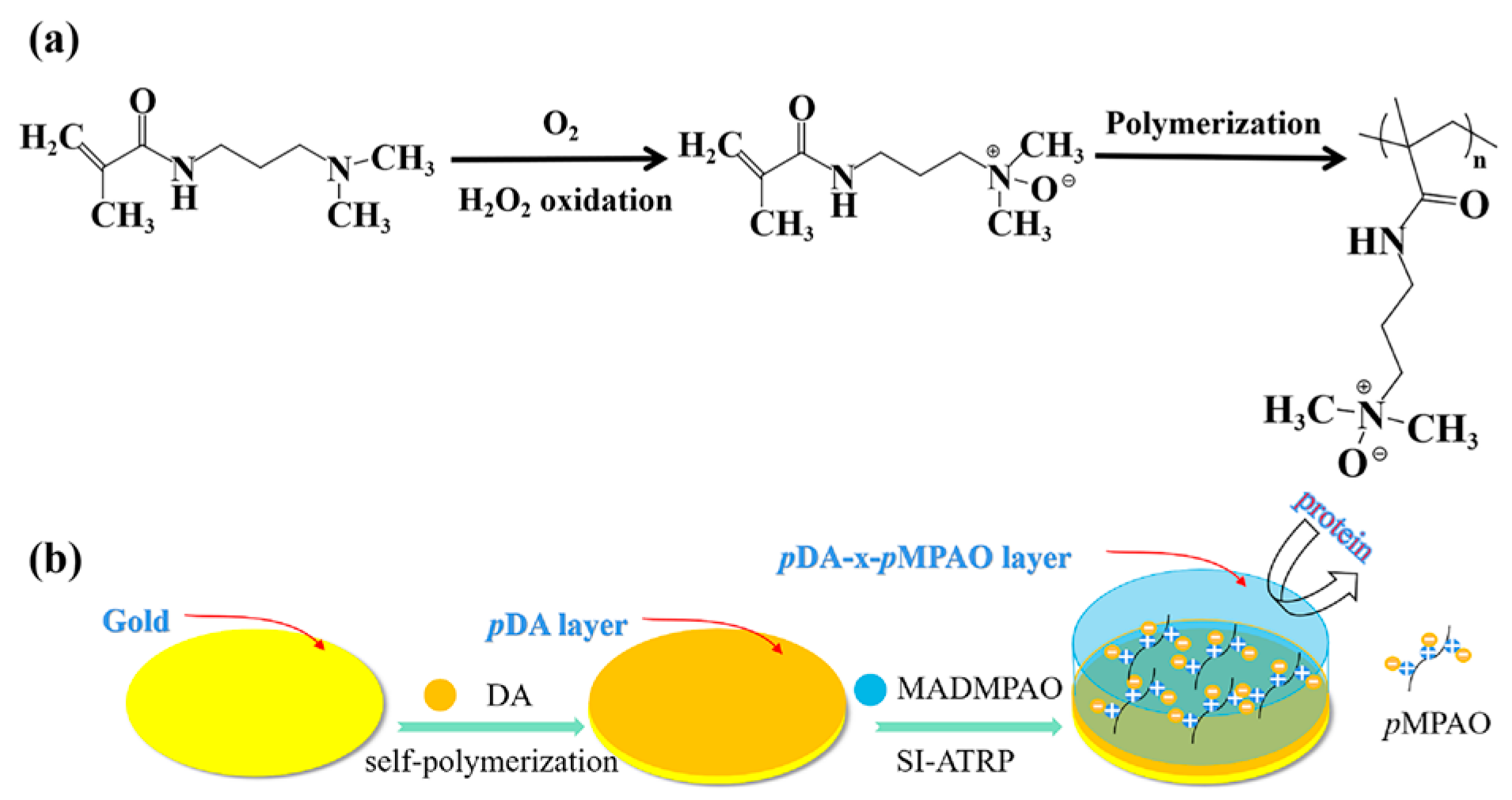
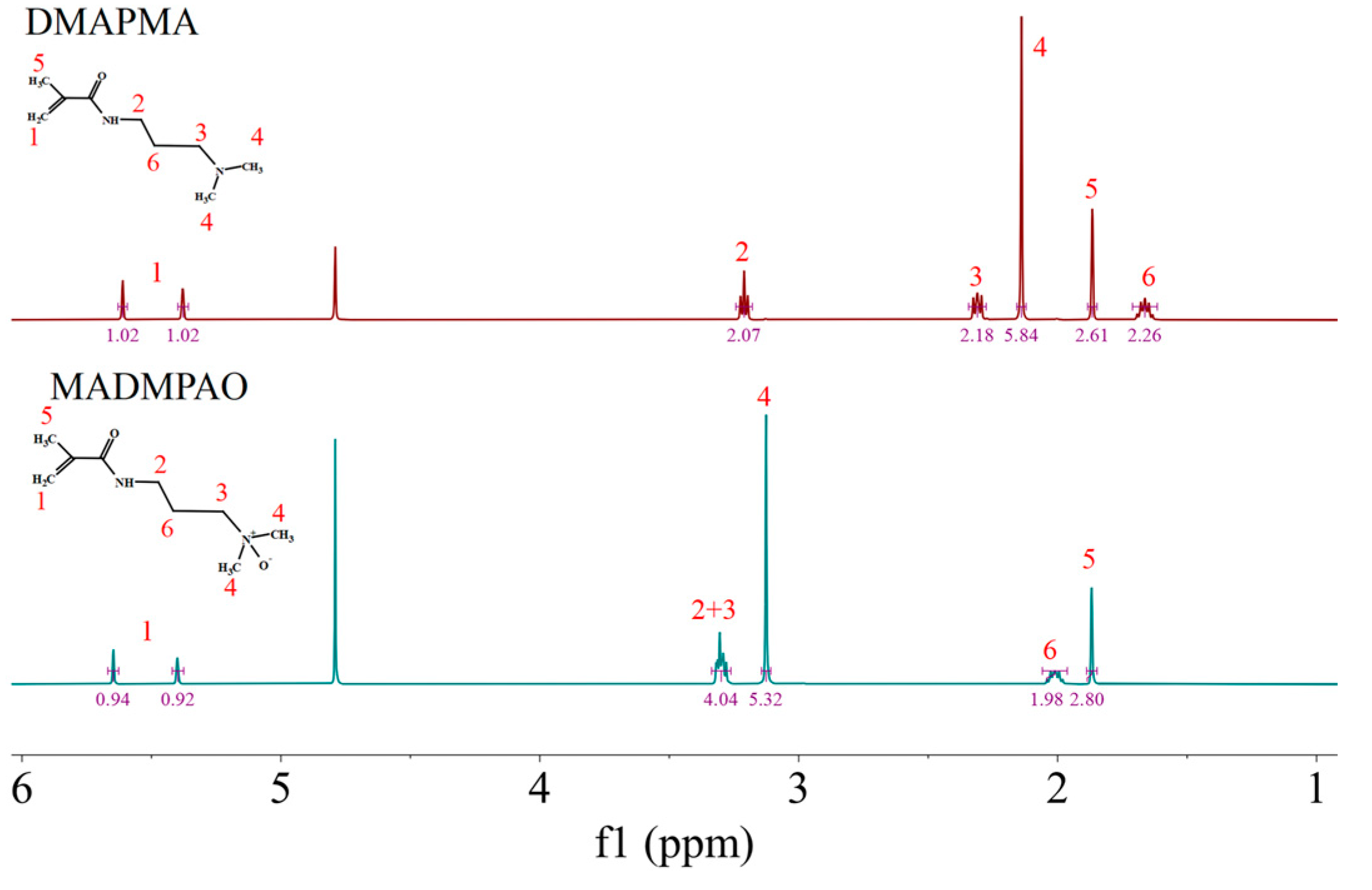
2.2. Synthesis of MADMPAO
2.3. Surface Coating on QCM Sensors by Polydopamine
2.4. Polymer Grafting on Au@pDA
2.5. Characterization
2.6. Ion and BSA Adsorption Study by QCM-D
3. Results
3.1. Synthesis of TMAO-Analog Monomer
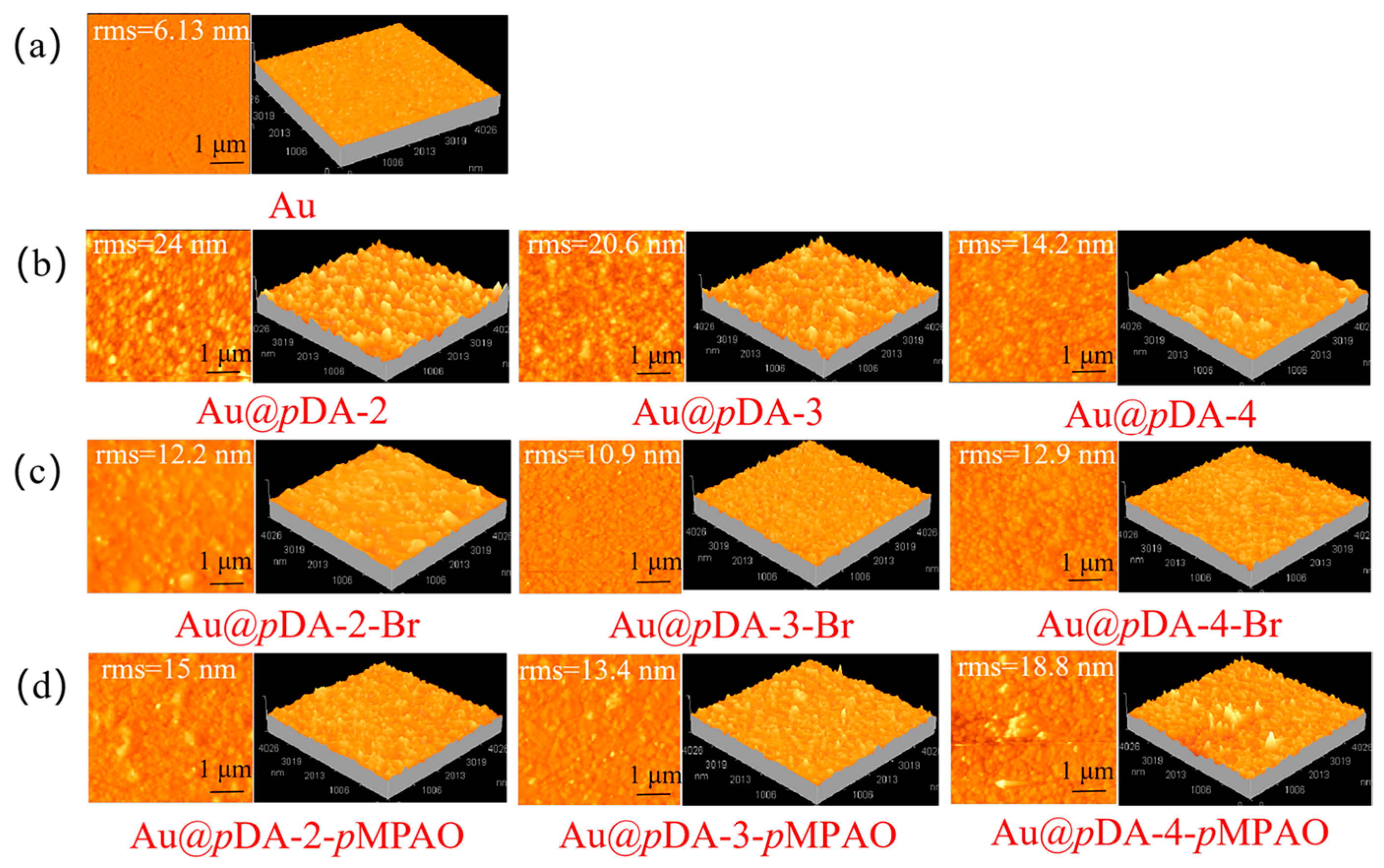
3.2. Surface Modification and Characterization
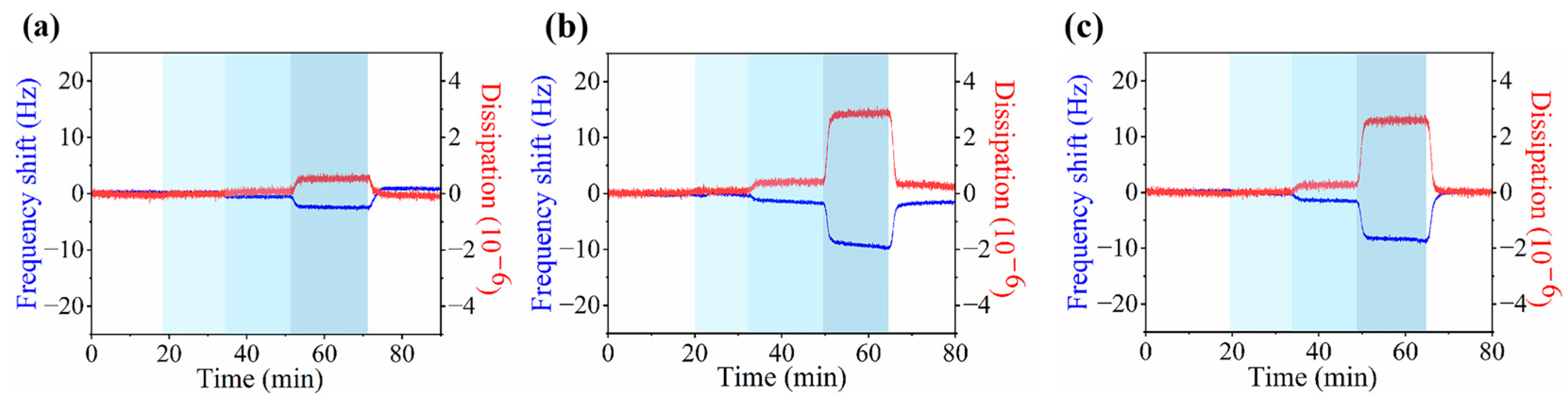
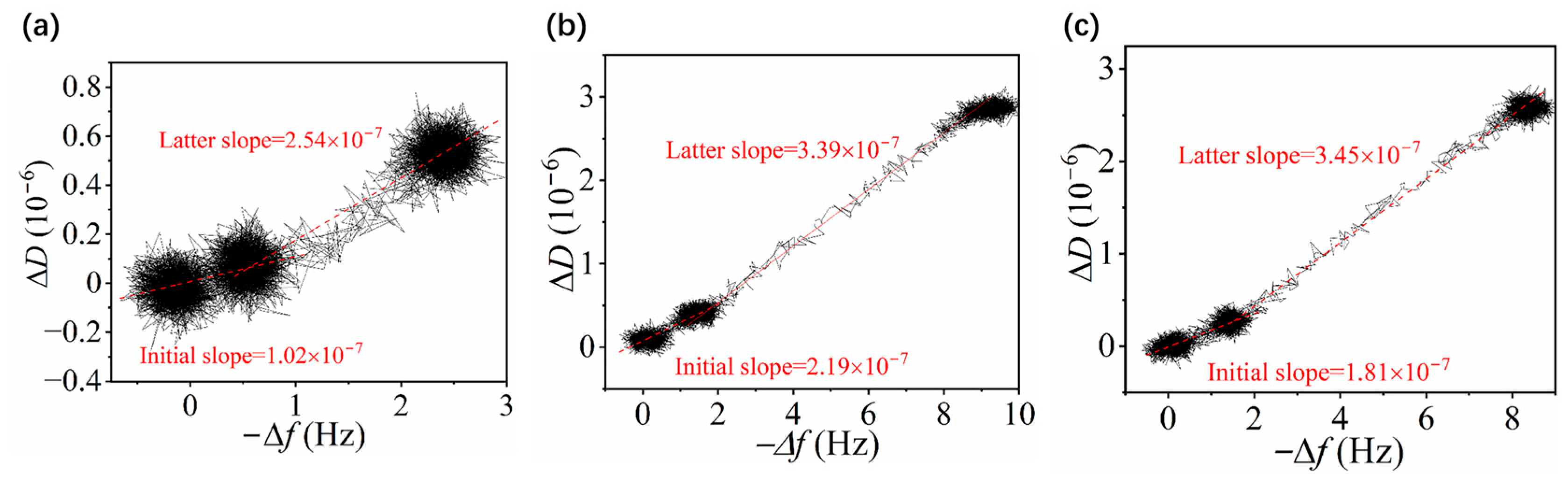
3.3. Adsorption of Ions on Zwitterionic Polymer
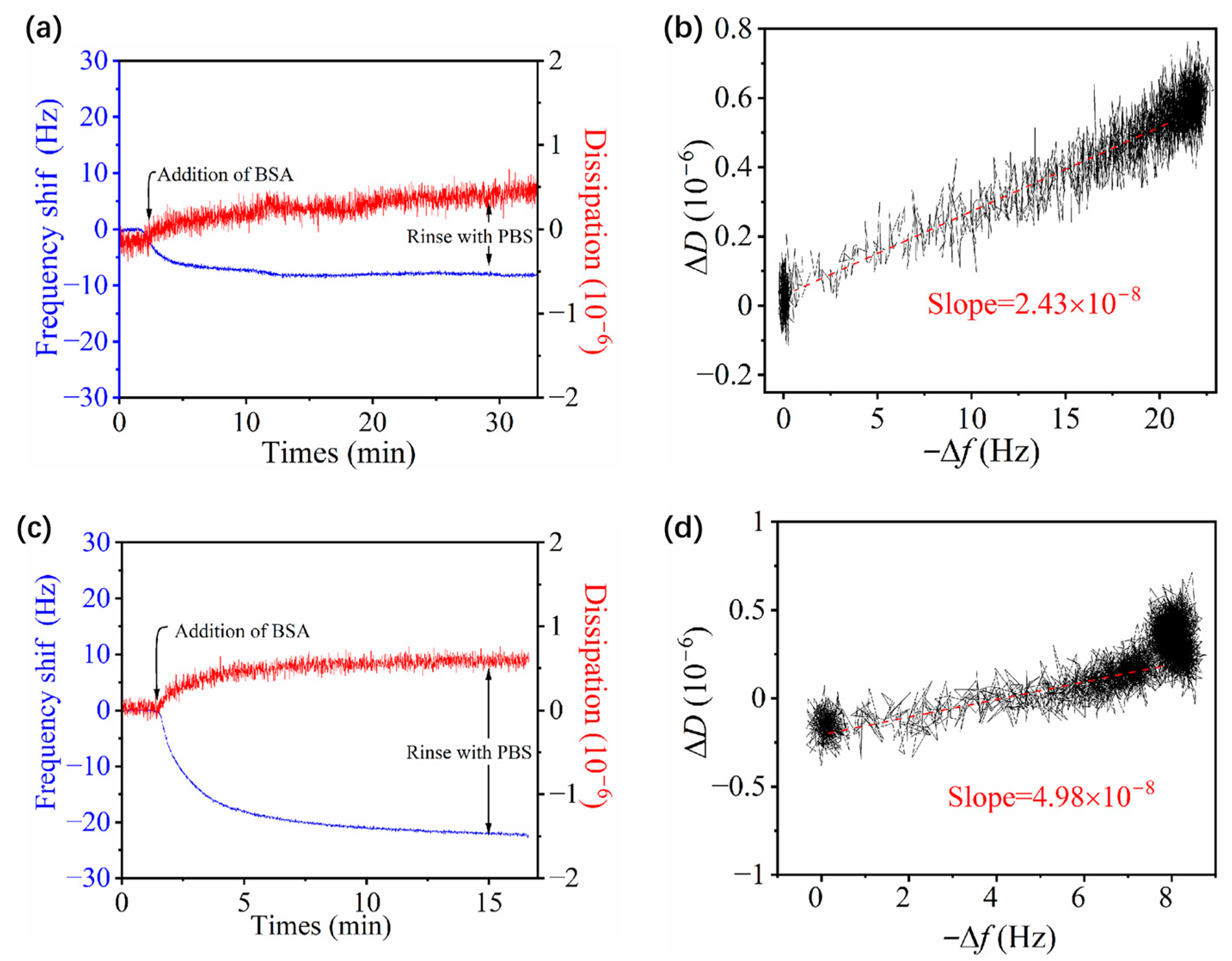
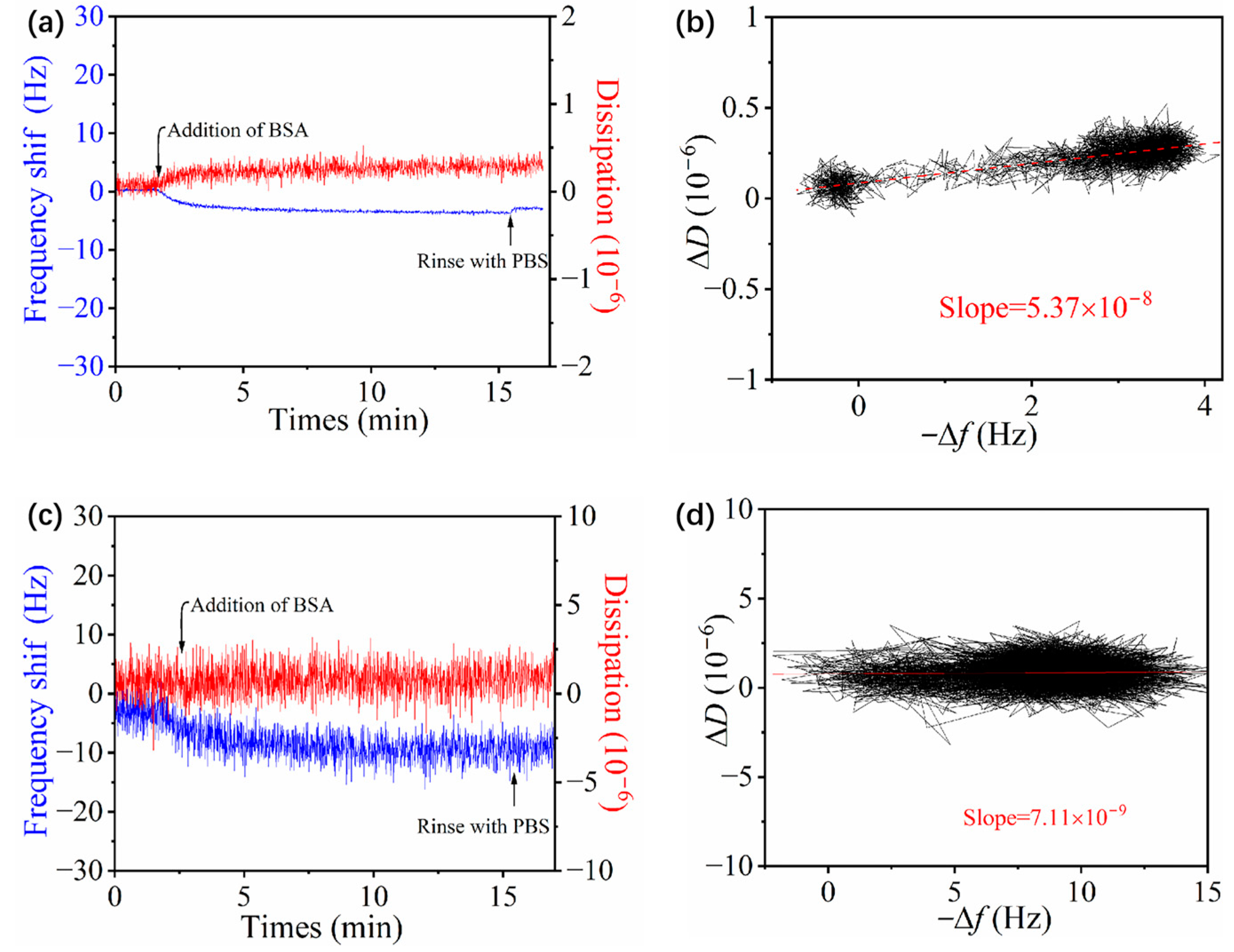
3.4. Adsorption of BSA on Zwitterionic pMPAO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.C.; Luo, Z.; Li, X.; Wang, R.; Chen, H.; Zhao, Y.C.; Wang, J.; Huang, N. Preparation, evaluation and functionalization of biomimetic block copolymer coatings for potential applications in cardiovascular implants. Appl. Surf. Sci. 2020, 502, 144085. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.J.; Zhang, H.X.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, P.S.; Ye, Z.P.; Wang, Y.P.; Wang, W.W.; Kong, D.L.; Zhang, J.H.; Deng, L.D.; Dong, A.J. Layer-by-layer zwitterionic modification of diverse substrates with durable anti-corrosion and anti-fouling properties. J. Mater. Chem. B 2019, 7, 6024–6034. [Google Scholar] [CrossRef]
- Zhao, X.T.; Zhang, R.N.; Liu, Y.N.; He, M.R.; Su, Y.L.; Gao, C.J.; Jiang, Z.Y. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Liu, Q.J.; Wu, C.S.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-Based Biosensors and Their Application in Biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Han, R.; Liu, N.Z.; Gao, F.X.; Luo, X.L. Electrochemical biosensors for the detection of carcinoembryonic antigen with low fouling and high sensitivity based on copolymerized polydopamine and zwitterionic polymer. Sens. Actuators B 2020, 319, 128253. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Schonemann, E.; Koc, J.; Karthauser, J.F.; Ozcan, O.; Schanzenbach, D.; Schardt, L.; Rosenhahn, A.; Laschewsky, A. Sulfobetaine Methacrylate Polymers of Unconventional Polyzwitterion Architecture and Their Antifouling Properties. Biomacromolecules 2021, 22, 1494–1508. [Google Scholar] [CrossRef]
- Yeon, D.K.; Ko, S.; Jeong, S.; Hong, S.P.; Kang, S.M.; Cho, W.K. Oxidation-Mediated, Zwitterionic Polydopamine Coatings for Marine Antifouling Applications. Langmuir 2019, 35, 1227–1234. [Google Scholar] [CrossRef]
- Li, B.W.; Jain, P.; Ma, J.R.; Smith, J.K.; Yuan, Z.F.; Hung, H.C.; He, Y.W.; Lin, X.J.; Wu, K.; Pfaendtner, J.; et al. Trimethylamine N-oxide-derived zwitterionic polymers: A new class of ultralow fouling bioinspired materials. Sci. Adv. 2019, 5, eaaw9562. [Google Scholar] [CrossRef]
- Lin, X.J.; Wu, K.; Zhou, Q.; Jain, P.; Boit, M.O.; Li, B.W.; Hung, H.C.; Creason, S.A.; Himmelfarb, J.; Ratner, B.D.; et al. Photoreactive Carboxybetaine Copolymers Impart Biocompatibility and Inhibit Plasticizer Leaching on Polyvinyl Chloride. ACS Appl. Mater. Interfaces 2020, 12, 41026–41037. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, D.; Ren, B.P.; Gong, X.; Xu, L.J.; Feng, Z.Q.; Chang, Y.; He, Y.; Zheng, J. Molecular simulations and understanding of antifouling zwitterionic polymer brushes. J. Mater. Chem. B 2020, 8, 3814–3828. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Cao, Z.Q. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Jin, X.X.; Yuan, J.; Shen, J. Zwitterionic polymer brushes via dopamine-initiated ATRP from PET sheets for improving hemocompatible and antifouling properties. Colloids Surf. B 2016, 145, 275–284. [Google Scholar] [CrossRef]
- Ma, W.Y.; Yang, P.; Li, J.G.; Li, S.Q.; Li, P.C.; Zhao, Y.C.; Huang, N. Immobilization of poly(MPC) brushes onto titanium surface by combining dopamine self-polymerization and ATRP: Preparation, characterization and evaluation of hemocompatibility in vitro. Appl. Surf. Sci. 2015, 349, 445–451. [Google Scholar] [CrossRef]
- Kobayashi, M.; Terayama, Y.; Kikuchi, M.; Takahara, A. Chain dimensions and surface characterization of superhydrophilic polymer brushes with zwitterion side groups. Soft Matter 2013, 9, 5138–5148. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Jana, S.; Lee, S.S.C.; Lim, C.S.; Teo, S.L.M.; Parthiban, A.; Vancso, J.G. Dual hydrophilic and salt responsive schizophrenic block copolymers—Synthesis and study of self-assembly behavior. Polym. Chem. 2015, 6, 599–606. [Google Scholar] [CrossRef]
- Wu, B.Z.; Zhang, L.X.; Huang, L.; Xiao, S.W.; Yang, Y.; Zhong, M.Q.; Yang, J. Salt-Induced Regenerative Surface for Bacteria Killing and Release. Langmuir 2017, 33, 7160–7168. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, D.; Zhang, L.X.; Wu, B.Y.; Xiao, S.W.; Chen, F.; Fan, P.; Zhong, M.Q.; Tan, J.; Chu, Y.H.; et al. Long-term stability and salt-responsive behavior of polyzwitterionic brushes with cross-linked structure. Prog. Org. Coat. 2019, 134, 153–161. [Google Scholar] [CrossRef]
- Lin, X.J.; Jain, P.; Wu, K.; Hong, D.; Hung, H.C.; O’Kelly, M.B.; Li, B.W.; Zhang, P.; Yuan, Z.F.; Jiang, S.Y. Ultralow Fouling and Functionalizable Surface Chemistry Based on Zwitterionic Carboxybetaine Random Copolymers. Langmuir 2019, 35, 1544–1551. [Google Scholar] [CrossRef]
- Saha, P.; Santi, M.; Emondts, M.; Roth, H.; Rahimi, K.; Grosskurth, J.; Ganguly, R.; Wessling, M.; Singha, N.K.; Pich, A. Stimuli-Responsive Zwitterionic Core-Shell Microgels for Antifouling Surface Coatings. ACS Appl. Mater. Interfaces 2020, 12, 58223–58238. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, X.Y.; Lu, Q.; Li, R.; Bai, Y.J.; Li, X.; Zhang, S.P.; Gong, Y.K. Anchorable phosphorylcholine copolymer synthesis and cell membrane mimetic antifouling coating fabrication for blood compatible applications. J. Mater. Chem. B 2020, 8, 4299–4309. [Google Scholar] [CrossRef]
- Lin, C.H.; Luo, S.C. Combination of AFM and Electrochemical QCM-D for Probing Zwitterionic Polymer Brushes in Water: Visualization of Ionic Strength and Surface Potential Effects. Langmuir 2021, 37, 12476–12486. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.C.; Crisci, R.; Lu, T.Y.; Hung, H.C.; Sajib, M.S.J.; Sarker, P.; Ma, J.R.; Wei, T.; Jiang, S.Y.; et al. Strong Surface Hydration and Salt Resistant Mechanism of a New Nonfouling Zwitterionic Polymer Based on Protein Stabilizer TMAO. J. Am. Chem. Soc. 2021, 143, 16786–16795. [Google Scholar] [CrossRef]
- Zhang, C.X.; Zhou, J.M.; Ye, X.Y.; Li, Z.; Wang, Y. Zwitterionization of Tertiary Amines in Nanoporous Block Copolymers: Toward Fouling-Resistant Ultrafiltration Membranes. Macromolecules 2021, 54, 4236–4245. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Cheng, W.J.; Ll, Y.; Li, X.Y.; Bai, W.J.; Liang, Y.X. Preparation and characterization of PDA/SiO2 nanofilm constructed macroporous monolith and its application in lipase immobilization. J. Taiwan Inst. Chem. Eng. 2019, 104, 351–359. [Google Scholar] [CrossRef]
- Wang, W.Q.; Julaiti, P.; Ye, G.; Huo, X.M.; Lu, Y.X.; Chen, J. Controlled Architecture of Glass Fiber/Poly(glycidyl methacrylate) Composites via Surface-Initiated ICAR ATRP Mediated by Mussel Inspired Polydopamine Chemistry. Ind. Eng. Chem. Res. 2017, 56, 11467–11476. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, C.; Yu, R.; Ding, Y.M. Application of polydopamine on the implant surface modification. Polym. Bull. 2022, 79, 5613–5633. [Google Scholar] [CrossRef]
- Tagaya, M.; Ikoma, T.; Hanagata, N.; Tanaka, J. Analytical Investigation of Protein Mediation between Biomaterials and Cells. Mater. Express 2012, 2, 1–22. [Google Scholar] [CrossRef]
- Tagaya, M. In situ QCM-D study of nano-bio interfaces with enhanced biocompatibility. Polym. J. 2015, 47, 599–608. [Google Scholar] [CrossRef]
- Dolatshahi-Pirouz, A.; Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Chevallier, J.; Besenbacher, F. Bovine serum albumin adsorption on nano-rough platinum surfaces studied by QCM-D. Colloids Surf. B 2008, 66, 53–59. [Google Scholar] [CrossRef]
- Kushiro, K.; Lee, C.H.; Takai, M. Simultaneous characterization of protein-material and cell-protein interactions using dynamic QCM-D analysis on SAM surfaces. Biomater. Sci. 2016, 4, 989–997. [Google Scholar] [CrossRef]
- You, F.F.; Shi, Q.H. Kinetic investigation of protein adsorption into polyelectrolyte brushes by quartz crystal microbalance with dissipation: The implication of the chromatographic mechanism. J. Chromatogr. A 2021, 1654, 462460. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.X.; Wan, L.S.; Ji, J.; Xu, Z.K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Moxey, M.; Alswieleh, A.; Morse, A.J.; Lewis, A.L.; Geoghegan, M.; Leggett, G.J. Effect of Salt on Phosphorylcholine-based Zwitterionic Polymer Brushes. Langmuir 2016, 32, 5048–5057. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.Y.; Li, B.; Deng, S.Y.; Zhang, X. Graphene oxide nanofiltration membrane with trimethylamine-N-oxide zwitterions for robust biofouling resistance. J. Membr. Sci. 2021, 640, 119855. [Google Scholar] [CrossRef]
- Li, N.; Li, T.; Qiao, X.Y.; Li, R.; Yao, Y.; Gong, Y.K. Universal Strategy for Efficient Fabrication of Blood Compatible Surfaces via Polydopamine-Assisted Surface-Initiated Activators Regenerated by Electron Transfer Atom-Transfer Radical Polymerization of Zwitterions. ACS Appl. Mater. Interfaces 2020, 12, 12337–12344. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.-T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Wei, Q.L.; Wu, C.X.; Zhang, X.F.; Xue, Q.H.; Zheng, T.R.; Cao, M.W. Superhydrophilicity and strong salt-affinity: Zwitterionic polymer grafted surfaces with significant potentials particularly in biological systems. Adv. Colloid Interface Sci. 2020, 278, 102141. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Feng, X.; Lu, X. Bioinspired N-Oxide-Based Zwitterionic Polymer Brushes for Robust Fouling-Resistant Surfaces. Environ. Sci. Technol. 2023, 57, 7298–7308. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carras, S. Dynamics of Contact Line Motion During the Wetting of Rough Surfaces and Correlation with Topographical Surface Parameters. Scanning 2011, 33, 370–377. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.; Feng, X.; Wang, W.; Xu, F.; Zhang, L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X.; Mao, Z. Lasting superhydrophobicity and antibacterial activity of Cu nanoparticles immobilized on the surface of dopamine modified cotton fabrics. Surf. Coat. Technol. 2017, 309, 149–154. [Google Scholar] [CrossRef]
- Luo, R.F.; Tang, L.L.; Zhong, S.; Yang, Z.L.; Wang, J.; Weng, Y.J.; Tu, Q.F.; Jiang, C.X.; Huang, N. In Vitro Investigation of Enhanced Hemocompatibility and Endothelial Cell Proliferation Associated with Quinone-Rich Polydopamine Coating. ACS Appl. Mater. Interfaces 2013, 5, 1704–1714. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, B.; Huang, L.; Shen, M.; Zhang, Y.; Zhong, M.; Yang, J.; Zheng, J. Salt-responsive zwitterionic polymer brushes with anti-polyelectrolyte property. Curr. Opin. Chem. Eng. 2018, 19, 86–93. [Google Scholar] [CrossRef]
- Moraleda, B.F.M.; San Roman, J.; Rodriguez-Lorenzo, L.M. Adsorption and conformational modification of fibronectin and fibrinogen adsorbed on hydroxyapatite. A QCM-D study. J. Biomed. Mater. Res. A 2016, 104, 2585–2594. [Google Scholar] [CrossRef]
- Ikoma, T.; Tagaya, M.; Hanagata, N.; Yoshioka, T.; Chakarov, D.; Kasemo, B.; Tanaka, J. Protein Adsorption on Hydroxyapatite Nanosensors with Different Crystal Sizes Studied In Situ by a Quartz Crystal Microbalance with the Dissipation Method. J. Am. Ceram. Soc. 2009, 92, 1125–1128. [Google Scholar] [CrossRef]
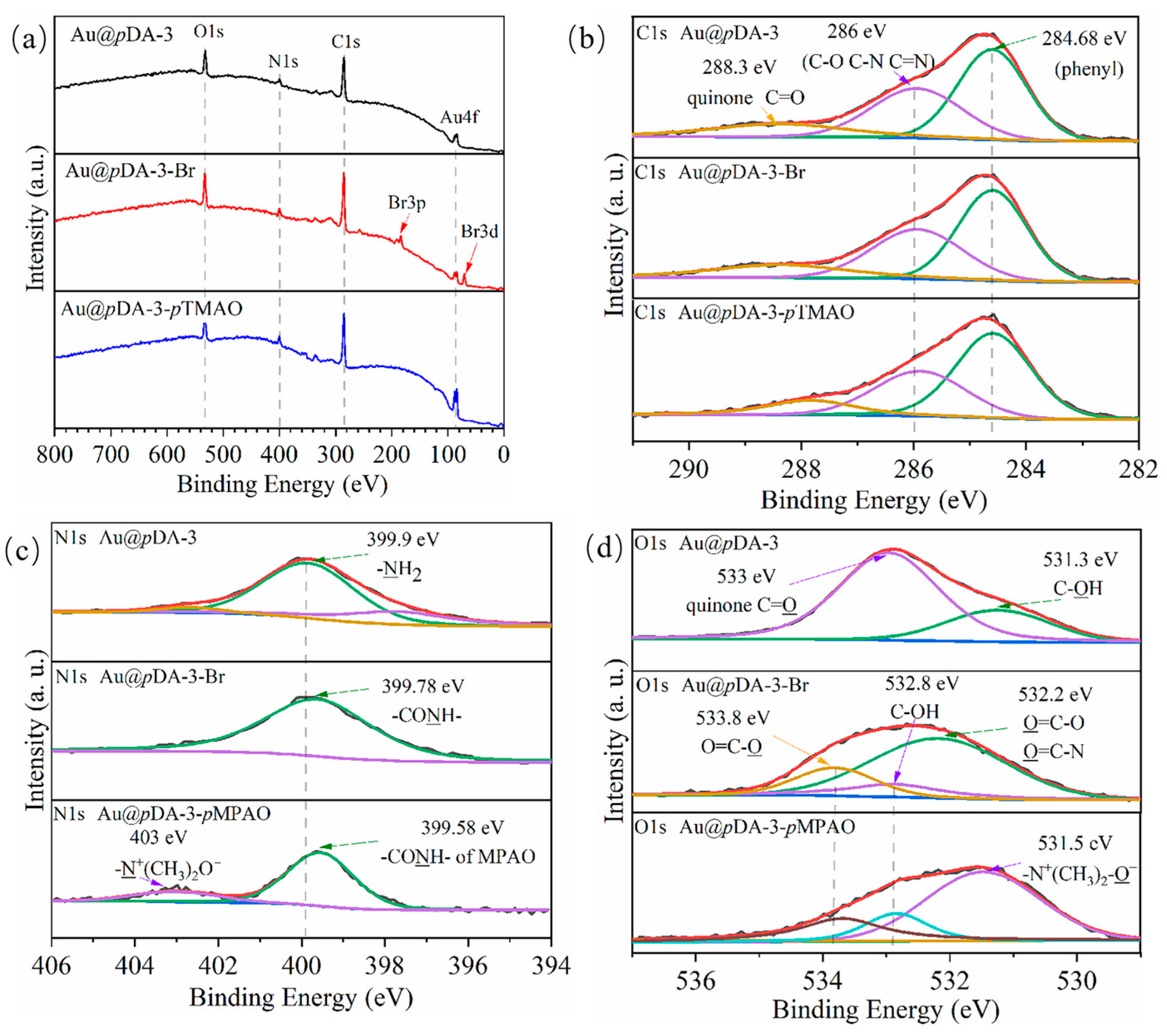
| Chips | XPS Surface Composition (at %) | |||
|---|---|---|---|---|
| C1s | N1s | O1s | Br3d | |
| Au@pDA-3 | 73.44 | 8.65 | 17.91 | 0 |
| Au@pDA-3-Br | 69.8 | 7.9 | 18.61 | 3.69 |
| Au@pDA-3-pMPAO | 72.61 | 8.05 | 19.34 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Shi, Q. Bioinspired Dopamine and N-Oxide-Based Zwitterionic Polymer Brushes for Fouling Resistance Surfaces. Polymers 2024, 16, 1634. https://doi.org/10.3390/polym16121634
Zhou Z, Shi Q. Bioinspired Dopamine and N-Oxide-Based Zwitterionic Polymer Brushes for Fouling Resistance Surfaces. Polymers. 2024; 16(12):1634. https://doi.org/10.3390/polym16121634
Chicago/Turabian StyleZhou, Zhen, and Qinghong Shi. 2024. "Bioinspired Dopamine and N-Oxide-Based Zwitterionic Polymer Brushes for Fouling Resistance Surfaces" Polymers 16, no. 12: 1634. https://doi.org/10.3390/polym16121634
APA StyleZhou, Z., & Shi, Q. (2024). Bioinspired Dopamine and N-Oxide-Based Zwitterionic Polymer Brushes for Fouling Resistance Surfaces. Polymers, 16(12), 1634. https://doi.org/10.3390/polym16121634






