Influence of Crosslinking Concentration on the Properties of Biodegradable Modified Cassava Starch-Based Films for Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
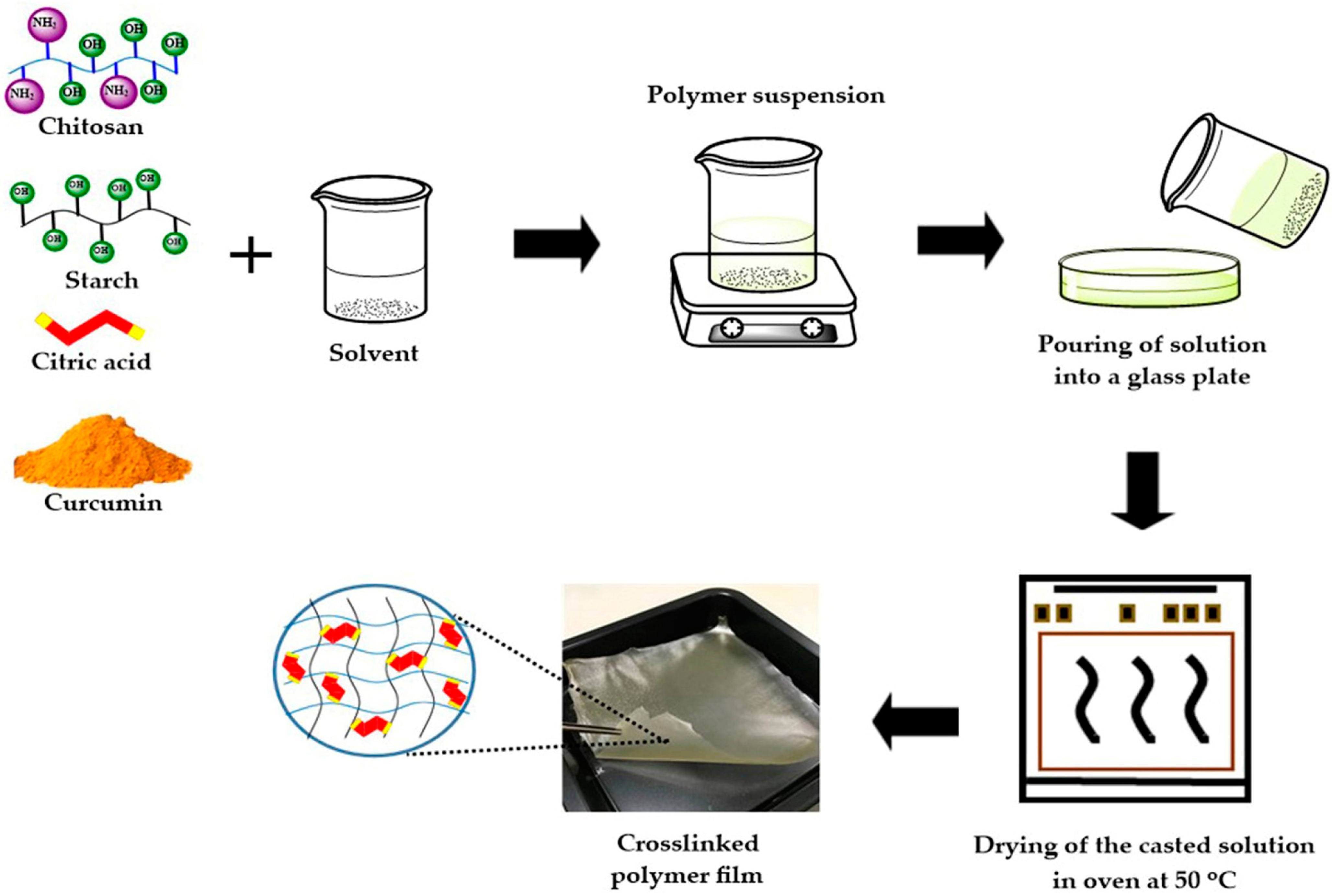
2.2. Sample Preparation
2.3. Film Characterization
2.3.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Water Vapor Permeability (WVP)
2.3.5. Moisture Content (MC), Swelling Degree (SD), and Water Solubility (WS)
2.3.6. Film Thickness
2.3.7. Mechanical Properties
2.3.8. Antioxidant Property
2.3.9. Statistical Analysis
3. Results
3.1. Structural Characterization
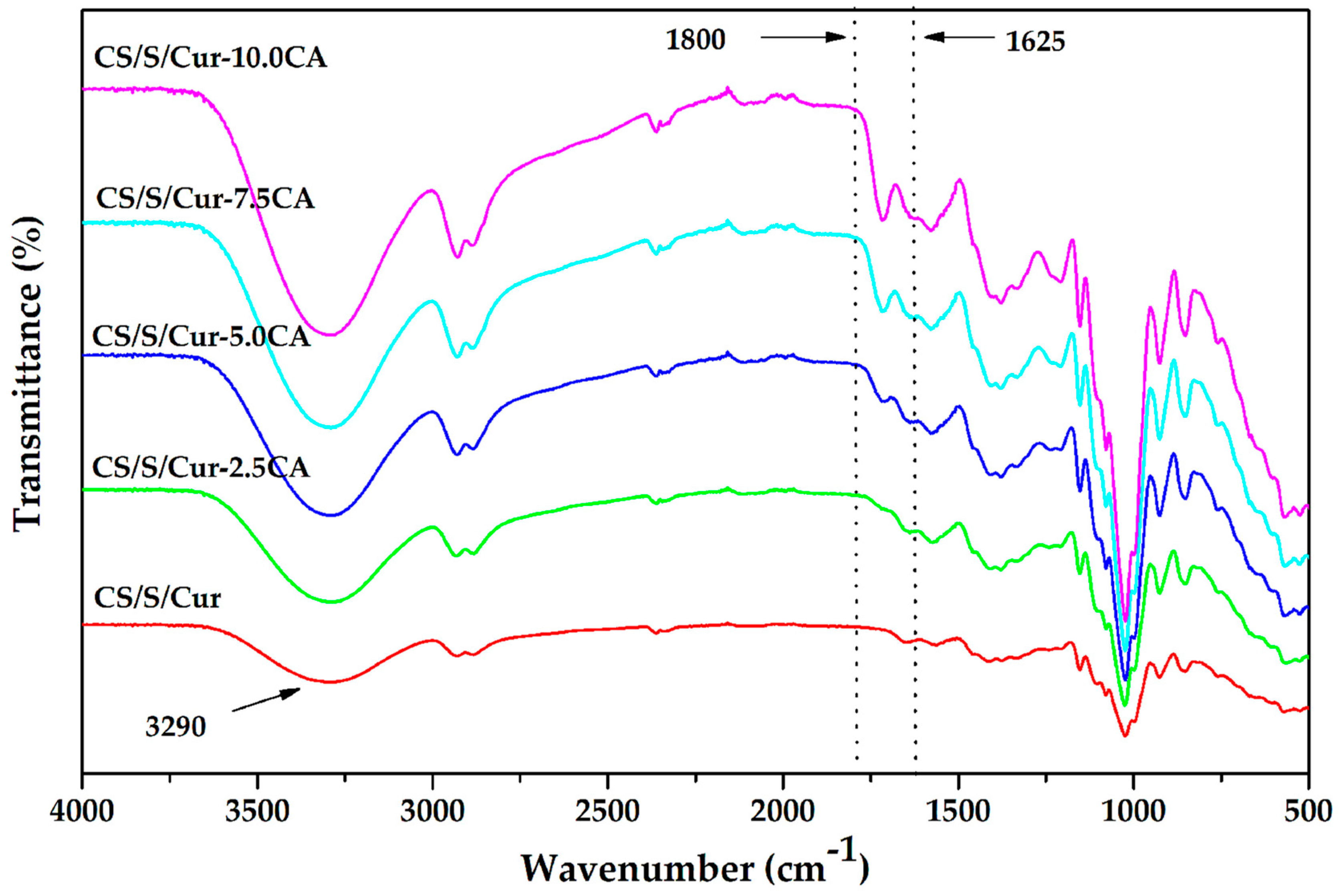
3.1.1. FTIR Spectra
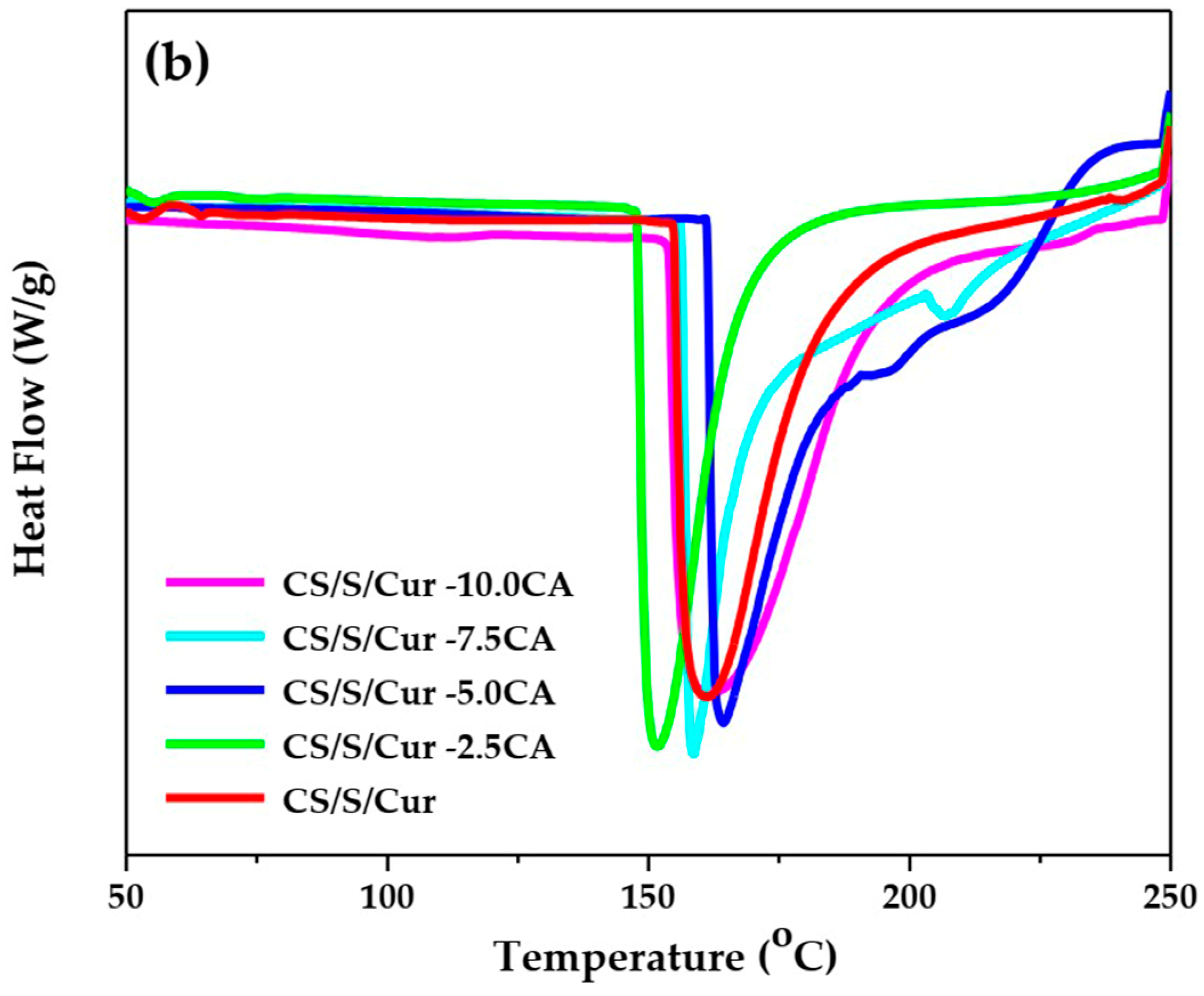
3.1.2. Thermal Analysis
3.2. Water Resistance Properties
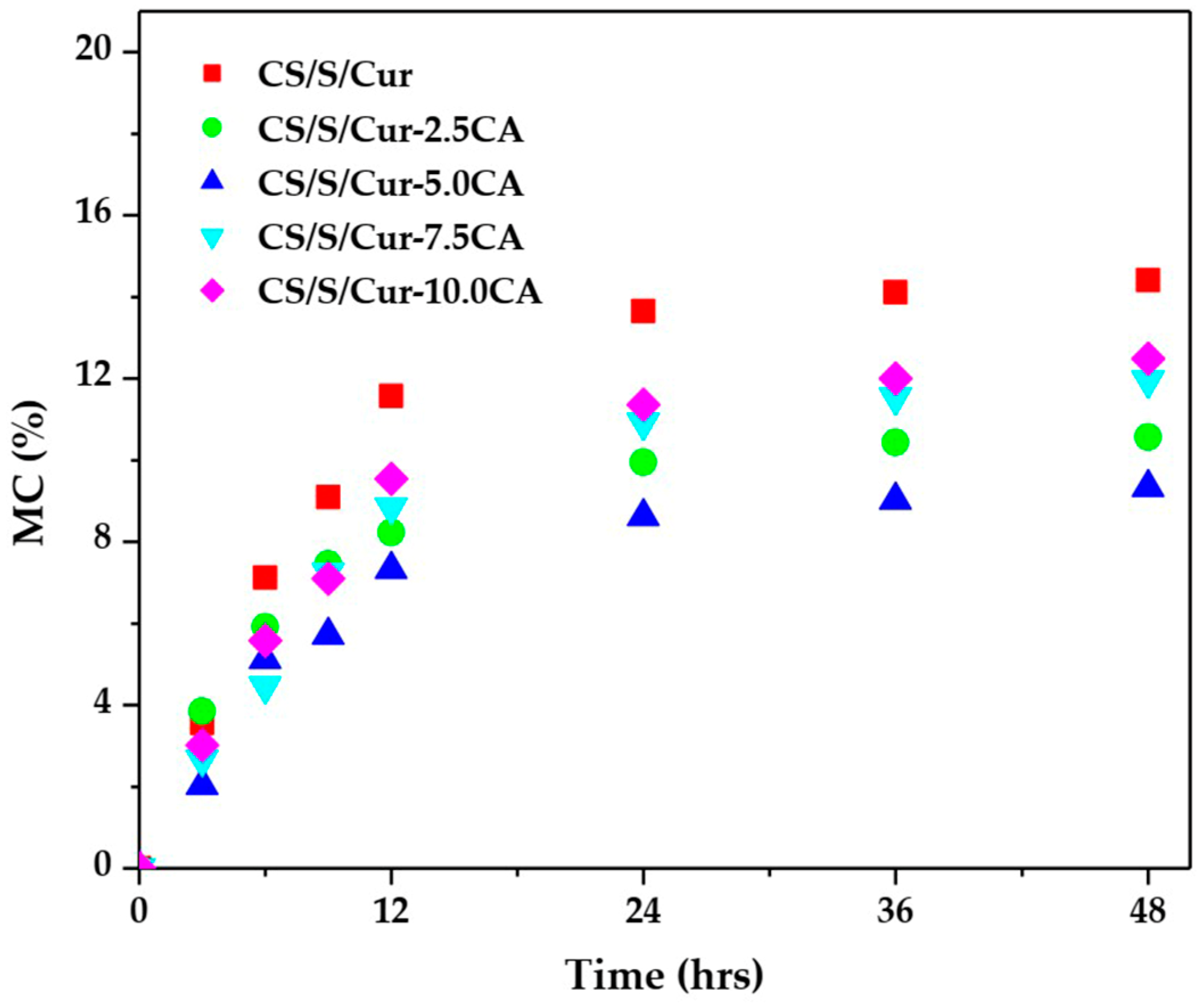
3.2.1. Moisture Content (MC), Swelling Degree (SD), and Water Solubility (WS)
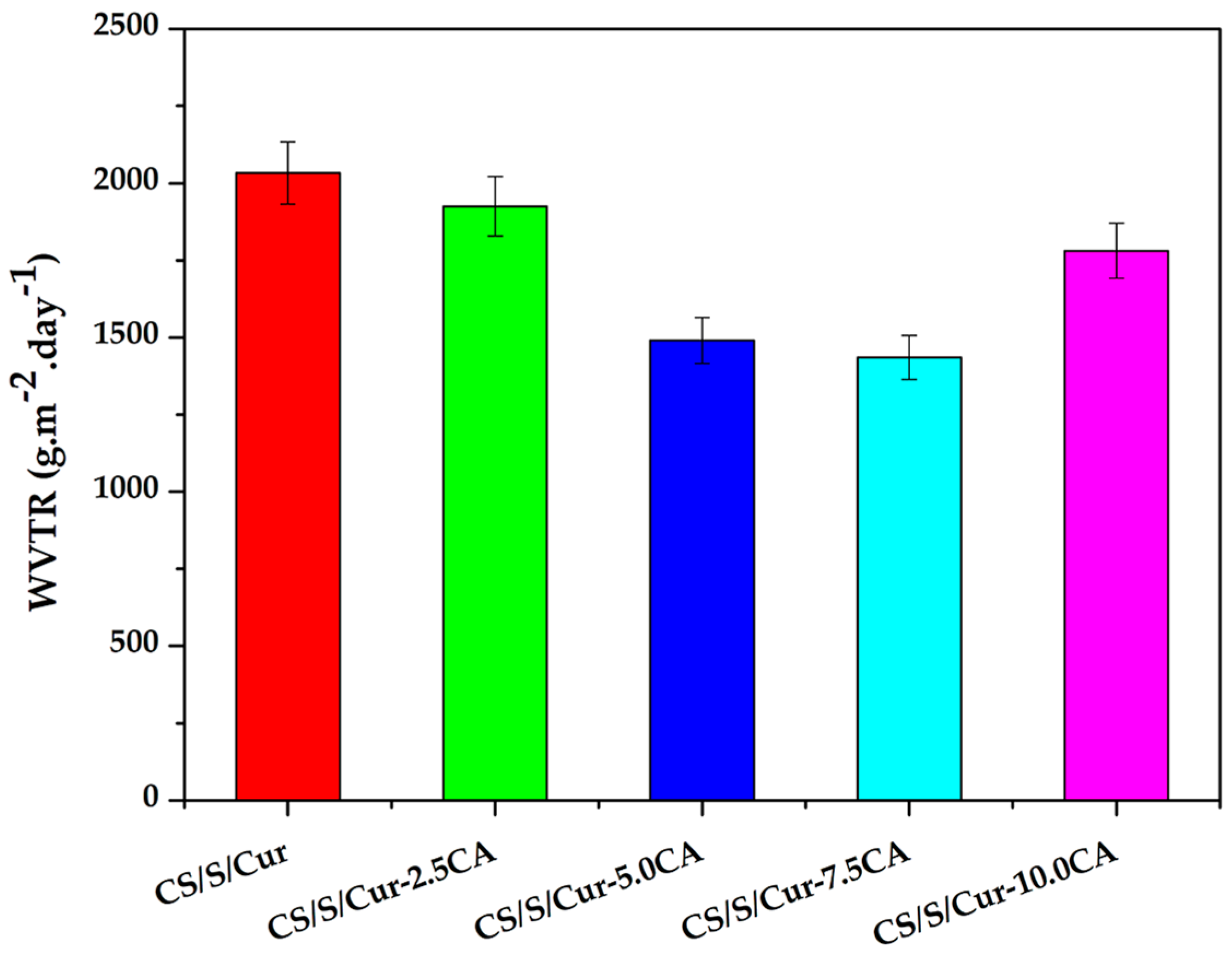
3.2.2. Water Vapor Permeability
3.3. Physical and Mechanical Properties
3.3.1. Thickness
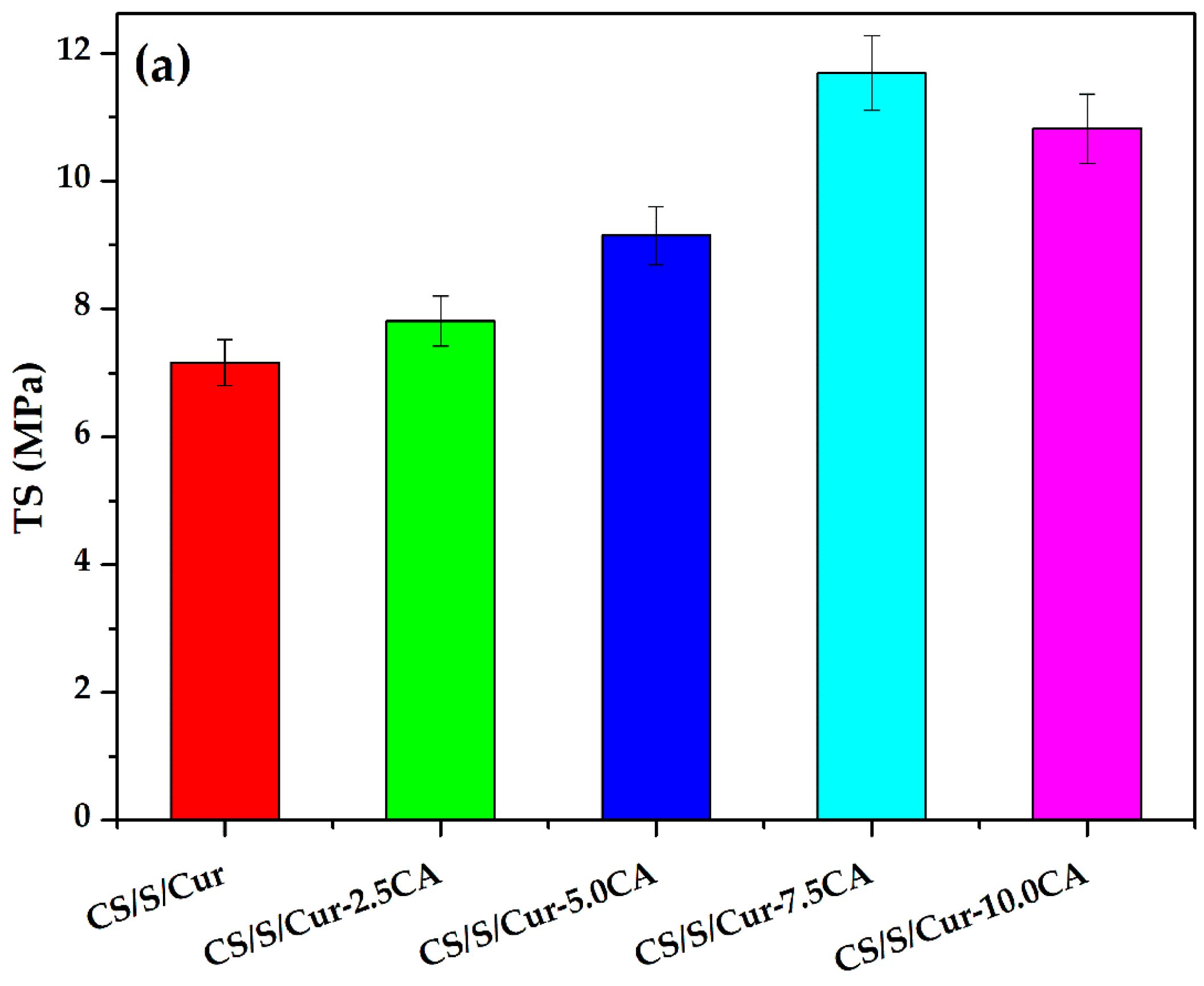
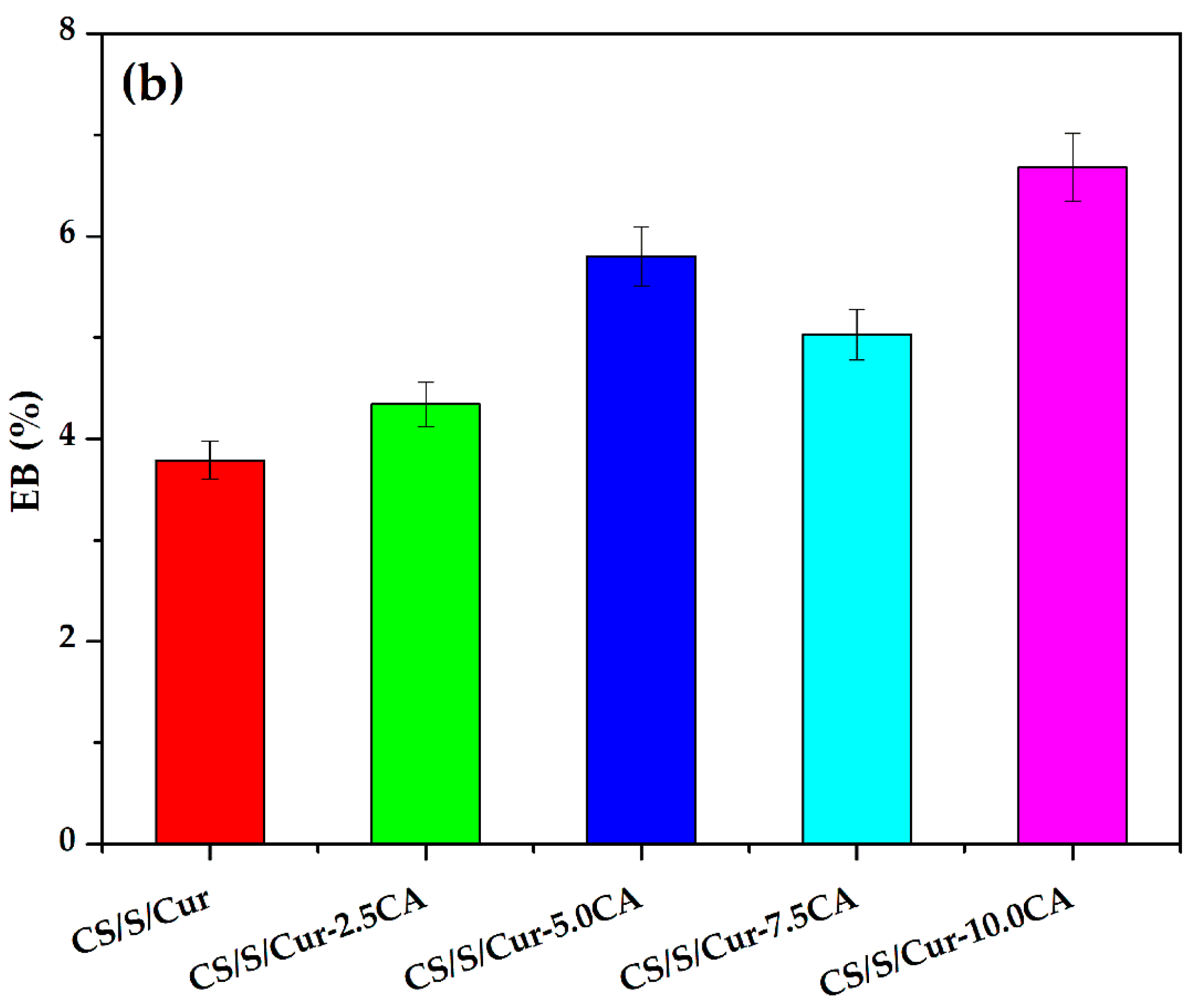
3.3.2. Mechanical Properties
3.4. Antioxidant Activity
DPPH Radical Scavenging Activity

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation; Springer: Boston, MA, USA, 1994; pp. 159–181. [Google Scholar]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Guha, P. Modelling the effect of guar gum on physical, optical, barrier and mechanical properties of potato starch based composite film. Carbohydr. Polym. 2018, 200, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Herniou-Julien, C.; Mendieta, J.R.; Gutiérrez, T.J. Characterization of biodegradable/non-compostable films made from cellulose acetate/corn starch blends processed under reactive extrusion conditions. Food Hydrocoll. 2019, 89, 67–79. [Google Scholar] [CrossRef]
- Noirod, P.; Lamangthong, J.; Ninjiaranai, P. Application of chitosan film for the removal of triclosan from aqueous solutions by adsorption. Key Eng. Mater. 2016, 675, 455–458. [Google Scholar]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of Curcumin Encapsulated Chitosan-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. J. Biomater. Nanobiotechnol. 2011, 2, 55–64. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food. Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Aljawish, A.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J.; Desobry, S. Characterization of films based on enzymatically modified chitosan derivatives with phenol compounds. Food Hydrocoll. 2016, 60, 551–558. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Khan, B.; Khan Niazi, M.B.; Jahan, Z.; Farooq, W.; Naqvi, S.R.; Ali, M.; Ahmed, I.; Hussain, A. Effect of ultra-violet cross-linking on the properties of boric acid and glycerol co-plasticized thermoplastic starch films. Food Packag. Shelf Life 2019, 19, 184–192. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch–chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Fan, H.Y.; Duquette, D.; Dumont, M.J.; Simpson, B.K. Salmon skin gelatin-corn zein composite films produced via crosslinking with glutaraldehyde: Optimization using response surface methodology and characterization. Int. J. Biol. Macromol. 2018, 120, 263–273. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Ammon1’, H.P.T.; Wahi’, M.A. Review Pharmacology of Curcuma longa. Planta Med. 1991, 157, 1–7. [Google Scholar] [CrossRef]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Van Vu, L.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. Springerplus 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Ninjiaranai, P. Biopolymer films based on chitosan and polyethylene glycol with pineapple leaf fiber for food packaging applications. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2015; pp. 294–298. [Google Scholar]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Monitoring freshness of chicken breast by using natural halochromic curcumin loaded chitosan/PEO nanofibers as an intelligent package. Int. J. Biol. Macromol. 2021, 170, 437–446. [Google Scholar] [CrossRef]
- Yildiz, E.; Ilhan, E.; Kahyaoglu, L.N.; Sumnu, G.; Oztop, M.H. The effects of crosslinking agents on faba bean flour–chitosan-curcumin films and their characterization. Legume Sci. 2022, 4, e121. [Google Scholar] [CrossRef]
- Likittrakulwong, W.; Chanburee, S.; Kitpot, T.; Ninjiaranai, P.; Pongpamorn, P. Phytochemical Properties, In Vitro Antimicrobial, and Bioactive Compounds of Banana Peel Extractions Using GC-MS. Nat. Life Sci. Commun. 2023, 22, e2023021. [Google Scholar] [CrossRef]
- Pavlovic, S.; Brandao, P.R.G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Miner. Eng. 2003, 16, 1117–1122. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Chen, M.; Ge, Y.; Ma, J.; Hu, Y.; Pang, J.; Yan, Z. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring. Food Hydrocoll. 2019, 95, 308–317. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch–chitosan blend films. Biopolym. Orig. Res. Biomol. 2006, 82, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Istiqomah, A.; Utami, M.R.; Firdaus, M.; Suryanti, V.; Kusumaningsih, T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022, 45, 101603. [Google Scholar] [CrossRef]
- Sanchez-Safont, E.L.; Aldureid, A.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Effect of the purification treatment on the valorization of natural cellulosic residues as fillers in PHB-based composites for short shelf-life applications. Waste Biomass Valori. 2021, 12, 2541–2556. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Sadik, T.; Pillon, C.; Carrot, C.; Reglero Ruiz, J.A. DSC studies on the decomposition of chemical blowing agents based on citric acid and sodium bicarbonate. Thermochim. Acta 2018, 659, 74–81. [Google Scholar] [CrossRef]
- Tavares, L.; Esparza Flores, E.E.; Rodrigues, R.C.; Hertz, P.F.; Norena, C.P.Z. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, A. Characterization of chickpea (Cicer arietinum L.) flour films: Effects of pH and plasticizer concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, B.J.; Weng, Y.M. Preparation and characterization of mung bean starch edible films using citric acid as cross-linking agent. Food Packag. Shelf Life 2022, 32, 100845. [Google Scholar] [CrossRef]
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe Vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Sowmya; Selvakumar, M.; Bhat, D.K. Miscibility studies of chitosan and starch blends in buffer solution. J. Macromol. Sci. Pure Appl. Chem. 2012, 49, 1099–1105. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Wang, Y.; Zhang, X.; Tong, Z. Comparison of chitosan/starch composite film properties before and after cross-linking. Int. J. Biol. Macromol. 2013, 52, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crop. Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.H. Mechanical and thermal characteristics of pea starch films plasticized with monosaccharides and polyols. J. Food Sci. 2006, 71, E109–E118. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Guerrero, P.; Muxika, A.; Zarandona, I.; de la Caba, K. Crosslinking of chitosan films processed by compression molding, Carbohydrate Polymers. Carbohydr. Polym. 2019, 206, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Emir, A.; Sumnu, G.; Kahyaoglu, L.N. Citric acid cross-linked curcumin/chitosan/chickpea flour film: An active packaging for chicken breast storage. Food Biosci. 2022, 50, 102121. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Kim, H.C.; Pham, D.H.; Alrobei, H.; Kim, J. Tannic-acid-cross-linked and TiO2-nanoparticle-reinforced chitosan-based nanocomposite film. Polymers 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]


| Film Types | Compositions | ||||
|---|---|---|---|---|---|
| Chitosan (CS) (g) | Modified Cassava Starch (S) (g) | Glycerol (%w/v) | Citric Acid (%w/w) * | Curcumin (mg/mL) | |
| CS/S/Cur | 2 | 2 | 20 | - | 50 |
| CS/S/Cur-2.5CA | 2 | 2 | 20 | 2.5 | 50 |
| CS/S/Cur-5.0CA | 2 | 2 | 20 | 5.0 | 50 |
| CS/S/Cur-7.5CA | 2 | 2 | 20 | 7.5 | 50 |
| CS/S/Cur-10.0CA | 2 | 2 | 20 | 10.0 | 50 |
| Film Types | Physicochemical Properties | ||
|---|---|---|---|
| Thickness (mm) | Swelling Degree (%) | Water Solubility (%) | |
| CS/S/Cur | 0.12 | 148 ± 6 | 22 ± 1 |
| CS/S/Cur-2.5CA | 0.13 | 76 ± 12 | 21 ± 1 |
| CS/S/Cur-5.0CA | 0.14 | 50 ± 7 | 19 ± 2 |
| CS/S/Cur-7.5CA | 0.14 | 40 ± 5 | 17 ± 2 |
| CS/S/Cur-10.0CA | 0.14 | 32 ± 1 | 21 ± 2 |
| Film Types | DPPH (% Scavenging Activity) |
|---|---|
| CS/S/Cur | 31 ± 0 |
| CS/S/Cur-2.5CA | 49 ± 1 |
| CS/S/Cur-5.0CA | 27 ± 1 |
| CS/S/Cur-7.5CA | 21 ± 1 |
| CS/S/Cur-10.0CA | 17 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadsai, S.; Janmanee, R.; Sam-Ang, P.; Nuanchawee, Y.; Rakitikul, W.; Mankhong, W.; Likittrakulwong, W.; Ninjiaranai, P. Influence of Crosslinking Concentration on the Properties of Biodegradable Modified Cassava Starch-Based Films for Packaging Applications. Polymers 2024, 16, 1647. https://doi.org/10.3390/polym16121647
Khadsai S, Janmanee R, Sam-Ang P, Nuanchawee Y, Rakitikul W, Mankhong W, Likittrakulwong W, Ninjiaranai P. Influence of Crosslinking Concentration on the Properties of Biodegradable Modified Cassava Starch-Based Films for Packaging Applications. Polymers. 2024; 16(12):1647. https://doi.org/10.3390/polym16121647
Chicago/Turabian StyleKhadsai, Sudarat, Rapiphun Janmanee, Pornpat Sam-Ang, Yossawat Nuanchawee, Waleepan Rakitikul, Wilawan Mankhong, Wirot Likittrakulwong, and Padarat Ninjiaranai. 2024. "Influence of Crosslinking Concentration on the Properties of Biodegradable Modified Cassava Starch-Based Films for Packaging Applications" Polymers 16, no. 12: 1647. https://doi.org/10.3390/polym16121647
APA StyleKhadsai, S., Janmanee, R., Sam-Ang, P., Nuanchawee, Y., Rakitikul, W., Mankhong, W., Likittrakulwong, W., & Ninjiaranai, P. (2024). Influence of Crosslinking Concentration on the Properties of Biodegradable Modified Cassava Starch-Based Films for Packaging Applications. Polymers, 16(12), 1647. https://doi.org/10.3390/polym16121647







