Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange
Abstract
:1. Introduction
2. Experimental Section
2.1. The Preparation of Activated Carbon
2.2. Characterization
3. Result and Discussion
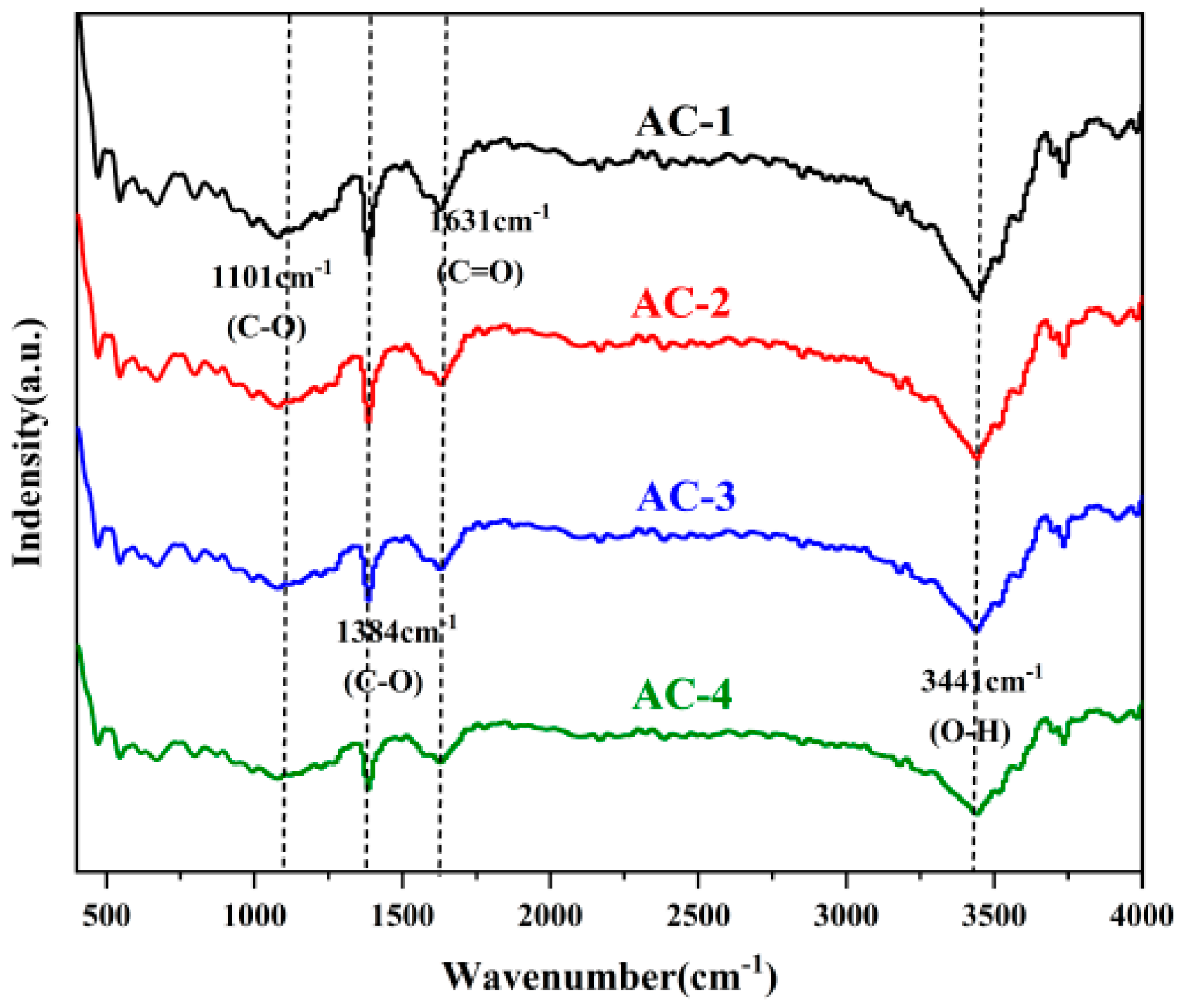
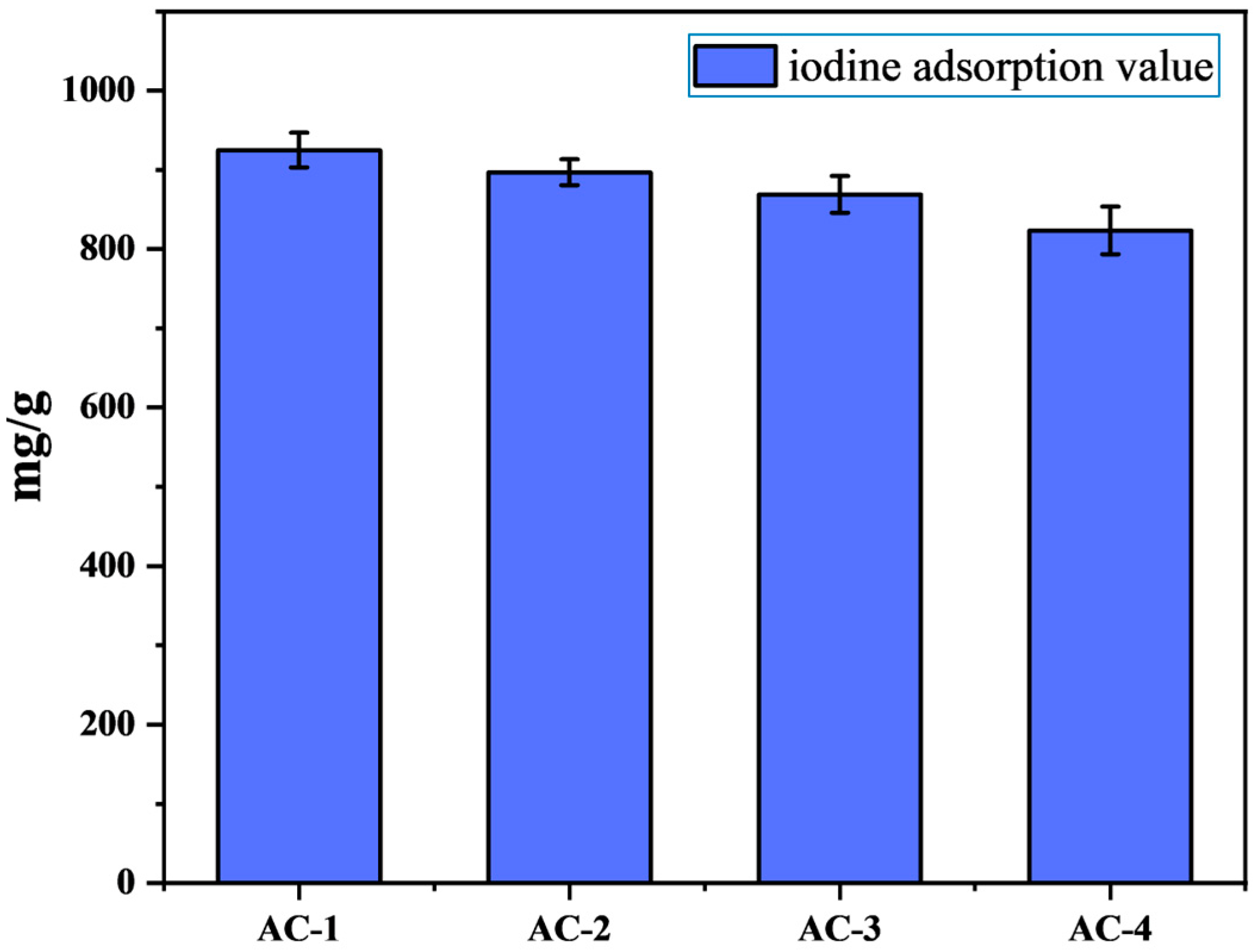
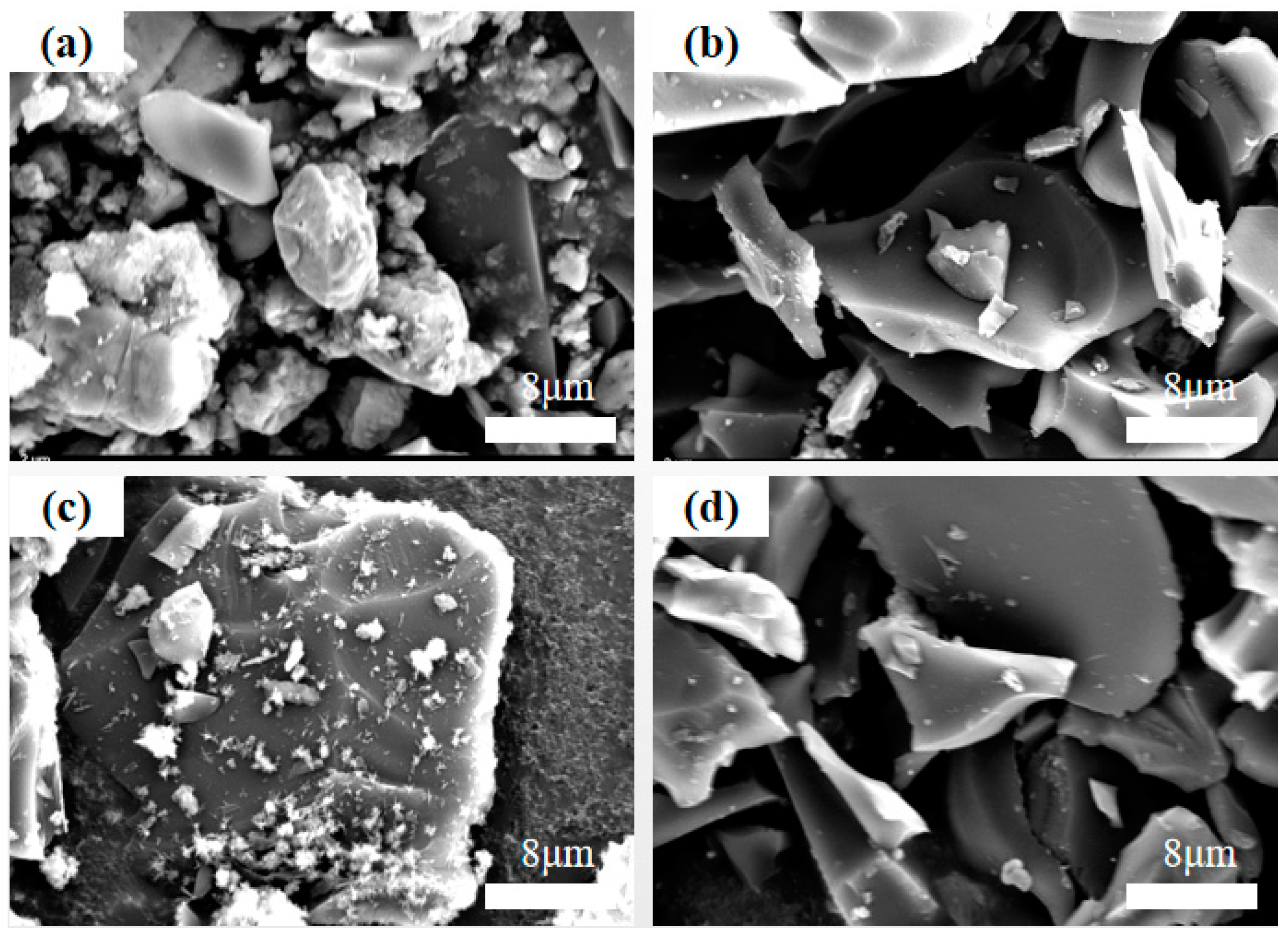
3.1. Structural Characterization of Activated Carbon
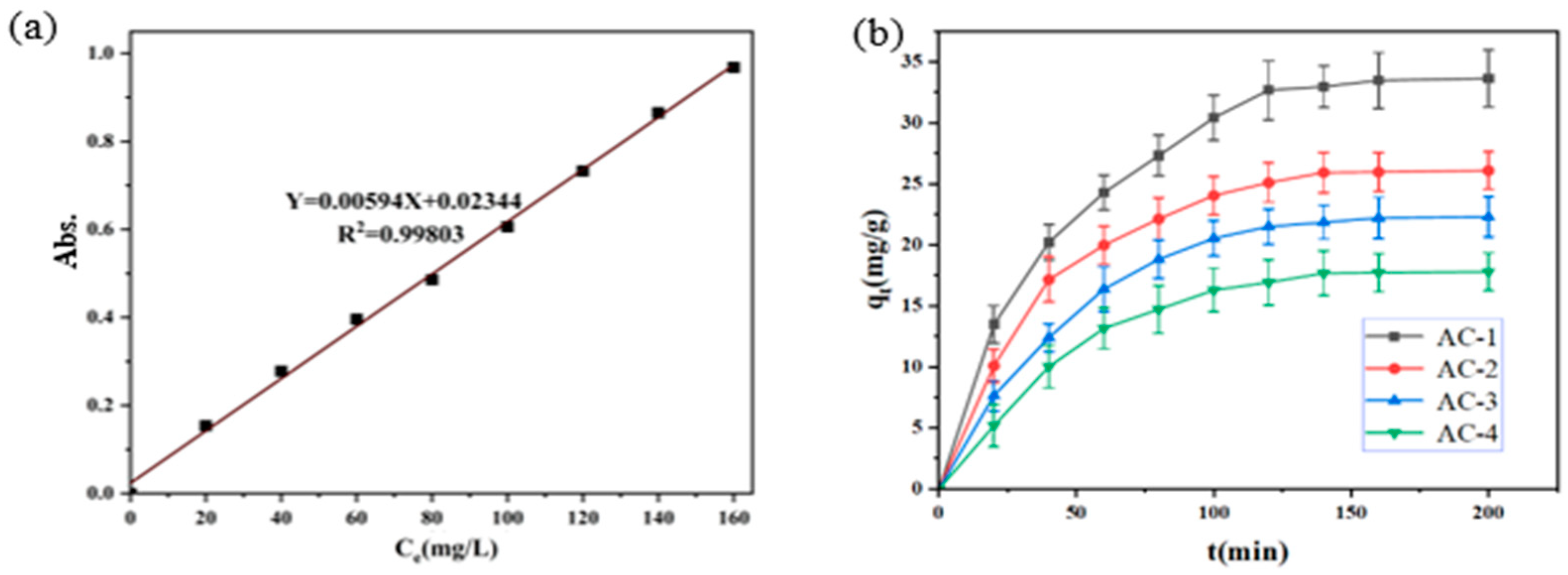
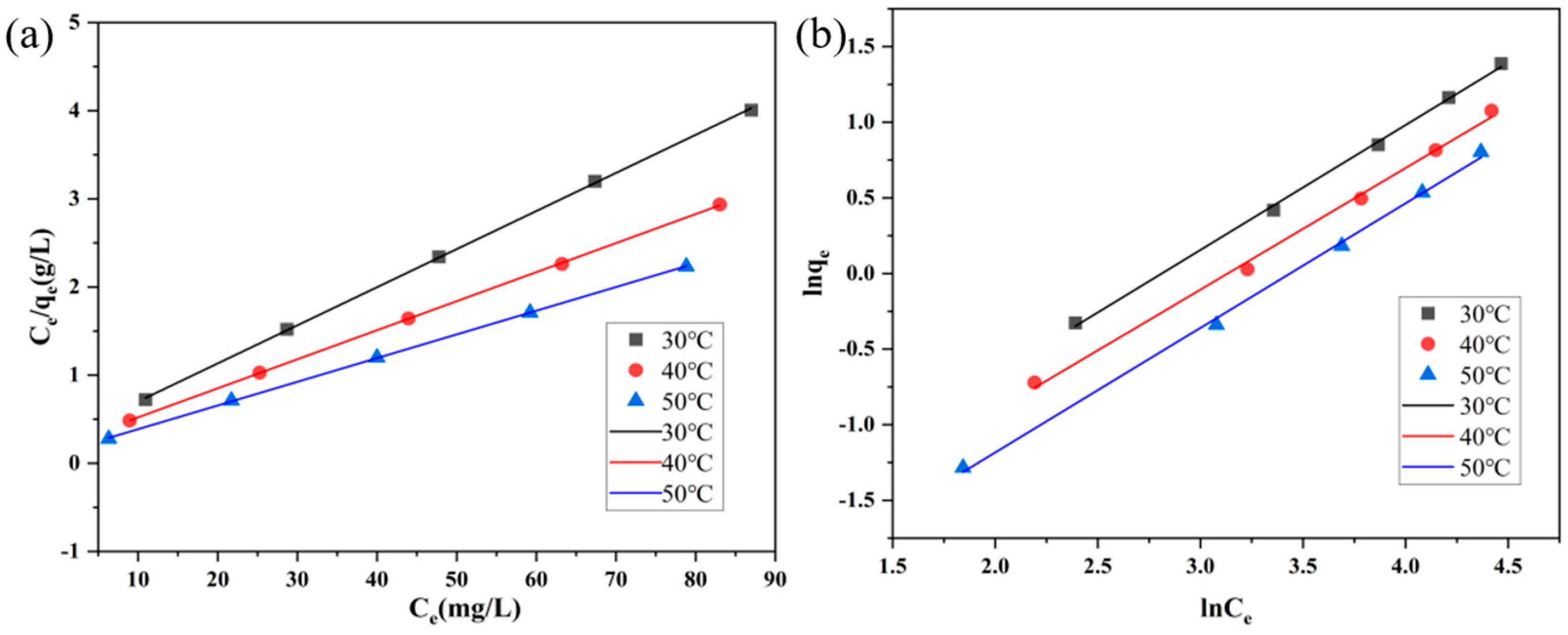
3.2. Adsorption Behavior of Methyl Orange on Activated Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Li, Q.; Qiu, J. Hydrothermal modification and recycling of nonmetallic particles from waste print circuit boards. Waste Manag. 2018, 74, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Ghosh, M.; Parhi, P.; Mukherjee, P.; Mishra, B. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Krzywinski, K.; Sadowski, L.; Piechówka-Mielnik, M. Engineering of composite materials made of epoxy resins modified with recycled fine aggregate. Sci. Eng. Compos. Mater. 2021, 28, 276–284. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Liang, Y.-X.; Wang, X.; Zhou, H.-J.; Li, S.-Y.; Zhang, J.; Feng, Y.; Lu, N.; Wang, Q.; Guo, Z. Strengthened epoxy resin with hyperbranched polyamine-ester anchored graphene oxide via novel phase transfer approach. Adv. Compos. Hybrid Mater. 2018, 1, 300–309. [Google Scholar] [CrossRef]
- Moe, A.K.; Chungprempree, J.; Preechawong, J.; Sapsrithong, P.; Nithitanakul, M. Recycling Waste Nonmetallic Printed Circuit Boards for Polyvinyl Chloride Composites. Polymers 2022, 14, 3531. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wei, L.; Pu, L.; Liu, J.; Liu, H.; Li, Y.; Fan, J.; Ding, T.; Guo, Z. Alternating Multilayer Structural Epoxy Composite Coating for Corrosion Protection of Steel. Macromol. Mater. Eng. 2019, 304, 1900374. [Google Scholar] [CrossRef]
- Hirayama, D.; Saron, C. Morphologic and mechanical properties of blends from recycled acrylonitrile-butadiene-styrene and high-impact polystyrene. Polymer 2018, 135, 271–278. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Cai, D.; Li, Y.; Wang, W.; Ma, Y.; Cao, N.; Zhang, J.; Pan, D.; Naik, N.; Wei, S.; Huang, M.; et al. Reinforcing and toughening blends of recycled acrylonitrile-butadiene-styrene/recycled high-impact polystyrene through ionic crosslinking. Surf. Interfaces 2022, 28, 101607. [Google Scholar] [CrossRef]
- Niu, B.; Shanshan, E.; Xu, Z.; Guo, J. How to efficient and high-value recycling of electronic components mounted on waste printed circuit boards: Recent progress, challenge, and future perspectives. J. Clean. Prod. 2023, 415, 137815. [Google Scholar] [CrossRef]
- Ogi, K.; Nishikawa, T.; Okano, Y.; Taketa, I. Mechanical properties of ABS resin reinforced with recycled CFRP. Adv. Compos. Mater. 2007, 16, 181–194. [Google Scholar] [CrossRef]
- Lester, E. Microwave heating as a means for carbon fibre recovery from polymer composites: A technical feasibility study. Mater. Res. Bull. 2004, 39, 1549–1556. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Korotta-Gamage, S.M.; Sathasivan, A. A review: Potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process. Chemosphere 2017, 167, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, Z.; Ma, C. Development of Resin Based Spherical Activated Carbon with High Performance Derived from Waste Ion-Exchange Resin and Its High Effective Removal of Tetracycline. Chem. Sel. 2023, 8, 2–8. [Google Scholar] [CrossRef]
- Marandi, A.; Kolvari, E.; Gilandoust, M.; Zolfigol, M.A. Immobilization of –OSO3H on activated carbon powder and its use as a heterogeneous catalyst in the synthesis of phthalazine and quinoline derivatives. Diam. Relat. Mater. 2022, 124, 108908. [Google Scholar] [CrossRef]
- Lidman Olsson, E.O.; Purnomo, V.; Glarborg, P.; Leion, H.; Dam-Johansen, K.; Wu, H. Thermal Conversion of Sodium Phytate Using the Oxygen Carrier llmenite Interaction with Na-Phosphate and Its Effect on Reactivity. Energy Fuels 2022, 36, 9423–9436. [Google Scholar] [CrossRef]
- Ning, P.; Wang, X.; Bart, H.-J.; Tian, S.; Zhang, Y.; Wang, X.-Q. Removal of phosphorus and sulfur from yellow phosphorus off-gas by metal-modified activated carbon. J. Clean. Prod. 2011, 19, 1547–1552. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Jiao, Y.; Yang, H.; Li, Y.; Zhang, J.; Gao, P. The graphene/lanthanum oxide nanocomposites as electrode materials of supercapacitors. J. Power Sources 2019, 419, 99–105. [Google Scholar] [CrossRef]
- Lin, C.; Fan, B.; Zhang, J.X.; Yang, X.; Zhang, H. Study on lead ion wastewater treatment of self-assembled film. Desalination Water Treat. 2016, 57, 21627–21633. [Google Scholar] [CrossRef]
- Tan, L.; Wang, J.; Cai, B.; Wang, C.; Ao, Z.; Wang, S. Nitrogen-rich layered carbon for adsorption of typical volatile organic compounds and low-temperature thermal regeneration. J. Hazard. Mater. 2022, 424, 127348. [Google Scholar] [CrossRef]
- Ye, M.; Tao, N.; Zhou, X.; Wang, X.; Jin, W.; Zhang, T.; Liu, X. A super-hydrophilic honeycomb activated carbon evaporator for simultaneous salt rejection and VOCs removal during solar-driven seawater desalination. Sep. Purif. Technol. 2023, 311, 123201. [Google Scholar] [CrossRef]
- Lin, C.; Liu, B.; Pu, L.; Sun, Y.; Xue, Y.; Chang, M.; Li, X.; Lu, X.; Chen, R.; Zhang, J. Photocatalytic oxidation removal of fluoride ion in wastewater by g-C3N4/TiO2 under simulated visible light. Adv. Compos. Hybrid Mater. 2021, 4, 339–349. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, S.; Liu, J.; Zhang, Y.; Wang, Y.; Yu, J.; Yuan, M.; Zhang, P.; Liu, W.; Zhang, J. C, F co-doping Ag/TiO2 with visible light photocatalytic performance toward degrading Rhodamine B. Environ. Res. 2023, 232, 116311. [Google Scholar] [CrossRef] [PubMed]
- Vedernikov, A.; Tucci, F.; Carlone, P.; Gusev, S.; Konev, S.; Firsov, D.; Akhatov, I.; Safonov, A. Effects of pulling speed on structural performance of L-shaped pultruded profiles. Compos. Struct. 2021, 255, 112967. [Google Scholar] [CrossRef]
- Serban, G.V.; Iancu, V.I.; Dinu, C.; Tenea, A.; Vasilache, N.; Cristea, I.; Niculescu, M.; Ionescu, I.; Chiriac, F.L. Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon. Sustainability 2023, 15, 12939. [Google Scholar] [CrossRef]
- Khattabi EH, E.; Rachdi, Y.; Bassam, R.; Mourid, E.H.; Naimi, Y.; Alouani, M.E.; Belaaouad, S. Enhanced Elimination of Methyl Orange and Recycling of an Eco-Friendly Adsorbent Activated Carbon from Aqueous Solution. Russ. J. Phys. Chem. B 2022, 15, S149–S159. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Wang, D.; Li, P. Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci. Technol. 2018, 77, 1303–1312. [Google Scholar] [CrossRef]
- Sayed, N.S.; Ahmed, A.S.; Abdallah, M.H.; Gouda, G.A. ZnO@ activated carbon derived from wood sawdust as adsorbent for removal of methyl red and methyl orange from aqueous solutions. Sci. Rep. 2024, 14, 5384. [Google Scholar] [CrossRef]
- Tang, J.; Gao, G.; Luo, W.; Dai, Q.; Wang, Y.; Elzilal, H.A.; Abo-Dief, H.M.; Algadi, H.; Zhang, J. Z-scheme metal organic framework@graphene oxide composite photocatalysts with enhanced photocatalytic degradation of tetracycline. Adv. Compos. Hybrid Mater. 2023, 6, 190. [Google Scholar] [CrossRef]
- Zheng, X.; Ni, C.; Xiao, W.; Liang, Y.; Li, Y. Ionic liquid grafted polyethersulfone nanofibrous membrane as recyclable adsorbent with simultaneous dye, heavy metal removal and antibacterial property. Chem. Eng. J. 2022, 428, 132111. [Google Scholar] [CrossRef]
- Alkan, M.; Doğan, M. Adsorption of Copper(II) onto Perlite. J. Colloid Interface Sci. 2001, 243, 280–291. [Google Scholar] [CrossRef]
- Akhatova, G.I.; Gus’kov, V.Y. Adsorption Isotherms of Enantiomer on Hippuric Acid Crystals Obtained under Viedma Ripening Conditions Using a Temperature Gradient. Prot. Met. Phys. Chem. Surf. 2024, 59, 1132–1138. [Google Scholar] [CrossRef]




| Samples | Quasi-Primary Kinetic Model | Quasi-Secondary Kinetic Model | ||||
|---|---|---|---|---|---|---|
| R2 | qe mg·g−1 | k1 | R2 | qe mg·g−1 | k2 × 10−3 | |
| AC-1 | 0.906 | 54.250 | 0.03381 | 0.993 | 41.051 | 0.63 |
| AC-2 | 0.918 | 44.646 | 0.03825 | 0.993 | 31.556 | 0.92 |
| AC-3 | 0.932 | 40.509 | 0.03882 | 0.984 | 28.121 | 0.81 |
| AC-4 | 0.915 | 34.531 | 0.03855 | 0.978 | 23.607 | 0.85 |
| Samples | Quasi-Primary Kinetic Model | Quasi-Secondary Kinetic Model | |||||
|---|---|---|---|---|---|---|---|
| R2 | qe mg·g−1 | k1 | R2 | qe mg·g−1 | k2 × 10−3 | ||
| AC-1 | 0.906 | 54.250 | 0.03381 | 0.993 | 41.051 | 0.63 | |
| AC | 0.9564 | 44.950 | −0.006 | 0.9992 | 100 | 46 | [26] |
| C-CA | 0.904 | 11.004 | 0.0491 | 0.97 | 96.15 | 0.0006 | [27] |
| CMC | 0.392 | 43.85 | 0.00084 | 0.999 | 38.61 | 0.0093 | [28] |
| ZnO@AC | 0.789 | 49.329 | 0.341 | 0.905 | 50.853 | 0.018 | [29] |
| T/K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
mg·g−1 | ||||||
| 303K | 23.137 | 0.1608 | 0.9997 | 1.2109 | 0.0981 | 0.9887 |
| 313K | 30.358 | 0.1724 | 0.9999 | 1.2137 | 0.0806 | 0.9859 |
| 333K | 37.202 | 0.2269 | 0.9998 | 1.2439 | 0.05893 | 0.9877 |
| (KJ·mol−1) | (J·K−1·mol−1) | |||
|---|---|---|---|---|
| 303 K | 313 K | 333 K | ||
| 18.09 | 43.54 | 4.85 | 4.57 | 3.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; He, J.; Wang, Q.; Zhang, D.; Qi, G.; Cai, X.; Li, P.; Zhang, J. Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange. Polymers 2024, 16, 1648. https://doi.org/10.3390/polym16121648
Zhu W, He J, Wang Q, Zhang D, Qi G, Cai X, Li P, Zhang J. Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange. Polymers. 2024; 16(12):1648. https://doi.org/10.3390/polym16121648
Chicago/Turabian StyleZhu, Wenfeng, Jiacheng He, Qianxi Wang, Dongna Zhang, Guoquan Qi, Xuehua Cai, Peipei Li, and Jiaoxia Zhang. 2024. "Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange" Polymers 16, no. 12: 1648. https://doi.org/10.3390/polym16121648
APA StyleZhu, W., He, J., Wang, Q., Zhang, D., Qi, G., Cai, X., Li, P., & Zhang, J. (2024). Activated Carbon Based on Recycled Epoxy Boards and Their Adsorption toward Methyl Orange. Polymers, 16(12), 1648. https://doi.org/10.3390/polym16121648







