A Comparison of Laboratory and Industrial Processes Reveals the Effect of Dwell Time and UV Pre-Exposure on the Behavior of Two Polymers in a Disintegration Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Differential Scanning Calorimetry (DSC)
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Gel Permeation Chromatography (GPC)
2.5. Tensile Test
2.6. Fiber Preparation
2.7. Quick Weathering
2.8. Laboratory-Scale Disintegration
3. Results
3.1. Mechanical Properties of the Spun Fibers
3.2. Change Due to Degradation
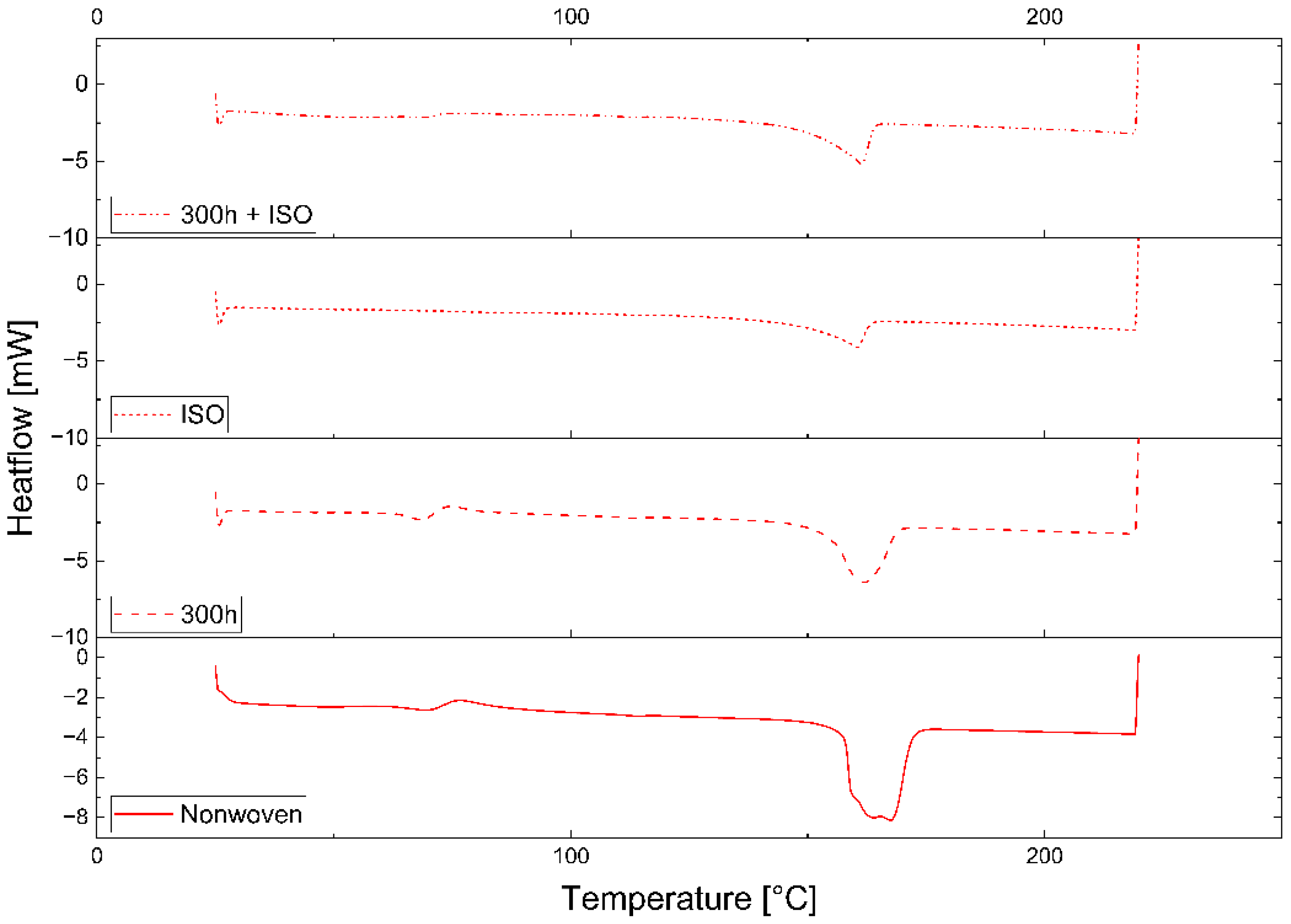
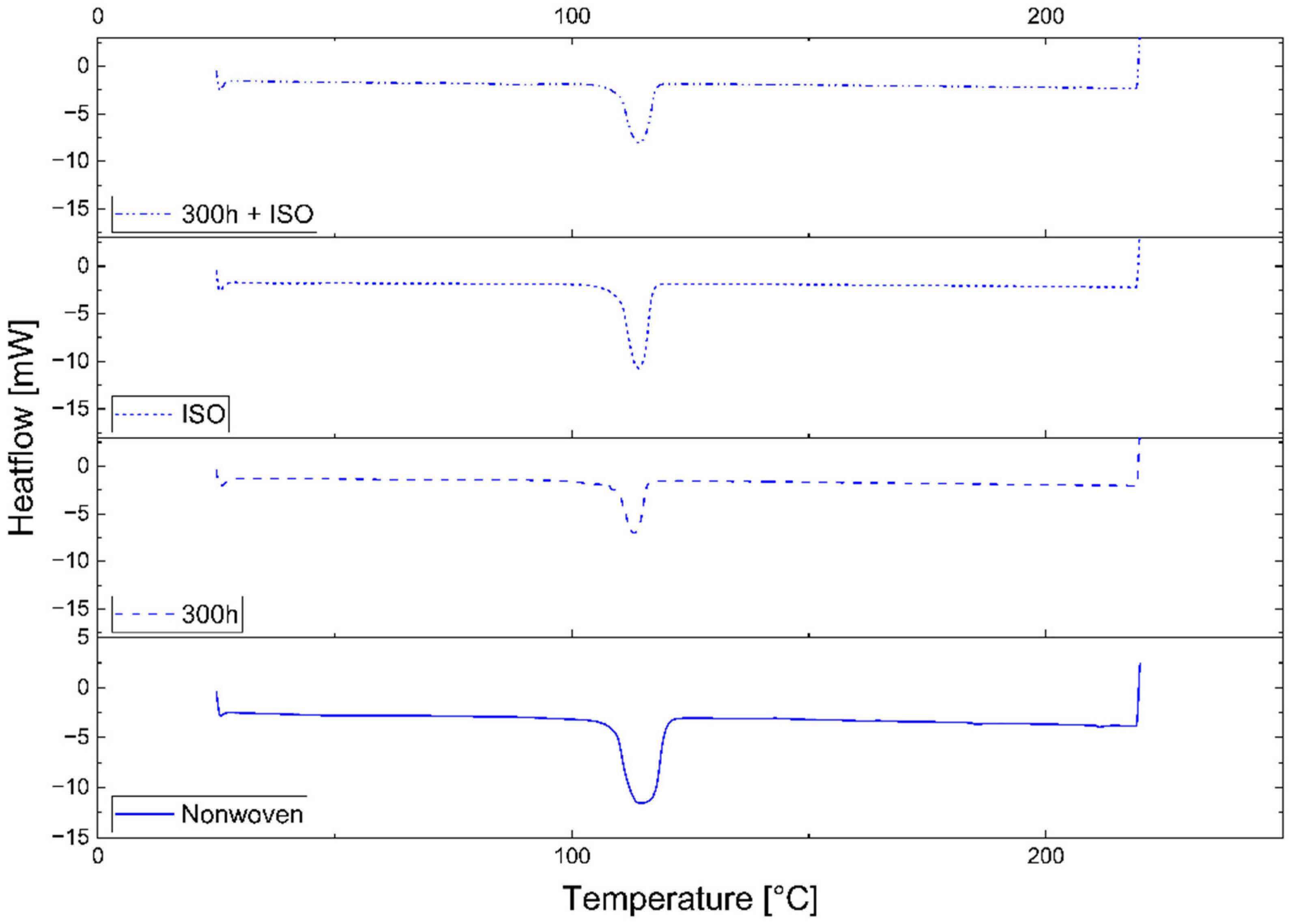
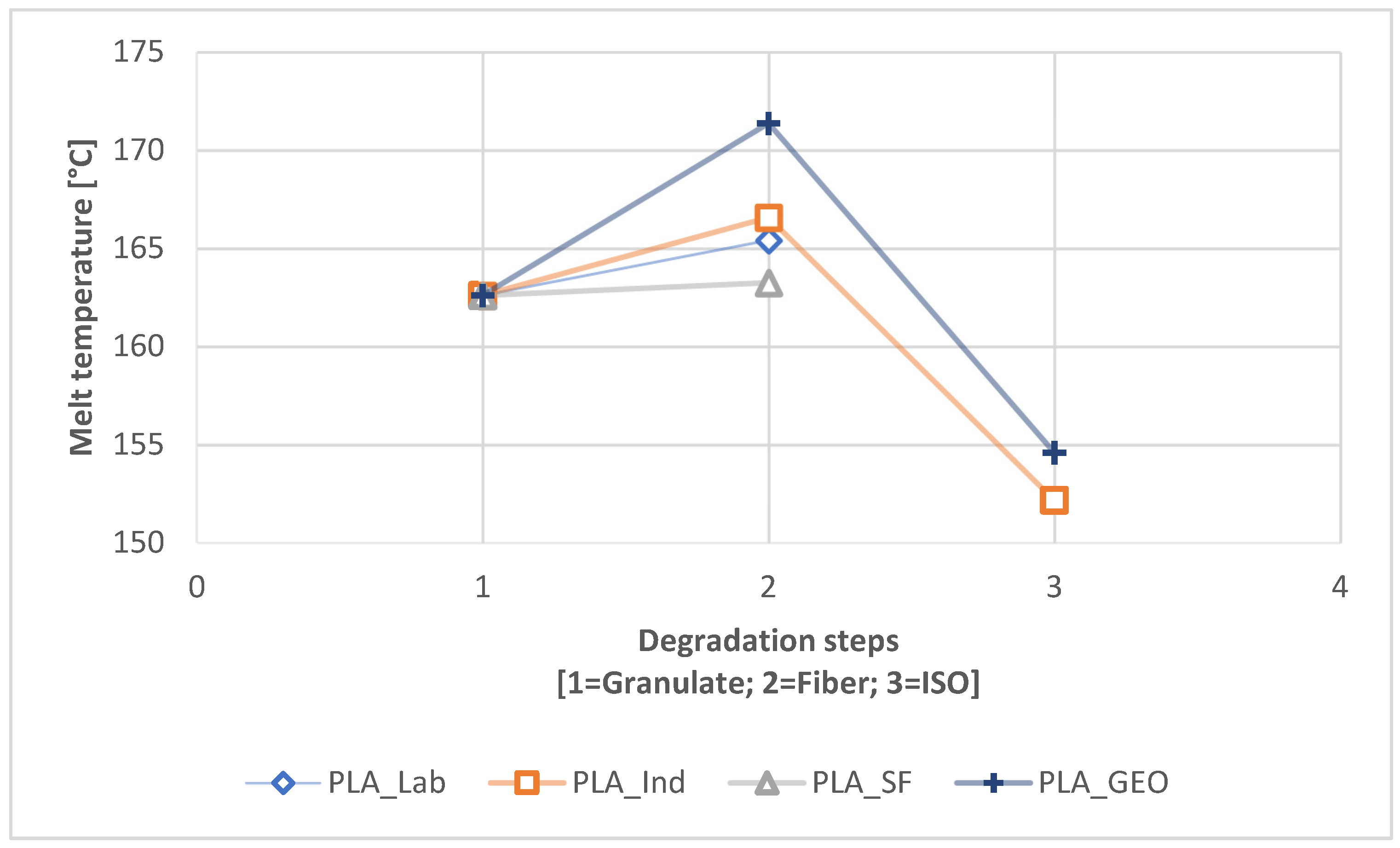
3.2.1. Differential Scanning Calorimetry
3.2.2. FTIR Spectroscopy
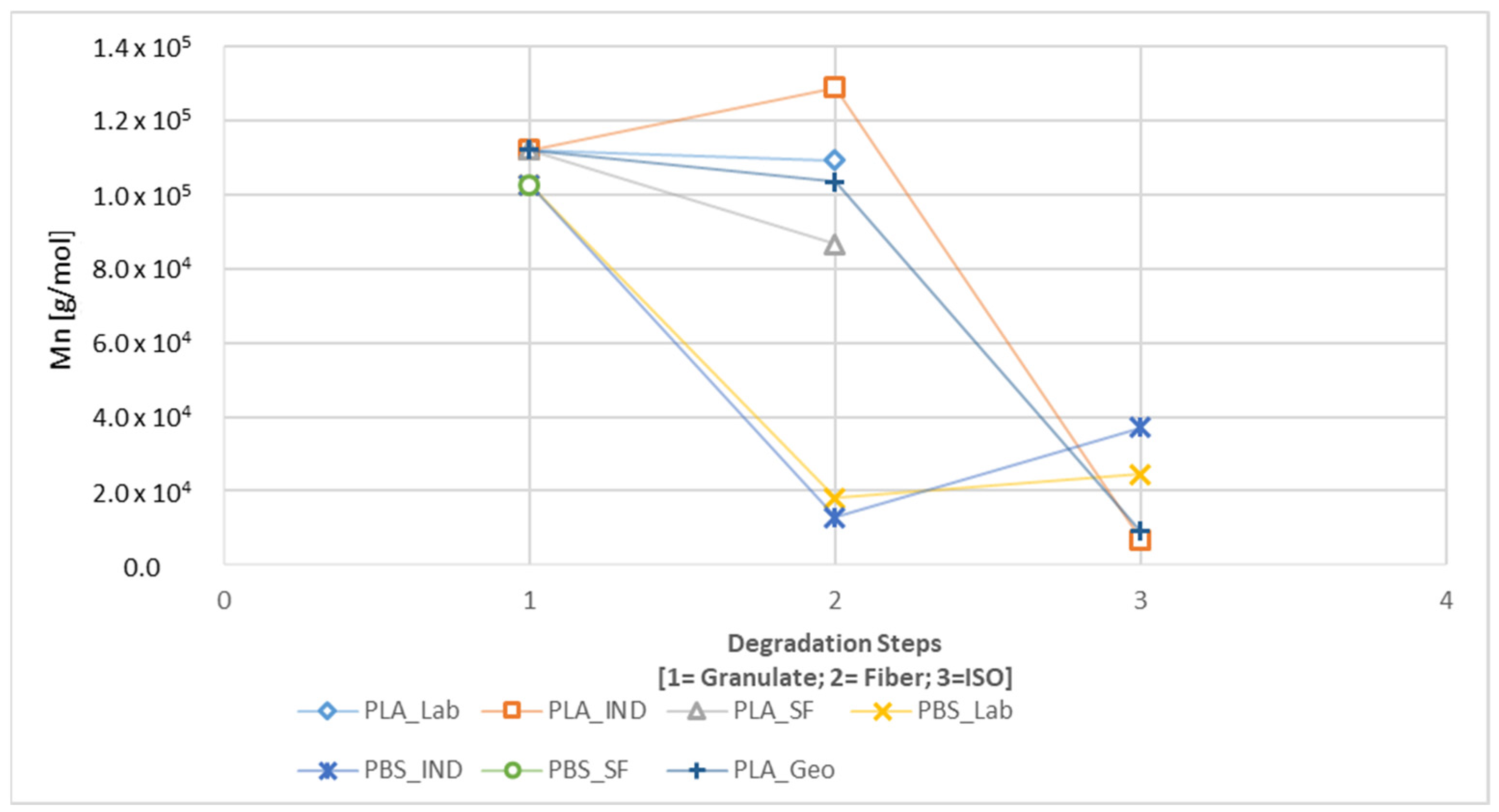
3.2.3. Gel Permeation Chromatography
4. Discussion
4.1. Fiber Properties
4.2. Change Due to Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Aguero, A.; Morcillo, M.D.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Bauchmüller, V.; Carus, M.; Chinthapalli, R.; Dammer, L.; Hark, N.; Partanen, A.; Ruiz, P.; Lajewski, S. BioSinn; Nova-Institut für Politische und Ökologische Innovation GmbH: Hürth, Deutschland, 2021. [Google Scholar]
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Biopolymer production and end of life comparisons using life cycle assessment. Resour. Conserv. Recycl. 2017, 122, 295–306. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Chen, W.Q.; Zhu, B.; Qu, S.; Xu, M. Critical review of global plastics stock and flow data. J. Ind. Ecol. 2021, 25, 1300–1317. [Google Scholar] [CrossRef]
- Brizga, J.; Hubacek, K.; Feng, K. The Unintended Side Effects of Bioplastics: Carbon, Land, and Water Footprints. One Earth 2020, 3, 45–53. [Google Scholar] [CrossRef]
- Market.Us. Biopolymer Maket Is Poised to Reach USD 120.5 Billion by 2033. 2024. Available online: https://www.globenewswire.com/news-release/2024/01/24/2814859/0/en/Biopolymer-Market-is-poised-to-reach-USD-120-5-billion-by-2033-fueled-by-the-escalating-demand-for-sustainable-alternatives-across-industries.html (accessed on 27 March 2024).
- Conference, E.B. Report Bioplastics Market Data 2021 short version. In Proceedings of the European Bioplastics Conference, Berlin, Germany, 30 November–1 December 2021. [Google Scholar]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 1997.
- Moni, S.M.; Mahmud, R.; High, K.; Carbajales-Dale, M. Life cycle assessment of emerging technologies: A review. J. Ind. Ecol. 2019, 24, 52–63. [Google Scholar] [CrossRef]
- Pellengahr, F.; Ghannadzadeh, A.; van der Meer, Y. How accurate is plastic end-of-life modeling in LCA? Investigating the main assumptions and deviations for the end-of-life management of plastic packaging. Sustain. Prod. Consum. 2023, 42, 170–182. [Google Scholar] [CrossRef]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the Degradation of Poly(lactic acid) during Melt Processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, M.; Patwa, R.; Kommadath, R.; Kotecha, P.; Katiyar, V. Silk nanocrystals stabilized melt extruded poly (lactic acid) nanocomposite films: Effect of recycling on thermal degradation kinetics and optimization studies. Thermochim. Acta 2016, 643, 41–52. [Google Scholar] [CrossRef]
- Gajjar, C.R.; Stallrich, J.W.; Pasquinelli, M.A.; King, M.W. Process-Property Relationships for Melt-Spun Poly(lactic acid) Yarn. ACS Omega 2021, 6, 15920–15928. [Google Scholar] [CrossRef] [PubMed]
- Arias-Nava, E.H.; Sullivan, B.P.; Valles-Rosales, D.J. Biopolymer Degradation Analysis: Accelerated Life Testing Study to Characterize Polylactic Acid Durability. Materials 2021, 14, 5730. [Google Scholar] [CrossRef] [PubMed]
- Paakinaho, K.; Ellä, V.; Syrjälä, S.; Kellomäki, M. Melt spinning of poly(l/d)lactide 96/4: Effects of molecular weight and melt processing on hydrolytic degradation. Polym. Degrad. Stab. 2009, 94, 438–442. [Google Scholar] [CrossRef]
- Brunšek, R.; Kopitar, D.; Schwarz, I.; Marasović, P. Biodegradation Properties of Cellulose Fibers and PLA Biopolymer. Polymers 2023, 15, 3532. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qi, Z.; Xu, J.; Guo, B.; Huang, Z. Study on the Photodegradation Stability of Poly(butylene Succinate-co-butylene Adipate)/TiO2 Nanocomposites. J. Chem. 2019, 2019, 5036019. [Google Scholar] [CrossRef]
- Fritz, M.; Lauschke, T.; Schlebrowski, T.; Beucher, L.; Schweyen, P.; Alenezi, B.; Hahn, B.; Dierkes, G.; Ternes, T.; Fischer, C.B. Photoaging phenomena of biodegradable polybutylene succinate and conventional low density polyethylene by artificial weathering—A comparative surface study. Appl. Surf. Sci. 2022, 590, 153058. [Google Scholar] [CrossRef]
- Shaiju, P.; Dorian, B.B.; Senthamaraikannan, R.; Padamati, R.B. Biodegradation of Poly (Butylene Succinate) (PBS)/Stearate Modified Magnesium-Aluminium Layered Double Hydroxide Composites under Marine Conditions Prepared via Melt Compounding. Molecules 2020, 25, 5766. [Google Scholar] [CrossRef]
- Ludwiczak, J.; Dmitruk, A.; Skwarski, M.; Kaczyński, P.; Makuła, P. UV resistance and biodegradation of PLA-based polymeric blends doped with PBS, PBAT, TPS. Int. J. Polym. Anal. Charact. 2023, 28, 366–382. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Tolga, S.; Kabasci, S.; Duhme, M. Progress of Disintegration of Polylactide (PLA)/Poly(Butylene Succinate) (PBS) Blends Containing Talc and Chalk Inorganic Fillers under Industrial Composting Conditions. Polymers 2020, 13, 10. [Google Scholar] [CrossRef]
- Yasin, M.; Holland, S.J.; Tighe, B.J. Polymers for biodegradable medical devices. Biomaterials 1989, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Aldhafeeri, T.; Alotaibi, M.; Barry, C.F. Impact of Melt Processing Conditions on the Degradation of Polylactic Acid. Polymers 2022, 14, 2790. [Google Scholar] [CrossRef] [PubMed]
- Borelbach, P.; Kopitzky, R.; Dahringer, J.; Gutmann, P. Degradation Behavior of Biodegradable Man-Made Fibers in Natural Soil and in Compost. Polymers 2023, 15, 2959. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Thomas, N.L. Blending poly(butylene succinate) with poly(lactic acid): Ductility and phase inversion effects. Eur. Polym. J. 2015, 71, 534–546. [Google Scholar] [CrossRef]
- Sabnis, S.; Block, L.H. Improved infrared spectroscopic method for the analysis of degree of N-deacetylation of chitosan. Polym. Bull. 1997, 39, 67–71. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.-S.; Sudesh, K.; Chow, W.S.; Ishak, Z.A.M. A study on the effects of organoclay content and compatibilizer addition on the properties of biodegradable poly(butylene succinate) nanocomposites under natural weathering. J. Compos. Mater. 2014, 49, 891–902. [Google Scholar] [CrossRef]
- Tyagi, P.; Agate, S.; Velev, O.D.; Lucia, L.; Pal, L. A Critical Review of the Performance and Soil Biodegradability Profiles of Biobased Natural and Chemically Synthesized Polymers in Industrial Applications. Environ. Sci. Technol. 2022, 56, 2071–2095. [Google Scholar] [CrossRef]
- DIN EN ISO 5079; Textilfasern—Bestimmung der Höchstzugkraft und Höchstzugkraftdehnung an einzelnen Fasern (ISO 5079:2020). Deutsches Institut für Normung: Geneva, Switzerland, 2020.
- DIN standard EN ISO 11341; Beschichtungsstoffe—Künstliches Bewittern und künstliches Bestrahlen—Beanspruchung durch gefilterte Xenonbogenstrahlung (ISO 11341:2004). Deutsches Institut für Normung: Geneva, Switzerland, 2004.
- ISO 4892-2; Kunststoffe—Künstliches Bestrahlen oder Bewittern in Geräten—Teil 2: Xenonbogenlampen (ISO 4892-2:2013). Deutsches Institut für Normung: Geneva, Switzerland, 2013.
- ISO 4892.3; Plastics—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps. International Organization for Standardization: Geneva, Switzerland, 2016.
- Pinto, J.; Dias, M.; Amaral, J.; Ivanov, M.; Paixao, J.A.; Coimbra, M.A.; Ferreira, P.; Pereira, E.; Goncalves, I. Influence of UV degradation of bioplastics on the amplification of mercury bioavailability in aquatic environments. Mar. Pollut. Bull. 2022, 180, 113806. [Google Scholar] [CrossRef]
- ISO 3310-1; Analysensiebe—Technische Anforderungen und Prüfung—Teil 1: Analysensiebe mit Metalldrahtgewebe (ISO 3310-1:2000). Deutsches Institut für Normung: Geneva, Switzerland, 2016.
- UNE-EN ISO 20200:2016; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test (ISO 20200:2015). AENOR: Madrid, Spain, 2016.
- Schick, S.; Groten, R.; Seide, G.H. Performance Spectrum of Home-Compostable Biopolymer Fibers Compared to a Petrochemical Alternative. Polymers 2023, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Dekeyser, A.; Cardon, L.; Degrieck, J. Quantification of thermal material degradation during the processing of biomedical thermoplastics. J. Appl. Polym. Sci. 2011, 120, 2872–2880. [Google Scholar] [CrossRef]
- Ostheller, M.E.; Balakrishnan, N.K.; Groten, R.; Seide, G. The Effect of Electrical Polarity on the Diameter of Biobased Polybutylene Succinate Fibers during Melt Electrospinning. Polymers 2022, 14, 2865. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Banerjee, S.; Biswas, C.; Guchhait, R.; Chatterjee, A.; Pramanick, K. Functional interplay between plastic polymers and microbes: A comprehensive review. Biodegradation 2021, 32, 487–510. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]



| Spinning Scale | Throughput [kg/h] | Nozzle Count | Dwell Time [min] |
|---|---|---|---|
| PLA_LAB | 1 | 829 | 5 |
| PLA_IND | 368 | 42.922 | 7 |
| PLA_SF | 30 | 10.005 | 36 |
| PBS_LAB | 1 | 829 | 5 |
| PBS_IND | 351 | 42.922 | 7 |
| PBS_SF | 23 | 10.005 | 22 |
| Sample | Throughput [kg/h] | Dwell Time [min] | Draw Ratio | Titer [dtex] | cN/tex | Elongation [%] | Crystallinity [%] |
|---|---|---|---|---|---|---|---|
| PLA_LAB | 1 | 5 | 2.4 | 3.91 | 19.28 | 43.78 | 39.03 |
| PLA_IND | 368 | 7 | 1.9 | 2.6 | 26.96 | 45.66 | 39.88 |
| PLA_SF | 30 | 36 | 1.82 | 2.19 | 23.73 | 39.55 | 33.84 |
| PBS_LAB | 1 | 5 | 3.2 | 4.18 | 23.41 | 116.21 | 54.3 |
| PBS_IND | 351 | 7 | 1.75 | 9.06 | 15.97 | 214.74 | 51.46 |
| PBS_SF | 23 | 22 | 1.76 | 4.24 | 12.86 | 184.51 | 64.51 |
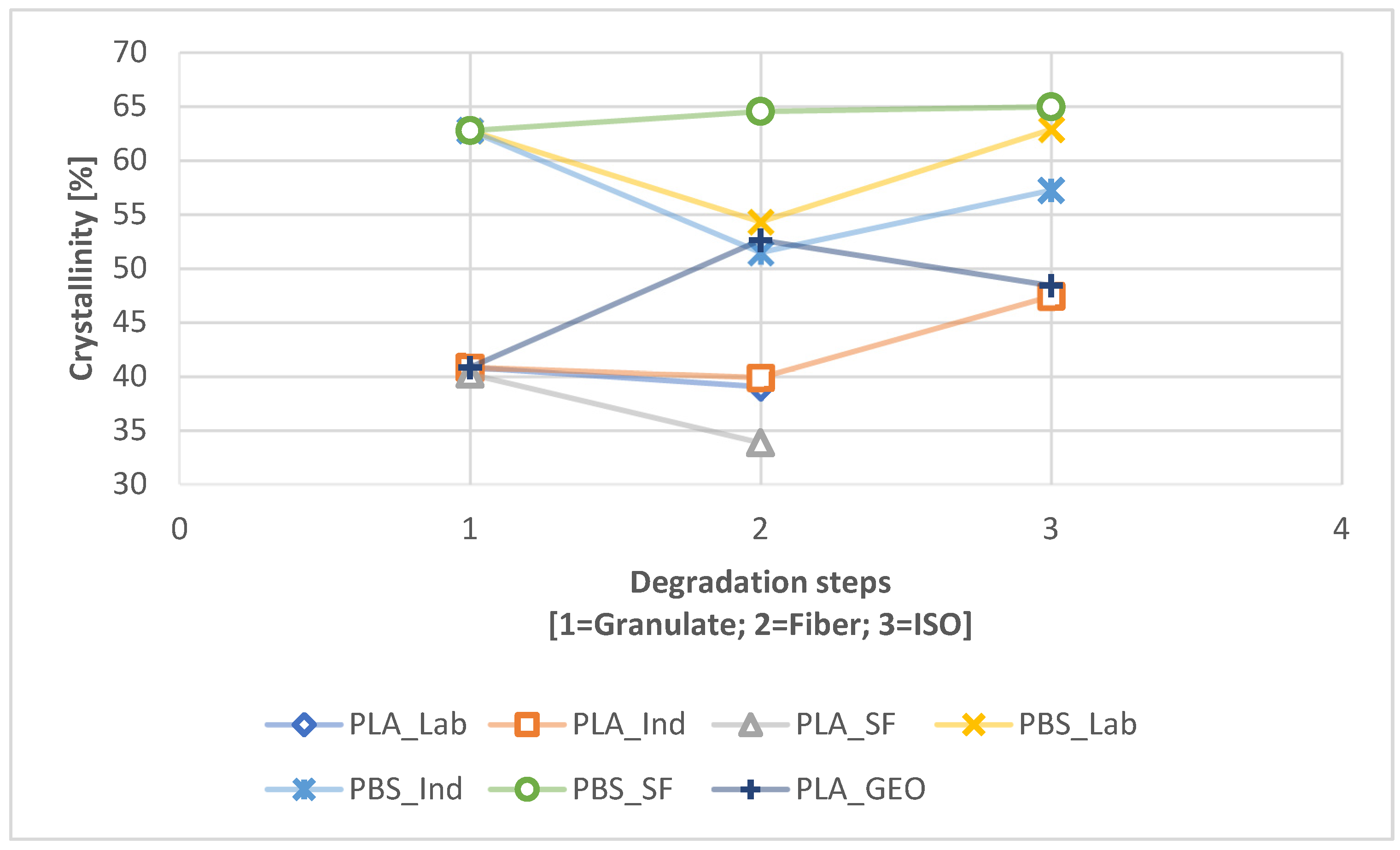
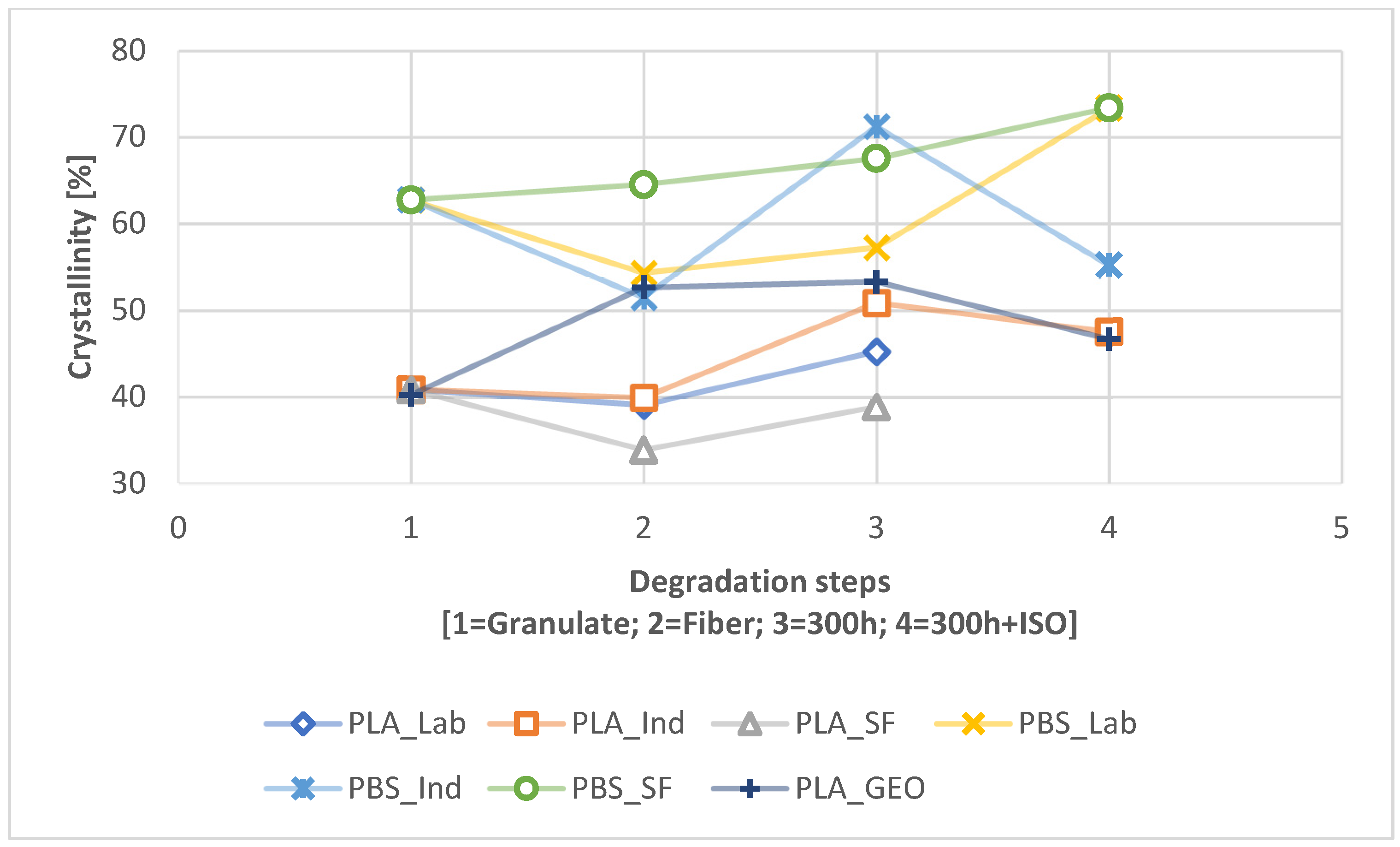
| Crystallinity | Path | PLA_LAB | PLA_IND | PLA_SF | PBS_LAB | PBS_IND | PBS_SF | PLA_GEO |
|---|---|---|---|---|---|---|---|---|
| Granulate | 40.82 | 40.82 | 40.82 | 62.74 | 62.74 | 62.74 | 40.82 | |
| Fiber | 39.03 | 39.88 | 33.84 | 54.30 | 51.46 | 64.51 | 52.61 | |
| ISO | 2.1 | N.A. | 47.40 | N.A. | 62.85 | 57.21 | 64.95 | 48.4 |
| 300h | 2.2 | 45.22 | 50.84 | 38.84 | 57.23 | 71.22 | 67.54 | 53.29 |
| 300h + ISO | 2.2 | N.A. | 47.50 | N.A. | 73.31 | 55.20 | 73.38 | 46.66 |
| Carbonyl Index | Path | PLA_GEO | PLA_LAB | PLA_IND | PLA_SF | PBS_LAB | PBS_IND | PBS_SF |
|---|---|---|---|---|---|---|---|---|
| Granulate | 1.94789 | 1.94789 | 1.94789 | 1.94789 | 5.01155 | 5.01155 | 5.01155 | |
| Fiber | 2.55801 | 7.50089 | 2.99638 | 2.23939 | 7.50089 | 6.59484 | 7.38402 | |
| ISO | 2.1 | 1.52021 | 1.70042 | 6.86914 | 6.24853 | 6.69622 | ||
| 300h | 2.2 | 2.88662 | 7.59869 | 2.41957 | 2.28803 | 7.59869 | 6.70654 | 7.15743 |
| 300h + ISO | 2.2 | 2.09586 | 1.99091 | 7.19465 | 6.57988 | 6.86802 |
| Hydroxyl Index | Path | PLA_GEO | PLA_LAB | PLA_IND | PLA_SF | PBS_LAB | PBS_IND | PBS_SF |
|---|---|---|---|---|---|---|---|---|
| Granulate | 0.20621 | 0.20621 | 0.20621 | 0.20621 | 0.04891 | 0.04891 | 0.04891 | |
| Fiber | 0.36899 | 0.20037 | 0.35473 | 0.41445 | 0.20037 | 0.21611 | 0.18229 | |
| ISO | 2.1 | 0.3296 | 0.43955 | 0.24062 | 0.26277 | 0.22467 | ||
| 300h | 2.2 | 0.46371 | 0.22207 | 0.40568 | 0.40011 | 0.22207 | 0.22213 | 0.20362 |
| 300h + ISO | 2.2 | 0.42325 | 0.48282 | 0.23186 | 0.22818 | 0.22155 |
| Vinyl Index | Path | PLA_GEO | PLA_LAB | PLA_IND | PLA_SF | PBS_LAB | PBS_IND | PBS_SF |
|---|---|---|---|---|---|---|---|---|
| Granulate | 1.78049 | 1.78049 | 1.78049 | 1.78049 | 2.82858 | 2.82858 | 2.82858 | |
| Fiber | 2.33928 | 2.92216 | 2.25796 | 2.1267 | 2.92216 | 2.95236 | 2.91245 | |
| ISO | 2.1 | 0.93223 | 0.71412 | 2.37826 | 2.37442 | 2.34196 | ||
| 300h | 2.2 | 2.90349 | 2.93925 | 2.21447 | 2.07426 | 2.93925 | 3.08622 | 2.97048 |
| 300h + ISO | 2.2 | 1 | 0.98249 | 2.5889 | 2.64028 | 2.50618 |
| Sample | Fiber | ISO |
|---|---|---|
| PLA_LAB | 1.01 | N.A. |
| PLA_IND | 0.857 | 18.842 |
| PLA_SF | 1.273 | N.A. |
| PBS_LAB | 5.67 | 4.19 |
| PBS_IND | 7.9 | 2.77 |
| PBS_SF | N.A | N.A. |
| PLA_GEO | 1.236 | 11.336 |
| Sample | Fiber | 300h | 300h + ISO |
|---|---|---|---|
| PLA_LAB | 1.01 | 1.604 | N.A. |
| PLA_IND | 0.857 | 1.536 | 8.81 |
| PLA_SF | 1.273 | 2.747 | N.A. |
| PBS_LAB | 5.67 | 5.77 | 10.72 |
| PBS_IND | 7.9 | 14.47 | 2.92 |
| PBS_SF | N.A | N.A. | N.A. |
| PLA_GEO | 1.236 | 1.446 | 10.000 |
| Parameter | Change Toward | Crystallinity | Tenacity | Elongation | CI | VI | HI | Mn | Degradability |
|---|---|---|---|---|---|---|---|---|---|
| Dwell time | ↑ | ↓ | ↘ | ↘ | ↗ | ↗ | ↔ | ↓ | ↑ |
| Draw ratio | ↗ | ↗ | ↗ | ↘ | ↗ | ↗ | ↘ | ↔ | ↘ |
| Fiber diameter | ↘ | ↗ | ↗ | ↓ | ↗ | ↘ | ↗ | ↔ | ↑ |
| Tenacity | ↗ | ↗ | ↘ | ↗ | ↗ | ↘ | ↔ | ↘ | |
| Elongation | ↘ | ↗ | ↗ | ↗ | ↗ | ↘ | ↔ | ↘ | |
| UV exposure before disintegration | yes | ↑ | ↗ | ↗ | ↗ | ↓ | ↑ |
| Parameter | Change Toward | Crystallinity | Tenacity | Elongation | CI | VI | HI | Mn | Degradability |
|---|---|---|---|---|---|---|---|---|---|
| Dwell time | ↑ | ↑ | ↘ | ↘ | ↘ | ↔ | ↔ | ↘ | ↘ |
| Draw ratio | ↗ | ↔ | ↑ | ↘ | ↗ | ↔ | ↔ | ↗ | ↗ |
| Fiber diameter | ↘ | ↗ | ↑ | ↘ | ↗ | ↘ | ↔ | ↗ | ↗ |
| Tenacity | ↗ | ↘ | ↘ | ↘ | ↗ | ↔ | ↗ | ↗ | |
| Elongation | ↘ | ↘ | ↗ | ↘ | ↗ | ↔ | ↗ | ↗ | |
| UV exposure before disintegration | yes | ↑ | ↑ | ↑ | ↘ | ↔ | ↗ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schick, S.; Groten, R.; Weinberger, A.; Seide, G.H. A Comparison of Laboratory and Industrial Processes Reveals the Effect of Dwell Time and UV Pre-Exposure on the Behavior of Two Polymers in a Disintegration Trial. Polymers 2024, 16, 1650. https://doi.org/10.3390/polym16121650
Schick S, Groten R, Weinberger A, Seide GH. A Comparison of Laboratory and Industrial Processes Reveals the Effect of Dwell Time and UV Pre-Exposure on the Behavior of Two Polymers in a Disintegration Trial. Polymers. 2024; 16(12):1650. https://doi.org/10.3390/polym16121650
Chicago/Turabian StyleSchick, Simon, Robert Groten, Andreas Weinberger, and Gunnar H. Seide. 2024. "A Comparison of Laboratory and Industrial Processes Reveals the Effect of Dwell Time and UV Pre-Exposure on the Behavior of Two Polymers in a Disintegration Trial" Polymers 16, no. 12: 1650. https://doi.org/10.3390/polym16121650
APA StyleSchick, S., Groten, R., Weinberger, A., & Seide, G. H. (2024). A Comparison of Laboratory and Industrial Processes Reveals the Effect of Dwell Time and UV Pre-Exposure on the Behavior of Two Polymers in a Disintegration Trial. Polymers, 16(12), 1650. https://doi.org/10.3390/polym16121650







