Synthesis and Evaluation of Plugging Gel Resistant to 140 °C for Lost Circulation Control: Effective Reduction in Leakage Rate in Drilling Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Experimental reagents: acrylamide (AM), analytical pure; N-vinyl pyrrolidone (NVP), containing 100 ppm NaOH stabilizer; N, N-methylene bisacrylamide (MBA), all purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (China); sodium styrene sulfonate (SSS), purchased from Shanghai Bohr Reagent Co., Ltd. (China); polyvinyl alcohol (PVA), purchased from Shanghai Chenqi Chemical Technology Co., Ltd. (China); ammonium persulfate (APS), analytical pure, purchased from Tianjin Damao Chemical Reagent Factory (China); distilled water, self-made in the laboratory.
- (2)
- Experimental instruments: vacuum drying oven, Shanghai Shangpu Instrument Equipment Co., Ltd. (China); magnetic stirrer and rotary stirrer, Jiangsu Jincheng Guosheng Experimental Instrument Factory (China); roller heating furnace, OFI Testing Equipment, (Houston, TX, USA); electric thermostatic water bath, Shanghai Yulong Instrument Equipment Co., Ltd. (China); pressure reducing valve, Qingdao Huaqing Automation Instrument Co., Ltd. (China); pressure gauge, Xi’an Automation Instrument Factory (China); three-type piston container and confining pressure pump, Nantong Yichuang Experimental Instrument Co., Ltd. (China); electronic balance, Harbin Zhonghui Weighing Apparatus Co., Ltd. (China); multi-function grinder, Beijing Yongguangming Medical Instrument Co., Ltd. (China); thermogravimetric analyzer, Nanjing Huicheng Instrument Co., Ltd. (China); infrared spectrometer, Brook Corporation, (Boston, MA, USA); cold field emission scanning electron microscope, HITACHI, Tokyo (Japan); MD-NJ5 (5961) gel strength tester, Zhejiang Fengyuan Electronics Co., Ltd. (China); visual sand bed sealing device and high temperature high pressure filtration instrument, Qingdao Chuangmeng Instrument Co., Ltd. (China).
2.2. Experimental Methods
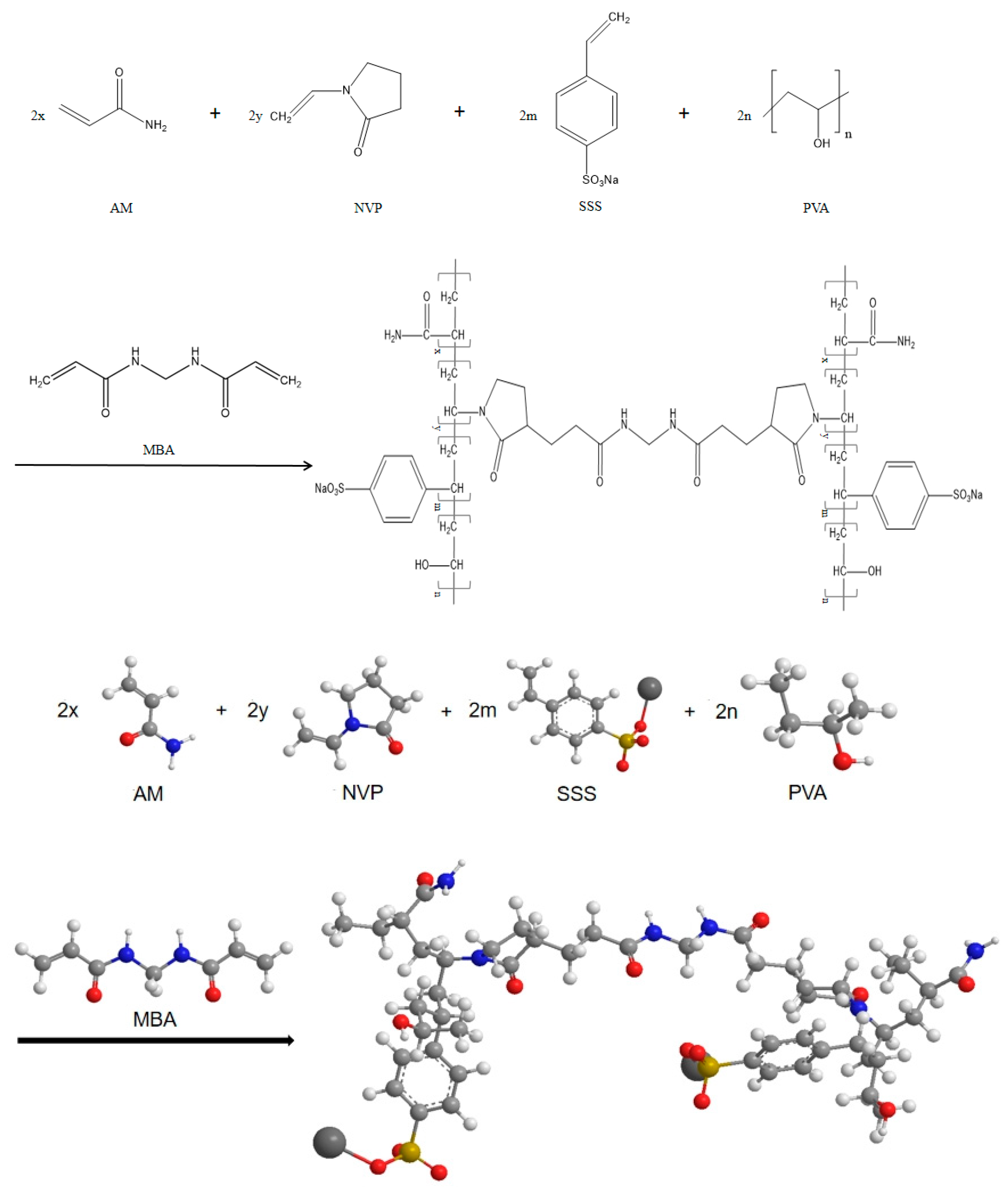
2.2.1. Synthesis Method of Gel
- (1)
- Weigh 7 g of 1788 mesh PVA and add it to 100 g of distilled water. Stir thoroughly at room temperature using a magnetic stirrer until fully dissolved, then set aside for later use;
- (2)
- Weigh 30 g of AM and dissolve it in 100 g of distilled water. Then, add 20 g of NVP and 10 g of SSS, stir well and dissolve;
- (3)
- Transfer the dissolved PVA solution and AM, NVP, SSS solution into a three-neck flask and mix them thoroughly. Add 0.5 g APS and 0.65 g MBA and react at 60 °C for 5 h under a nitrogen-protected environment. Maintain a rotation speed of 600 r/min during the experiment.
2.2.2. Characterization of Gel
- (1)
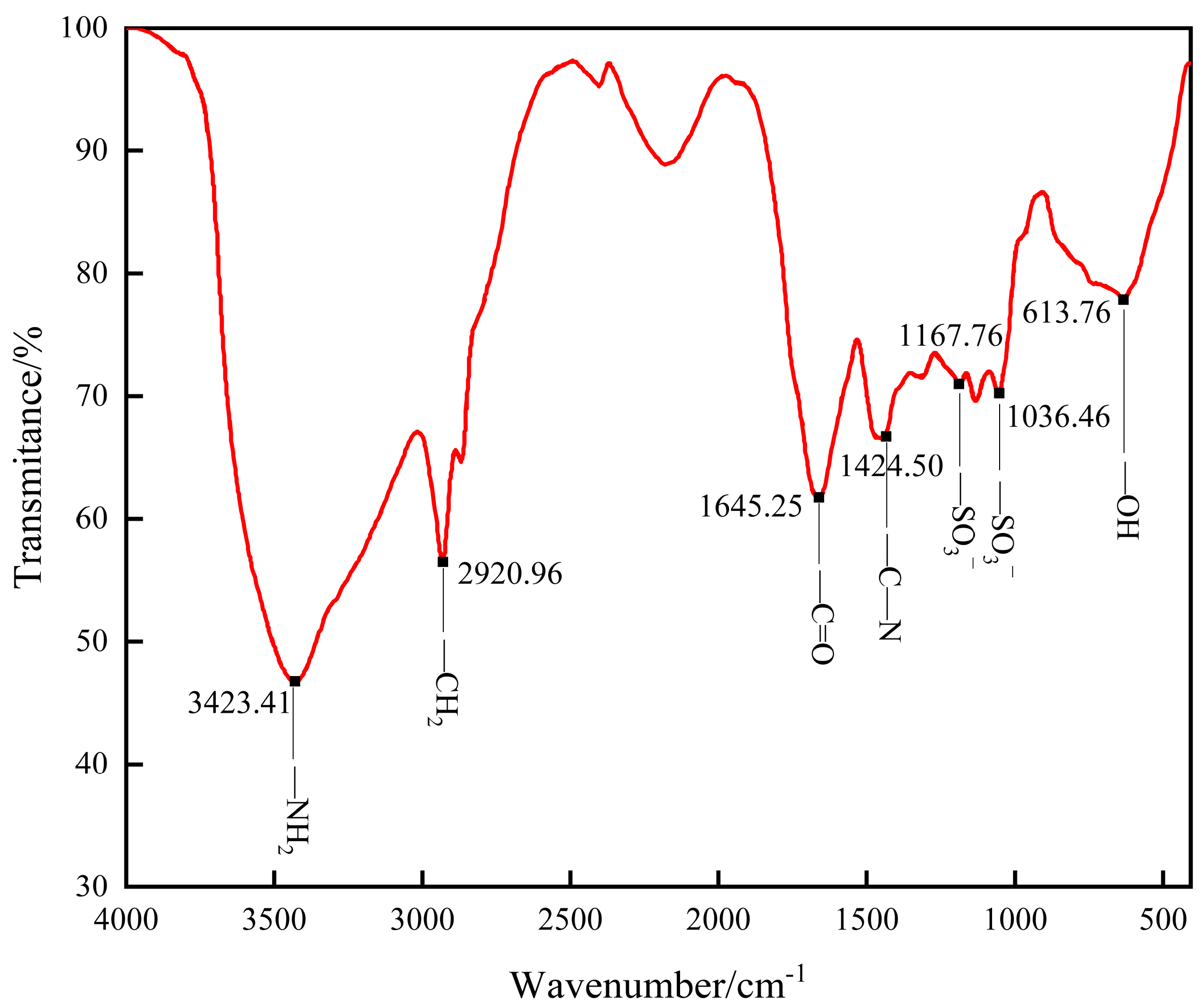
- Infrared characterization of gel (FT-IR)
- (2)
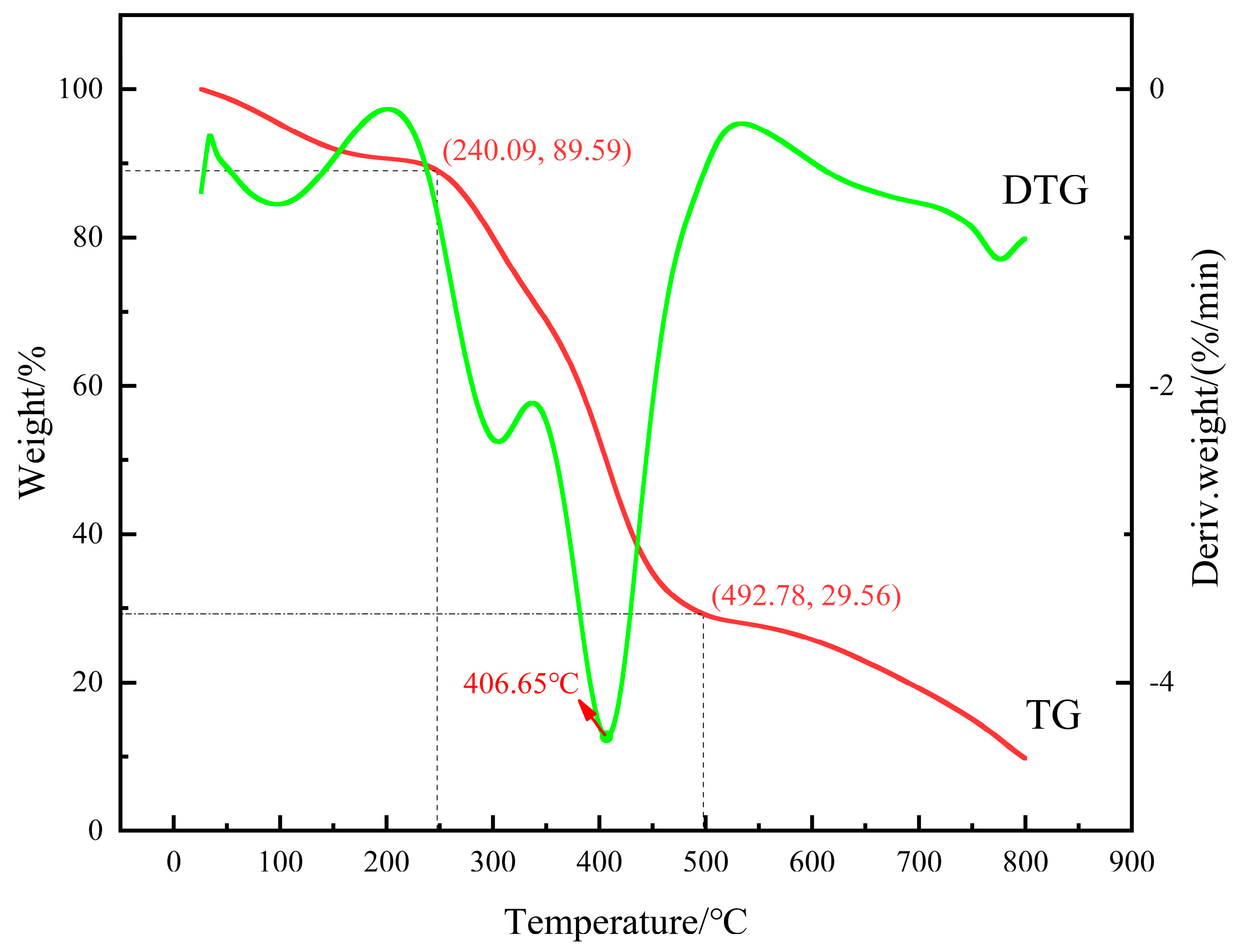
- Thermogravimetric analysis of gel
- (3)
- Gel scanning electron microscope analysis
2.2.3. Evaluation of Water-Swelling Performance of Gel
- (1)
- Evaluation of water absorption of gel
- (2)
- Gel strength analysis after aging
2.2.4. Evaluation of the Filtration Property of Gel
- (1)
- Temperature range test of the gel
- (2)
- Evaluation of gel blocking performance
- (3)
- Gel drag reduction evaluation
- (4)
- High-temperature and high-pressure filtration performance evaluation of gel
2.2.5. Polymerization Mechanism of Gel
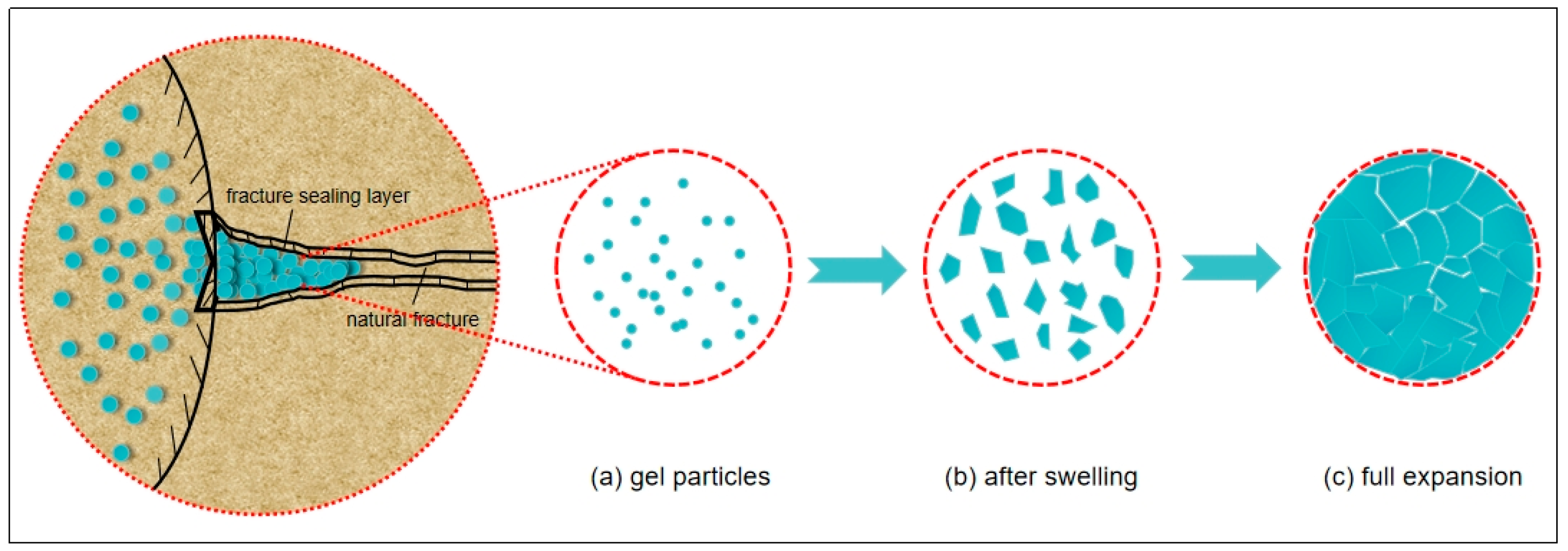
2.2.6. Plugging Mechanism of Gel
3. Results and Discussion
3.1. Characteristics of Gel
3.1.1. FT-IR
3.1.2. TGA
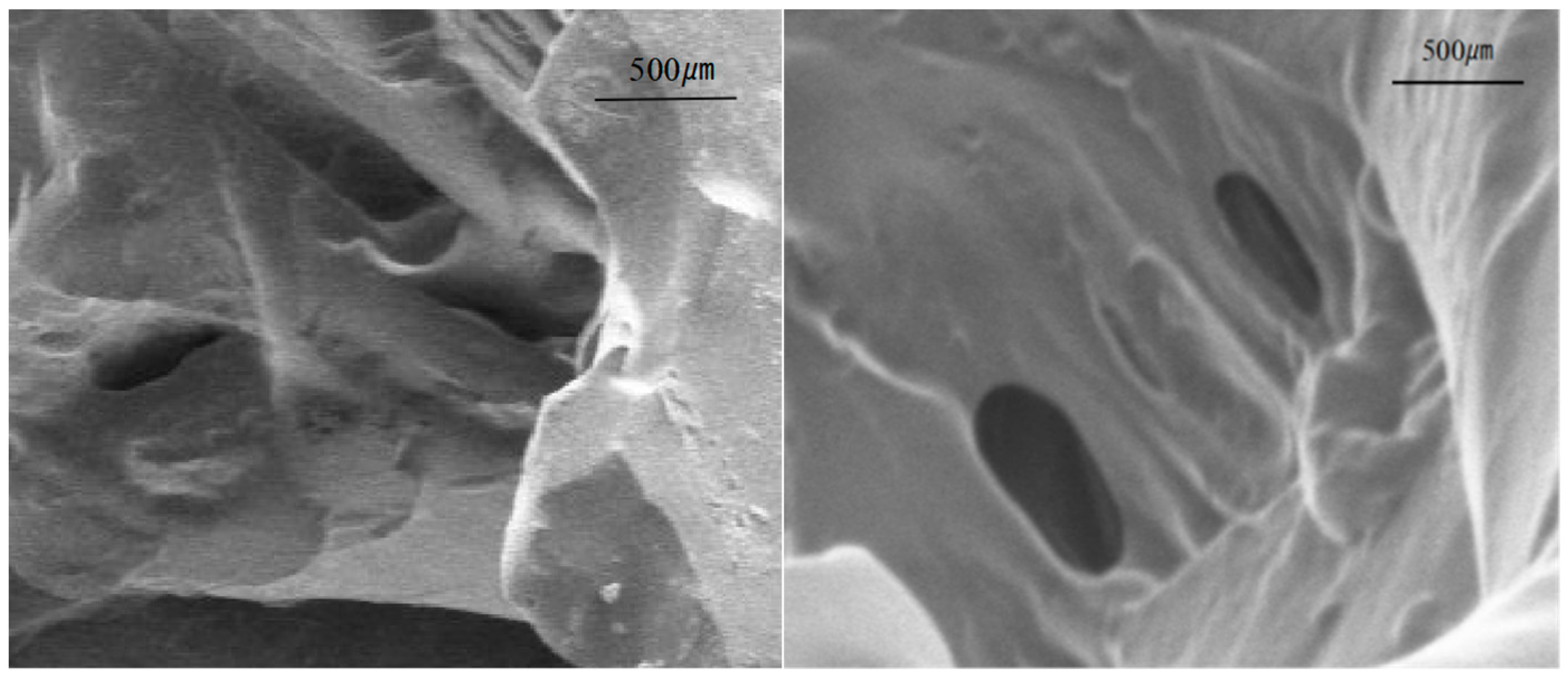
3.1.3. Gel Scanning Electron Microscopy Analysis
3.2. Analysis of Water-Swelling Performance of Gel
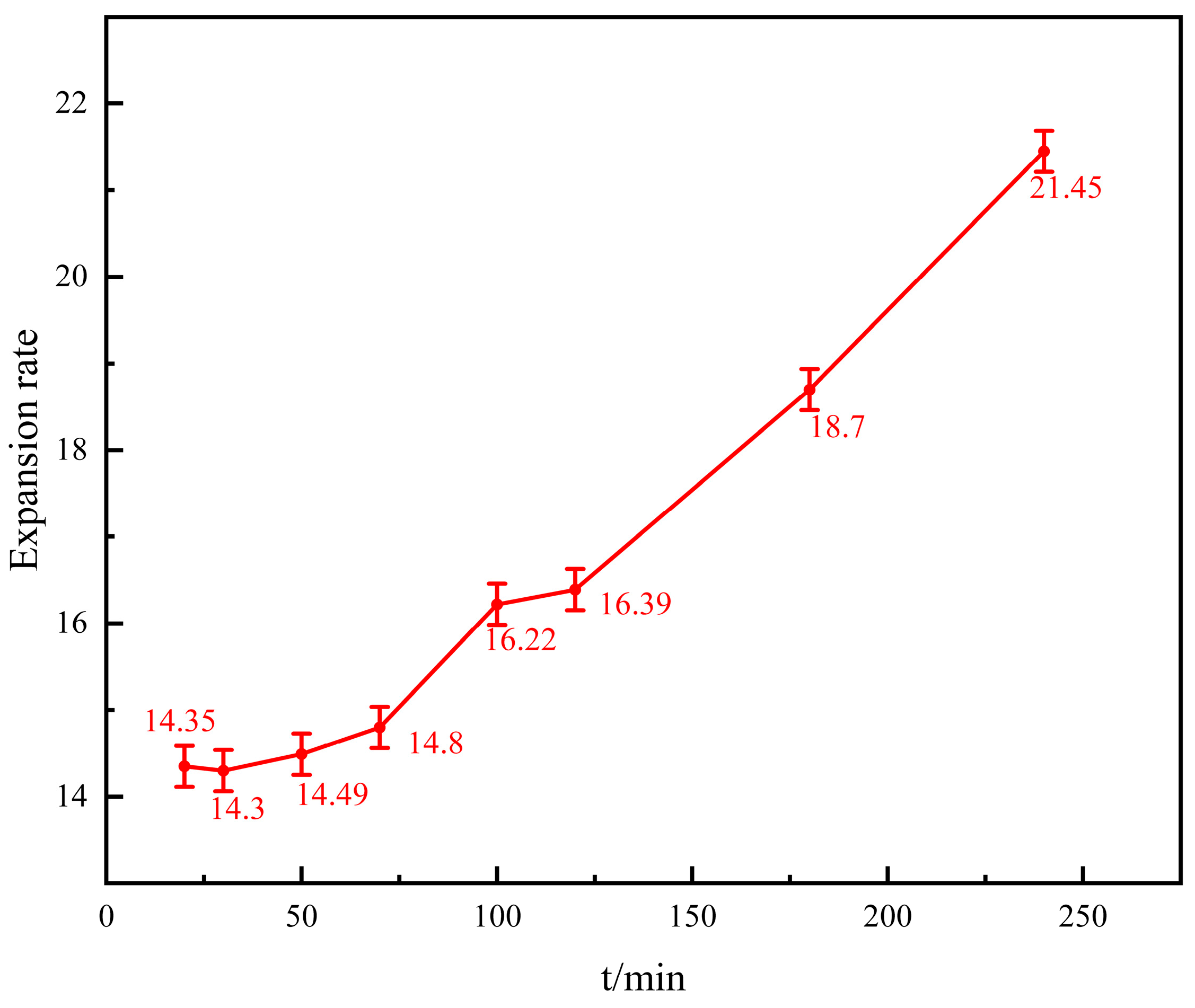
3.2.1. Water Absorption and Expansion of Gel
3.2.2. Strength Analysis of Gels after Water Absorption
3.3. Filtration Property of Gel
3.3.1. Effect of the Temperature
3.3.2. Evaluation of Sand Bed Plugging Performance
3.3.3. Evaluation of Drag Reduction in Simulated Fractured Pipelines
3.3.4. High-Temperature and High-Pressure Filtration Loss Evaluation
4. Conclusions
- (1)
- An anti-140 °C high-temperature gel plugging material was synthesized by aqueous solution polymerization with acrylamide, N-vinylpyrrolidone, sodium p-styrene sulfonate, and polyvinyl alcohol as polymerization monomers, N,N-methylenebisacrylamide as the crosslinking agent, and ammonium persulfate as the initiator.
- (2)
- The gel material has a good high-temperature water absorption and expansion. The size of the gel particles increases significantly after high-temperature water absorption. The expansion rate after water absorption can reach dozens of times more than before water absorption, and the gel strength performance is relatively stable. After 1 h, 2 h, and 4 h of high-temperature water absorption, the gel strength changes little and is stable between 0.4 and 0.6 MPa.
- (3)
- The gel material has a good temperature resistance and plugging performance. After aging at 140 °C for 24 h, it basically does not degrade. It has long-term temperature resistance and can maintain effective plugging for a long time at high temperatures. After 18 h, 24 h, and 30 h of aging, the plugging effect is significantly improved compared with the base slurry.
- (4)
- After aging, the gel material has good drag reduction, which is 97.55% higher than that of clear water. It can fully contact and retain with the fracture wall, squeeze and accumulate, and finally form a plugging layer to achieve the purpose of plugging. The gel material also has a significant filtration reduction effect on drilling fluid under high-temperature and high-pressure conditions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.Q.; Liu, S.H.; Liu, J.H.; Li, D.Q. Development of A Novel Two-step Crosslinking Gel with High Temperature Resistance for Plugging Lost Circulation. Explor. Eng. 2016, 43, 103–106. [Google Scholar]
- Zhang, P. Drilling fluid plugging materials and leakage prevention and plugging technology. China Pet. Chem. Ind. Stand. Qual. 2023, 43, 164–166. [Google Scholar]
- Liu, X.Z.; Lv, R.K.; Zhang, Y.M. Analysis of drilling fluid plugging materials and exploration of leakage prevention and plugging technology. Agric. Sci. Technol. 2018, 15, 41–42. [Google Scholar]
- Lu, Z.Q. Analysis of drilling fluid plugging materials and discussion on leakage prevention and plugging technology. Sci. Technol. Innov. Appl. 2019, 28, 157–158. [Google Scholar]
- Chen, Z.H.; Shang, W.T.; Wang, X. Analysis of drilling fluid plugging materials and discussion on leakage prevention and plugging technology. China Pet. Chem. Ind. Stand. Qual. 2020, 40, 207–208. [Google Scholar]
- Nie, T.Y.; Guo, J.B.; Liu, F. Analysis of drilling fluid plugging materials and discussion on leakage prevention and plugging technology. Chem. Des. Newsl. 2020, 46, 197–198. [Google Scholar]
- Tan, M.B.; He, S.M. Anti-leakage and plugging technology for drilling in low-pressure fractured formation of Xiangguosi underground gas storage. Nat. Gas Ind. 2014, 34, 97–101. [Google Scholar]
- Liu, G.H.; Liu, S.T.; Liu, Z. Research and application analysis of ultra-low permeability drilling fluid leakage prevention and plugging technology. Technol. Enterp. 2018, 25, 52–53. [Google Scholar]
- Zhang, X.W.; Li, S.; Zhang, J. Research progress of drilling fluid plugging materials and plugging technology. Drill. Fluid Complet. Fluid 2009, 26, 74–76+79+97. [Google Scholar]
- Hao, H.J.; Liu, P.; Yan, J.T.; Ma, C.Y.; He, B.; Huan, W.Y. Research progress of polymer gel plugging. Drill. Fluid Complet. Fluid 2022, 39, 661–667+676. [Google Scholar]
- Wang, L.Z. Research on Drilling Leakage Prevention and Plugging Technology in Beisi Area of Daqing Oilfield; Daqing Petroleum Institute: Daqing, China, 2008. [Google Scholar]
- Zhou, P.; Li, Q.D.; Li, H.; Li, S.J. Advances in bulk swelling plugging materials and their plugging mechanisms. Oilfield Chem. 2009, 26, 111–114. [Google Scholar]
- Wang, Z.B. Construction and Plugging Mechanism of Interpenetrating Network Polymer Plugging Agent; China University of Geosciences: Beijing, China, 2020. [Google Scholar]
- Chen, A.; Luo, Y.; Wu, Y.B. Synthesis and performance evaluation of a heat-resistant and salt-resistant pre-crosslinked hydrophobic water swellable body. Fine Petrochem. Prog. 2009, 10, 1–4. [Google Scholar]
- Vasouez, J.; Dalrymple, E.D.; Reddy, B.R. Development and evaluation of high-temperature conformance polymer systems. In Proceedings of the International Symposium on Oilfield Chemistry, SPE-93156-MS, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Salunkhe, B.; Schuman, T.; Al Brahim, A. Ultra-high temperature resistant preformed particle gels for enhanced oil recovery. Chem. Eng. J. 2021, 426, 130712. [Google Scholar] [CrossRef]
- Hutchins, R.D.; Dovan, H.T.; Sandiford, B.B. Field applications of high temperature organic gels for water control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Sun, J.S.; Bai, Y.R. High temperature resistant fiber reinforced gel particle plugging agent. Drill. Fluid Complet. Fluid 2020, 37, 269–274. [Google Scholar]
- Tan, L. Experimental Study on New Fracture Plugging Agent for Plugging Old Fractures in W Block of Huabei Oilfield; Southwest Petroleum University: Chengdu, China, 2017. [Google Scholar]
- Jiang, M.; Li, Z.Q.; Duan, G.F. The influence of hydraulic fracture conductivity on the productivity of deep shale gas. Xinjiang Oil Gas 2023, 19, 35–41. [Google Scholar]
- Luo, T.Y.; Fang, G.; Liang, L.J. New research progress and application of chemical temporary plugging agent in petroleum engineering. Xinjiang Oil Gas 2019, 15, 79–87. [Google Scholar]
- GB/T 6040-2002; General Rules for Infrared Analysis. National Standards of the People’s Republic of China: Beijing, China, 2002.
- NB/SH/T0859-2013; Standard Test Method for the Thermal Stability of Chemicals by Thermal Analysis. National Standards of the People’s Republic of China: Beijing, China, 2013.
- GB/T 36422-2018; Man-Made Fiber—Test Method for Micro Morphology and Diameter—Scanning Electron Microscope Method. National Standards of the People’s Republic of China: Beijing, China, 2018.
- Wu, X.L.; Yan, L.L.; Wang, L.H. Research Progress of Filtrate Reducer for Environmentally Friendly Drilling Fluid. Drill. Fluid Complet. Fluid 2018, 35, 8–16. [Google Scholar]
- Amir, Z.; Said, I.M.; Jan, B.M. In situ organically cross-linked polymer gel for high-temperature reservoir conformance control. A review. Polym. Adv. Technol. 2019, 30, 13–39. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Du, K. Development of a new high-temperature and high-strength polymer gel for plugging fractured reservoirs. Upstream Oil Gas Technol. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Zhang, D.J.; Gao, J.C. Research on AM-AA copolymer/SA composite ball plugging agent. Petrochem. Ind. 2014, 43, 1315–1318. [Google Scholar]
- Zhong, J.X.; Chen, Y.; Tan, H. Solution properties of AM/NVP binary copolymer. Polym. Mater. Sci. Eng. 2005, 220–223. [Google Scholar] [CrossRef]
- Song, L.H.; Cai, L.; Qiao, K. Synthesis and performance evaluation of a novel oil recovery chemical additive NVP–AM copolymer. Chem. Times 2009, 23, 10–14. [Google Scholar]
- Albonico, P.L.; Lockhart, T.P. Divalent Ion-Resistant Polymer Gels for High-Temperature Applications. Syneresis Inhibiting Additives. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar] [CrossRef]
- Schuman, T.; Salunkhe, B.; Al, B.; Bai, B.J. Evaluation of Ultrahigh-Temperature-Resistant Preformed Particle Gels for Conformance Control in North Sea Reservoirs. SPE J. 2022, 27, 3660–3673. [Google Scholar] [CrossRef]
- Hu, J.M.; Xie, D.H.; Shen, Y. Synthesis of terpolymer of PVA with AA and AM. Plast. Ind. 1988, 19–22+2. [Google Scholar]
- Yan, J.F.; Jiang, B.; Zhang, T.D. Research progress on high temperature plugging materials and evaluation instruments for drilling. Sichuan Geol. J. 2022, 42 (Suppl. S1), 7–12. [Google Scholar]
- Zhang, C.; Lv, K.H.; Zeng, C.M. Preparation and performance evaluation of 140 °C high temperature pre-crosslinked gel. Oilfield Chem. 2011, 28, 399–401. [Google Scholar]
- Bai, Y.R.; Dai, L.Y.; Sun, J.S. Plugging performance and mechanism of an oil-absorbing gel for lost circulation control while drilling in fractured formations. Pet. Sci. 2022, 19, 2941–2958. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Yu, J.; Xie, L. Synthesis and Evaluation of Plugging Gel Resistant to 140 °C for Lost Circulation Control: Effective Reduction in Leakage Rate in Drilling Process. Polymers 2024, 16, 1658. https://doi.org/10.3390/polym16121658
Xu P, Yu J, Xie L. Synthesis and Evaluation of Plugging Gel Resistant to 140 °C for Lost Circulation Control: Effective Reduction in Leakage Rate in Drilling Process. Polymers. 2024; 16(12):1658. https://doi.org/10.3390/polym16121658
Chicago/Turabian StyleXu, Peng, Jun Yu, and Lingzhi Xie. 2024. "Synthesis and Evaluation of Plugging Gel Resistant to 140 °C for Lost Circulation Control: Effective Reduction in Leakage Rate in Drilling Process" Polymers 16, no. 12: 1658. https://doi.org/10.3390/polym16121658




