Experimental Investigation of the Effect of Surfactant–Polymer Flooding on Enhanced Oil Recovery for Medium Crude Oil
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Setup and Procedures
- Soxhlet Extractor: a single unit extractor from Vinici Technologies, Nanterre, France this used to remove water and oil from core samples and restore the core to its original state or prepare it for flooding purposes. It has the capacity to heat 250 mL of water to about 450 °C.
- Manual Saturator: The manual core saturator from Vinici Technologies, Nanterre, France is used to obtain remarkable fluid saturations of dry core samples. It has a cell diameter and height of 58 mm and 300 mm respectively.
- Pycnometer: this is used to determine the original densities of the crude oil sample and polymeric surfactant mixtures.
- Glass capillary viscometer: The OFITE viscometer, (Product Code: 130-10-L-99, Houston, TX 77065 USA) is used to measure the viscosity of fluid samples.
- Desiccator: its sole purpose is to dry the core samples in a vacuum. The system includes a heated desiccator and a vacuum pump.
- Reservoir Permeability Tester: The OFITE Permeability tester (Product Code: 127-00, Houston, TX 77065 USA) has various functions such as testing core samples and measuring their permeabilities, and core flooding analysis such as water flooding, gas injection, and enhanced oil recovery options. These enable the parameters like water and oil saturations, oil recoveries, and residual saturations to be measured. The schematic for the equipment is shown in Figure 3 and can be configured to suit various functions [6].
- Confining pressure (to maintain the pressure of the core and helps to avoid the displacing fluids from going outside the core).
- Drive pressure (indicated by 9 which controls water pumps and hydraulic fluids used in pushing fluids into the core).
- Back pressure which is set to keep the displacing fluids from boiling at the test temperatures.
2.2. Sulfonation Method
2.3. Characterization of the Core Samples
2.4. Characterization of Jatropha Oil
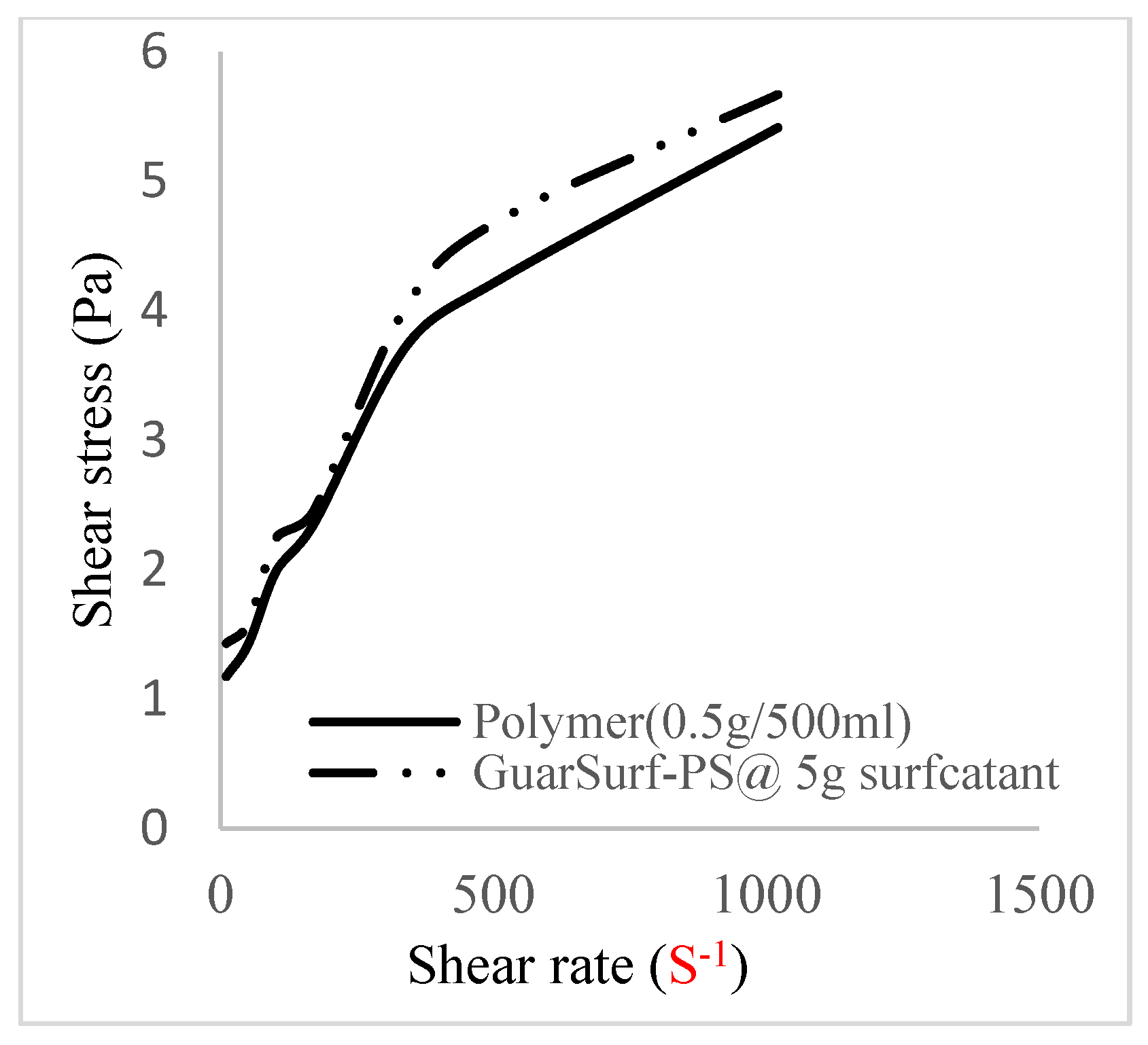
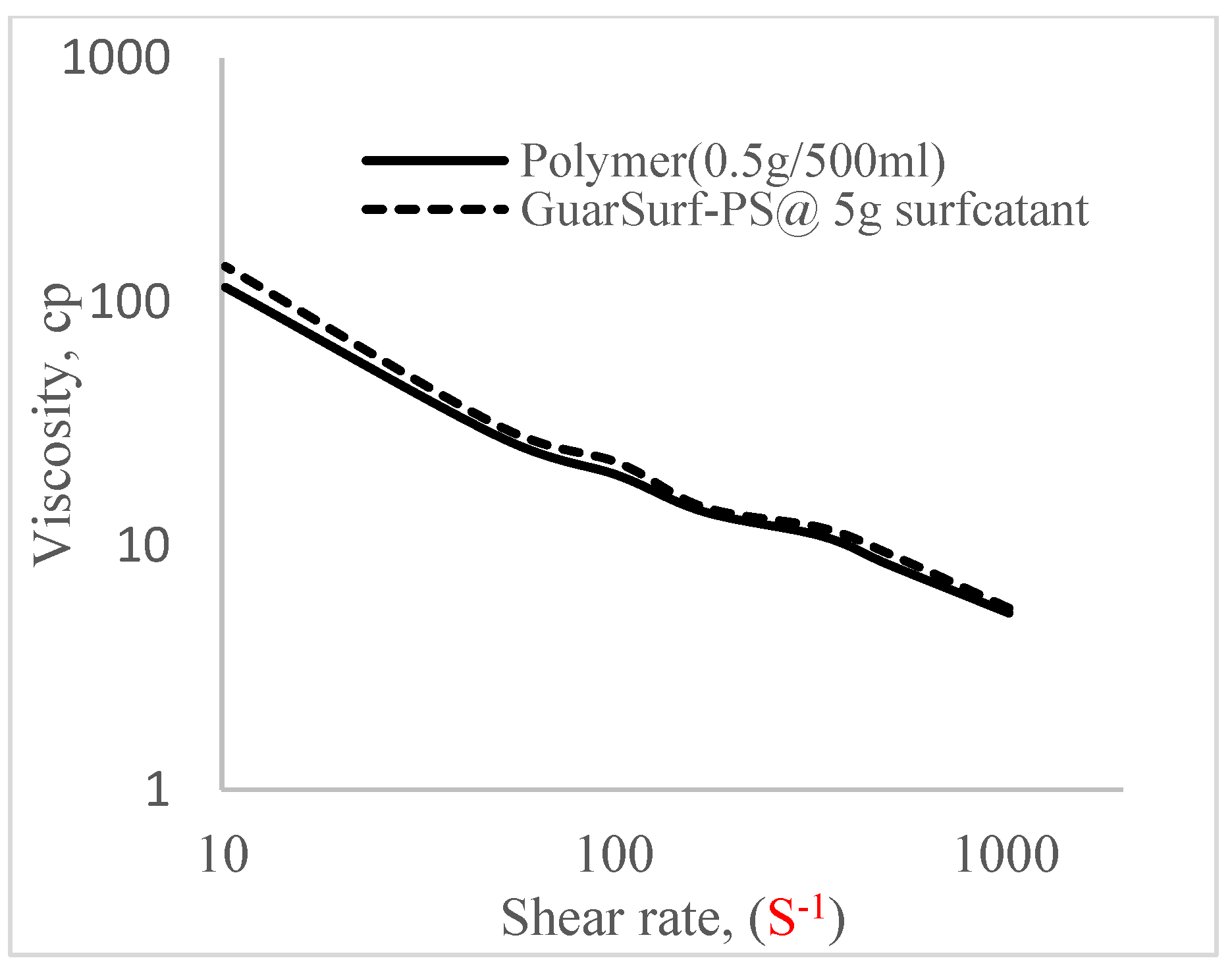
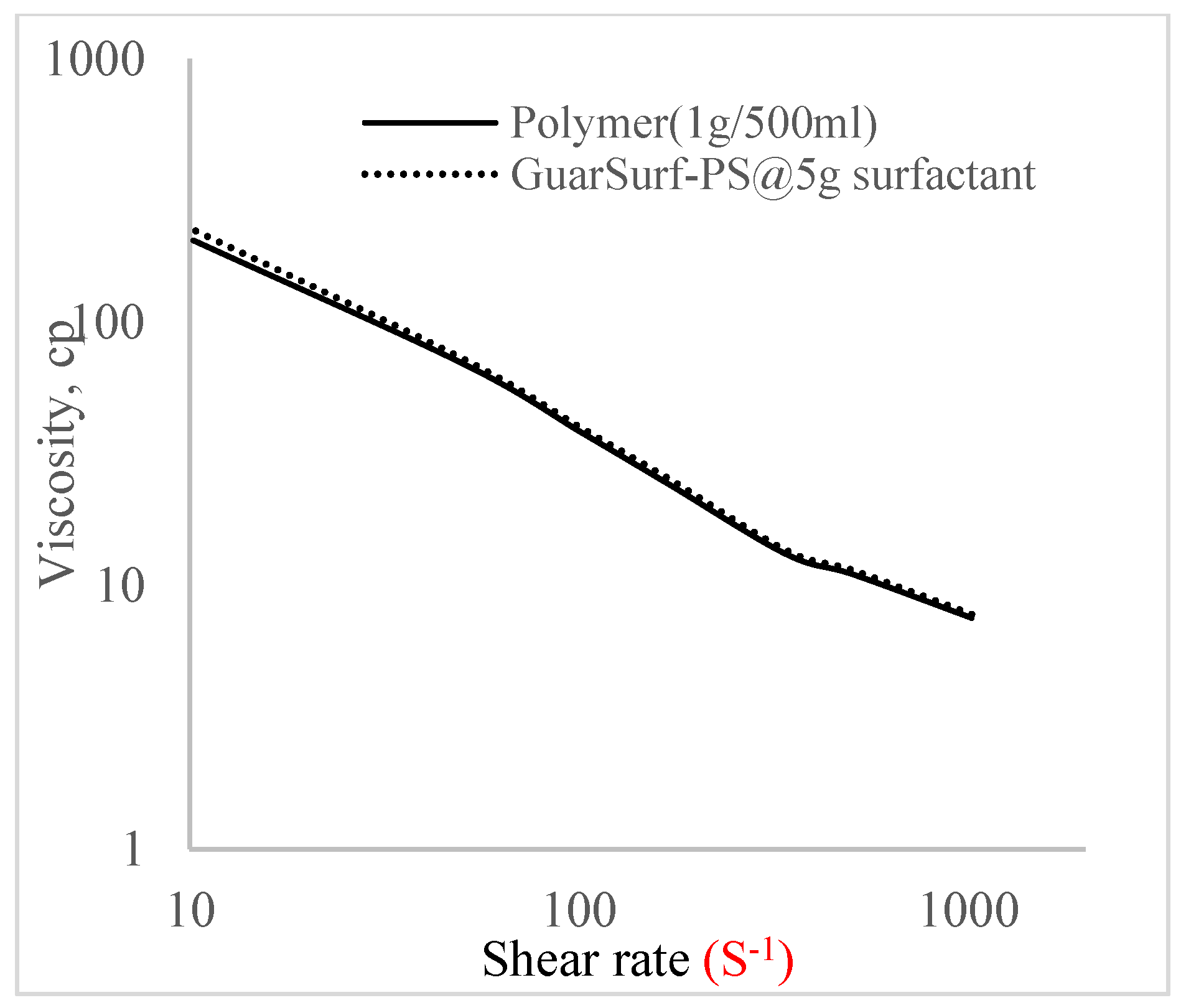
2.5. Rheological Properties of Biopolymers and Biosurfactants
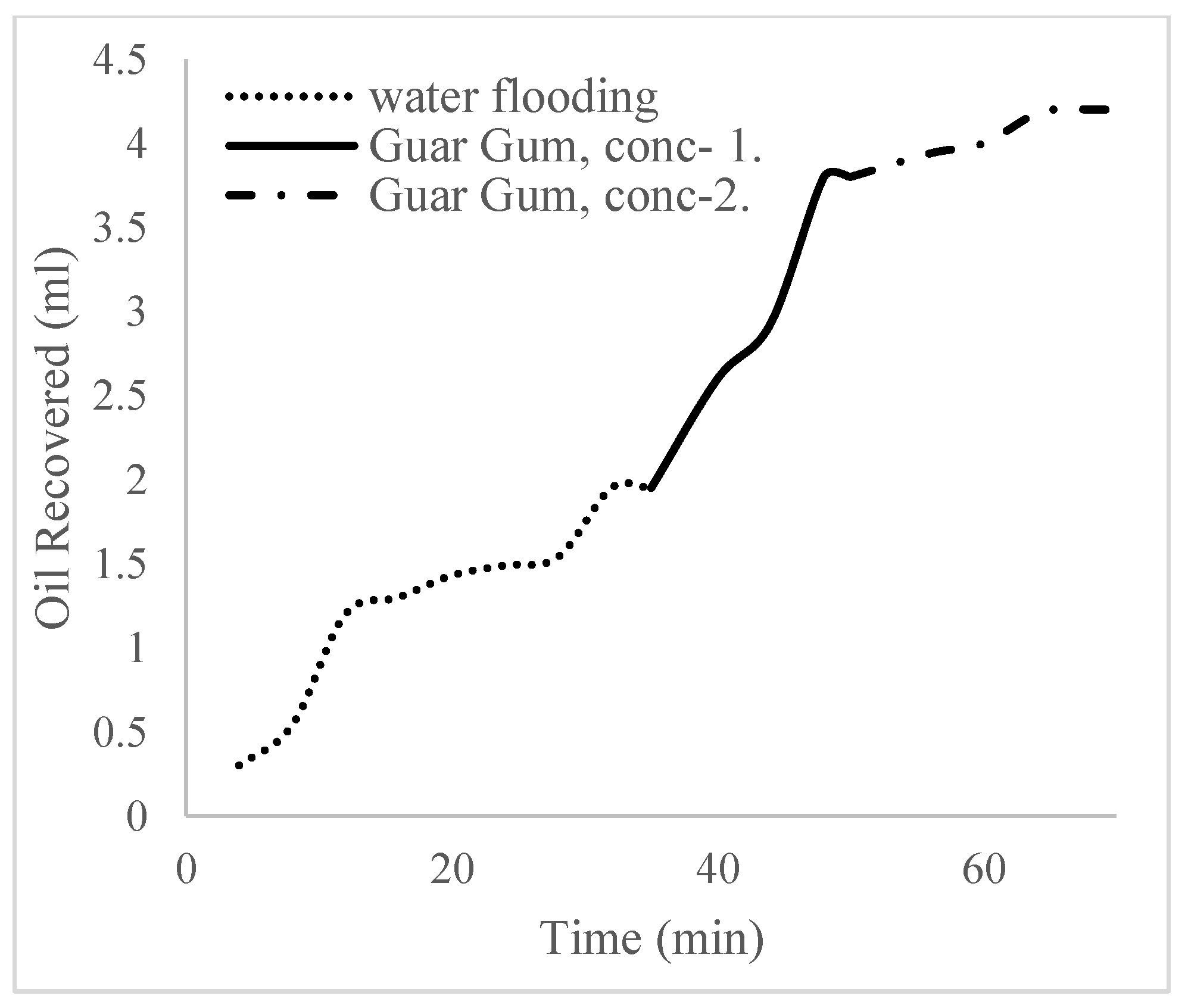
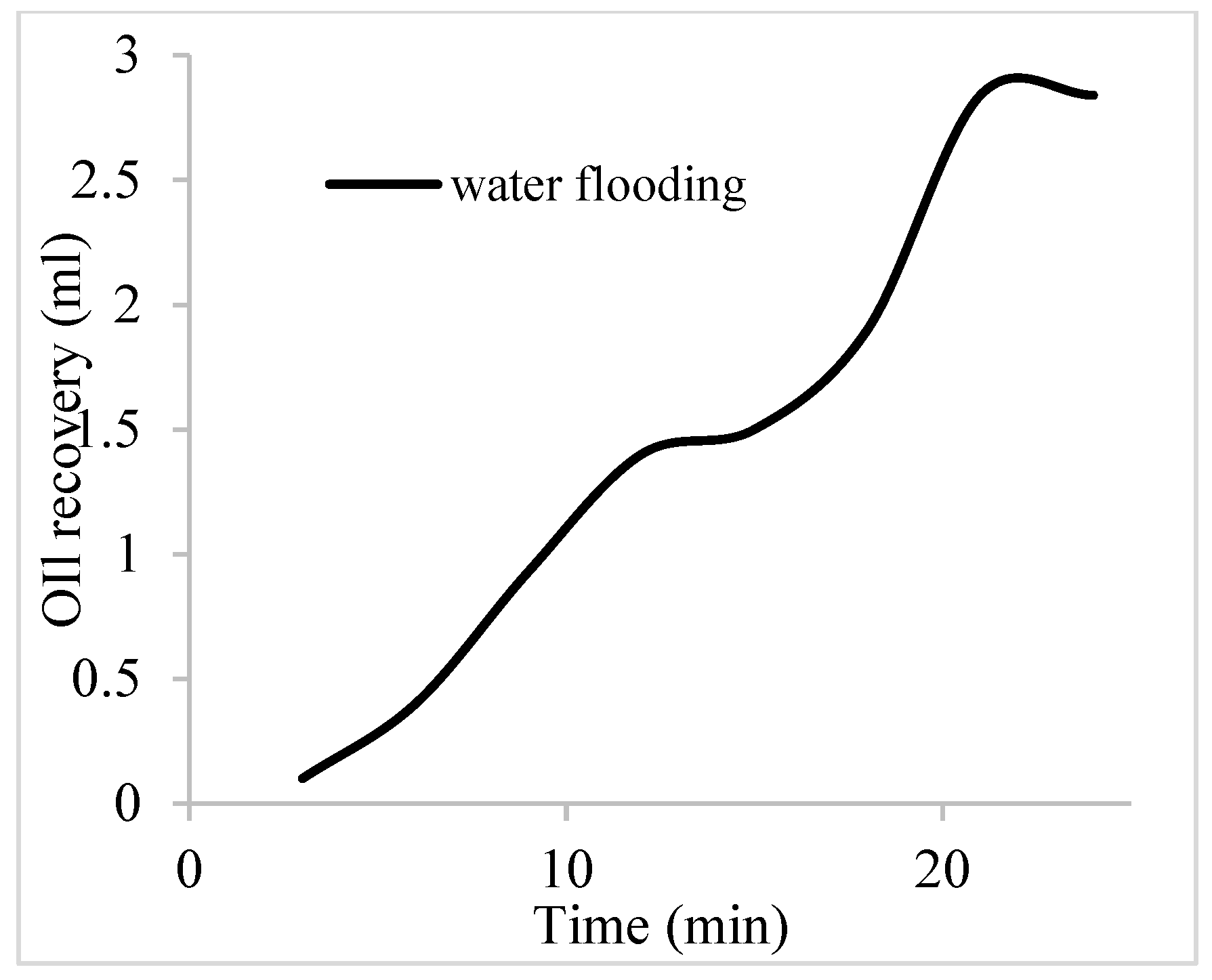
2.6. Water Flooding Procedure
2.7. Case Studies
- Polymer flooding used for core Z (at 1 wt%, 1.5 wt%, and 2 wt% of polymer).
- Polymeric surfactant flooding used for core C (at 5 wt% of surfactant and 1 wt%, 1.5 wt%, and 2 wt% of polymer).
- Polymeric surfactant flooding used for K (at 10 wt% of surfactant and 1 wt%, 1.5 wt%, and 2 wt% of polymer).
3. Results
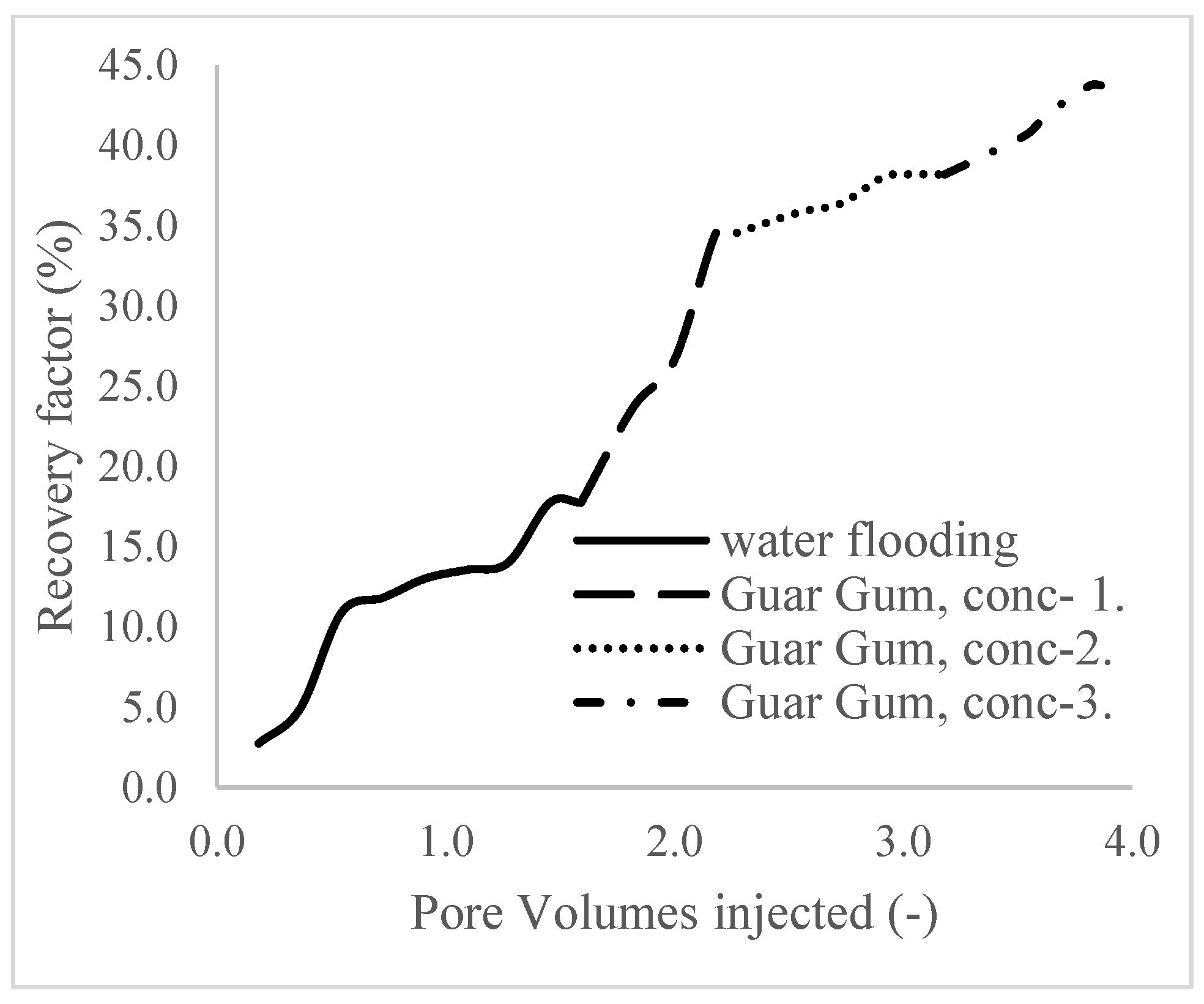
3.1. Case 1
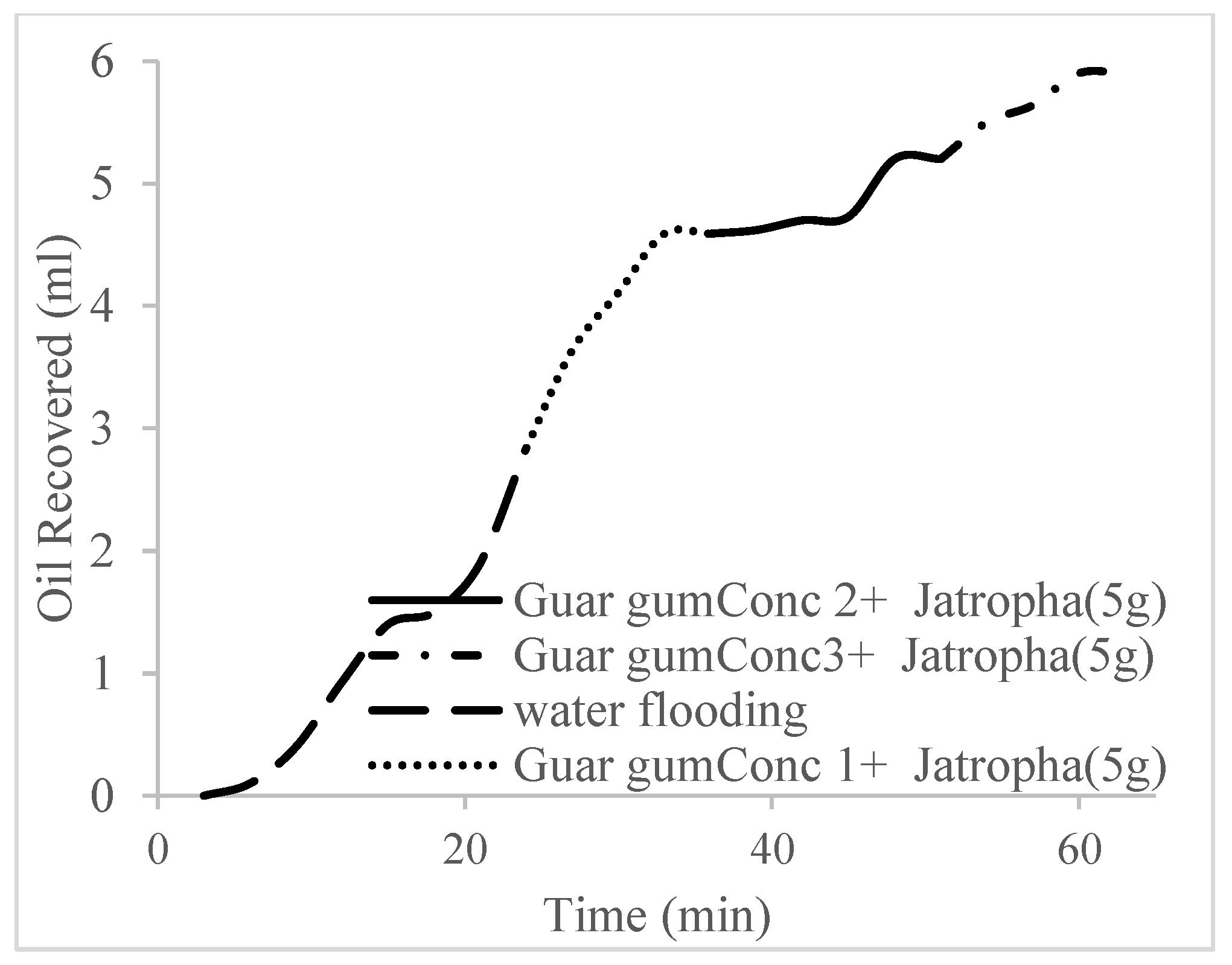
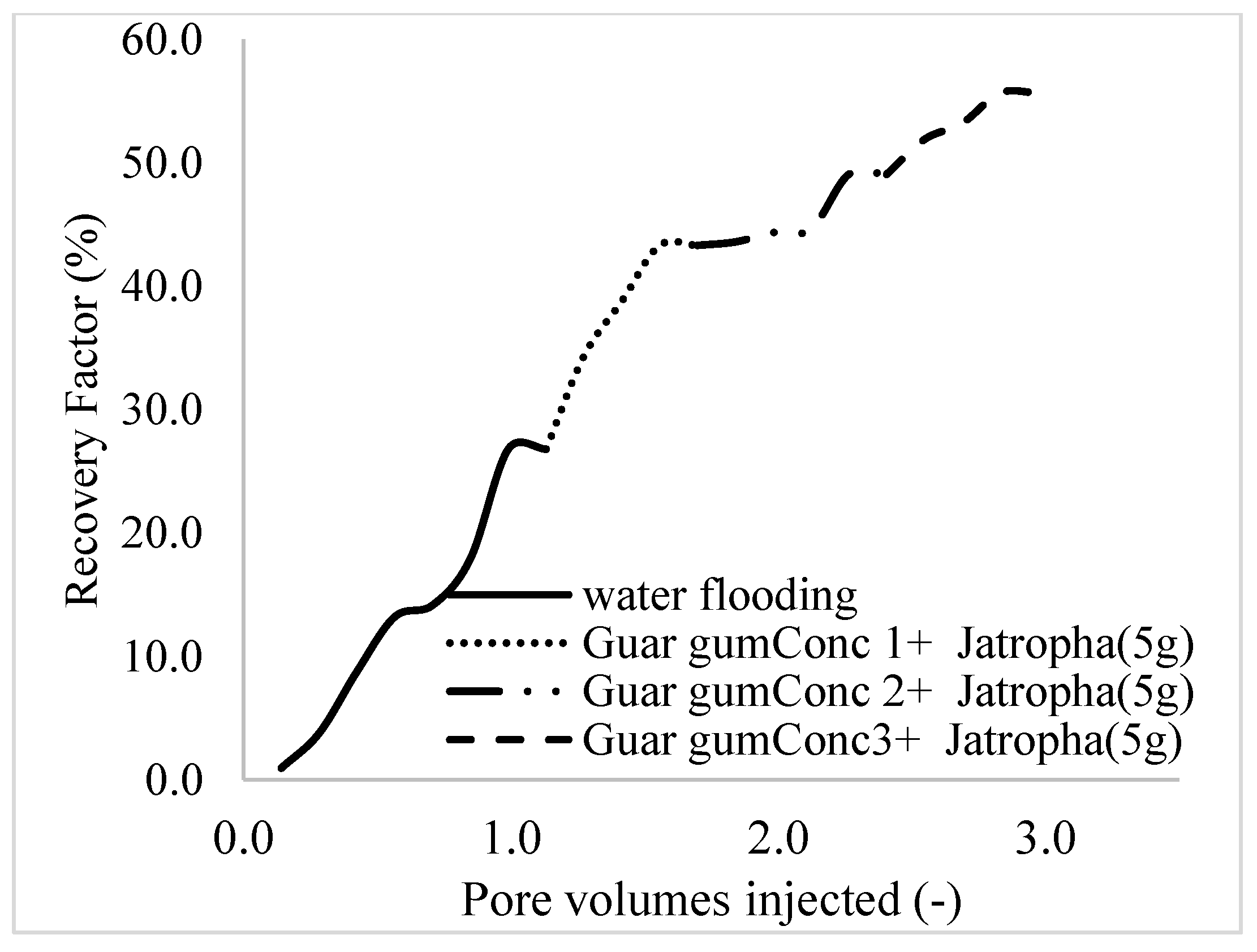
3.2. Case 2
3.3. Case 3
4. Conclusions and Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khojastehmehr, M.; Madani, M.; Daryasafar, A. Screening of enhanced oil recovery techniques for Iranian oil reservoirs using TOPSIS algorithm. Energy Rep. 2019, 5, 529–544. [Google Scholar] [CrossRef]
- Campbell, D.A. Enhanced Oil-recovery and Its Environmental and Economic Implications in the United States. Environ. Conserv. 1981, 8, 5–18. [Google Scholar] [CrossRef]
- Kovscek, A.R. Emerging challenges and potential futures for thermally enhanced oil recovery. J. Pet. Sci. Eng. 2012, 98, 130–143. [Google Scholar] [CrossRef]
- Hirasaki, G.J. Recent Advances in Surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Hirasaki, G.; Zhang, D.L. Surface Chemistry of Oil Recovery from Fractured, Oil-Wet, Carbonate Formations. SPE J. 2004, 9, 151–162. [Google Scholar] [CrossRef]
- Samanta, A.; Ojha, K.; Sarkar, A.; Mandal, A. Surfactant and Surfactant-Polymer Flooding for Enhanced Oil Recovery. Adv. Pet. Explor. Dev. 2011, 2, 13–18. [Google Scholar]
- Mokheimer, E.M.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. Technol. 2019, 141, 030801. [Google Scholar] [CrossRef]
- Lazar, I.; Petrisor, I.G.; Yen, T.F. Microbial Enhanced Oil Recovery (MEOR). Pet. Sci. Technol. 2007, 25, 1353–1366. [Google Scholar] [CrossRef]
- Hadi, S.; Clarence, A.M.; Michael, S.W.; James, M.T.; Rafael, V. Polymer Coated Nanoparticles for Enhanced Oil Recovery. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Abraham, V.D.; Orodu, O.D.; Efeovbokhan, V.E.; Olabode, O.A.; Ojo, T.I. The Influence of Surfactant Concentration and Surfactant Type on the Interfacial Tension of Heavy Crude Oil/Brine/Surfactant System. Pet. Coal. 2020, 62, 292–298. [Google Scholar]
- Olabode, O.A. Production forecast for Niger delta oil rim synthetic reservoirs. Data Brief 2018, 19, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Olabode, O.; Ojo, T.; Oguntade, T.; Oduwole, D. Recovery potential of biopolymer (B-P) formulation from Solanum tuberosum (waste) starch for enhancing recovery from oil reservoirs. Energy Rep. 2020, 6, 1448–1455. [Google Scholar] [CrossRef]
- Tabary, R.; Bazin, B.; Douarche, F.; Moreau, P.; Oukhemanou-Destremaut, F. Surfactant Flooding in Challenging Conditions: Towards Hard Brines and High Temperatures. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10–13 March 2013; p. SPE-164359-MS. [Google Scholar] [CrossRef]
- Arash, R.; Milad, S.; Ali, N.; Abdolnabi, H.; Shahab, A. Core flooding tests to investigate the effects of IFT reduction and wettability alteration on oil recovery during MEOR process in an Iranian oil reservoir. Appl. Microb. Cell Physiol. 2013, 97, 5979–5991. [Google Scholar]
- Liu, X. Experimental evaluation of polymer performance for tertiary oil recovery in oil field. IOP Conf. Ser. Earth Environ. Sci. 2021, 671, 012012. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- John, M.F.; Olabode, O.A.; Egeonu, G.I.; Ojo, T.I. Enhanced Oil Recovery of Medium Crude Oil (31Api) Using Nanoparticles and Polymer. Int. J. Appl. Eng. Res. 2017, 12, 8425–8435. [Google Scholar]
- Temiouwa, O.; Oluwasanmi, O.; Ifeanyi, S.; Tomiwa, O. Nano Augumented Biosurfactant Formulation for Oil Recovery in Medium Oil Reservoirs. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2018; p. SPE-193485-MS. [Google Scholar] [CrossRef]
- Zhang, J.; Misra, R.D.K. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core–shell nanoparticle carrier and drug release response. Acta Biomater. 2007, 3, 838–850. [Google Scholar] [CrossRef]
- Sidiq, H.; Abdulsalam, V.; Nabaz, Z. Reservoir simulation study of enhanced oil recovery by sequential polymer flooding method. Adv. Geo-Energy Res. 2019, 3, 115–121. [Google Scholar] [CrossRef]
- Olabode, O.; Akinsanya, O.; Daramola, O.; Sowunmi, A.; Osakwe, C.; Benjamin, S.; Samuel, I. Effect of Salt Concentration on Oil Recovery during Polymer Flooding: Simulation Studies on Xanthan Gum and Gum Arabic. Polymers 2023, 15, 4013. [Google Scholar] [CrossRef]
- Skauge, T.; Hetland, S.; Spildo, K.; Skauge, A. Nano-sized particles for EOR. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; p. SPE-129933. [Google Scholar]
- Sensoy, T.; Chenevert, M.E.; Sharma, M.M. Minimizing water invasion in shale using nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; p. SPE-124429. [Google Scholar]
- Alvani, A.; Jouyban, A.; Shayanfar, A. The effect of surfactant and polymer on solution stability and solubility of tadalafil-methylparaben cocrystal. J. Mol. Liq. 2019, 281, 86–92. [Google Scholar] [CrossRef]
- Gogoi, S.B. Adsorption–Desorption of Surfactant for Enhanced Oil Recovery. Transp. Porous Media 2011, 90, 589–604. [Google Scholar] [CrossRef]
- Madani, M. Fundamental investigation of an environmentally friendly surfactant agent for chemical enhanced oil recovery. Fuel 2019, 238, 186–197. [Google Scholar] [CrossRef]
- Elraies, K.A.; Tan, I.M.; Fathaddin, M.T.; Abo-Jabal, A. Development of a New Polymeric Surfactant for Chemical Enhanced Oil Recovery. Pet. Sci. Technol. 2011, 29, 1521–1528. [Google Scholar] [CrossRef]
- Saxena, N. Bio-based surfactant for enhanced oil recovery: Interfacial properties, emulsification and rock-fluid interactions. J. Pet. Sci. Eng. 2019, 176, 299–311. [Google Scholar] [CrossRef]
- Pal, N. Phase behaviour and characterization of micro emulsion stabilized by a novel synthesized surfactant: Implications for enhanced oil recovery. Fuel 2019, 235, 995–1009. [Google Scholar] [CrossRef]
- Ayoub, M. A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability 2021, 13, 788. [Google Scholar] [CrossRef]
- Faria, D. Extraction of radish seed oil (Raphanus sativus L.) and evaluation of its potential in biodiesel production. AIMS Energy 2018, 6, 551–565. [Google Scholar] [CrossRef]
- Muentes, S.A.G.; Ávila, M.G.G.; Vázquez, B.L.L.; del Campo Laffita, A.E.S. The production of biodiesel from Jatropha curca and its social impact. Int. Res. J. Eng. IT Sci. Res. 2017, 3, 89–98. [Google Scholar] [CrossRef]
- Castañeda, I.; Bojacá, V.; Ramos Borda, G.E.; Navarro, S.; Marìa, A.; Acevedo Pabon, P.A. Study of the eco-efficiency of biodiesel production from the fruit of the Jatropha curcas plant. Chem. Eng. 2017, 58, 493–498. [Google Scholar]
- Huang, J.; Jiang, P.; Wen, Y.; Deng, J.; He, J. Soy-castor oil-based polyurethanes with octaphenylsilsesquioxanetetraol double-decker silsesquioxane in the main chains. RSC Adv. 2016, 6, 69521–69529. [Google Scholar] [CrossRef]
- Awang, M.; Diyanti, I. The Production of Surfactant by Direct Reaction of Pyrolysis Oil for Enhanced Oil Recovery. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1540–1546. [Google Scholar] [CrossRef]
- Kumar, S. Synthesis and evaluation of physicochemical properties of anionic polymeric surfactant derived from Jatropha oil for application in enhanced oil recovery. J. Ind. Eng. Chem. 2016, 43, 106–116. [Google Scholar] [CrossRef]
- Fei, D.; Guo, J.; Xiong, R.; Zhang, X.; Kang, C.; Kiyingi, W. Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery. Polymers 2023, 15, 4606. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Koopal, L.K. Adsorption of Cationic and Anionic Surfactants on Metal Oxide Surfaces: Surface Charge Adjustment and Competition Effects. J. Colloid Interface Sci. 1996, 177, 478–489. [Google Scholar] [CrossRef]
- Guo, J.; Wang, F.; Zhao, Y.; Wang, P.; Wang, T.; Yang, J.; Yang, B.; Ma, L. A Laboratory Experimental Study on Enhancing the Oil Recovery Mechanisms of Polymeric Surfactants. Molecules 2024, 29, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.D.K.; Megawati, R.; Setyaningrum, V.K.; Putri, E.W.; Handayani, A.S.; Solikhah, M.D.; Chafidz, A. Investigating potential application of bio-based polymeric surfactant using methyl ester from palm oil for chemical enhanced oil recovery (CEOR). Commun. Sci. Technol. 2023, 8, 235–242. [Google Scholar] [CrossRef]
- Wibowo, A.D.K.; Yoshi, L.A.; Handayani, A.S.; Joelianingsih. Synthesis of polymeric surfactant from palm oil methyl ester for enhanced oil recovery application. Colloid Polym. Sci. 2021, 299, 81–92. [Google Scholar] [CrossRef]
- Li, B.; Liu, Z.; Fei, C.; Lv, J.; Chen, X.; Li, Y. Polymeric Surfactant for Enhanced Oil Recovery-Microvisual, Core-Flood Experiments and Field Application. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar] [CrossRef]
- Babu, K.; Pal, N.; Saxena, V.K.; Mandal, A. Synthesis and characterization of a new polymeric surfactant for chemical enhanced oil recovery. Korean J. Chem. Eng. 2016, 33, 711–719. [Google Scholar] [CrossRef]
- Olabode, O.A. Effect of water and gas injection schemes on synthetic oil rim models. J. Pet. Explor. Prod. Technol. 2020, 10, 1343–1358. [Google Scholar] [CrossRef]
- Sharma, T.; Suresh Kumar, G.; Sangwai, J.S. Enhanced oil recovery using oil-in-water (o/w) emulsion stabilized by nanoparticle, surfactant, and polymer in the presence of NaCl. Geosyst. Eng. 2014, 17, 195–205. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Olabode, O.A.; Isehunwa, S.; Orodu, O.; Rotimi, T.; Mamudu, A. Effect of foam and WAG (water alternating gas) injection on performance of thin oil rim reservoirs. J. Pet. Sci. Eng. 2018, 171, 1443–1454. [Google Scholar] [CrossRef]
- Yan, L. Impact of water salinity differential on a crude oil droplet constrained in a capillary: Pore-scale mechanisms. Fuel 2020, 274, 11779. [Google Scholar] [CrossRef]
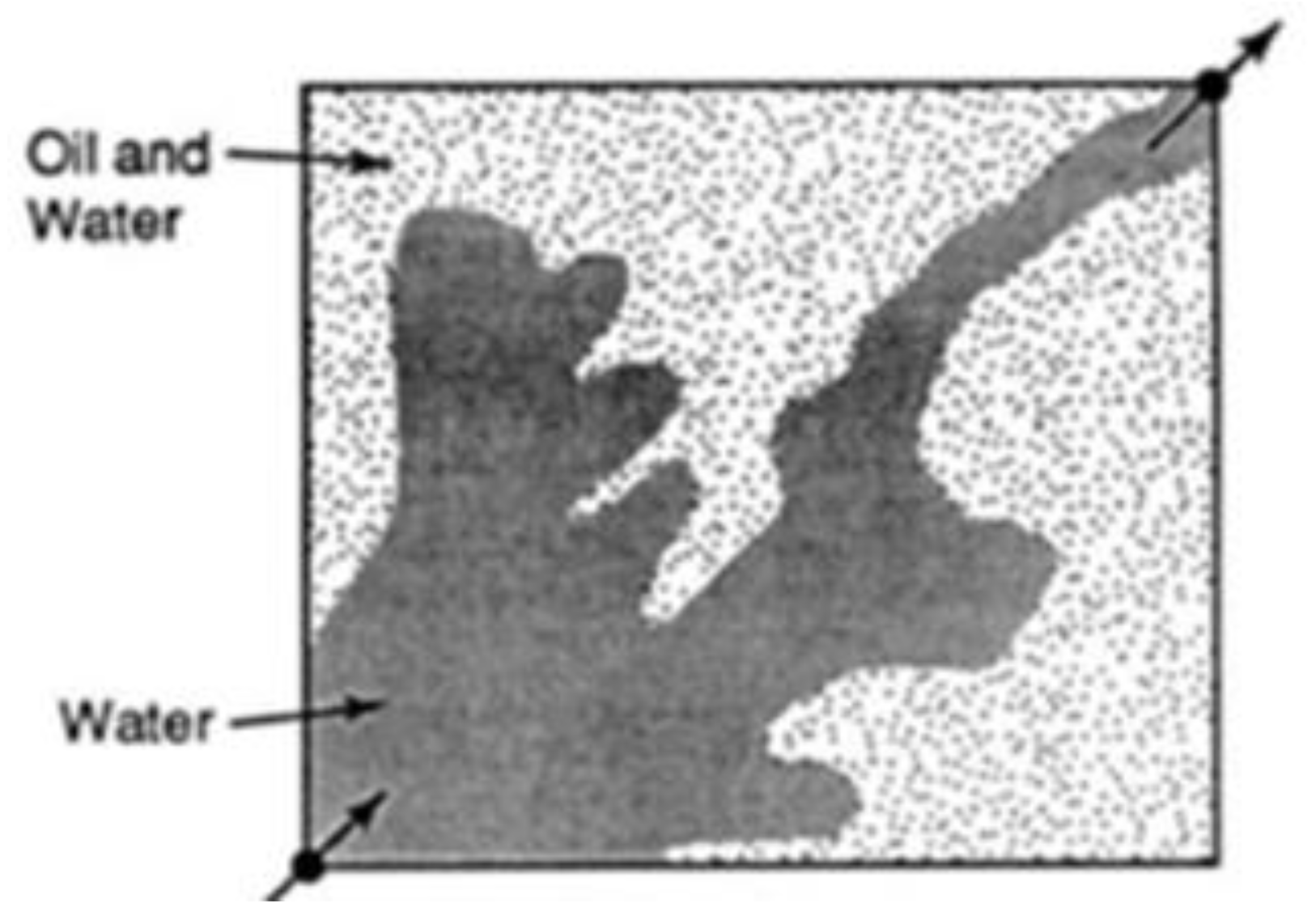
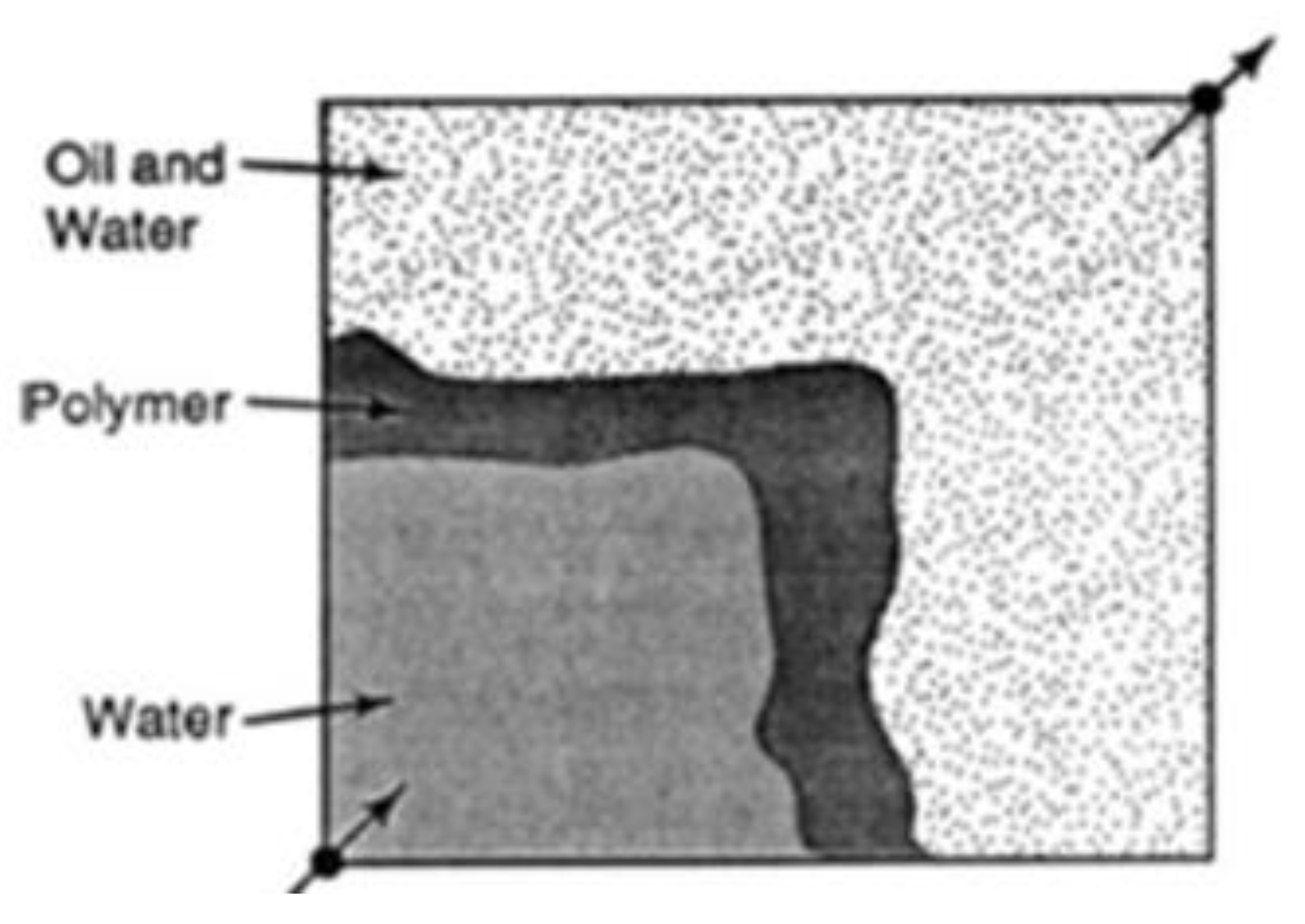
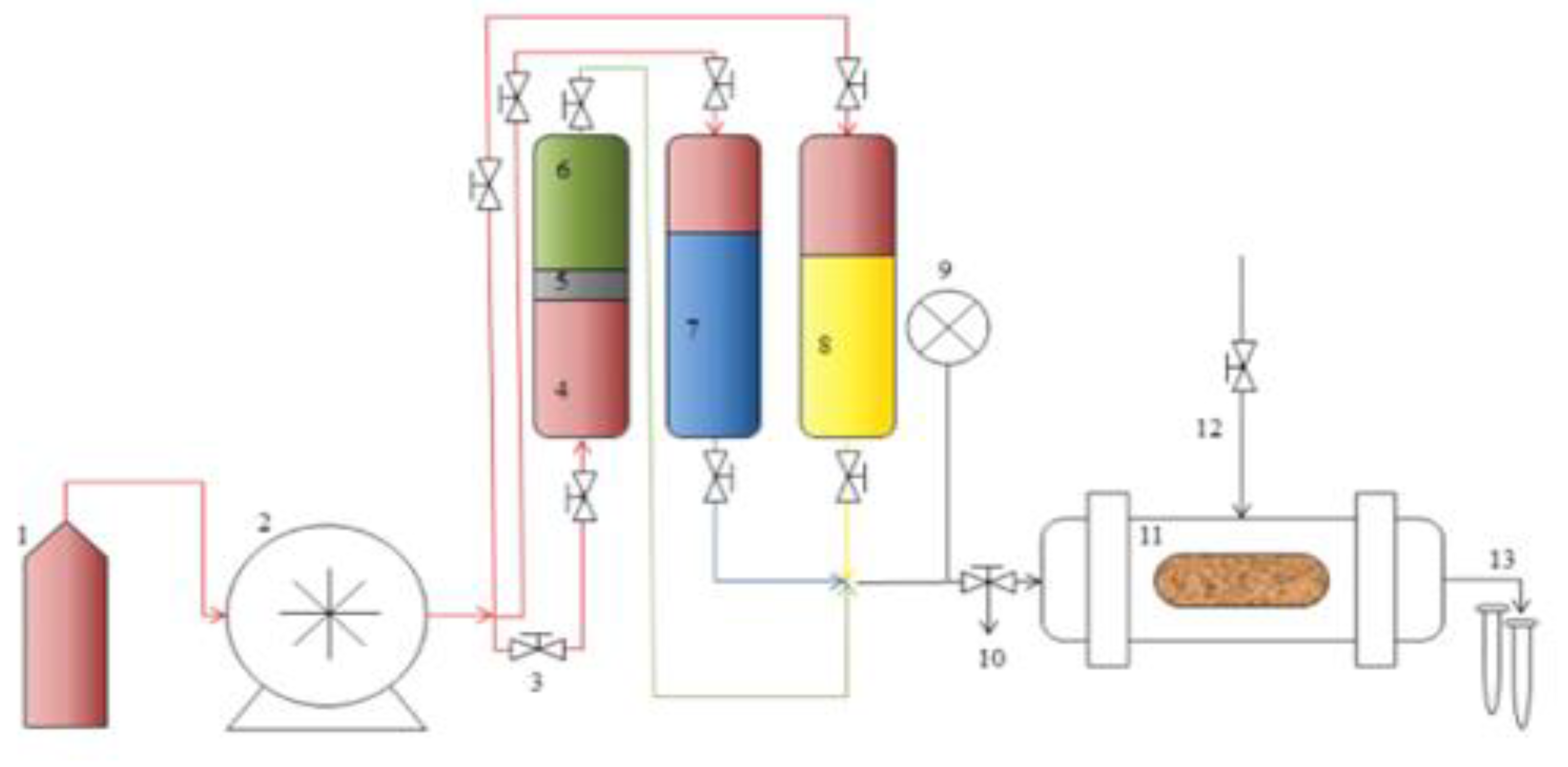

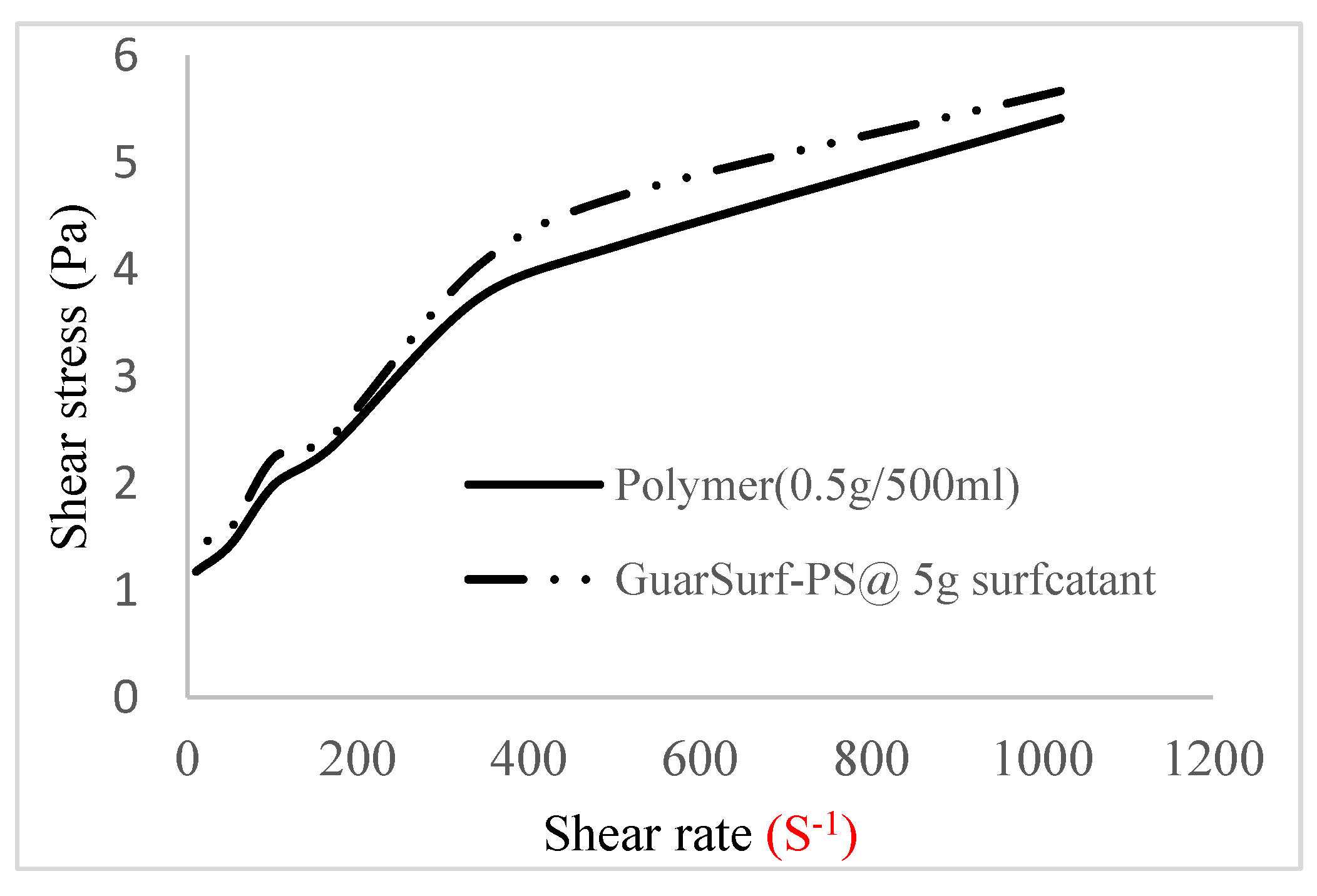










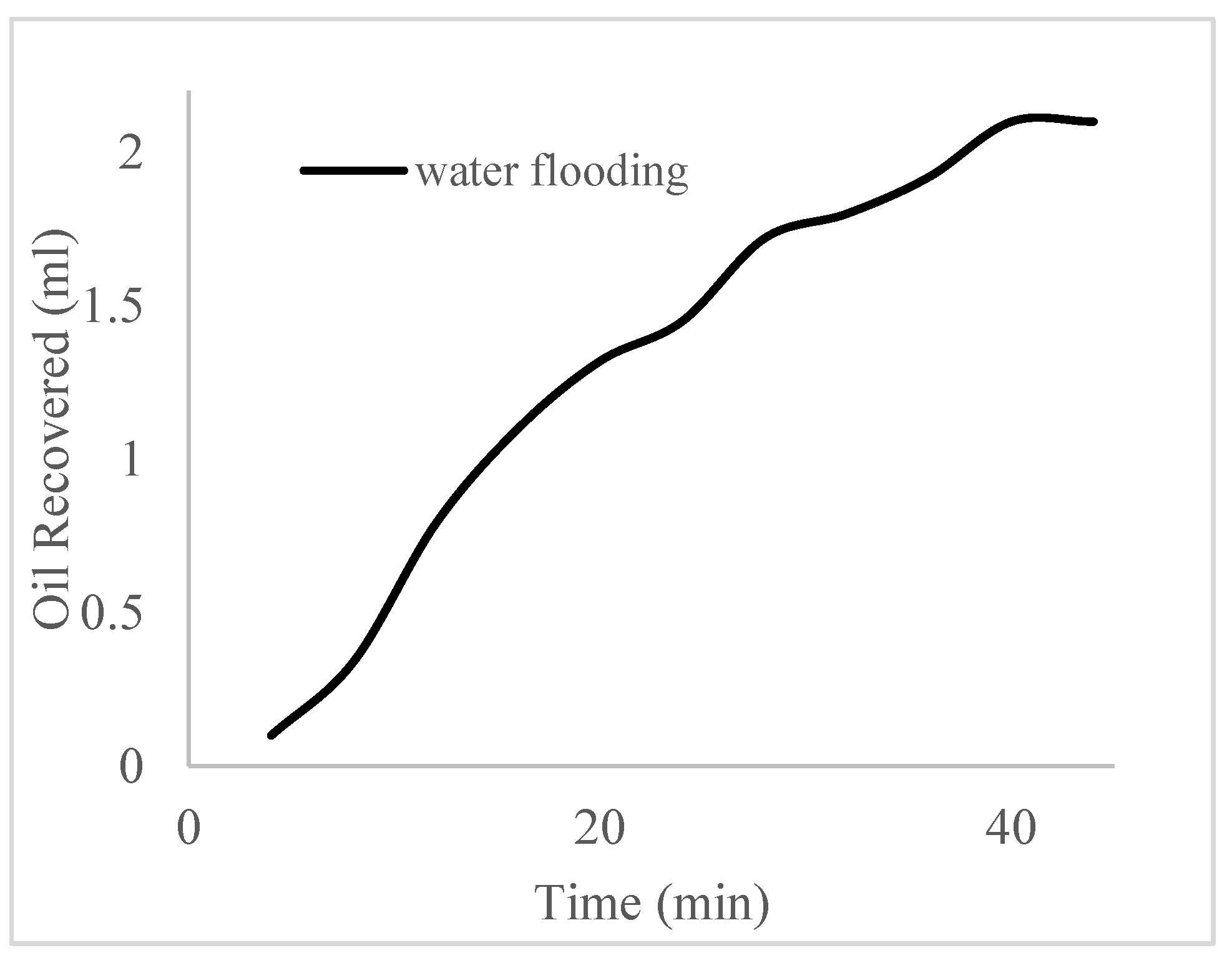
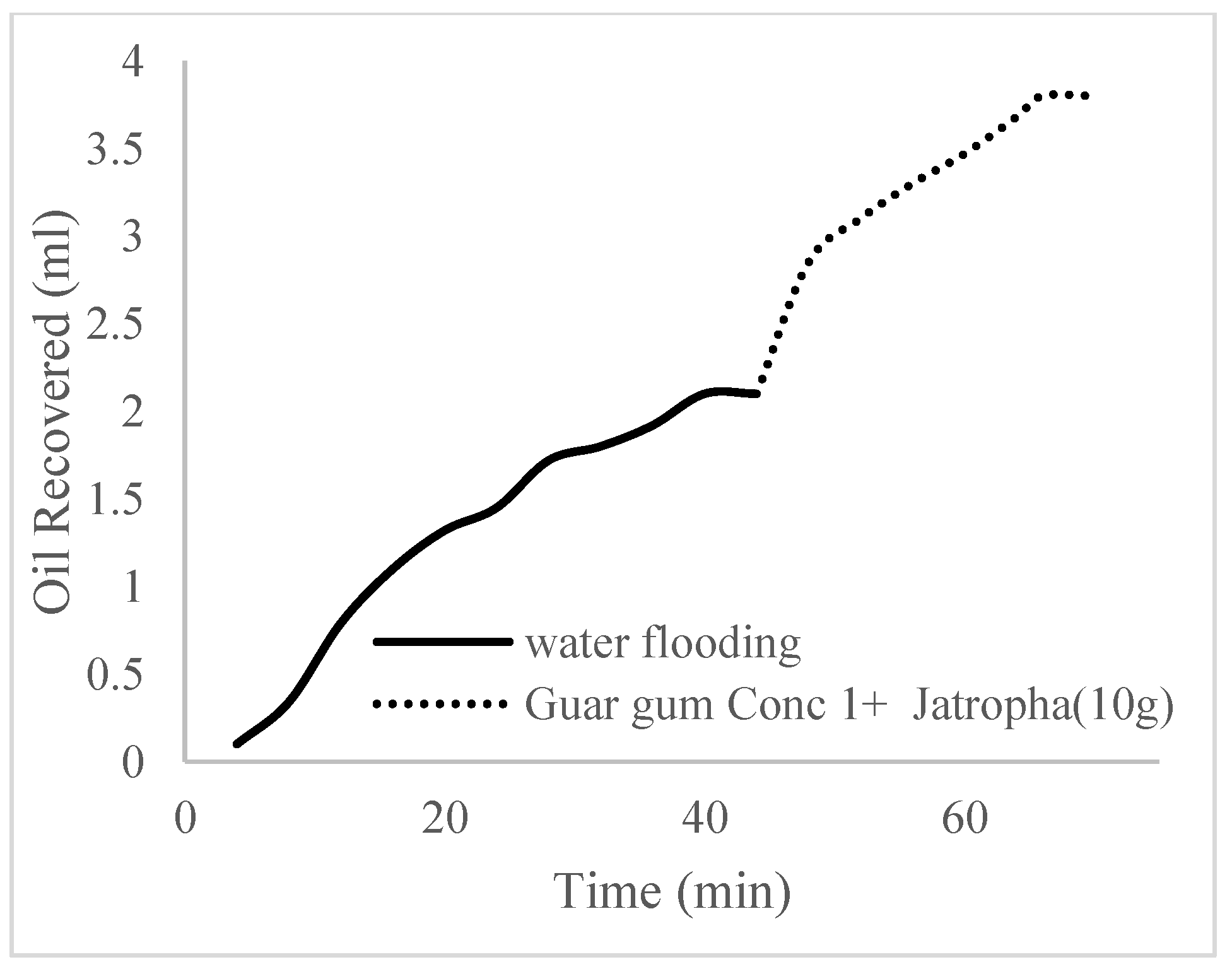
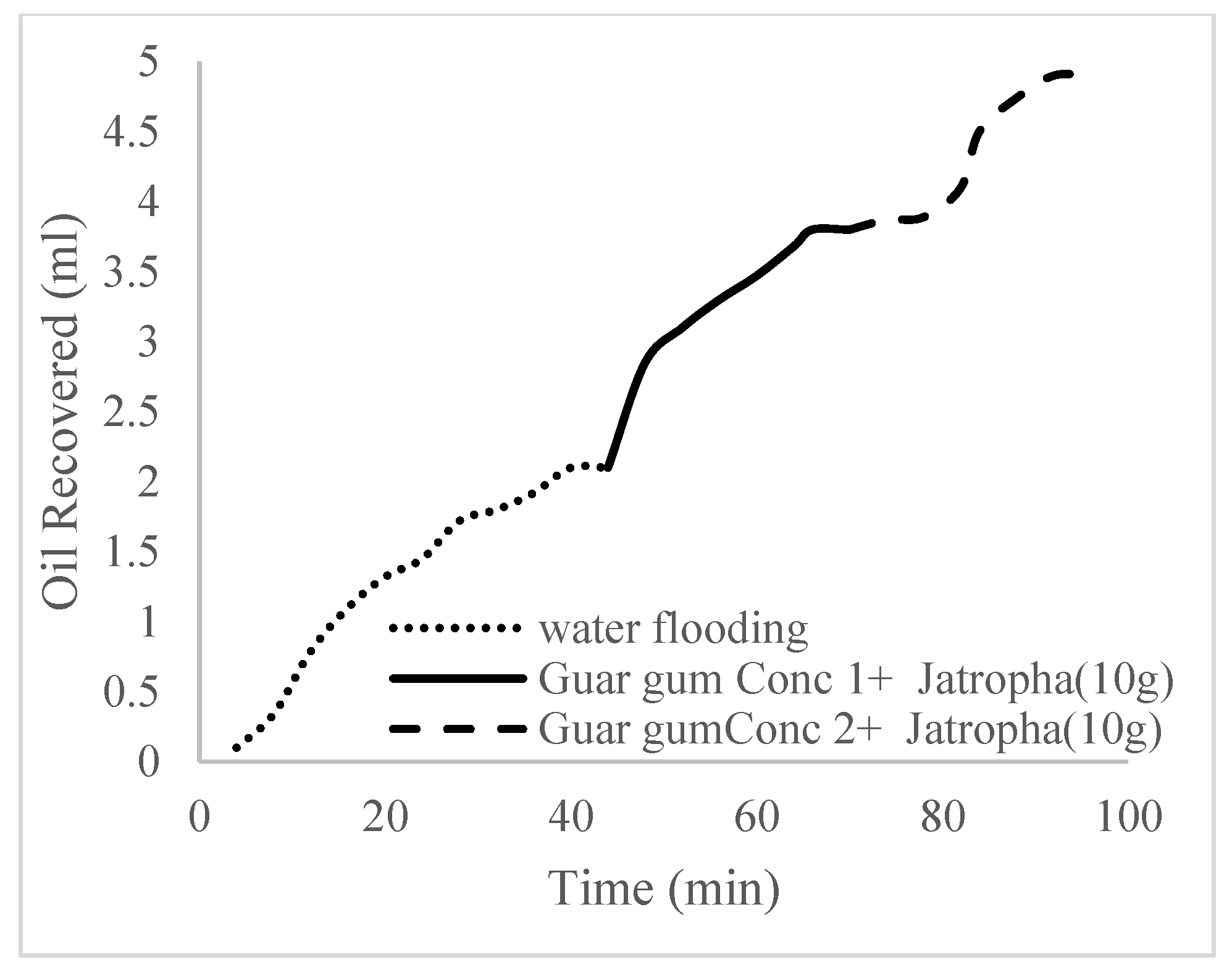

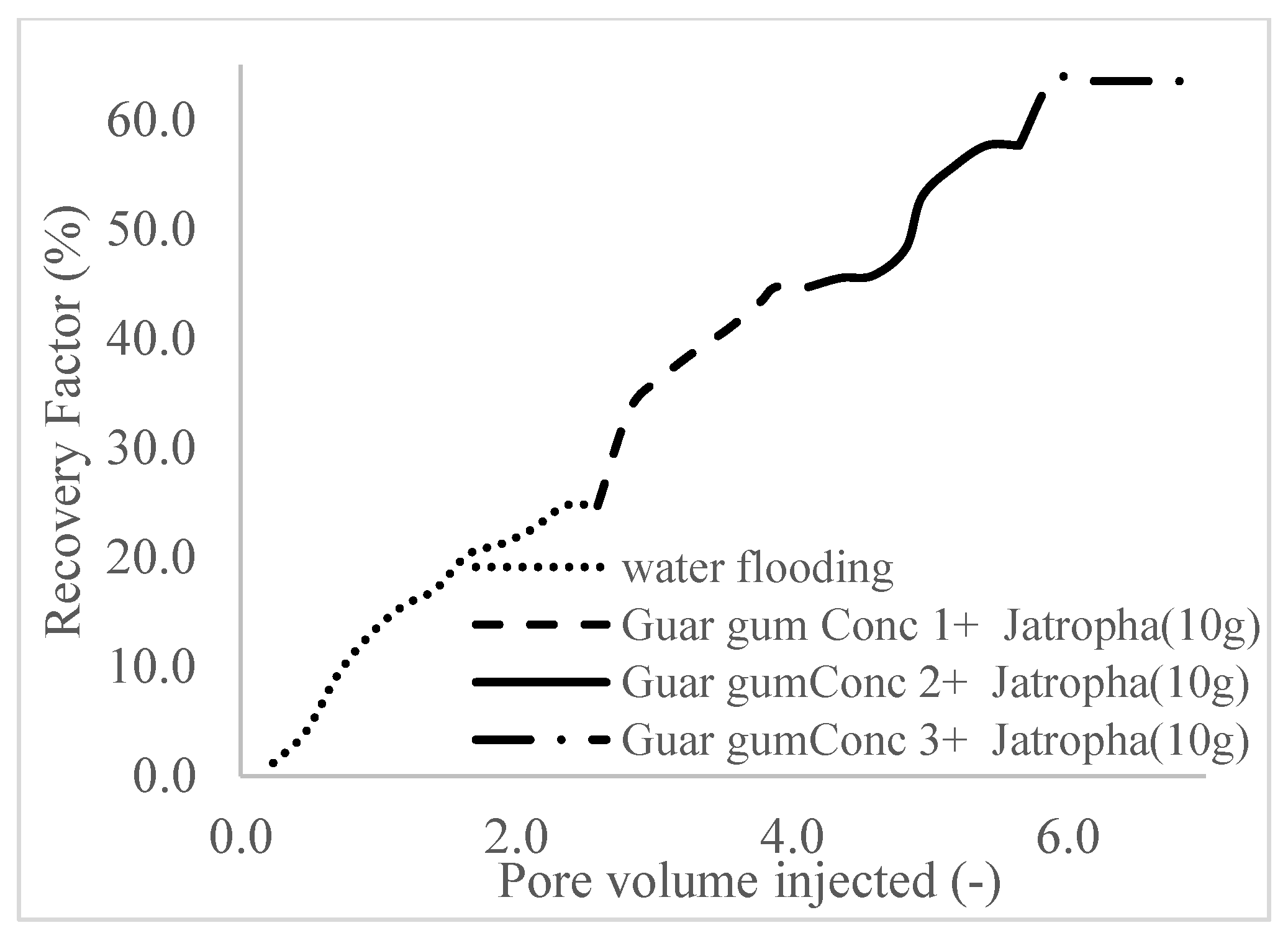

| Polymer Concentrations (g/cc) | 2.0 | 1.5 | 1.0 | 0.5 |
| Sample | Length (cm) | Diameter (cm) | Pore Vol. (mL) | Bulk Vol. (mL) | Poro. | Perm. | Initial Oil Vol. | So | Sw |
|---|---|---|---|---|---|---|---|---|---|
| Core C | 4.7 | 3.5 | 10.6 | 45.2 | 0.23 | 285 | 23.13 | 0.51 | 0.49 |
| Core K | 2.2 | 3.5 | 9 | 21.1 | 0.42 | 290 | 11.69 | 0.55 | 0.45 |
| Core Z | 5 | 3.5 | 11.5 | 48.1 | 0.2 | 158 | 26.78 | 0.56 | 0.44 |
| Property | Value |
|---|---|
| Acid value (mg KOH/g of oil) | 6.04 |
| Color | Golden-yellow |
| Odor | Unpleasant |
| pH | 6.34 |
| Saponification value (mg KOH/g of oil) | 198.69 |
| Specific gravity | 0.92 |
| Viscosity at room temperature (cp) | 36 |
| Saturated | Fatty Acid | Percentage (%) |
|---|---|---|
| C16:0 | Palmitic acid | 13.75 |
| C18:0 | Stearic acid | 6.68 |
| Unsaturated | ||
| C18:1 | Oleic acid | 44.16 |
| C18:2 | Linoleic acid | 34.75 |
| C16:1 | Palmitoleic acid | 0.65 |
| Oil Recovery (%) | Incremental Oil Recovery (%) | Pore Vol. Injected (mL) | Oil Recovered (mL) | Residual Vol. (mL) | |
|---|---|---|---|---|---|
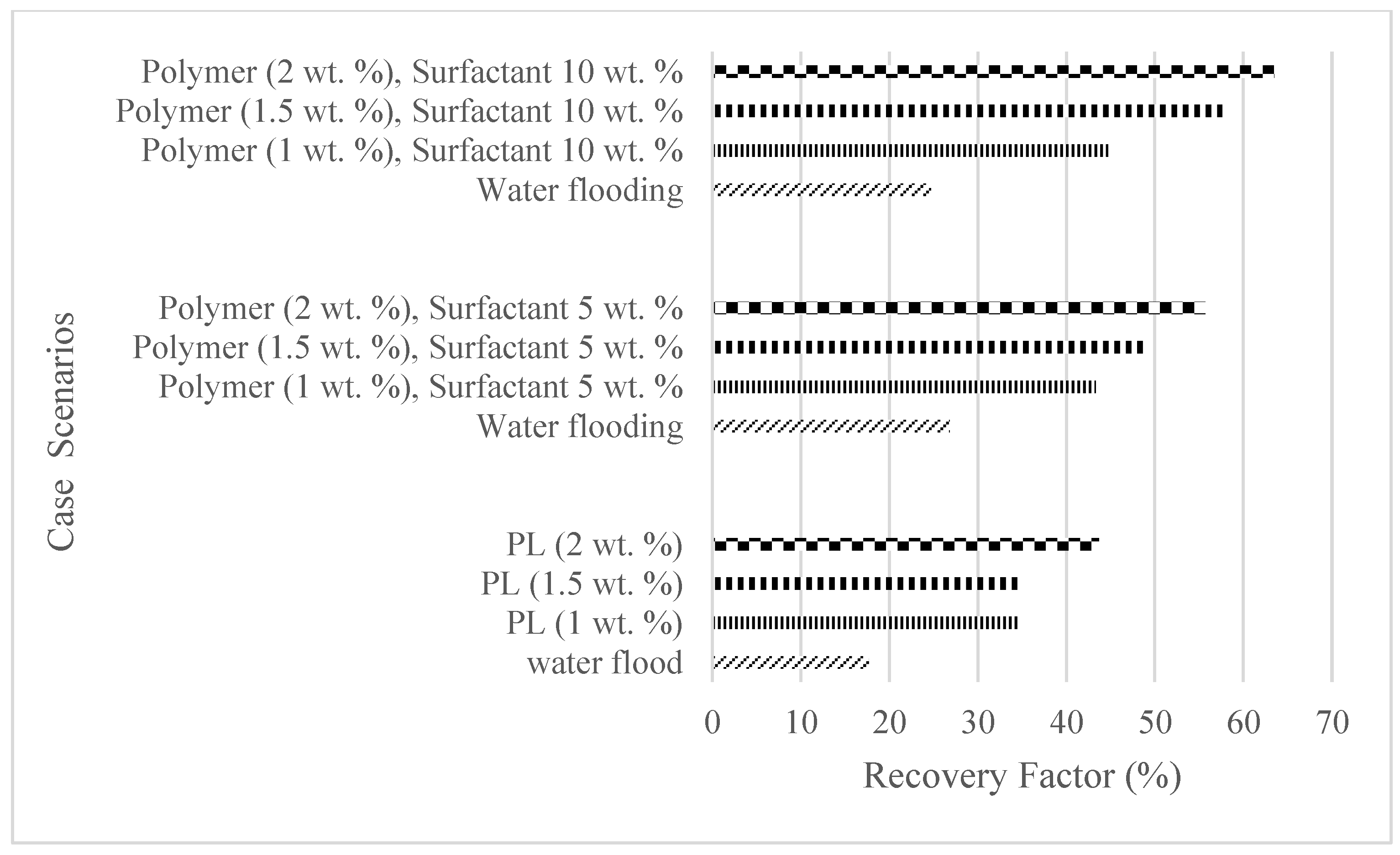
| Water flooding | 17.7 | 0 | 1.6 | 1.95 | 24.83 |
| Polymer (1 wt%) | 34.5 | 16.8 | 2.3 | 3.8 | 22.98 |
| Polymer (1.5 wt%) | 34.7 | 17 | 3.2 | 4.2 | 22.58 |
| Polymer (2 wt%) | 43.7 | 26 | 3.9 | 4.81 | 21.97 |
| Oil Recovery (%) | Incremental Oil Recovery (%) | Pore Volume Injected | Oil Recovered (mL) | Residual Volume | |
|---|---|---|---|---|---|
| Water flooding | 26.8 | 0 | 1.1 | 2.84 | 20.29 |
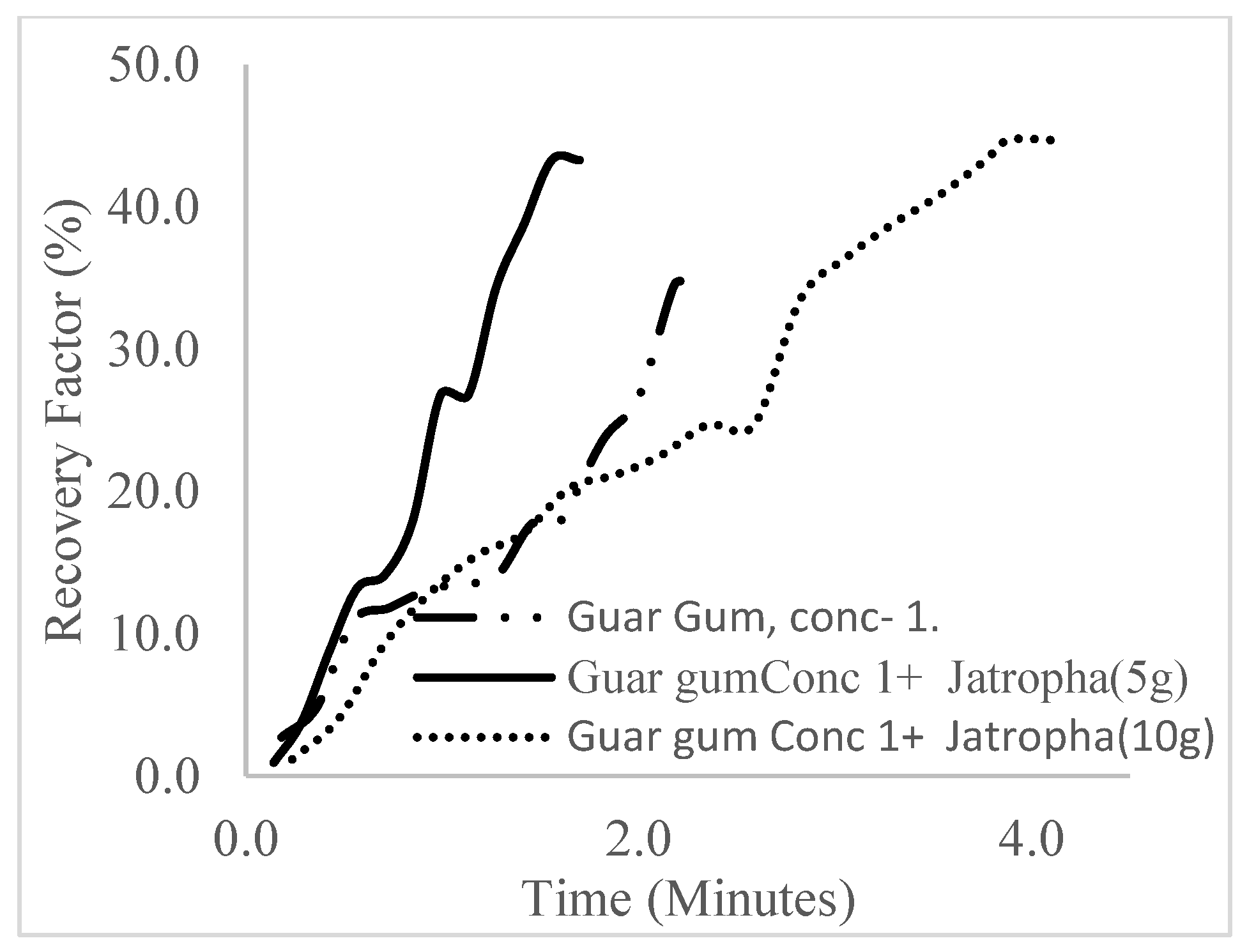
| Polymer (1 wt%), Surfactant (5 wt%) | 43.3 | 16.5 | 1.7 | 4.59 | 18.54 |
| Polymer (1.5 wt%), Surfactant (5 wt%) | 49.1 | 22.3 | 2.4 | 5.2 | 17.93 |
| Polymer (2 wt%), Surfactant (5 wt%) | 55.7 | 28.9 | 3.0 | 5.9 | 17.23 |
| Oil Recovery (%) | Incremental Oil Recovery (%) | Pore Vol. Inj. | Oil Recovery (mL) | Residual Volume | |
|---|---|---|---|---|---|
| Water flooding | 24.7 | 0 | 2.6 | 2.1 | 9.59 |
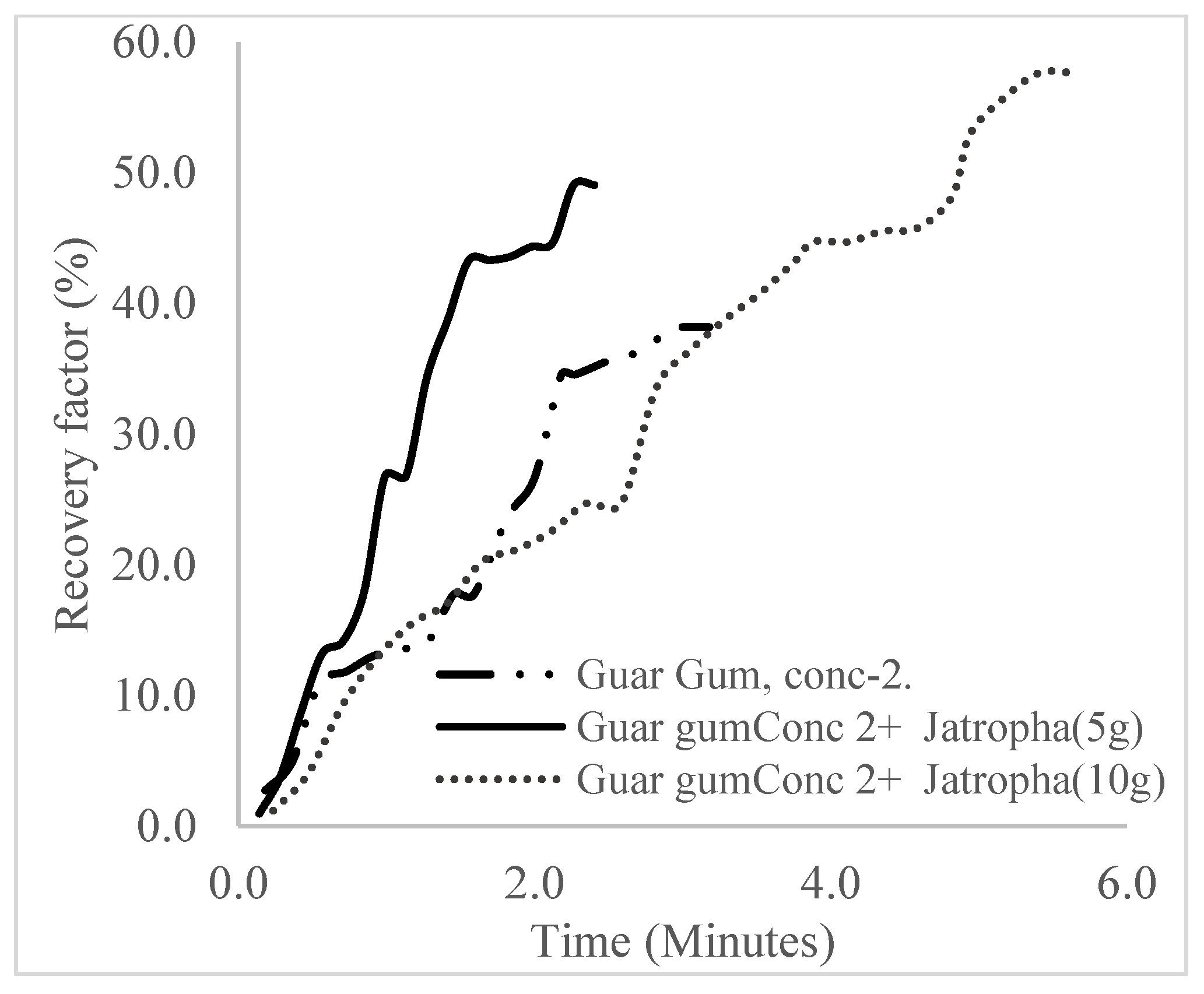
| Polymer (1 wt%), Surfactant (10 wt%) | 44.7 | 20 | 4.1 | 3.8 | 7.89 |
| Polymer (1.5 wt%), Surfactant (10 wt%) | 57.6 | 32.9 | 5.6 | 4.9 | 6.79 |
| Polymer (2 wt%), Surfactant (10 wt%) | 63.5 | 38.8 | 6.8 | 5.4 | 6.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabode, O.; Dike, H.; Olaniyan, D.; Oni, B.; Faleye, M. Experimental Investigation of the Effect of Surfactant–Polymer Flooding on Enhanced Oil Recovery for Medium Crude Oil. Polymers 2024, 16, 1674. https://doi.org/10.3390/polym16121674
Olabode O, Dike H, Olaniyan D, Oni B, Faleye M. Experimental Investigation of the Effect of Surfactant–Polymer Flooding on Enhanced Oil Recovery for Medium Crude Oil. Polymers. 2024; 16(12):1674. https://doi.org/10.3390/polym16121674
Chicago/Turabian StyleOlabode, Oluwasanmi, Humphrey Dike, Damilola Olaniyan, Babalola Oni, and Michael Faleye. 2024. "Experimental Investigation of the Effect of Surfactant–Polymer Flooding on Enhanced Oil Recovery for Medium Crude Oil" Polymers 16, no. 12: 1674. https://doi.org/10.3390/polym16121674






