Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts
Abstract
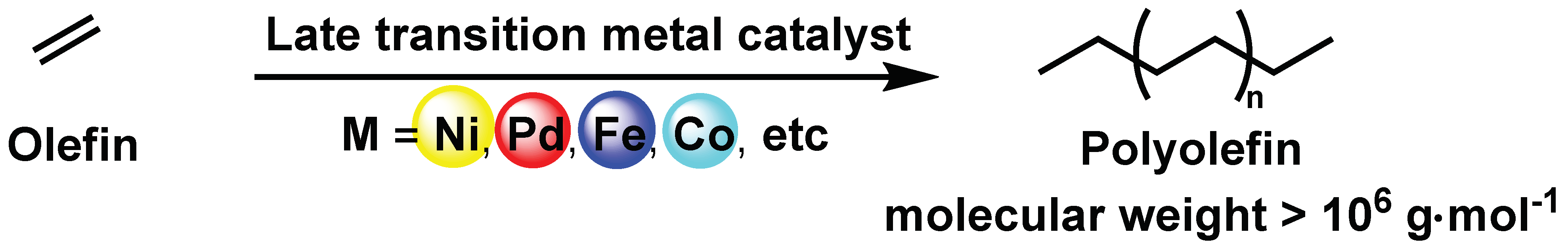
:1. Introduction
2. Late Transition Metal Catalysts
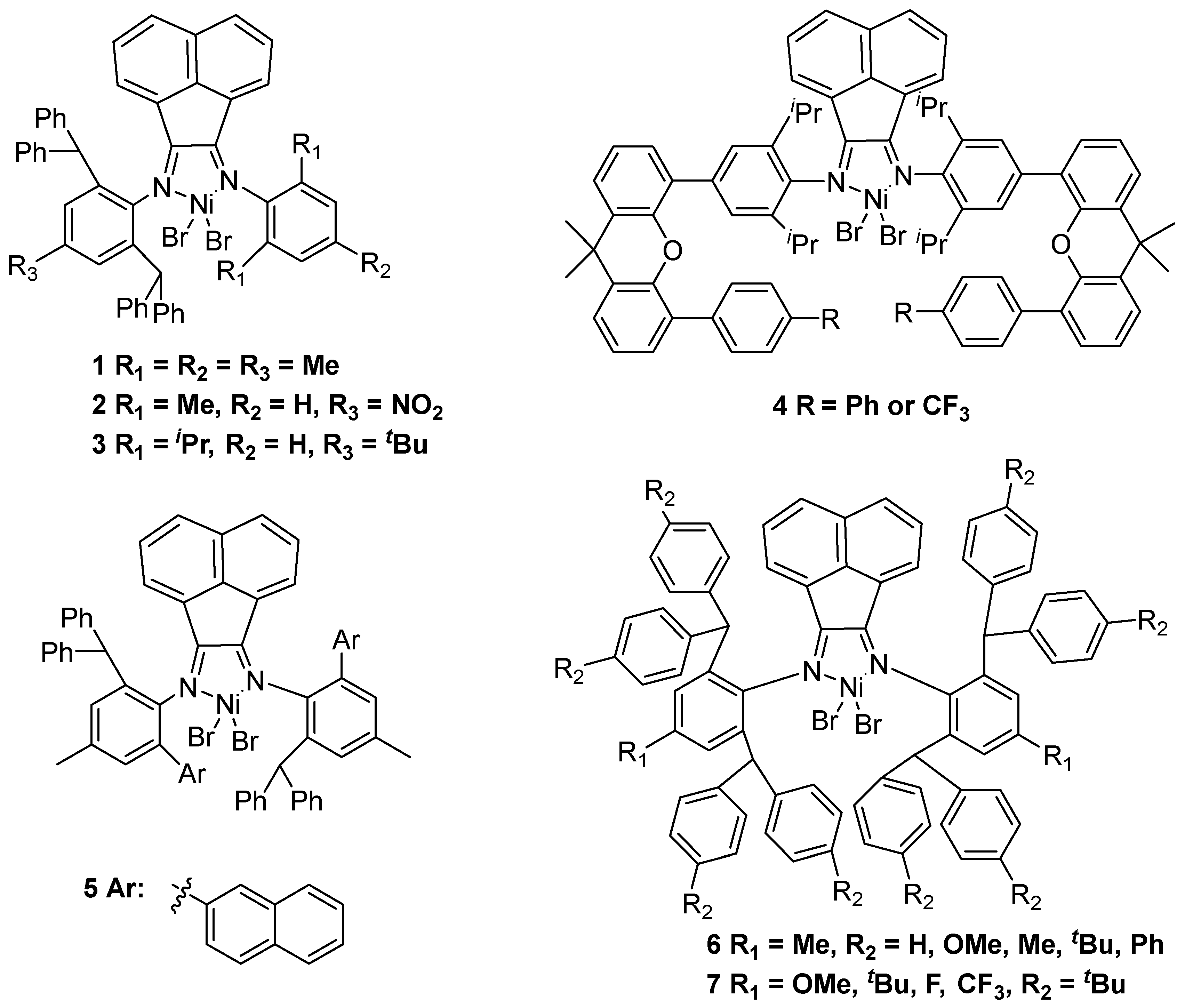
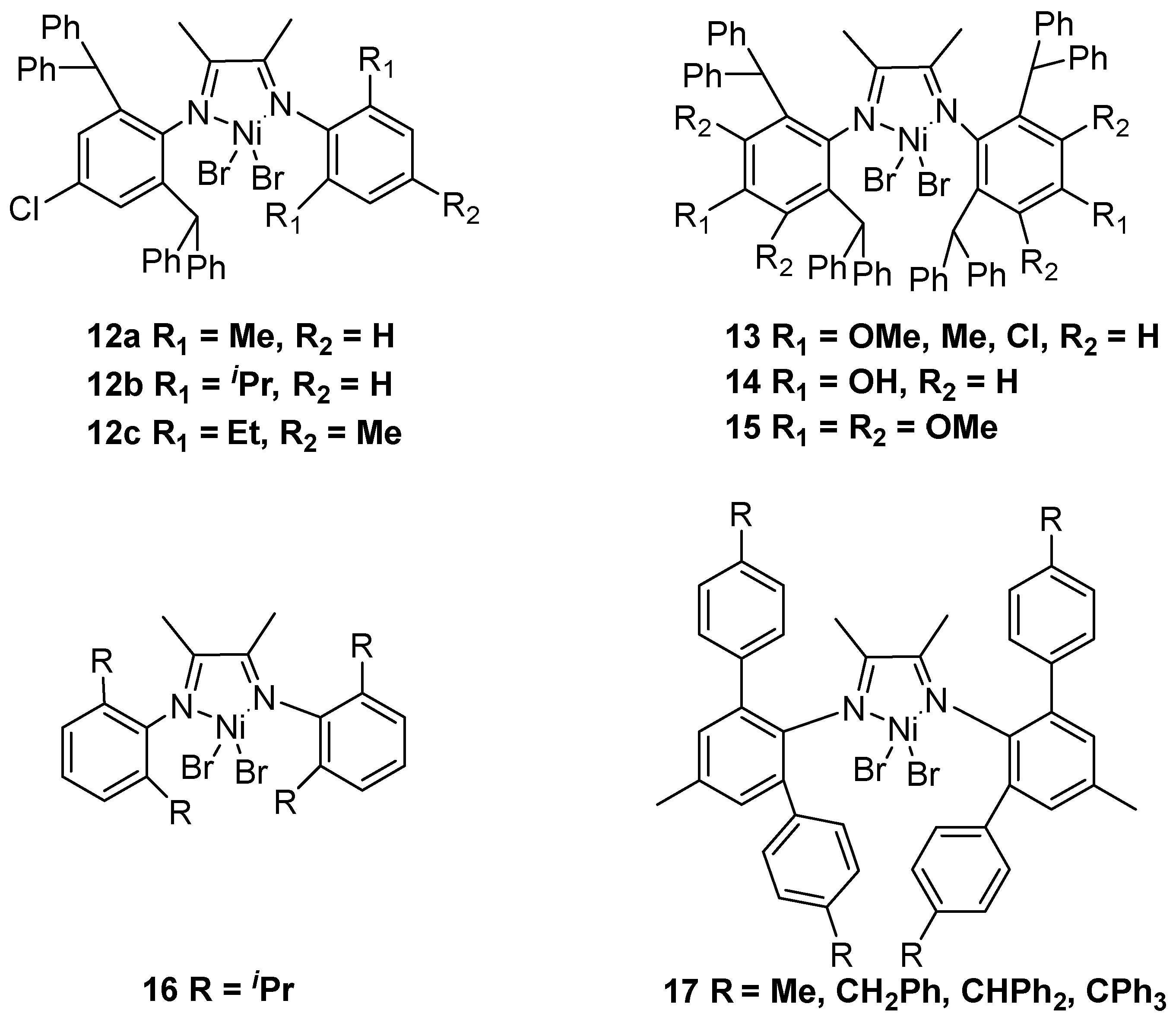
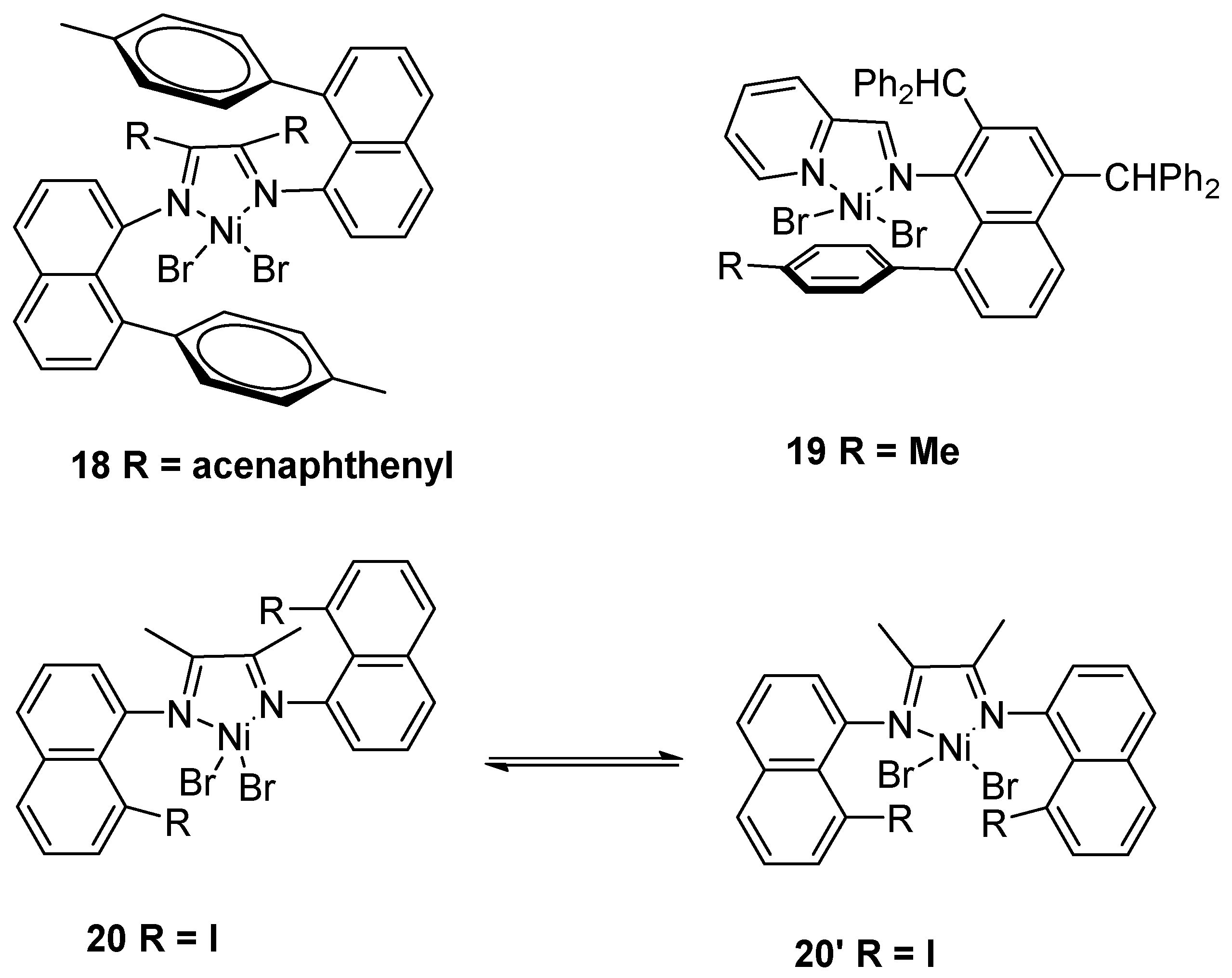
2.1. Nickel-Based Catalysts with N,N′ Ligands
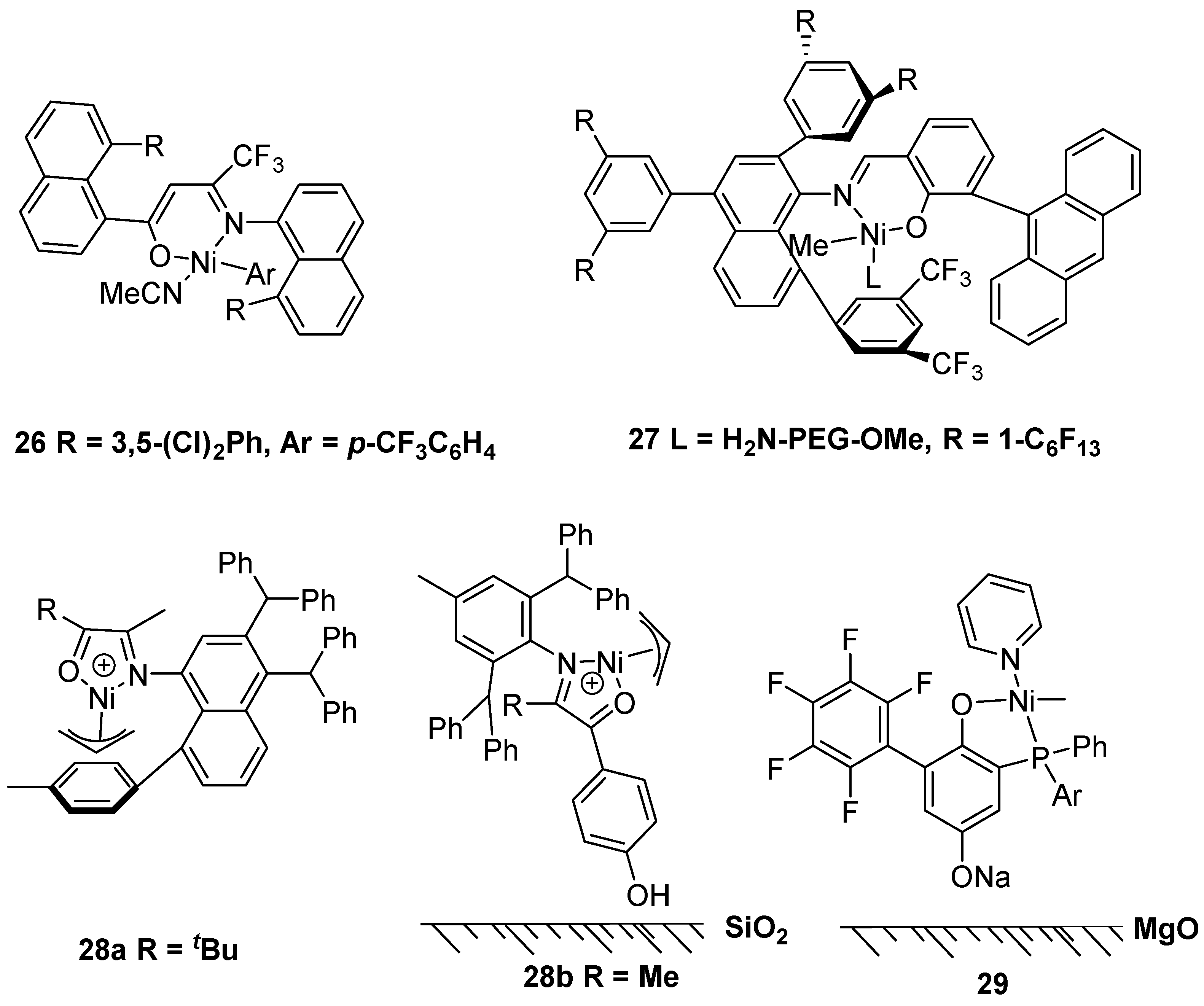
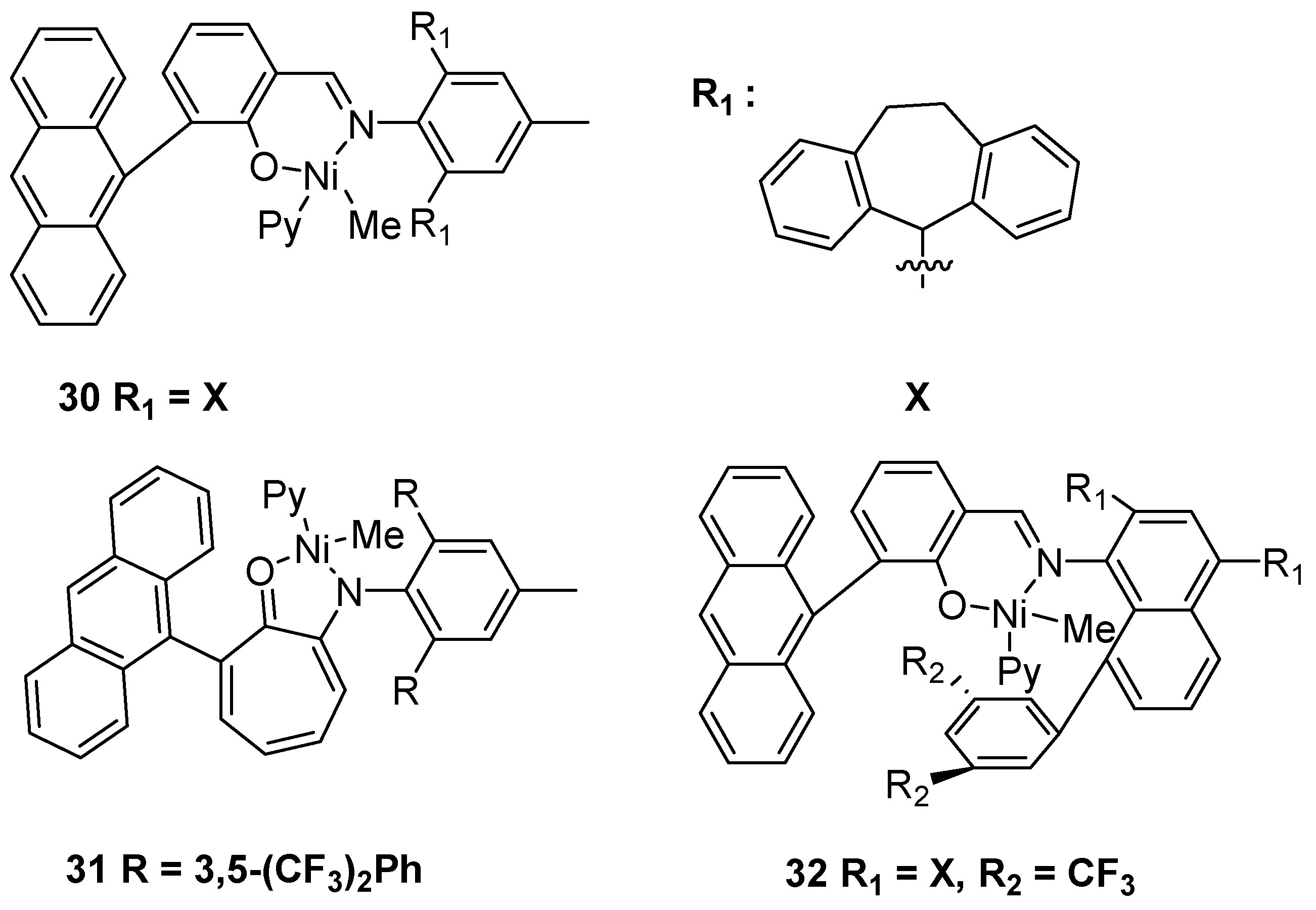
2.2. Nickel-Based Catalysts with N,O Ligands
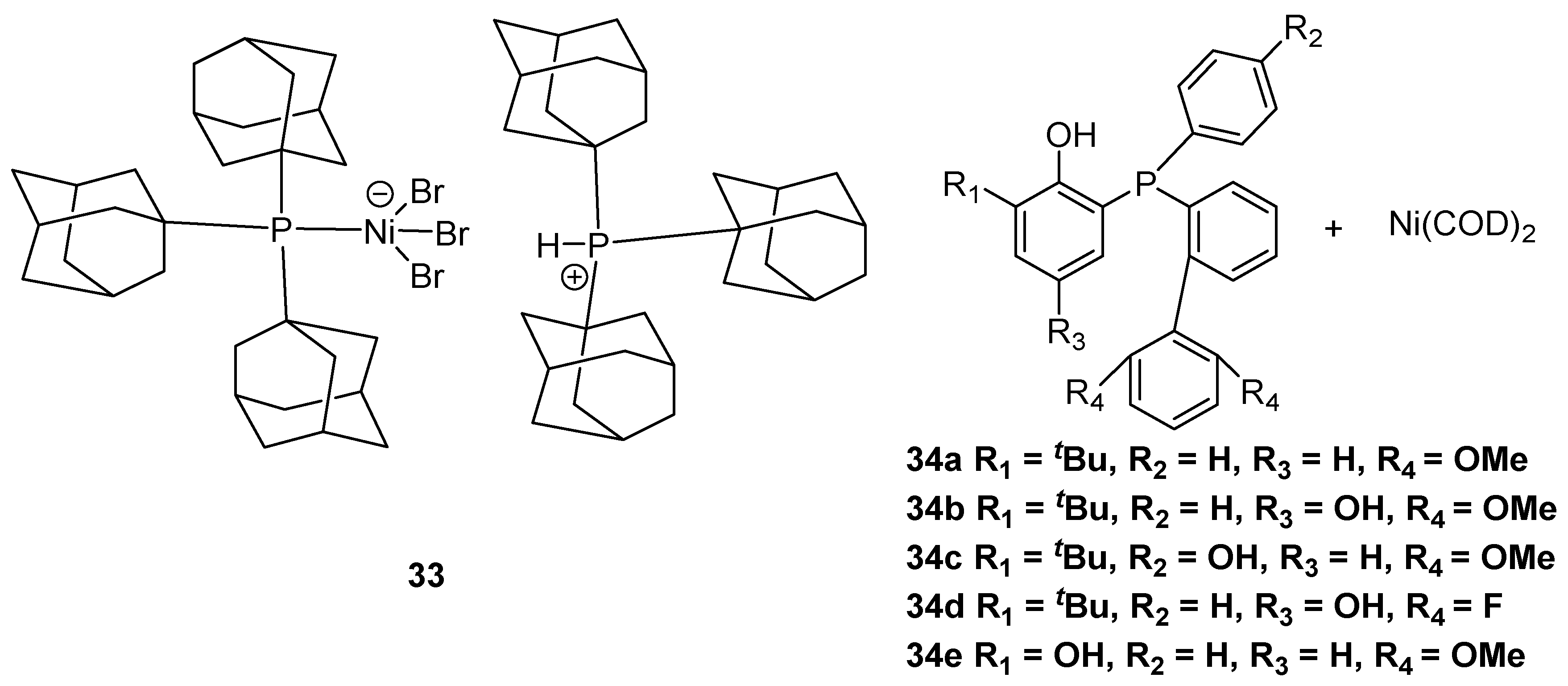
2.3. Nickel-Based Catalysts with P Ligands
2.4. Palladium-Based Catalysts with N,N′ Ligands
2.5. Iron and Cobalt-Based Catalysts with N,N′ Ligands
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-high molecular weight polyethylene: Influence of the chemical, physical and mechanical properties on the wear behavior. A review. Materials 2017, 10, 791. [Google Scholar] [CrossRef]
- Yang, H.G.; Yilmaz, G.; Jiang, J.; Xie, J.; Langstraat, T.; Chu, R.; van Es, M.; Garg, P.; Turng, L.S. Pelletizing ultra-high molecular weight polyethylene (UHMWPE) powders with a novel tapered die and addition of high density polyethylene (HDPE): Processing, morphology, and properties. Polymer 2022, 256, 125171–125181. [Google Scholar] [CrossRef]
- Amurin, L.G.; Felisberto, M.D.; Ferreira, F.L.Q.; Soraes, P.H.V.; Oliveira, P.N.; Santos, B.F.; Valeriano, J.C.S.; de Miranda, D.C.; Silva, G.G. Multifunctionality in ultra high molecular weight polyethylene nanocomposites with reduced graphene oxide: Hardness, impact and tribological properties. Polymer 2022, 240, 124475–124489. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-high-molecular-weight-polyethylene (UHMWPE) as a promising polymer material for biomedical applications: A concise review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh molecular weight polyethylene: Catalysis, structure, properties, processing and applications. Prog. Polym. Sci. 2020, 109, 101290–101310. [Google Scholar] [CrossRef]
- Bodkhe, D.V.; Chikkali, S.H. Ti-iminocarboxylate catalyzed polymerization of ethylene to highly crystalline, disentangled, ultrahigh molecular weight polyethylene. Eur. Polym. J. 2023, 182, 111725–111733. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Sarma, K.R.; Sharma, S. Synthesis of Ultrahigh Molecular Weight Polyethylene Using Traditional Heterogeneous Ziegler-Natta Catalyst Systems. Ind. Eng. Chem. Res. 2009, 48, 4866–4871. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polym. J. 2021, 142, 110162–110188. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Nickel catalysts for the synthesis of ultra-high molecular weight polyethylene. Sci. Bull. 2020, 65, 1137–1138. [Google Scholar] [CrossRef]
- Song, Z.; Wang, S.; Gao, R.; Wang, Y.; Gou, Q.; Zheng, G.; Feng, H.; Fan, G.; Lai, J. Recent Advancements in Mechanistic Studies of Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Polymers 2023, 15, 4343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd (II)- and Ni (II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; Hao, X.; Redshaw, C.; Huang, W.; Sun, W.-H. 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-methylbenzenamine Nickel Dibromides: Synthesis, Characterization, and Ethylene Polymerization. Organometallics 2011, 30, 2418–2424. [Google Scholar] [CrossRef]
- Mahmood, Q.; Zeng, Y.; Wang, X.; Sun, Y.; Sun, W.-H. Advancing polyethylene properties by incorporating NO2 moiety in 1,2-bis(arylimino)acenaphthylnickel precatalysts: Synthesis, characterization and ethylene polymerization. Dalton Trans. 2017, 46, 6934–6947. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Zeng, Y.; Yue, E.; Solan, G.A.; Liang, T.; Sun, W.-H. Ultra-high molecular weight elastomeric polyethylene using an electronically and sterically enhanced nickel catalyst. Polym. Chem. 2017, 8, 6416–6430. [Google Scholar] [CrossRef]
- Lian, K.; Zhu, Y.; Li, W.; Dai, S.; Chen, C. Direct Synthesis of Thermoplastic Polyolefin Elastomers from Nickel-Catalyzed Ethylene Polymerization. Macromolecules 2017, 50, 6074–6080. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.; Li, Y.; Chen, C. Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 9, 4143–4149. [Google Scholar] [CrossRef]
- Guo, L.; Lian, K.; Kong, W.; Xu, S.; Jiang, G.; Dai, S. Synthesis of Various Branched Ultra-High-Molecular-Weight Polyethylenes Using Sterically Hindered Acenaphthene-Based α-Diimine Ni(II) Catalysts. Organometallics 2018, 37, 2442–2449. [Google Scholar] [CrossRef]
- Zhu, N.; Khan, M.A.; Pang, W.; Behzadi, S.; Qasim, M. Synthesis of Ultra-High molecular weight polyethylene elastomers by para-tert-Butyl dibenzhydryl functionalized α-Diimine nickel catalysts at elevated temperature. Eur. Polym. J. 2022, 178, 111497–111506. [Google Scholar] [CrossRef]
- Zada, M.; Guo, L.; Zhang, R.; Zhang, W.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Moderately branched ultra-high molecular weight polyethylene by using N,N′-nickel catalysts adorned with sterically hindered dibenzocycloheptyl groups. Appl. Organometal. Chem. 2019, 33, e4749–e4766. [Google Scholar] [CrossRef]
- Guo, L.; Sun, W.; Li, S.; Xu, G.; Dai, S. Bulky yet flexible substituents in insertion polymerization with α-diimine nickel and palladium systems. Polym. Chem. 2019, 10, 4866–4871. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Gong, Q.; Zhang, S.; Liu, B.; Dai, S. Systematic Investigations of Ligand Steric Effects on α-Diimine Nickel Catalyzed Olefin Polymerization and Copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Xia, J.; Kou, S.; Zhang, Y.; Jian, Z. Strategies cooperation on designing nickel catalysts to access ultrahigh molecular weight polyethylenes. Polymer 2022, 240, 124478–124484. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, W.; Liu, W.; Wang, L.; Redshaw, C.; Sun, W.-H. Unsymmetrical α-diiminonickel bromide complexes: Synthesis, characterization and their catalytic behavior toward ethylene. Catal. Sci. Technol. 2013, 3, 2737–2745. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Chen, C. Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Pang, W.-M.; Chen, C. A Phenol-containing α-Diimine Ligand for Nickel- and Palladium-Catalyzed Ethylene Polymerization. Chinese J. Polym. Sci. 2019, 37, 974–980. [Google Scholar] [CrossRef]
- Muhammad, Q.; Tan, C.; Chen, C. Concerted steric and electronic effects on a-diimine nickel- and palladium-catalyzed ethylene polymerization and copolymerization. Sci. Bull. 2020, 65, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y.; Li, W.; Dong, C.; Liang, P.; Wang, N.; Jiang, B.; Wang, J.; Yang, Y. Selective distribution and contribution of nickel based pre-catalyst in the multisite catalyst for the synthesis of desirable bimodal polyethylene. Eur. Polym. J. 2020, 135, 109878–109886. [Google Scholar] [CrossRef]
- Hu, X.; Kang, X.; Zhang, Y.; Jian, Z. Facile Access to Polar-Functionalized Ultrahigh Molecular Weight Polyethylene at Ambient Conditions. CCS Chem. 2022, 4, 1680–1694. [Google Scholar] [CrossRef]
- Zhang, D.; Nadres, E.T.; Brookhart, M.; Daugulis, O. Synthesis of Highly Branched Polyethylene Using “Sandwich” (8-p-Tolyl naphthyl α-diimine)nickel(II) Catalysts. Organometallics 2013, 32, 5136–5143. [Google Scholar] [CrossRef]
- Dai, S.; Sui, X.; Chen, C. Synthesis of high molecular weight polyethylene using iminopyridyl nickel catalysts. Chem. Commun. 2016, 52, 9113–9116. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Daugulis, O.; Brookhart, M. Ethylene Polymerization with Ni(II) Diimine Complexes Generated from 8-Halo-1-naphthylamines: The Role of Equilibrating Syn/Anti Diastereomers in Determining Polymer Properties. Organometallics 2019, 38, 4658–4668. [Google Scholar] [CrossRef]
- Chen, Z.; Mesgar, M.; White, P.S.; Daugulis, O.; Brookhart, M. Synthesis of Branched Ultrahigh-Molecular-Weight Polyethylene Using Highly Active Neutral, Single-Component Ni(II) Catalysts. ACS Catal. 2015, 5, 631–636. [Google Scholar] [CrossRef]
- Kenyon, P.; Wörner, M.; Mecking, S. Controlled Polymerization in Polar Solvents to Ultrahigh Molecular Weight Polyethylene. J. Am. Chem. Soc. 2018, 140, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.; Mecking, S. Pentafluorosulfanyl Substituents in Polymerization Catalysis. J. Am. Chem. Soc. 2017, 139, 13786–13790. [Google Scholar] [CrossRef] [PubMed]
- Schnitte, M.; Staiger, A.; Casper, L.A.; Mecking, S. Uniform shape monodisperse single chain nanocrystals by living aqueous catalytic polymerization. Nat. Commun. 2019, 10, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Dai, S.; Chen, C. Ethylene Polymerization and Copolymerization Using Nickel 2-Iminopyridine-N-oxide Catalysts: Modulation of Polymer Molecular Weights and Molecular-Weight Distributions. Macromolecules 2018, 51, 49–56. [Google Scholar] [CrossRef]
- Tran, Q.H.; Brookhart, M.; Daugulis, O. New Neutral Nickel and Palladium Sandwich Catalysts: Synthesis of Ultra-High Molecular Weight Polyethylene (UHMWPE) via Highly Controlled Polymerization and Mechanistic Studies of Chain Propagation. J. Am. Chem. Soc. 2020, 142, 7198–7206. [Google Scholar] [CrossRef]
- Schnitte, M.; Scholliers, J.S.; Riedmiller, K.; Mecking, S. Remote Perfluoroalkyl Substituents are Key to Living Aqueous Ethylene Polymerization. Angew. Chem. Int. Ed. 2020, 59, 3258–3263. [Google Scholar] [CrossRef]
- Antonov, A.A.; Sun, W.-H.; Bryliakov, K.P. New family of single-component Ni catalysts for ethylene polymerization to high and ultrahigh molecular weight polyethylene. Sci. China. Chem. 2020, 63, 753–754. [Google Scholar] [CrossRef]
- Liang, T.; Goudari, S.B.; Chen, C. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372–380. [Google Scholar] [CrossRef]
- Zou, C.; Si, G.; Chen, C. A general strategy for heterogenizing olefin polymerization catalysts and the synthesis of polyolefins and composites. Nat. Commun. 2022, 13, 1954–1966. [Google Scholar] [CrossRef]
- Wang, C.; Kang, X.; Dai, S.; Cui, F.; Li, Y.; Mu, H.; Mecking, S.; Jian, Z. Efficient Suppression of Chain Transfer and Branching via Cs-Type Shielding in a Neutral Nickel(II) Catalyst. Angew. Chem. Int. Ed. 2021, 60, 4018–4022. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mu, H.; Kang, X.; Jian, Z. Suppression of Chain Transfer and Promotion of Chain Propagation in Neutral Anilinotropone Nickel Polymerization Catalysis. Macromolecules 2022, 55, 2533–2541. [Google Scholar] [CrossRef]
- Wang, C.; Kang, X.; Mu, H.; Jian, Z. Positive Effect of Polar Solvents in Olefin Polymerization Catalysis. Macromolecules 2022, 55, 5441–5447. [Google Scholar] [CrossRef]
- Kocen, A.L.; Brookhart, M.; Daugulis, O. A highly active Ni(II)-triadamantylphosphine catalyst for ultrahigh-molecular-weight polyethylene synthesis. Nat. Commun. 2019, 10, 438–444. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Zou, C.; Chen, C. Synthesis of Ultra-High-Molecular-Weight Polyethylene by Transition-Metal-Catalyzed Precipitation Polymerization. Precis. Chem. 2024, 2, 63–69. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic Investigations of Ligand Steric Effects on α-Diimine Palladium Catalyzed Olefin Polymerization and Copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 128, 13475–13479. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. A Self-Supporting Strategy for Gas-Phase and Slurry-Phase Ethylene Polymerization using Late-Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2020, 59, 14884–14890. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Mecking, S.; Jian, Z. Ultrahigh Branching of Main-Chain-Functionalized Polyethylenes by Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, X.; Jian, Z. Selective branch formation in ethylene polymerization to access precise ethylenepropylene copolymers. Nat. Commun. 2022, 13, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zhang, S.; Li, B.; Hao, X.; Redshaw, C.; Li, Y.-S.; Sun, W.-H. Ferrous and cobaltous chloride complexes bearing 2-(1-(arylimino)methyl)-8-(1Hbenzimidazol-2-yl)quinolines: Synthesis, characterization and catalytic behavior in ethylene polymerization. Polymer 2011, 52, 5803–5810. [Google Scholar] [CrossRef]
- Cao, X.; He, F.; Zhao, W.; Cai, Z.; Hao, X.; Shiono, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-chlorophenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyliron(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polymer 2012, 53, 1870–1880. [Google Scholar] [CrossRef]
- Chen, P.; Yang, H.; Chen, T.; Li, W. Weakly Entangled Ultra-high Molecular Weight Polyethylene Prepared via Ethylene Extrusion Polymerization. Ind. Eng. Chem. Res. 2015, 54, 11024–11032. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Q.; Gao, R.; Song, Z.; Gou, Q. Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts. Polymers 2024, 16, 1688. https://doi.org/10.3390/polym16121688
Yue Q, Gao R, Song Z, Gou Q. Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts. Polymers. 2024; 16(12):1688. https://doi.org/10.3390/polym16121688
Chicago/Turabian StyleYue, Qiang, Rong Gao, Zhihui Song, and Qingqiang Gou. 2024. "Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts" Polymers 16, no. 12: 1688. https://doi.org/10.3390/polym16121688
APA StyleYue, Q., Gao, R., Song, Z., & Gou, Q. (2024). Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts. Polymers, 16(12), 1688. https://doi.org/10.3390/polym16121688






