Optimization of Starch–Tannin Adhesives for Solid Wood Gluing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Adhesive
2.3. Plasma Treatment of the Wooden Surfaces
2.4. Sample Glueing
2.5. Mechanical Shear Test
2.6. Water Exposure
2.7. TMA—Thermomechanical Analysis
2.8. Chemical Investigation
3. Results and Discussion
3.1. Dilution Effect
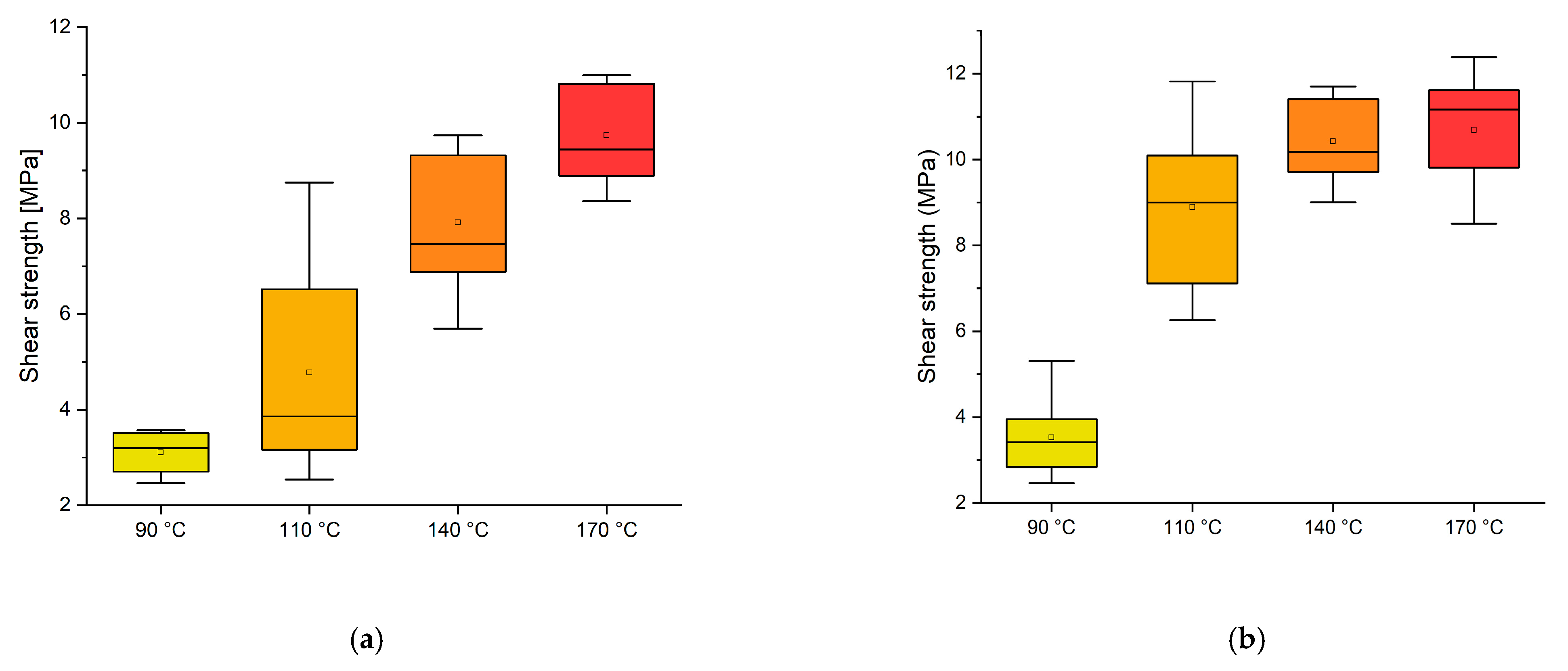
3.2. Effect of the Temperature and Time
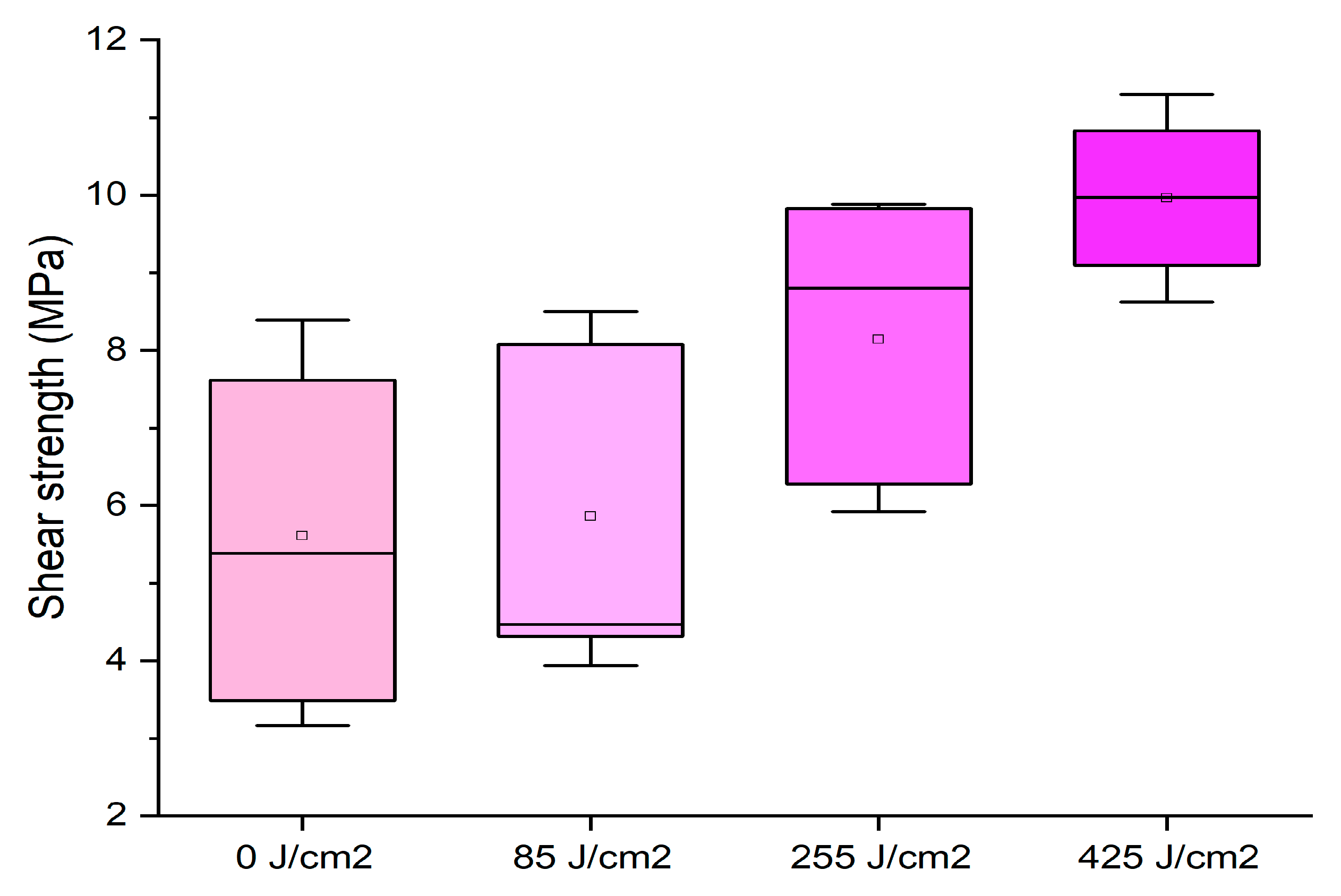
3.3. Effect of Plasma Treatment
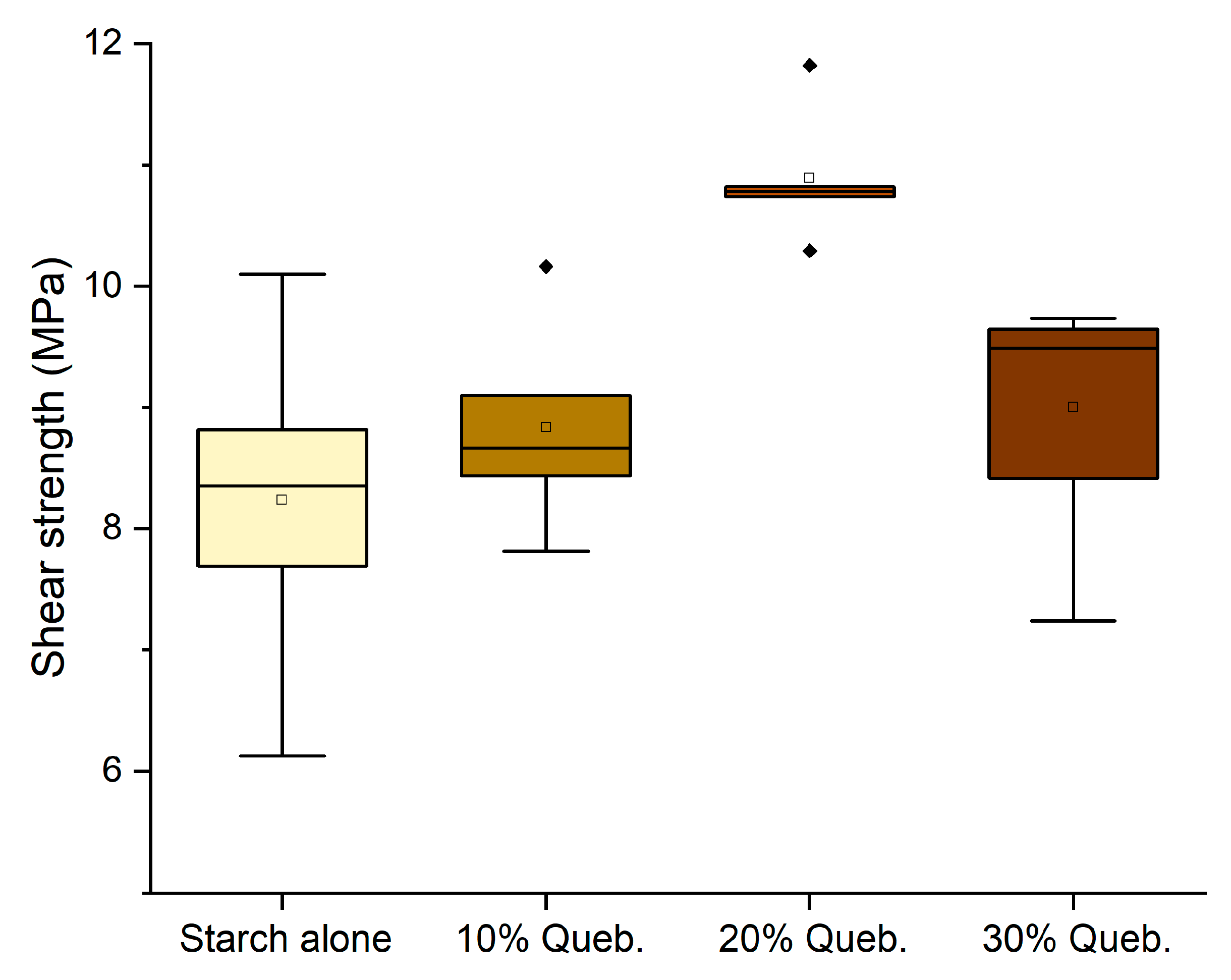
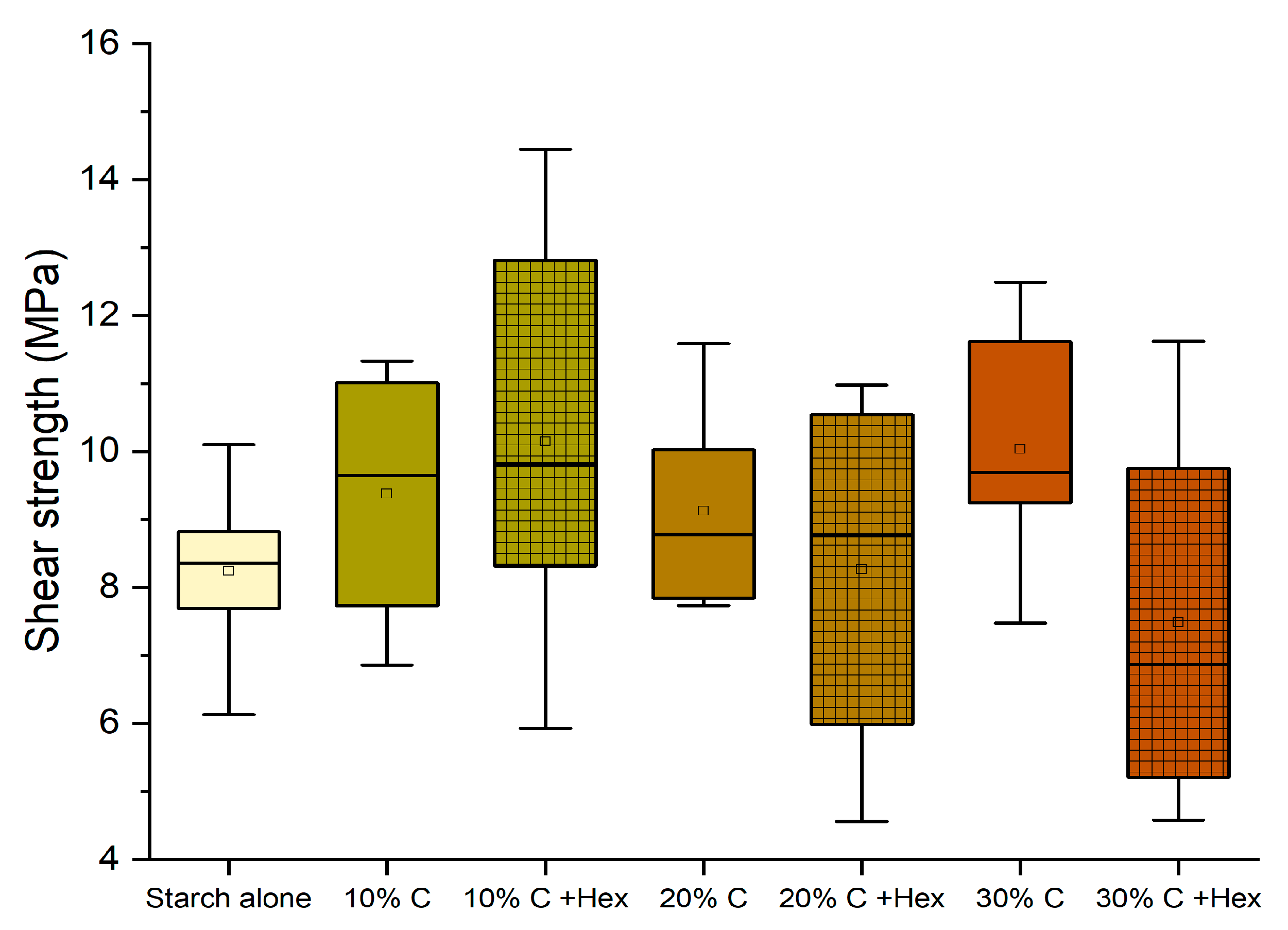
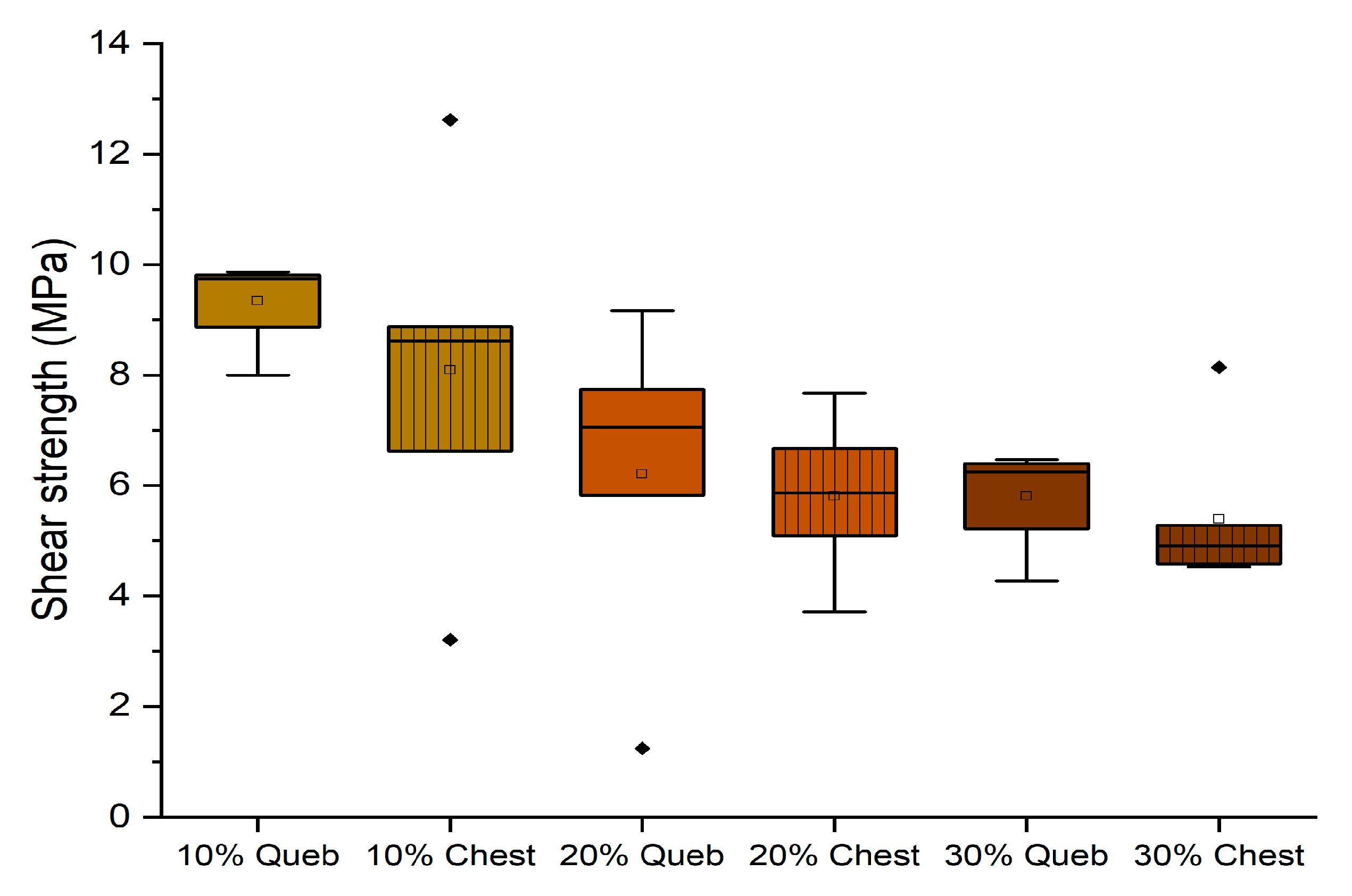
3.4. Effect of Tannin Concentration
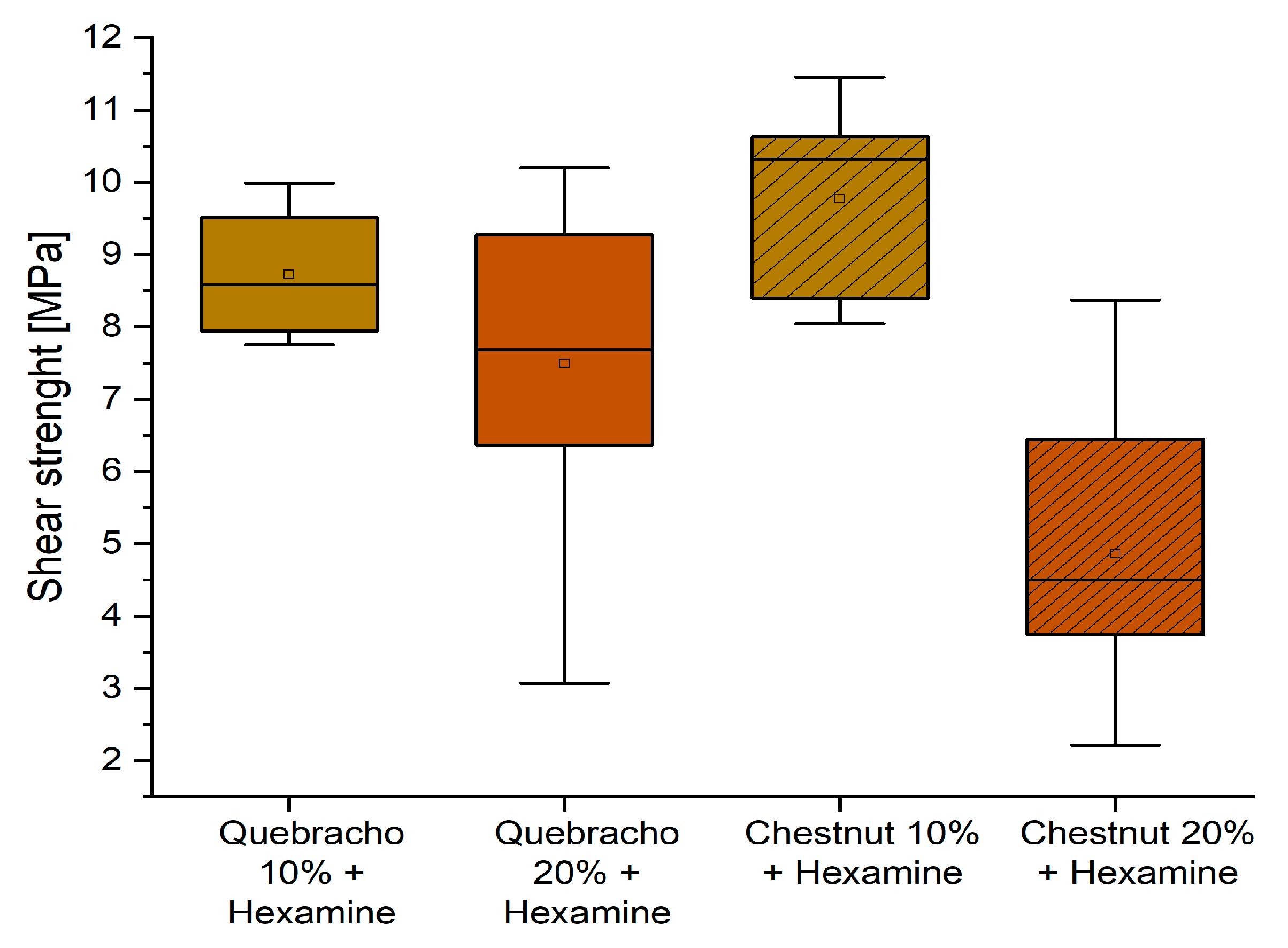
3.5. Performance after Water Exposure
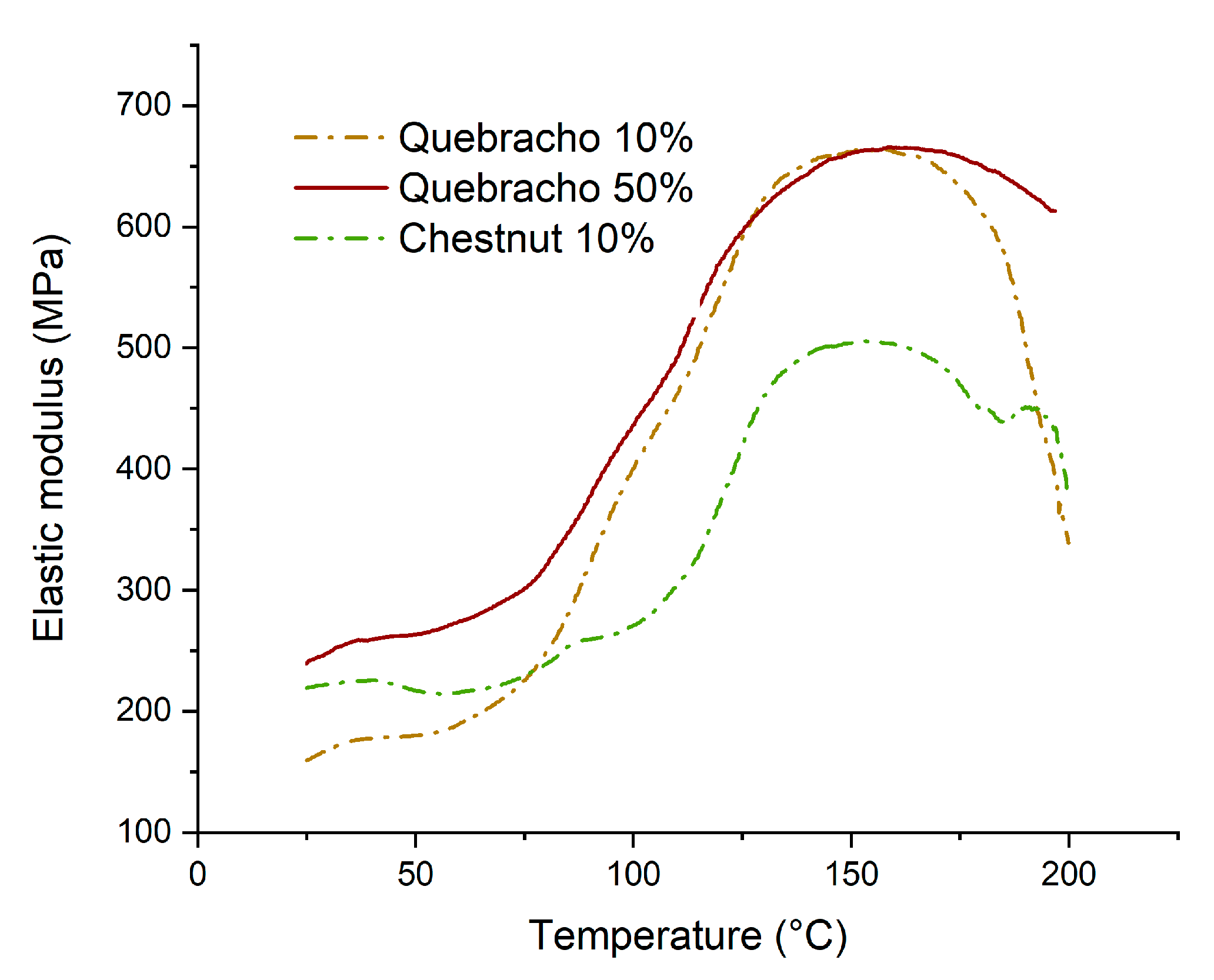
3.6. Thermomechanical Analysis
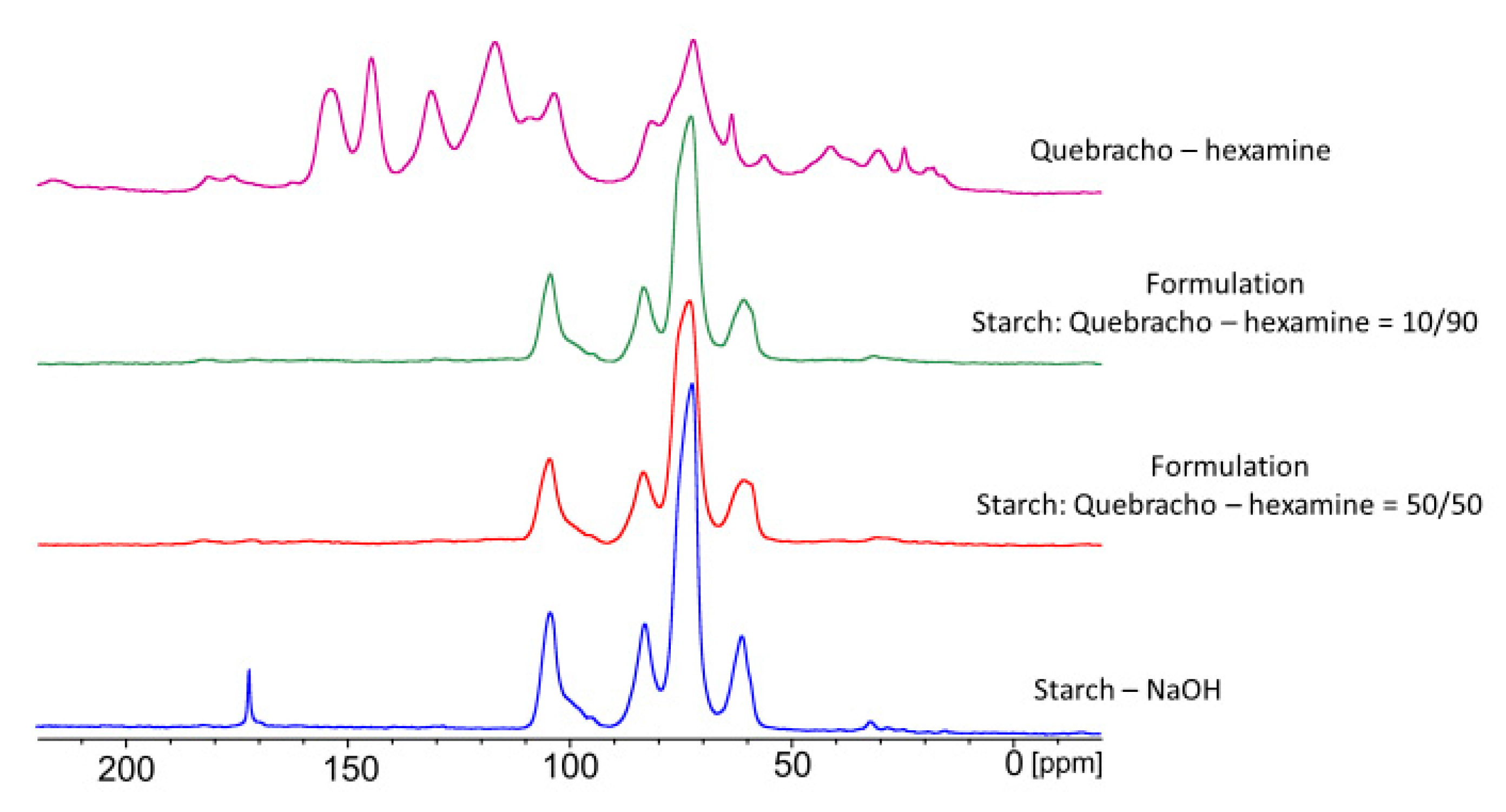
3.7. Chemical Investigation
4. Conclusions
- (i)
- Starch–tannin adhesives from previous studies can reach the D1 classification according to EN205 when diluted at 22% by using 13 min and 140 °C or more as press conditions.
- (ii)
- When used as a concentrated resin (65%), intensive plasma treatment (425 J/cm2) of the wooden substrate is required to enhance the bonding strength.
- (iii)
- Enhancing the amount of tannin until 30% can be beneficial independently on the tannin type (quebracho or chestnut) and on the presence of hexamine as a hardener. D1 classification (10 MPa) was achieved by six formulations.
- (iv)
- The presence of hexamine in 10% quebracho and chestnut formulations also allowed for reaching the D2 classification when testing these adhesives after leaching (3 h). These values were never registered previously for starch-based adhesives.
- (v)
- Starch–tannin adhesives are thermosetting adhesives that start curing at around 75 °C, reaching their maximum at around 150 °C, but they start to lose their properties if exposed to excessive heat (>170 °C). Quebracho tannin enables faster and tougher curing than chestnuts.
- (vi)
- Solid state 13C-NMR spectroscopy could not highlight any newer signal between starch and tannin, but a possible coordination effect is suspected due to the impossibility of significantly detecting the polyphenols when in the presence of starch. The hypothesis of having new covalent bonds could not be proven, but the coordination observed could be the main reason for the extension of the water resistance of some of the tannin-containing starch formulations. Indeed, only formulations containing hexamine were able to reach the D2 level, meaning that this crosslinking agent plays a major role in the tightness of the glue line.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Trisatya, D.R.; Santoso, A.; Abdurrachman, A.; Alva, D. Performance of Six-Layered Cross Laminated Timber of Fast-Growing Species Glued with Tannin Resorcinol Formaldehyde. J. Korean Wood Sci. Technol. 2023, 51, 81–97. [Google Scholar] [CrossRef]
- Cesprini, E.; Šket, P.; Causin, V.; Zanetti, M.; Tondi, G. Development of Quebracho (Schinopsis balansae) Tannin-Based Thermoset Resins. Polymers 2021, 13, 4412. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Neykov, N. Sustainable Bio-Based Adhesives for Eco-Friendly Wood Composites a Review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Li, J. Reactivity of Larch and Valonia Tannins in Synthesis of Tannin-Formaldehyde Resins. BioResources 2016, 11, 2256–2268. [Google Scholar] [CrossRef]
- Moubarik, A.; Pizzi, A.; Allal, A.; Charrier, F.; Charrier, B. Cornstarch and Tannin in Phenol-Formaldehyde Resins for Plywood Production. Ind. Crops Prod. 2009, 30, 188–193. [Google Scholar] [CrossRef]
- Trosa, A.; Mondovi, S.M.; Pizzi, A. Stability and Performance of Tannin-Accelerated PF Resins for Plywood. Holz als Roh-und Werkstoff 1997, 55, 306. [Google Scholar] [CrossRef]
- Cesprini, E.; Jorda, J.; Paolantoni, M.; Valentini, L.; Šket, P.; Causin, V.; Bedolla, D.E.; Zanetti, M.; Tondi, G. Bio-Based Tannin-Furanic-Silk Adhesives: Applications in Plywood and Chemical Cross-Linking Mechanisms. ACS Appl. Polym. Mater. 2023, 5, 4468–4476. [Google Scholar] [CrossRef]
- Jorda, J.; Cesprini, E.; Barbu, M.-C.; Tondi, G.; Zanetti, M.; Král, P. Quebracho Tannin Bio-Based Adhesives for Plywood. Polymers 2022, 14, 2257. [Google Scholar] [CrossRef]
- Cesprini, E.; Causin, V.; De Iseppi, A.; Zanetti, M.; Marangon, M.; Barbu, M.C.; Tondi, G. Renewable Tannin-Based Adhesive from Quebracho Extract and Furfural for Particleboards. Forests 2022, 13, 1781. [Google Scholar] [CrossRef]
- de Carvalho Araújo, C.K.; Salvador, R.; Moro Piekarski, C.; Sokulski, C.C.; de Francisco, A.C.; de Carvalho Araújo Camargo, S.K. Circular Economy Practices on Wood Panels: A Bibliographic Analysis. Sustainability 2019, 11, 1057. [Google Scholar] [CrossRef]
- Ansell, M.P. Wood Composites. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 281–301. [Google Scholar] [CrossRef]
- Pizzi, A. Types, Processing and Properties of Bioadhesives for Wood and Fibers. Adv. Biorefineries 2014, 736–770. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future Opportunities for Bio-Based Adhesives-Advantages beyond Renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the Role of Lignin for Preparing the Lignin-Based Wood Adhesive: A Review. Ind. Crops Prod. 2022, 187, 115388. [Google Scholar] [CrossRef]
- Hendrik, J.; Hadi, Y.S.; Santoso, A. Properties of Laminated Composite Panels Made from Fast-Growing Species Glued with Mangium Tannin. BioResources 2016, 11, 5949–5960. [Google Scholar] [CrossRef]
- Kumar, C.; Leggate, W. An overview of bio-adhesives for engineered wood products. Int. J. Adhes. Adhes. 2022, 118, 103187. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Evaluation of starch as an environmental-friendly bioresource for the development of wood bioadhesives. Molecules 2021, 26, 4526. [Google Scholar] [CrossRef]
- Maulana, M.I.; Lubis, M.A.R.; Febrianto, F.; Hua, L.S.; Iswanto, A.H.; Antov, P.; Todaro, L. Environmentally friendly starch-based adhesives for bonding high-performance wood composites: A review. Forests 2022, 13, 1614. [Google Scholar] [CrossRef]
- Dunky, M. Wood Adhesives Based on Natural Resources: A Critical Review: Part II. Carbohydrate-Based Adhesives. Prog. Adhes. Adhes. 2021, 6, 337–382. [Google Scholar]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, Structure, Functionality and Applications of Rice Starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Wimmer, T.; Schnabel, T.; Petutschnigg, A. Starch-sugar synergy in wood adhesion science: Basic studies and particleboard production. Eur. J. Wood Wood Prod. 2012, 70, 271–278. [Google Scholar] [CrossRef]
- Li, C.; Hou, D.; Lei, H.; Xi, X.; Du, G.; Zhang, H.; Cao, M.; Tondi, G. Effective and eco-friendly safe self-antimildew strategy to simultaneously improve the water resistance and bonding strength of starch-based adhesive. Int. J. Biol. Macromol. 2023, 248, 125889. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Bonding strength and water resistance of starch-based wood adhesive improved by silica nanoparticles. Carbohydr. Polym. 2011, 86, 72–76. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y. Effects of montmorillonite addition on the performance of starch-based wood adhesive. Carbohydr. Polym. 2015, 115, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Moubarik, A.; Allal, A.; Pizzi, A.; Charrier, F.; Charrier, B. Preparation and mechanical characterization of particleboard made from maritime pine and glued with bio-adhesives based on cornstarch and tannins. Maderas Ciencia y Tecnología 2010, 12, 189–197. [Google Scholar] [CrossRef]
- Pichelin, F.; Kamoun, C.; Pizzi, A. Hexamine hardener behaviour: Effects on wood glueing, tannin and other wood adhesives. Holz als Rohund Werkstoff 1999, 57, 305–317. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Q.; Xi, X.; Lei, H.; Cao, M.; Du, G.; Wu, Z. Tannin-based wood adhesive with good water resistance crosslinked by hexanediamine. Int. J. Biol. Macromol. 2023, 234, 123644. [Google Scholar] [CrossRef]
- Arbenz, A.; Averous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-84735-3. [Google Scholar]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- EN 205:2016; Adhesives—Wood Adhesives for Nonstructural Applications—Determination of Tensile Shear Strength of Lap Joints. Comite Europeen de Normalisation: Brussels, Belgium, 2016.
- EN 204:2017; Classification of Thermoplastic Wood Adhesives for Non-Structural Applications. Comite Europeen de Normalisation: Brussels, Belgium, 2017.
- Acda, M.N.; Devera, E.E.; Cabangon, R.J.; Ramos, H.J. Effects of plasma modification on adhesion properties of wood. Int. J. Adhes. Adhes. 2012, 32, 70–75. [Google Scholar] [CrossRef]
- Žigon, J.; Saražin, J.; Šernek, M.; Kovač, J.; Dahle, S. The effect of ageing on bonding performance of plasma treated beech wood with urea-formaldehyde adhesive. Cellulose 2021, 28, 2461–2478. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Effects of heat pretreatment of starch on graft copolymerization reaction and performance of resulting starch-based wood adhesive. Int. J. Biol. Macromol. 2017, 96, 11–18. [Google Scholar] [CrossRef]
- Qiao ZhiBang, Q.Z.; Gu JiYou, G.J.; Lv ShanShan, L.S.; Cao Jun, C.J.; Tan HaiYan, T.H.; Zhang YanHua, Z.Y. Preparation and properties of normal temperature cured starch-based wood adhesive. Bioresources 2016, 11, 4839–4849. [Google Scholar]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Cheng, L.; Gu, Z.; Hong, Y.; Kowalczyk, A. Improving the performance of starch-based wood adhesive by using sodium dodecyl sulfate. Carbohydr. Polym. 2014, 99, 579–583. [Google Scholar] [CrossRef]



| Formulation | Starch (g) | Tannin * (g) | Hexamine (g) | NaOH (g) | Water (g) |
|---|---|---|---|---|---|
| 1 | 6.5 | 0.65 Q | 0.1 | 5 | 10 |
| 2 | 6.5 | 0.65 Q | 0.1 | 5 | 30 |
| 3 | 6.5 | 0.65 Q | 0.1 | 5 | 50 |
| 4 | 6.5 | 0.65 Q | 0.1 | 5 | 100 |
| 5 | 7.15 | 0 | 0 | 5 | 30 |
| 6 | 5.85 | 1.3 Q | 0.2 | 5 | 30 |
| 7 | 5.2 | 1.95 Q | 0.3 | 5 | 30 |
| 8 | 6.5 | 0.65 Q | 0 | 5 | 30 |
| 9 | 5.85 | 1.3 Q | 0 | 5 | 30 |
| 10 | 5.2 | 1.95 Q | 0 | 5 | 30 |
| 11 | 6.5 | 0.65 C | 0.1 | 5 | 30 |
| 12 | 5.85 | 1.3 C | 0.2 | 5 | 30 |
| 13 | 5.2 | 1.95 C | 0.3 | 5 | 30 |
| 14 | 6.5 | 0.65 C | 0 | 5 | 30 |
| 15 | 5.85 | 1.3 C | 0 | 5 | 30 |
| 16 | 5.2 | 1.95 C | 0 | 5 | 30 |
| 17 | 6.5 | 0.65 Q | 0.2 | 5 | 30 |
| 18 | 6.5 | 0.65 C | 0.2 | 5 | 30 |
| 19 | 5.85 | 1.3 Q | 0.4 | 5 | 30 |
| 20 | 5.85 | 1.3 C | 0.4 | 5 | 30 |
| Adhesive Formulations | Dry Shear Strength (MPa) | Wet Shear Strength (MPa) | EN 204 Classification |
|---|---|---|---|
| 2–10% Quebracho + hexamine | 10.42 (0.95) | 8.73 (0.99) | D2 |
| 11–10% Chestnut + hexamine | 10.14 (2.65) | 9.77 (1.48) | D2 |
| 7–30% Quebracho + hexamine | 11.12 (1.17) | 5.99 (1.89) | D1 |
| 6–20% Quebracho + hexamine | 10.93 (1.95) | 7.49 (2.29) | D1 |
| 9–20% Quebracho (no hexamine) | 10.89 (0.56) | 6.20 (3.03) | D1 |
| 16–30% Chestnut (no hexamine) | 10.03 (1.78) | 5.39 (1.37) | D1 |
| 14–10% Chestnut (no hexamine) | 9.37 (1.82) | 8.09 (3.09) | - |
| 15–20% Chestnut (no hexamine) | 9.12 (1.47) | 5.81 (1.36) | - |
| 10–30% Quebracho (no hexamine) | 9.00 (0.98) | 5.81 (1.03) | - |
| 8–10% Quebracho (no hexamine) | 8.83 (0.87) | 9.34 (0.89) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnabosco, A.; Kulyk, I.; Avancini, M.; Šket, P.; Eckardt, J.; Cesprini, E.; Marinello, F.; Tondi, G. Optimization of Starch–Tannin Adhesives for Solid Wood Gluing. Polymers 2024, 16, 1694. https://doi.org/10.3390/polym16121694
Magnabosco A, Kulyk I, Avancini M, Šket P, Eckardt J, Cesprini E, Marinello F, Tondi G. Optimization of Starch–Tannin Adhesives for Solid Wood Gluing. Polymers. 2024; 16(12):1694. https://doi.org/10.3390/polym16121694
Chicago/Turabian StyleMagnabosco, Annalisa, Illya Kulyk, Maurizio Avancini, Primož Šket, Jonas Eckardt, Emanuele Cesprini, Francesco Marinello, and Gianluca Tondi. 2024. "Optimization of Starch–Tannin Adhesives for Solid Wood Gluing" Polymers 16, no. 12: 1694. https://doi.org/10.3390/polym16121694








