Tuning the Structure–Property Relationships in Binary and Ternary Blends of PLA/PBAT/PHBH
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Blend Preparation
2.3. Rheological Analysis
2.4. Mechanical Properties Analysis
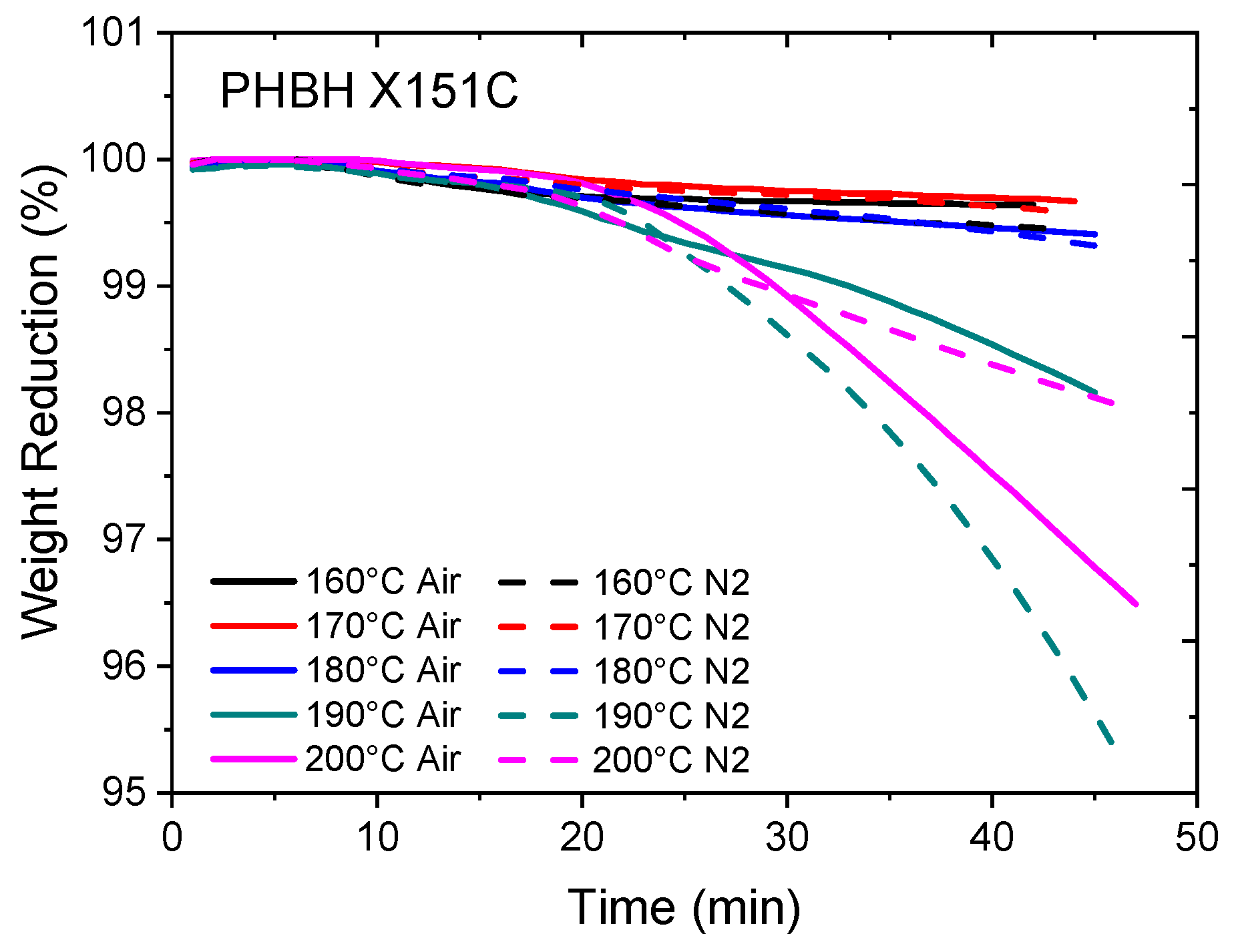
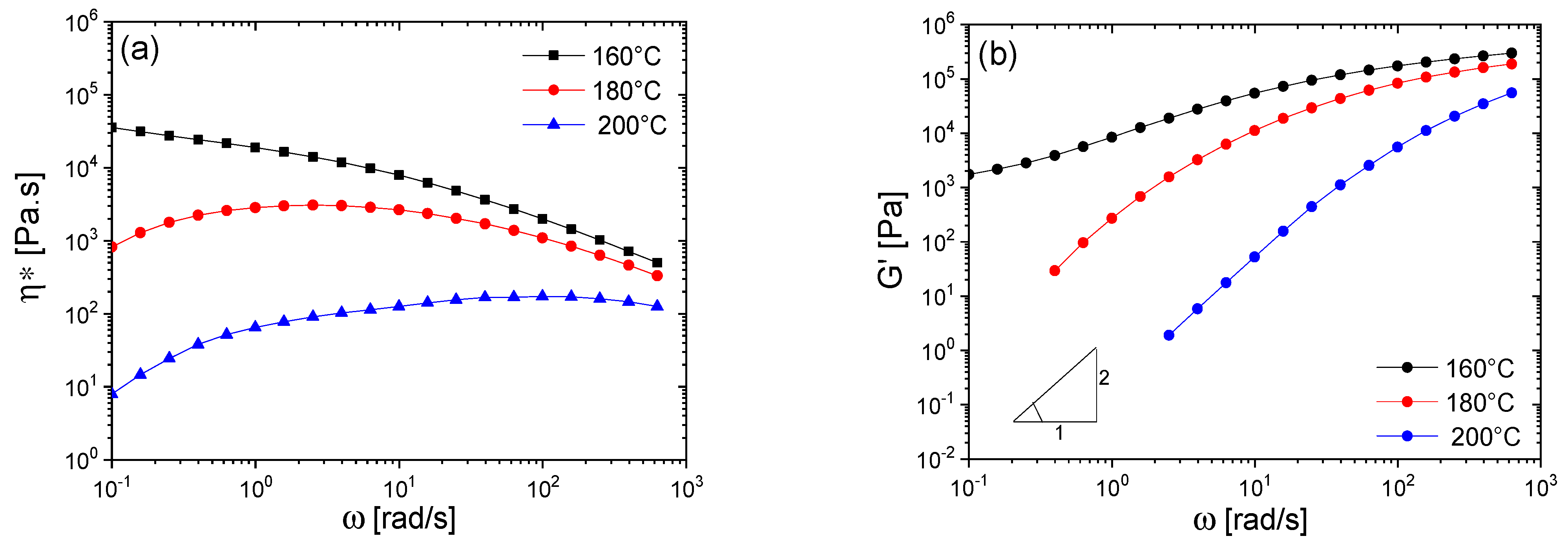
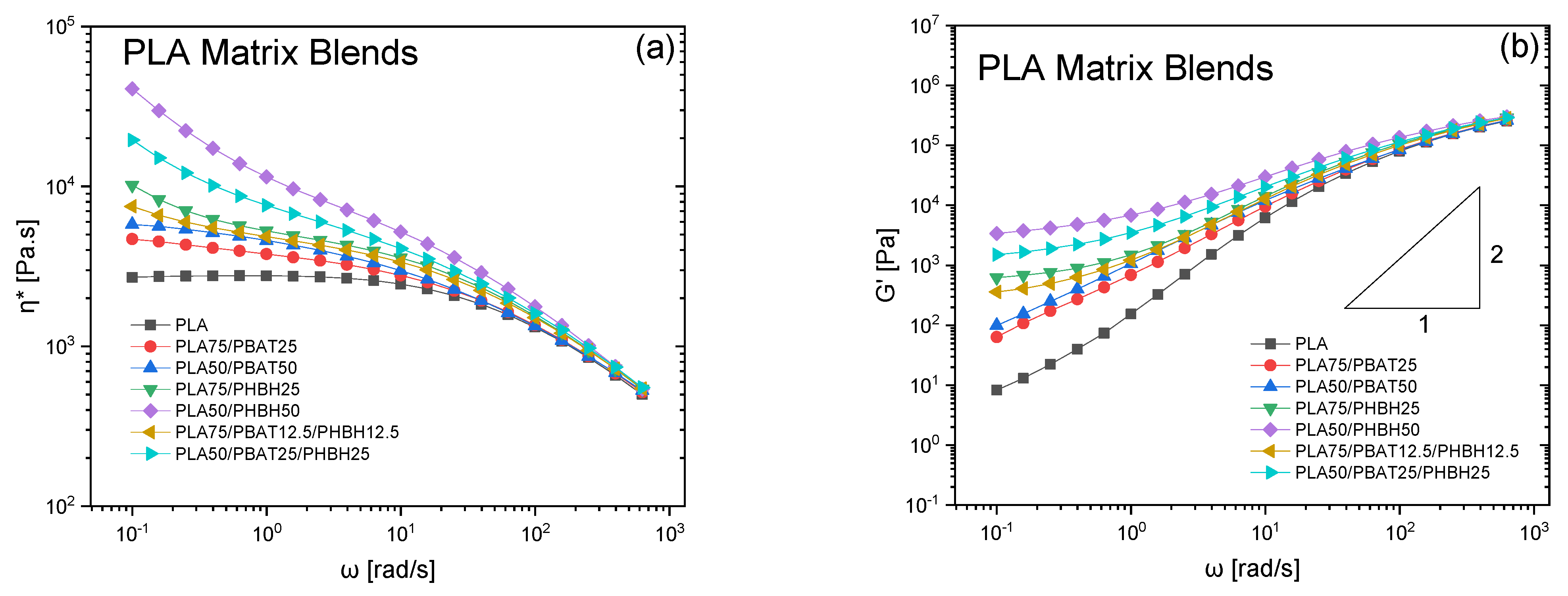
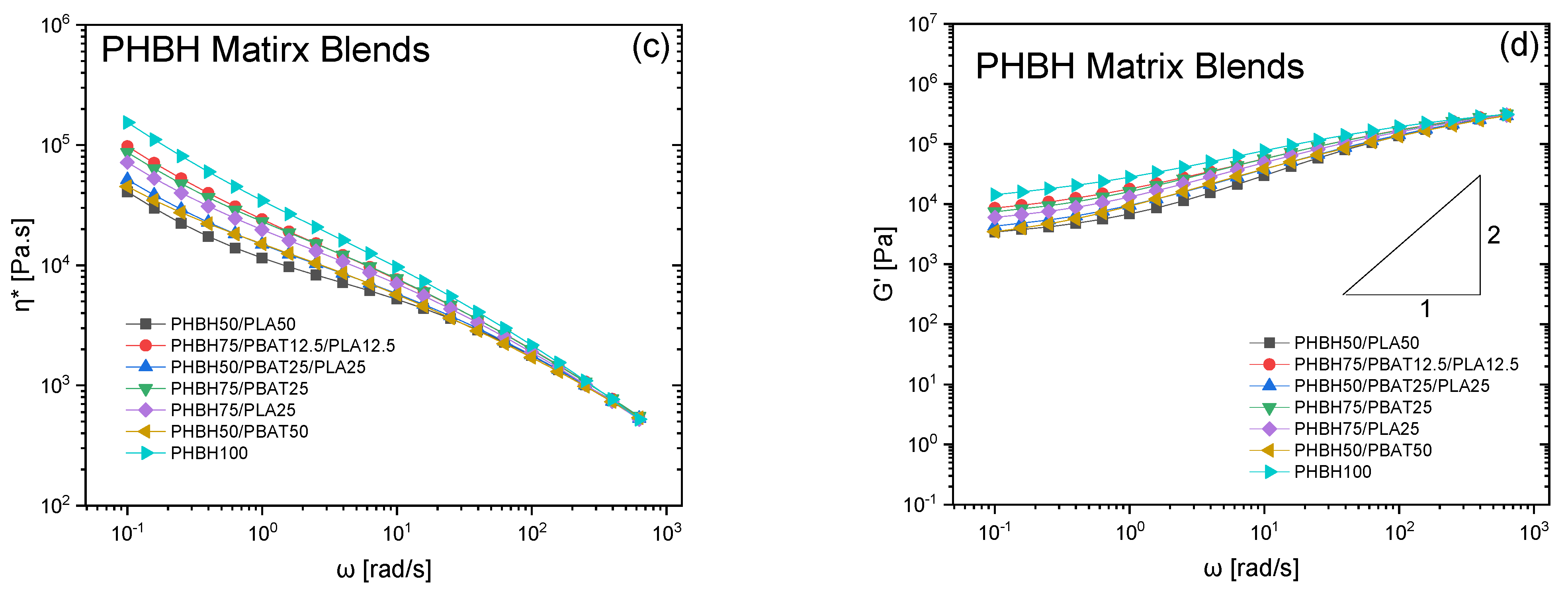
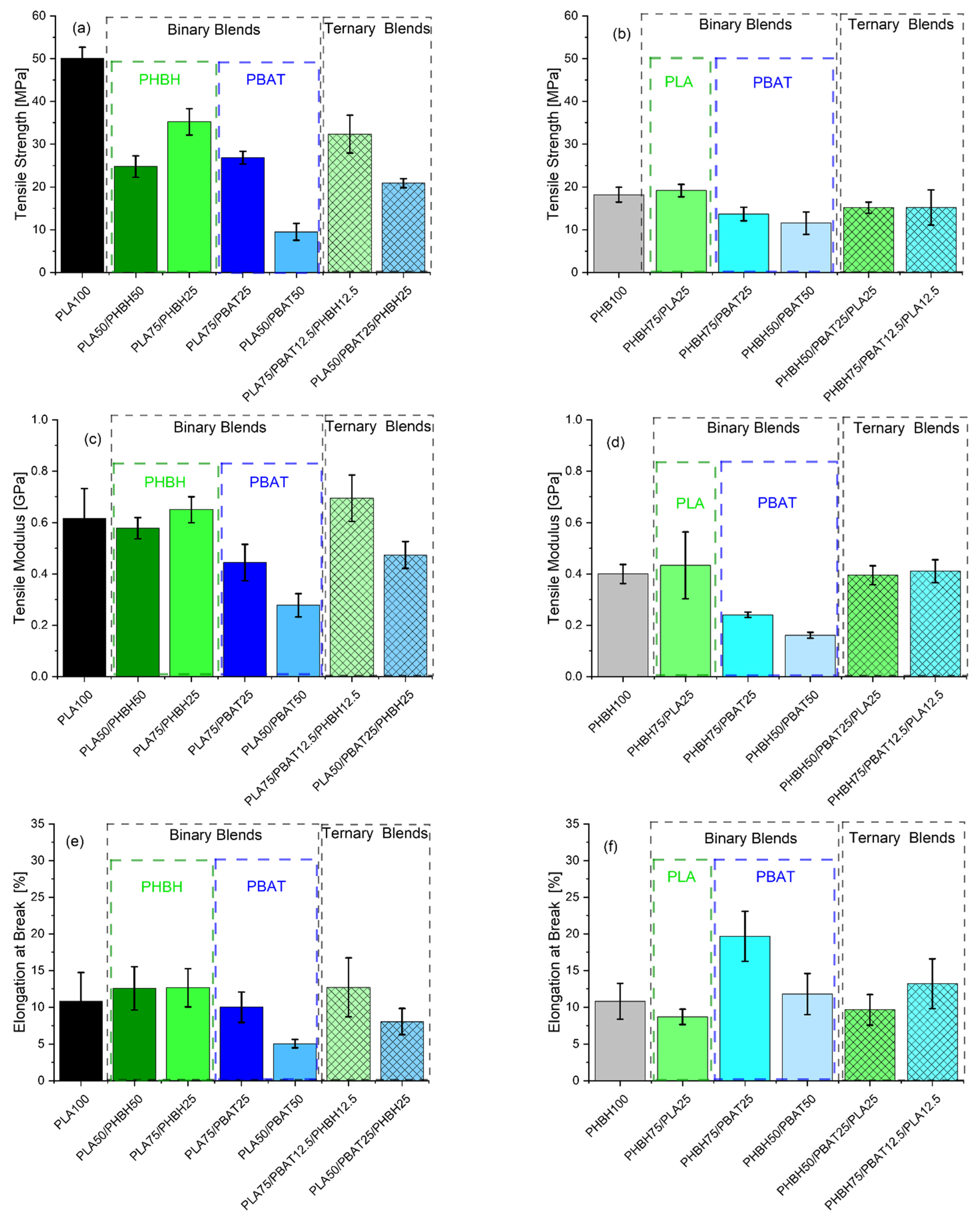
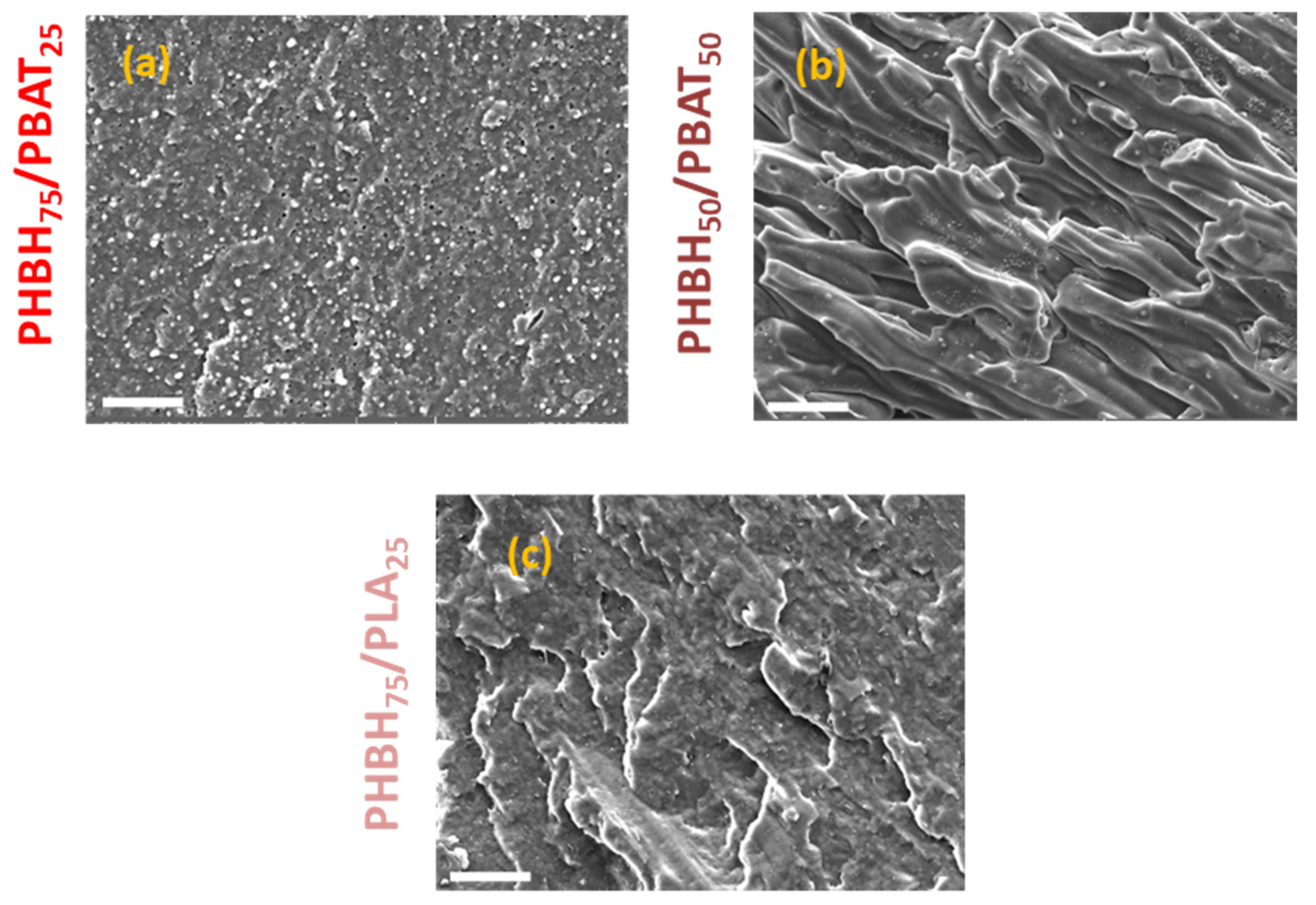
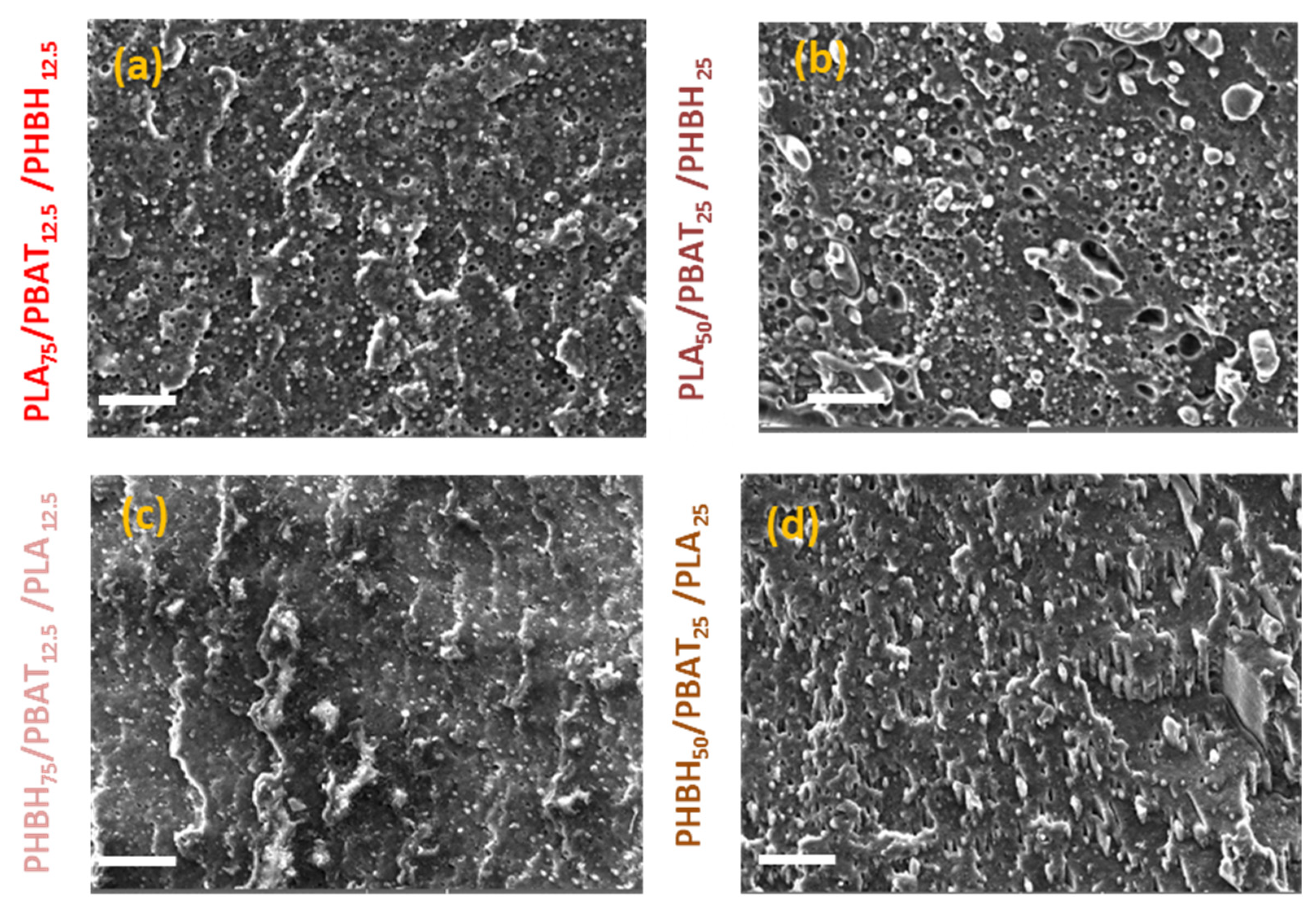
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanda, N.; Bharadvaja, N. Algal bioplastics: Current market trends and technical aspects. Clean Technol. Environ. Policy 2022, 24, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Global Production Capacities of Bioplastics 2023–2028 Global Production Capacities of Bioplastics 2023 by Material Type Global Production Capacities of Bioplastics 2028 by Material Type December 2023. Available online: http://www.european-bioplastics.org/news/publica- (accessed on 30 December 2023).
- Gironi, F.; Piemonte, V. Bioplastics and Petroleum-based Plastics: Strengths and Weaknesses. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Rieger, B.; Künkel, A.; Coates, G.W.; Reichardt, R.; Dinjus, E.; Zevaco, T.A. (Eds.) Synthetic Biodegradable Polymers; Springer: Berlin/Heidelberg, Germany, 2012; Volume 245. [Google Scholar]
- Eraslan, K.; Aversa, C.; Nofar, M.; Barletta, M.; Gisario, A.; Salehiyan, R.; Goksu, Y.A. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH): Synthesis, properties, and applications—A review. Eur. Polym. J. 2022, 167, 111044. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.J.; Palade, L.I.; Cicero, J. Polylactides: Properties and prospects of an environmentally benign plastic from renewable resources. Macromol. Symp. 2001, 175, 55–66. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Multiphase Polylactide Blends; Elsevier: Amsterdam, The Netherlands, 2021.
- Atalay, S.E.; Bezci, B.; Özdemir, B.; Göksu, Y.A.; Ghanbari, A.; Jalali, A.; Nofar, M. Thermal and Environmentally Induced Degradation Behaviors of Amorphous and Semicrystalline PLAs Through Rheological Analysis. J. Polym. Environ. 2021, 29, 3412–3426. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, C.; Weng, Y. Effect of multi-functional epoxy chain extender on the weathering resistance performance of Poly(butylene adipate-co-terephthalate) (PBAT). Polym. Test. 2021, 99, 107204. [Google Scholar] [CrossRef]
- Brzeska, J.; Tercjak, A.; Sikorska, W.; Mendrek, B.; Kowalczuk, M.; Rutkowska, M. Degradability of Polyurethanes and Their Blends with Polylactide, Chitosan and Starch. Polymers 2021, 13, 1202. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Pius, A. Biopolymer blends and composites. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–147. [Google Scholar]
- Sarul, D.S.; Arslan, D.; Vatansever, E.; Kahraman, Y.; Durmus, A.; Salehiyan, R.; Nofar, M. Preparation and characterization of PLA/PBAT/CNC blend nanocomposites. Colloid Polym. Sci. 2021, 299, 987–998. [Google Scholar] [CrossRef]
- Nofar, M.; Maani, A.; Sojoudi, H.; Heuzey, M.C.; Carreau, P.J. Interfacial and rheological properties of PLA/PBAT and PLA/PBSA blends and their morphological stability under shear flow. J. Rheol. 2015, 59, 317–333. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Mechanical and bead foaming behavior of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Nofar, M.; Oguz, H.; Ovalı, D. Effects of the matrix crystallinity, dispersed phase, and processing type on the morphological, thermal, and mechanical properties of polylactide-based binary blends with poly[(butylene adipate)-co-terephthalate] and poly[(butylene succinate). J. Appl. Polym. Sci. 2019, 136, 47636. [Google Scholar] [CrossRef]
- Bolton, R.J.; Bolton, R.J.; Hand, D.J. Unsupervised Profiling Methods for Fraud Detection. Credit. Scoring Credit. Control VII 2001, 5–7. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.24.5743 (accessed on 1 December 2021).
- Cheng, M.-L.; Chen, P.-Y.; Lan, C.-H.; Sun, Y.-M. Structure, mechanical properties and degradation behaviors of the electrospun fibrous blends of PHBHHx/PDLLA. Polymer 2011, 52, 1391–1401. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: Compression moulding vs. cast film extrusion. Compos. Part B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Pawar, S.P.; Misra, A.; Bose, S.; Chatterjee, K.; Mittal, V. Enzymatically degradable and flexible bio-nanocomposites derived from PHBV and PBAT blend: Assessing thermal, morphological, mechanical, and biodegradation properties. Colloid Polym. Sci. 2015, 293, 2921–2930. [Google Scholar] [CrossRef]
- Harkins, W.D.; Feldman, A. Films. The spreading of liquids and the spreading coefficient. J. Am. Chem. Soc. 1922, 44, 2665–2685. [Google Scholar] [CrossRef]
- Harkins, W.D. A General Thermodynamic Theory of the Spreading of Liquids to Form Duplex Films and of Liquids or Solids to Form Monolayers. J. Chem. Phys. 1941, 9, 552–568. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S. Tuning the Conductivity of Nanocomposites through Nanoparticle Migration and Interface Crossing in Immiscible Polymer Blends: A Review on Fundamental Understanding. Macromol. Mater. Eng. 2019, 304, 1800431. [Google Scholar] [CrossRef]
- Dil, E.J.; Favis, B.D. Localization of micro- and nano-silica particles in heterophase poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer 2015, 76, 295–306. [Google Scholar] [CrossRef]
- Yıldırım, M.A.; Demirbilek, M.; Gürsu, H.; Şahin, Y.; Türkoğlu, N. Manipulating cell behavior on a bacterial macro-polymer poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) via tuning the S-doped graphene ratio. Int. J. Biol. Macromol. 2021, 182, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Clint, J.H. Solid Wettability from Surface Energy Components: Relevance to Pickering Emulsions. Langmuir 2002, 18, 1270–1273. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Routledge: London, UK, 2017. [Google Scholar]
- Taylor, G.I. The viscosity of a fluid containing small drops of another fluid. In Proceedings of the Royal Society of London; Series A, Containing Papers of a Mathematical and Physical Character; Royal Society: London, UK, 1932; Volume 138, pp. 41–48. [Google Scholar] [CrossRef]
- Salehiyan, R.; Nofar, M.; Makwakwa, D.; Ray, S.S. Shear-Induced Carbon Nanotube Migration and Morphological Development in Polylactide/Poly(vinylidene fluoride) Blend Nanocomposites and Their Impact on Dielectric Constants and Rheological Properties. J. Phys. Chem. C 2020, 124, 9536–9547. [Google Scholar] [CrossRef]


| Components | Surface Energy at (mJ/m²) 25 °C | Surface Energy at (mJ/m²) 150 °C | ||||
|---|---|---|---|---|---|---|
| γ | γd | γp | γ | γd | γp | |
| PLA [1] | 39.4 | 33.6 | 5.8 | 31.04286 | 26.45714 | 4.514286 |
| PLA [1] | 50 | 37 | 13 | 39.42857 | 29.14286 | 10.21429 |
| PLA [1] | 51.1 | 34.9 | 16.1 | 40.24286 | 27.75714 | 12.67143 |
| PLA [1] | 53.5 | 37 | 16.5 | 42.14286 | 29.14286 | 13 |
| PLA [1] | 40.7 | 32.5 | 8.2 | 32.05714 | 25.57143 | 6.414286 |
| PLA [1] | 53.5 | 26.8 | 24.6 | 42.14286 | 22.15714 | 19.38571 |
| PLA [1] | 43.5 | 39.6 | 3.9 | 34.28571 | 31.17143 | 3.042857 |
| PBAT [1] | 38.4 | 32.1 | 6.3 | 30.25714 | 25.31429 | 4.942857 |
| PHBH [2] | 42.2 | 33.7 | 8.5 | 33.24895 | 26.55189 | 6.697064 |
| γ (PLA/PBAT) (mJ/m²) | γ(PLA/PHBH) (mJ/m²) | γ(PHBH/PBAT) (mJ/m²) | |||
|---|---|---|---|---|---|
| Harmonic | Geometric | Harmonic | Geometric | Harmonic | Geometric |
| 0.12 | 0.09 | 0.49 | 0.28 | 0.29 | 0.15 |
| 2.17 | 1.15 | 0.92 | 0.50 | ||
| 3.32 | 1.65 | 1.68 | 0.77 | ||
| 3.89 | 2.04 | 2.14 | 1.09 | ||
| 0.26 | 017 | 0.09 | 0.08 | ||
| 9.38 | 5.45 | 7.17 | 4.09 | ||
| 1.13 | 0.60 | 1.81 | 0.97 | ||
| PLA/PBAT/PHBH | |||||
|---|---|---|---|---|---|
| λ PLA/PBAT (mJ/m²) | λ PBAT/PHBH (mJ/m²) | λ PHBH/PLA (mJ/m²) | |||
| Harmonic | Geometric | Harmonic | Geometric | Harmonic | Geometric |
| −0.32 | −0.23 | 0.09 | 0.04 | −0.67 | −0.34 |
| −2.80 | −1.51 | −1.54 | −0.80 | 0.96 | 0.50 |
| −4.71 | −2.28 | −1.93 | −1.03 | 1.34 | 0.74 |
| −5.73 | −2.99 | −2.04 | −1.10 | 1.46 | 0.80 |
| −0.07 | −0.10 | −0.46 | −0.23 | −0.13 | −0.06 |
| −16.26 | −9.40 | −2.51 | −1.51 | 1.92 | 1.22 |
| −2.65 | −1.43 | 0.39 | 0.22 | −0.98 | −0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nofar, M.; Salehiyan, R.; Barletta, M. Tuning the Structure–Property Relationships in Binary and Ternary Blends of PLA/PBAT/PHBH. Polymers 2024, 16, 1699. https://doi.org/10.3390/polym16121699
Nofar M, Salehiyan R, Barletta M. Tuning the Structure–Property Relationships in Binary and Ternary Blends of PLA/PBAT/PHBH. Polymers. 2024; 16(12):1699. https://doi.org/10.3390/polym16121699
Chicago/Turabian StyleNofar, Mohammadreza, Reza Salehiyan, and Massimiliano Barletta. 2024. "Tuning the Structure–Property Relationships in Binary and Ternary Blends of PLA/PBAT/PHBH" Polymers 16, no. 12: 1699. https://doi.org/10.3390/polym16121699
APA StyleNofar, M., Salehiyan, R., & Barletta, M. (2024). Tuning the Structure–Property Relationships in Binary and Ternary Blends of PLA/PBAT/PHBH. Polymers, 16(12), 1699. https://doi.org/10.3390/polym16121699







