Investigation of Recycled Expanded Polyamide Beads through Artificial Ageing and Mechanical Recycling as a Proof of Concept for Circular Economy
Abstract
:1. Introduction
2. Scientific Background
3. Materials and Methods
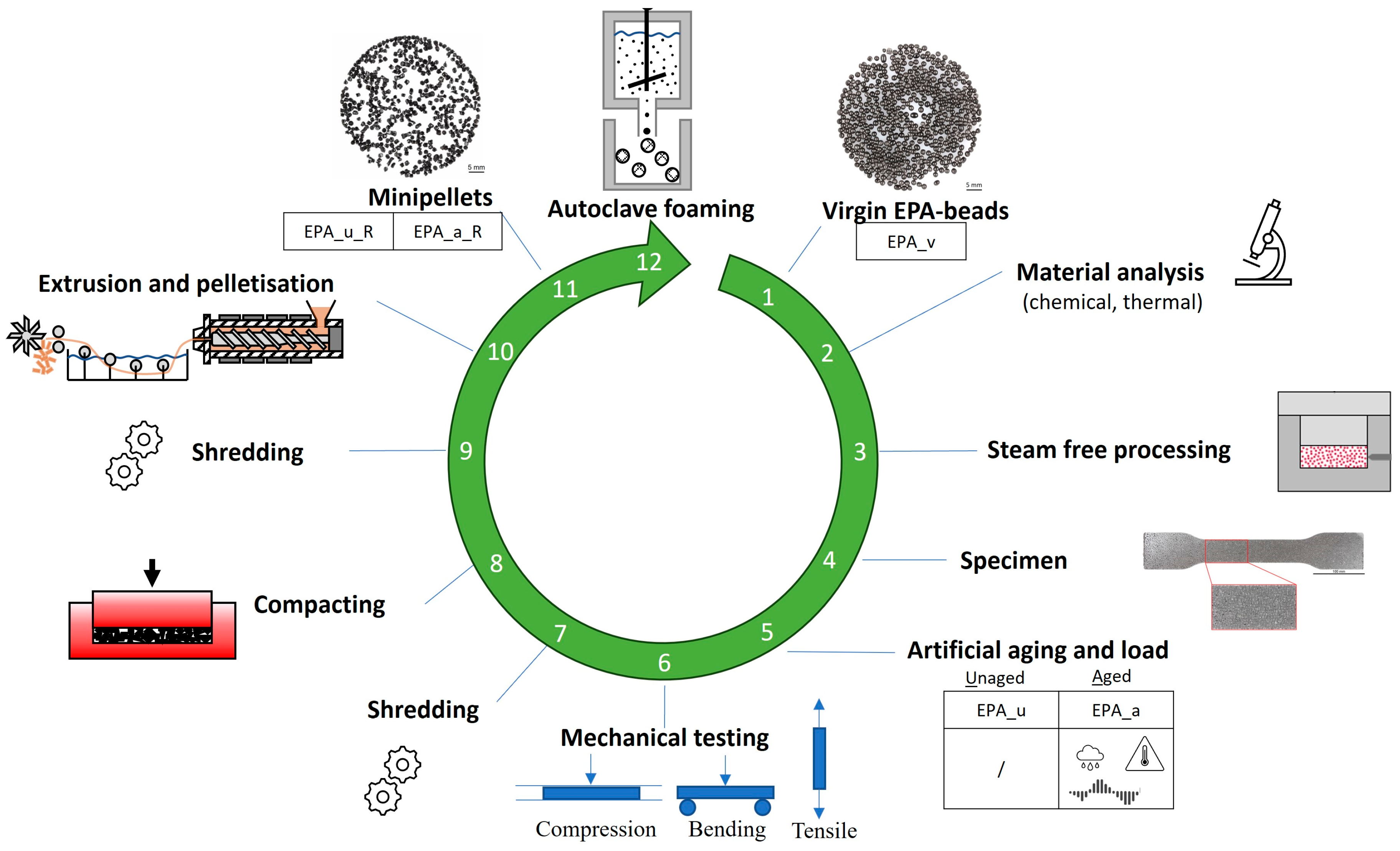
3.1. Materials
3.2. Steam-Free Processing and Artificial Ageing
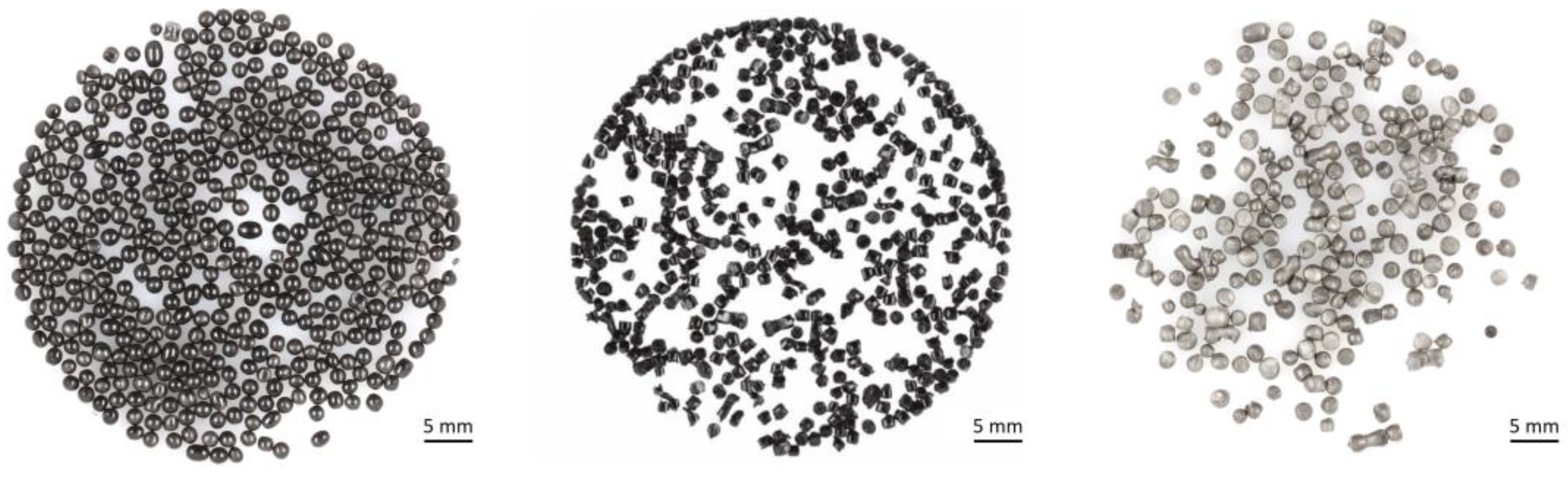
3.3. Mechanical Recycling
3.4. Autoclave Foaming of Recycled Beads
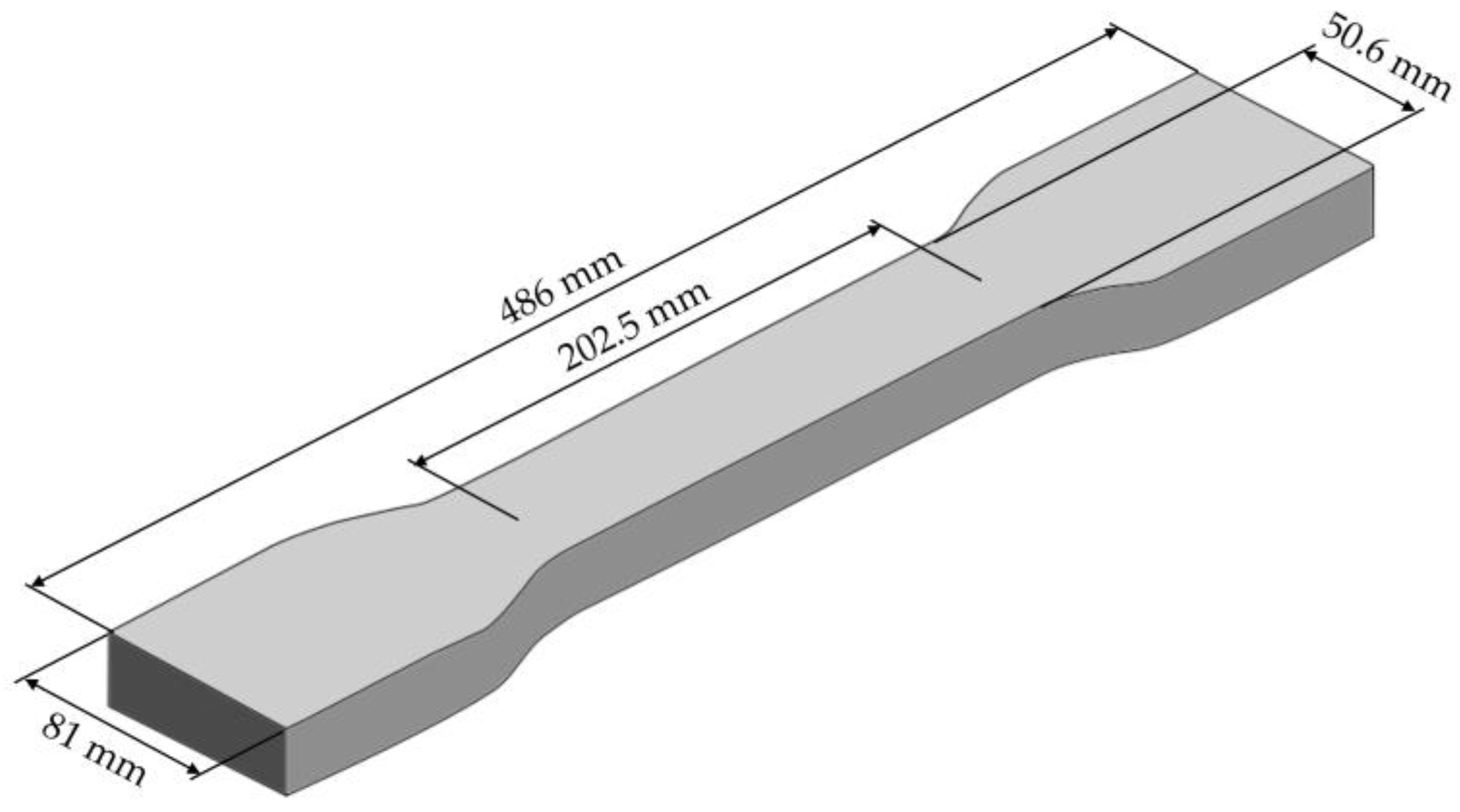
3.5. Material Properties
4. Results and Discussion
4.1. Change in Chemical Properties
4.2. Change in Thermal Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Proposal for a Regulation on Circularity Requirements for Vehicle Design and on Management of End-of-Life Vehicles; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Avalle, M.; Belingardi, G.; Montanini, R. Characterization of polymeric structural foams under compressive impact loading by means of energy-absorption diagram. Int. J. Impact Eng. 2001, 25, 455–472. [Google Scholar] [CrossRef]
- Rumianek, P.; Dobosz, T.; Nowak, R.; Dziewit, P.; Aromiński, A. Static Mechanical Properties of Expanded Polypropylene Crushable Foam. Materials 2021, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, H.; He, Q.; Wan, C.; Liu, T.; Zhao, L.; Park, C.B. Chain extension of polyamide 6 using multifunctional chain extenders and reactive extrusion for melt foaming. Eur. Polym. J. 2017, 96, 210–220. [Google Scholar] [CrossRef]
- Kriha, O.; Hahn, K.; Desbois, P.; Warzelhan, V.; Ruckdäschel, H.; Hofmann, M.; Exner, C.; Hingmann, R. Expandable Polyamide Granules. WO 2011/134996 A1, 3 November 2011. [Google Scholar]
- Raps, D.; Hossieny, N.; Park, C.B.; Altstädt, V. Past and present developments in polymer bead foams and bead foaming technology. Polymer 2015, 56, 5–19. [Google Scholar] [CrossRef]
- Srivastava, V.; Srivastava, R. A review on manufacturing, properties and application of expanded polypropylen. MIT Int. J. Mech. Eng. 2014, 4, 22–28. [Google Scholar]
- Zhai, W.; Kim, Y.-W.; Park, C.B. Steam-Chest Molding of Expanded Polypropylene Foams. 1. DSC Simulation of Bead Foam Processing. Ind. Eng. Chem. Res. 2010, 49, 9822–9829. [Google Scholar] [CrossRef]
- Dörr, D.; Kuhnigk, J.; Altstädt, V. Bead Foams. In Polymeric Foams: Innovations in Technologies and Environmentally Friendly Materials; Lee, S.T., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 51–90. [Google Scholar]
- Prissok, F.; Harms, M.; Schuette, M. Method for Producing Particle Foams Based on Thermoplastic Elastomers, by Thermal Bonding Using Microwaves. BASF SE. WO2016146537A1, 22 September 2016. [Google Scholar]
- Zubair, M.; Ferrari, R.; Alagha, O.; Mu’azu, N.D.; Blaisi, N.I.; Ateeq, I.S.; Manzar, M.S. Microwave Foaming of Materials: An Emerging Field. Polymers 2020, 12, 2477. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Gothe, B.; Drexler, M.; Siltamaeki, J.; Weiger, H.; Seefried, A.; Drummer, D. The effect of dielectric and thermal properties of plastic mold materials on the high frequency welding of three-dimensional foam components. Polym. Eng. Sci. 2022, 62, 3400–3411. [Google Scholar] [CrossRef]
- Romanov, V. Vorrichtung und Verfahren zur Herstellung Eines Partikelschaumstoffteils. Kurtz GmbH WO2017125412A1, 27 July 2017. [Google Scholar]
- Shojaei, S.; Rostami-Tapeh-Esmaeil, E.; Rodrigue, D. Polymer Foams Waste Management: A Focus on Mechanical and Chemical Recycling. In Polymeric Foams: Applications of Polymeric Foams; ACS Publications: Washington, DC, USA, 2023; Volume 2, pp. 289–318. [Google Scholar]
- Noguchi, T.; Miyashita, M.; Inagaki, Y.; Watanabe, H. A new recycling system for expanded polystyrene using a natural solvent. Part 1. A new recycling technique, Packag. Technol. Sci. 1998, 11, 19–27. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Bolzan, A.; Machado, R.A.F. A closed-loop process design for recycling expanded polystyrene waste by dissolution and polymerization. Polymer 2020, 209, 122940. [Google Scholar] [CrossRef]
- Weingart, N.; Raps, D.; Lamka, M.; Demleitner, M.; Altstädt, V.; Ruckdäschel, H. Influence of thermo-oxidative aging on the mechanical properties of the bead foams made of polycarbonate and polypropylene. J. Polym. Sci. 2023, 61, 2742–2757. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Liu, W.-H.; Huang, Y.-M. Carbon Dioxide-Blown Expanded Polyamide Bead Foams with Bimodal Cell Structure. Ind. Eng. Chem. Res. 2019, 58, 2958–2969. [Google Scholar] [CrossRef]
- Kuhnigk, J.; Standau, T.; Dörr, D.; Brütting, C.; Altstädt, V.; Ruckdäschel, H. Progress in the development of bead foams—A review. J. Cell. Plast. 2022, 1002, 0021955X2210876. [Google Scholar] [CrossRef]
- Dörr, D.; Raps, D.; Kirupanantham, D.; Holmes, C.; Altstädt, V. Expanded polyamide 12 bead foams (ePA) thermo-mechanical properties of molded parts. In Proceedings of the 35th International Conference of the Polymer Processing Society (PPS-35), Cesme-Izmir, Turkey, 26–30 May 2019; p. 20037. [Google Scholar]
- Vohlídal, J. Polymer degradation: A short review. Chem. Teach. Int. 2021, 3, 213–220. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Pliquet, M.; Rapeaux, M.; Delange, F.; Bussiere, P.O.; Therias, S.; Gardette, J.L. Multiscale analysis of the thermal degradation of polyamide 6,6: Correlating chemical structure to mechanical properties. Polym. Degrad. Stab. 2021, 185, 109496. [Google Scholar] [CrossRef]
- Deshoulles, Q.; Le Gall, M.; Dreanno, C.; Arhant, M.; Priour, D.; Le Gac, P.Y. Chemical coupling between oxidation and hydrolysis in polyamide 6—A key aspect in the understanding of microplastic formation. Polym. Degrad. Stab. 2022, 197, 109851. [Google Scholar] [CrossRef]
- Su, K.-H.; Lin, J.-H.; Lin, C.-C. Influence of reprocessing on the mechanical properties and structure of polyamide 6. J. Mater. Process. Technol. 2007, 192–193, 532–538. [Google Scholar] [CrossRef]
- Lozano-González, M.J.; Rodriguez-Hernandez, M.T.; Los Santos, E.A.G.-D.; Villalpando-Olmos, J. Physical-mechanical properties and morphological study on nylon-6 recycling by injection molding. J. Appl. Polym. Sci. 2000, 76, 851–858. [Google Scholar] [CrossRef]
- David, C.; Trojan, M.; Daro, A.; Demarteau, W. Photodegradation of polyethylene: Comparison of various photoinitiators in natural weathering conditions. Polym. Degrad. Stab. 1992, 37, 233–245. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Crystallization behavior of nylon 6 nanocomposites. Polymer 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Khanna, Y.P. Overview of transition phenomenon in nylon 6. Macromolecules 1992, 25, 3298–3300. [Google Scholar] [CrossRef]
- ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Beuth Verlag GmbH: Berlin, Germany, 2012.
- Preda, F.M. Dynamics of Polyamide in the Solid State in Presence of Solvents and in the Molten Staate. Materials and Structures in. Mechanics Dissertation, Université de Lyon, Lyon, France, 2016. [Google Scholar]
- Liu, X.; Wang, Y.; Wang, Z.; Cavallo, D.; Müller, A.J.; Zhu, P.; Zhao, Y.; Dong, X.; Wang, D. The origin of memory effects in the crystallization of polyamides: Role of hydrogen bonding. Polymer 2020, 188, 122117. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Spektroskopische Daten zur Strukturaufklärung Organischer Verbindungen; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Brütting, C.; Standau, T.; Meuchelböck, J.; Schreier, P.; Ruckdäschel, H. A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties. e-Polymers 2023, 23, 20230092. [Google Scholar] [CrossRef]






| Load/Aging Type | Series 1 | Series 2 |
|---|---|---|
| Climate changing | / | ✔ |
| High frequency | / | ✔ |
| Abbreviation | EPA_u | EPA_a |
| This Work | Su et al. [25] | Lozano-Gonzalez et al. [26] | |||||
|---|---|---|---|---|---|---|---|
| EPA_v | EPA_u_R | EPA_a_R | Virgin PA | PA Recycled Once | Virgin PA | PA Recycled Once | |
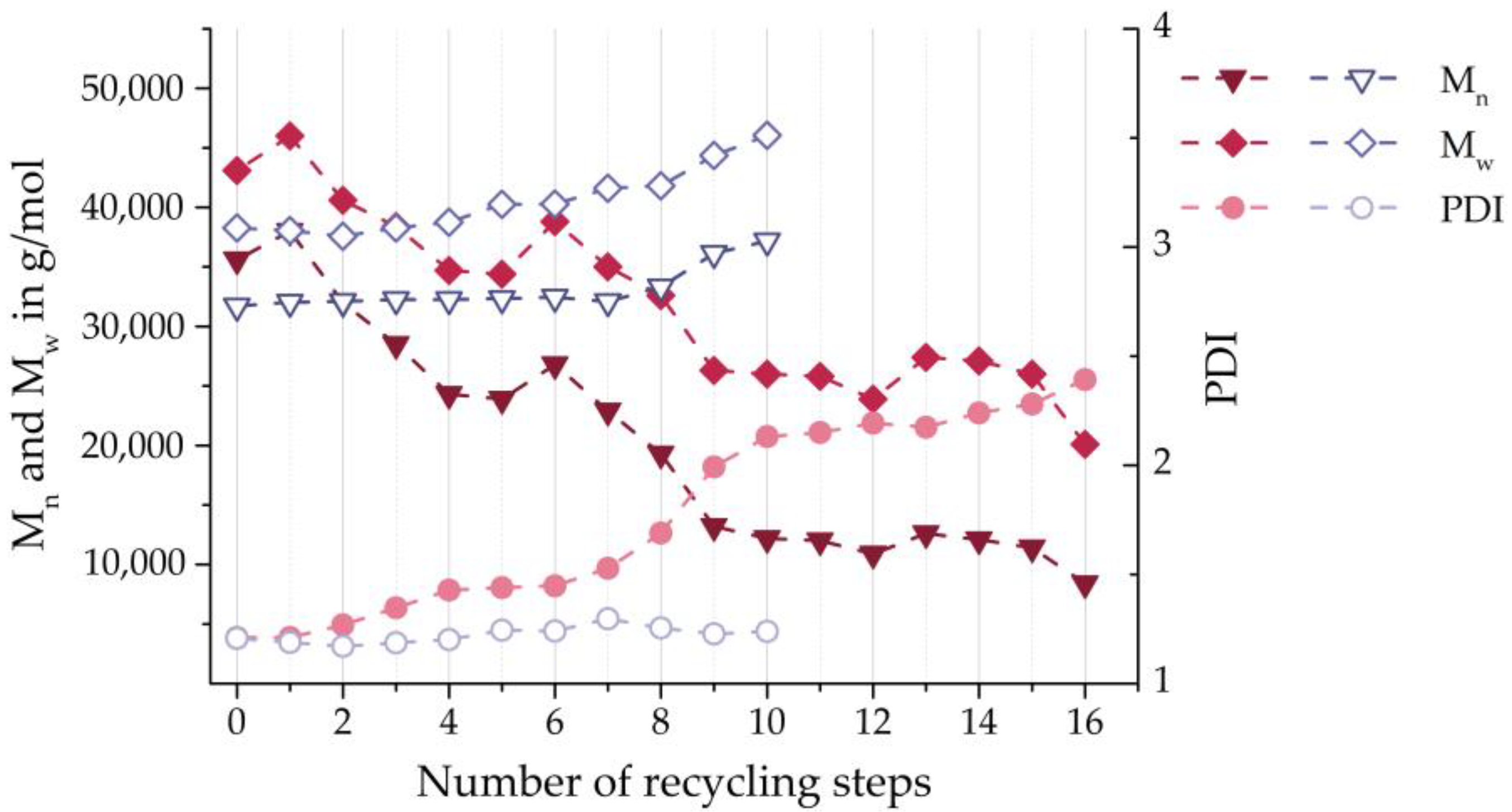
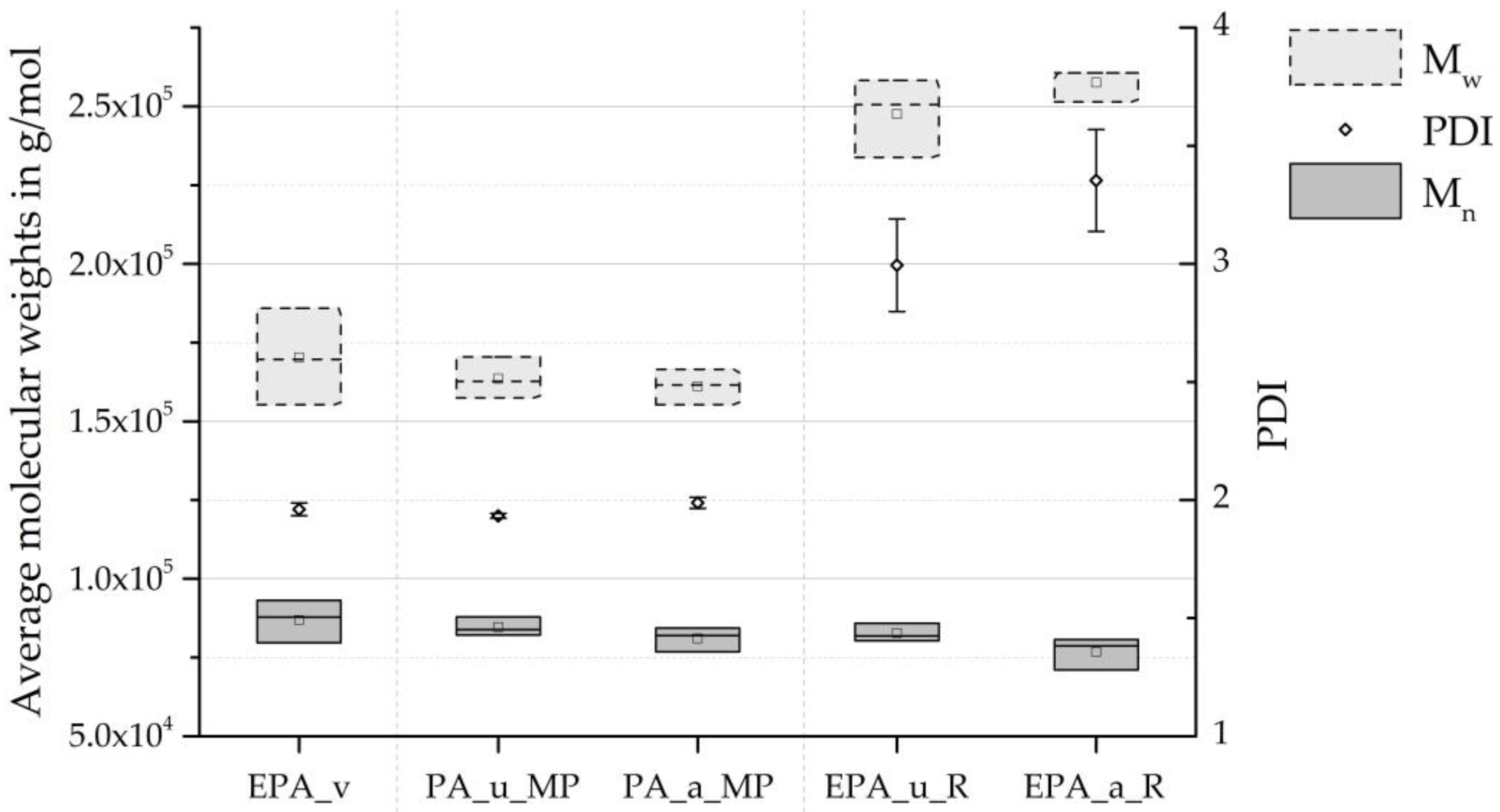
| [g/mol] | 86,870 | 82,675 (−4.83%) | 76,812 (−11.58%) | 35,600 | 38,000 (+6.74%) | 31,750 | 32,000 (+0.79%) |
| [g/mol] | 170,300 | 247,540 (+45.36%) | 257,550 (+51.23%) | 43,100 | 46,000 (+6.73%) | 38,250 | 38,000 (−0.65%) |
| 1.96 | 3.00 (+52.73%) | 3.36 (+71.04%) | 1.21 | 1.21 (±0%) | 1.20 | 1.18 (−1.43%) | |
| Species | Wavenumber in cm−1 | Source |
|---|---|---|
| “Free” carboxylic acids | 1756 | [10,11] |
| C=O stretching | 1745 | [12,13] |
| Imides | 1734 | [10,11] |
| “Bonded” carboxylic acids | 1716 | [11] |
| 1st Heating | 2nd Heating | 1st Cooling | |||||
|---|---|---|---|---|---|---|---|
| Tm low (°C) | Tm high (°C) | Δ Hm (J/g) | Tm (°C) | Δ Hm (J/g) | Tc (°C) | Δ Hc (J/g) | |
| EPA_v | 194.6 ± 0.2 | 46.39 ± 2.11 | 191.2 ± 0.5 | 43.29 ± 1.53 | 160.0 ± 0.3 | 36.0 ± 1.51 | |
| PA_u_MP | 196.0 ± 0.3 | 30.29 ± 0.74 | 191.8 ± 0.1 | 35.27 ± 0.46 | 160.0 ± 0.1 | 29.4 ± 0.42 | |
| PA_a_MP | 196.1 ± 0.2 | 31.23 ± 0.49 | 191.8 ± 0.1 | 34.14 ± 1.44 | 159.3 ± 0.1 | 30.1 ± 0.17 | |
| EPA_u_R | 187.4 ± 0.1 | 207.0 ± 0.2 | 68.74 ± 7.90 | 191.1 ± 0.2 | 49.66 ± 5.52 | 160.9 ± 0.1 | 46.0 ± 5.48 |
| EPA_a_R | 186.2 ± 0.2 | 206.5 ± 0.1 | 71.24 ± 6.66 | 190.5 ± 0.2 | 49.89 ± 4.11 | 161.1 ± 0.1 | 47.7 ± 4.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handtke, S.; Brömstrup, L.; Hain, J.; Fischer, F.; Ossowski, T.; Hartwig, S.; Dröder, K. Investigation of Recycled Expanded Polyamide Beads through Artificial Ageing and Mechanical Recycling as a Proof of Concept for Circular Economy. Polymers 2024, 16, 1730. https://doi.org/10.3390/polym16121730
Handtke S, Brömstrup L, Hain J, Fischer F, Ossowski T, Hartwig S, Dröder K. Investigation of Recycled Expanded Polyamide Beads through Artificial Ageing and Mechanical Recycling as a Proof of Concept for Circular Economy. Polymers. 2024; 16(12):1730. https://doi.org/10.3390/polym16121730
Chicago/Turabian StyleHandtke, Sören, Lena Brömstrup, Jörg Hain, Fabian Fischer, Tim Ossowski, Sven Hartwig, and Klaus Dröder. 2024. "Investigation of Recycled Expanded Polyamide Beads through Artificial Ageing and Mechanical Recycling as a Proof of Concept for Circular Economy" Polymers 16, no. 12: 1730. https://doi.org/10.3390/polym16121730
APA StyleHandtke, S., Brömstrup, L., Hain, J., Fischer, F., Ossowski, T., Hartwig, S., & Dröder, K. (2024). Investigation of Recycled Expanded Polyamide Beads through Artificial Ageing and Mechanical Recycling as a Proof of Concept for Circular Economy. Polymers, 16(12), 1730. https://doi.org/10.3390/polym16121730







