Crystallization Behavior and Mechanical Property of Biodegradable Poly(butylene succinate-co-2-methyl succinate)/Cellulose Nanocrystals Composites
Abstract
:1. Introduction
2. Experimental
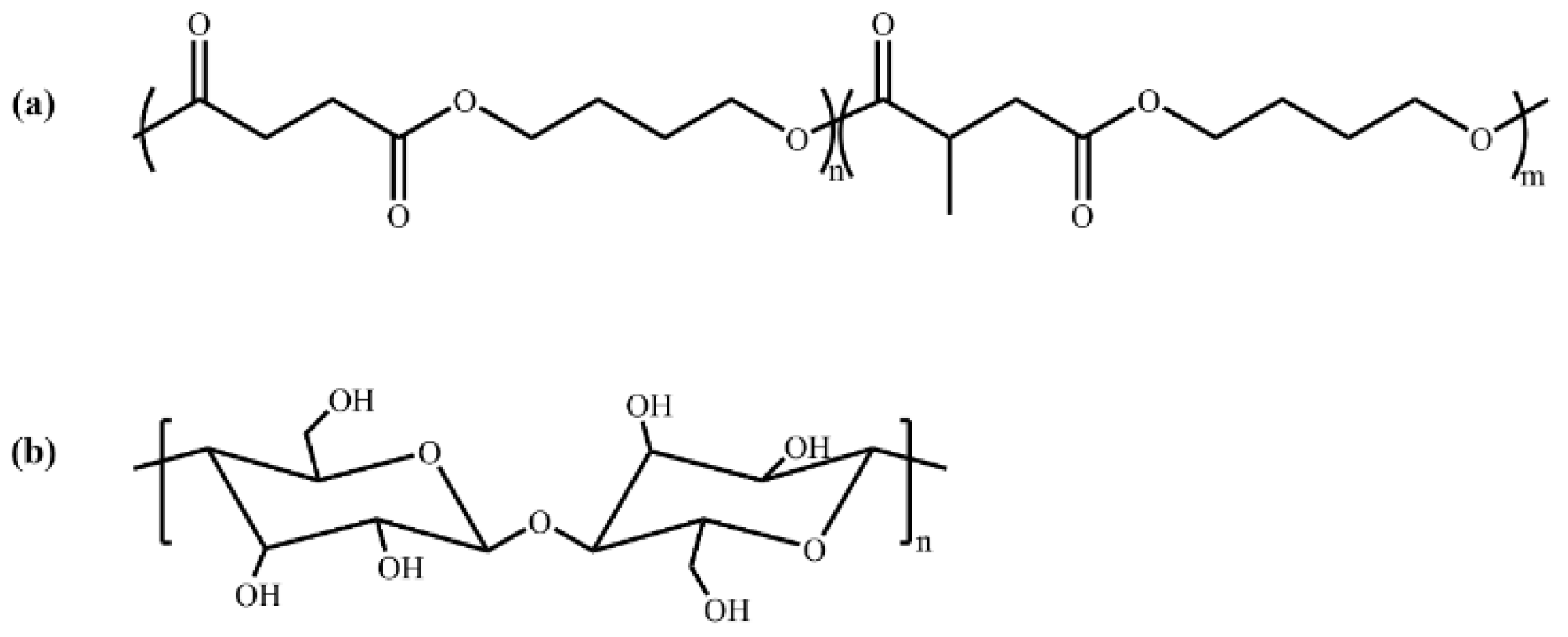
2.1. Materials and Preparation of PBSMS/CNC Composites
2.2. Characterizations
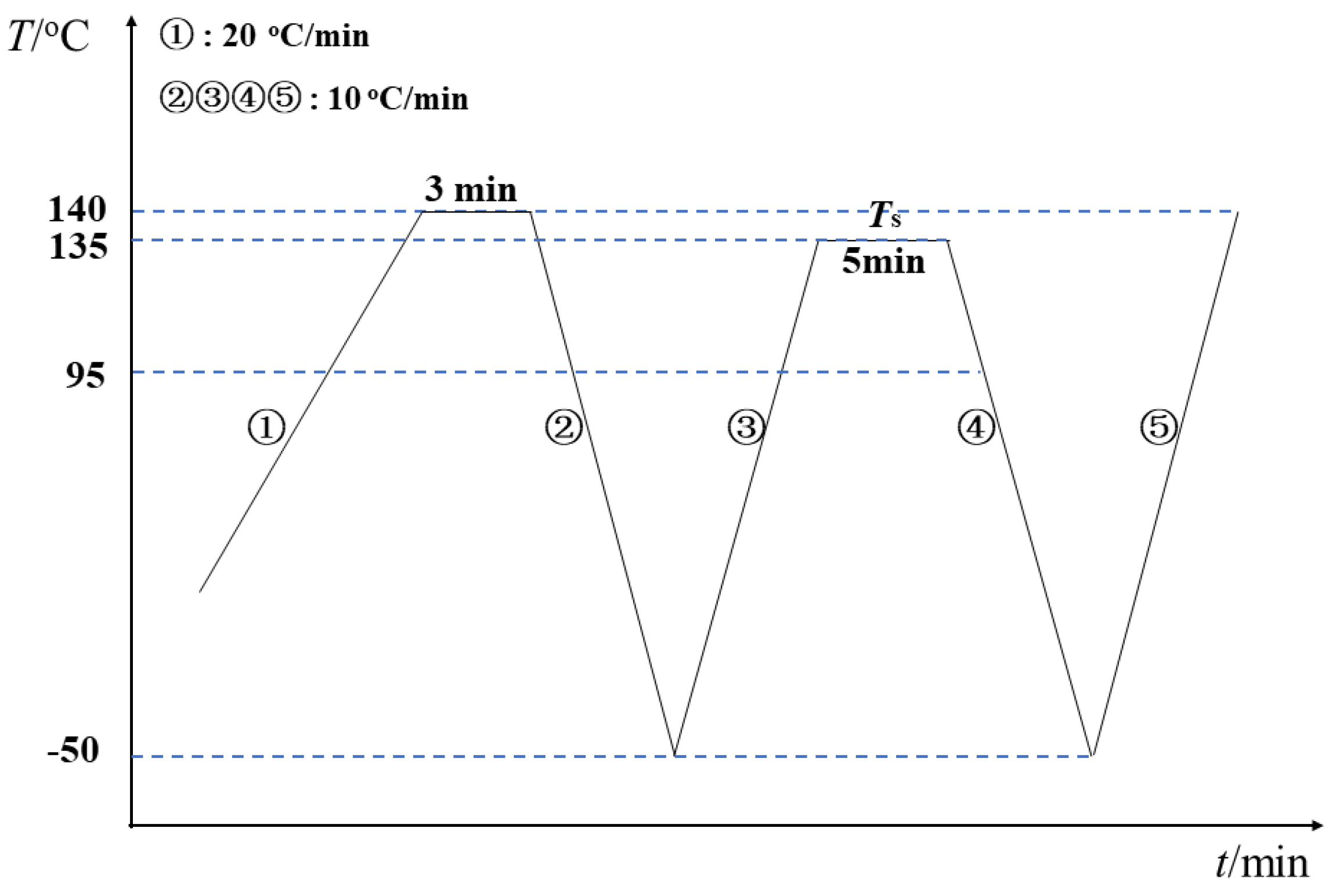
- The sample was melted at 140 °C for 3 min to remove the previous thermal history.
- The creation of the standard semicrystalline state was achieved by cooling the meltdown to −50 °C at a rate of 10 °C/min.
- Then, the sample was heated to an indicated self-nucleation temperature (Ts) at a rate of 10 °C/min and held at this temperature for 5 min. The self-nucleation temperature determined the various states that the sample went through, such as melting, self-nucleation, and annealing.
- The subsequent crystallization further occurred by cooling the sample at a rate of 10 °C/min.
- The sample was finally heated again to 140 °C at 10 °C/min to investigate the melting behavior.
3. Result and Discussion
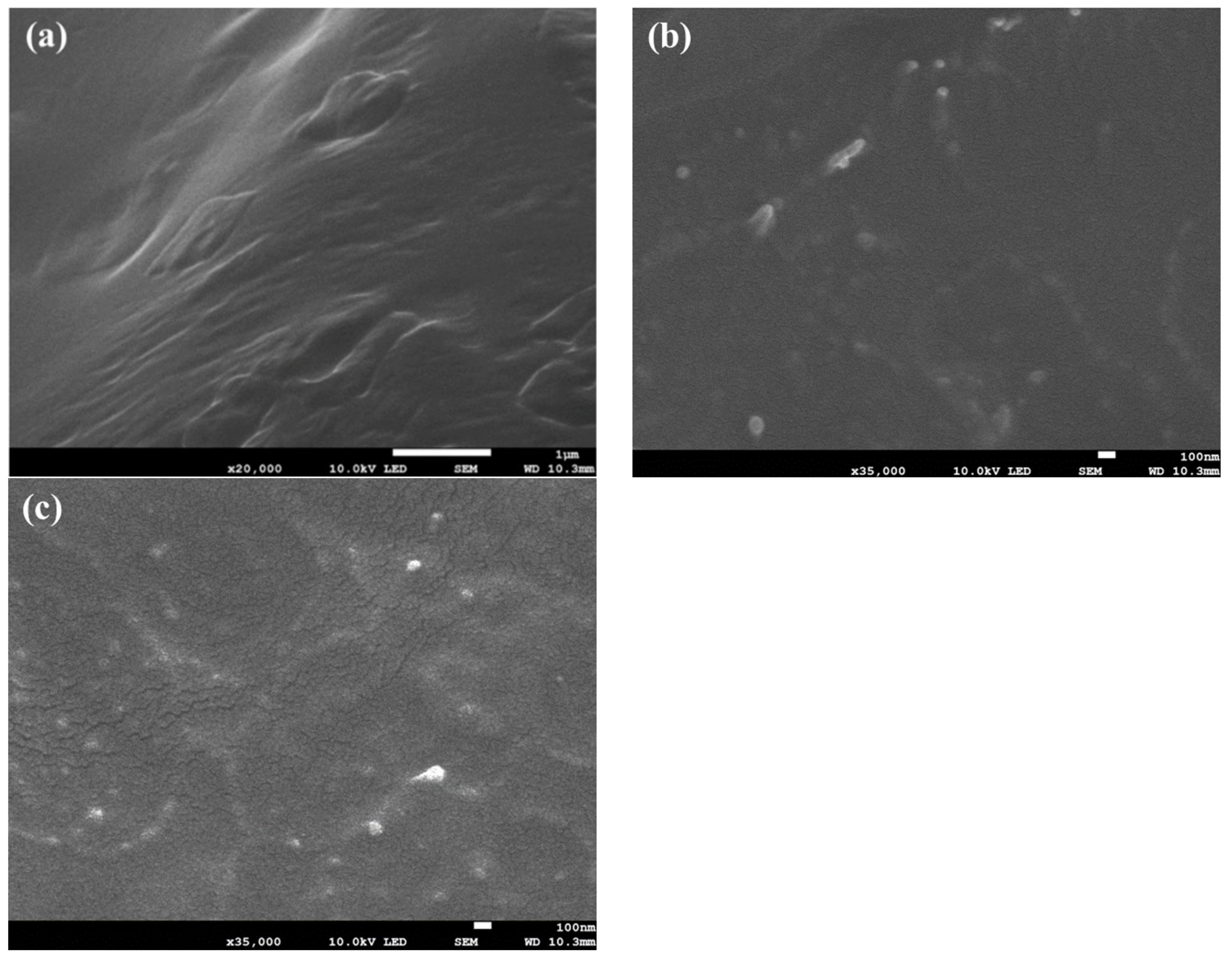
3.1. Dispersion of CNC in PBSMS Matrix
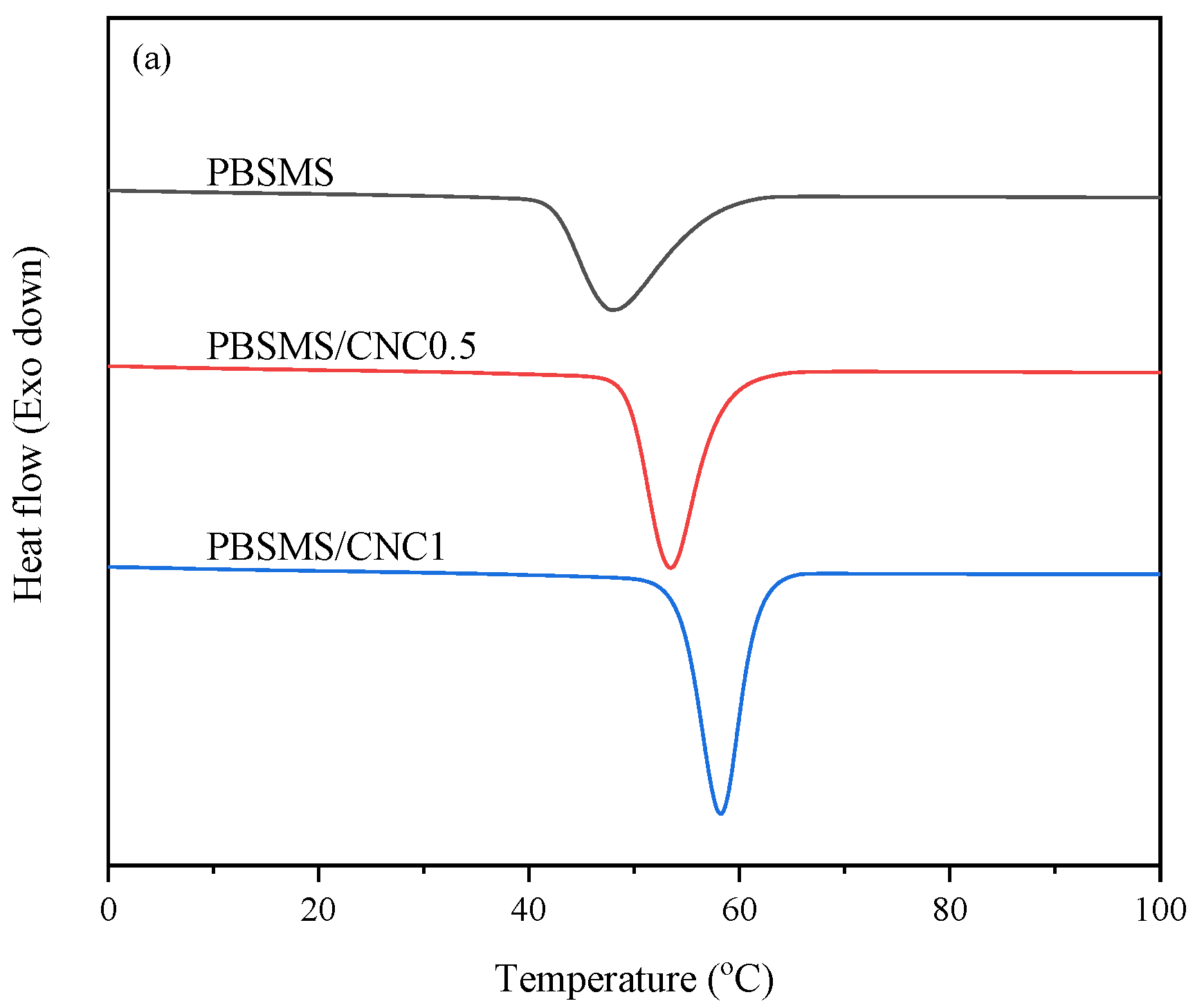
3.2. Nonisothermal Melt Crystallization Behavior Study
3.3. Isothermal Crystallization Kinetics Study
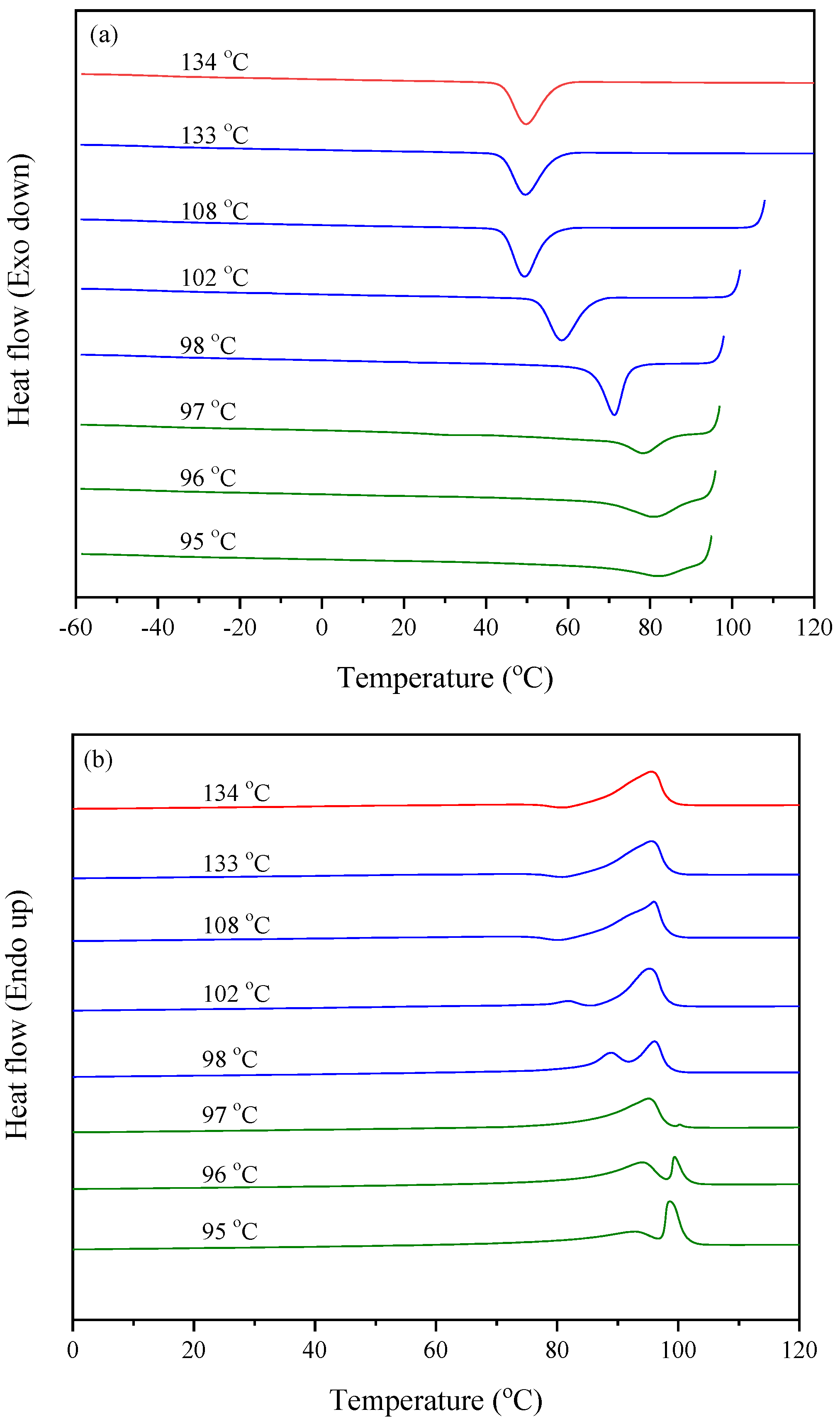
3.4. Nucleation Study
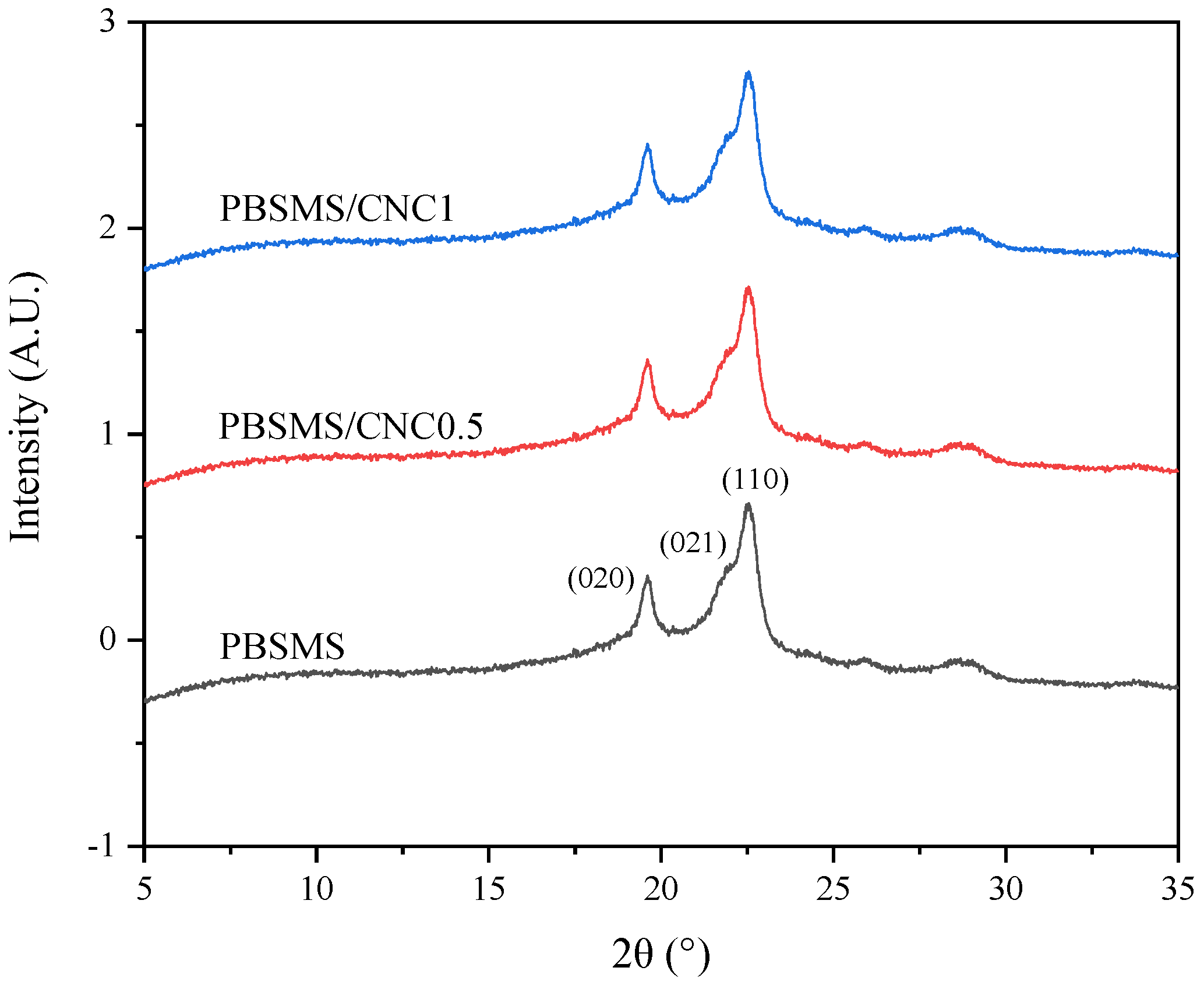
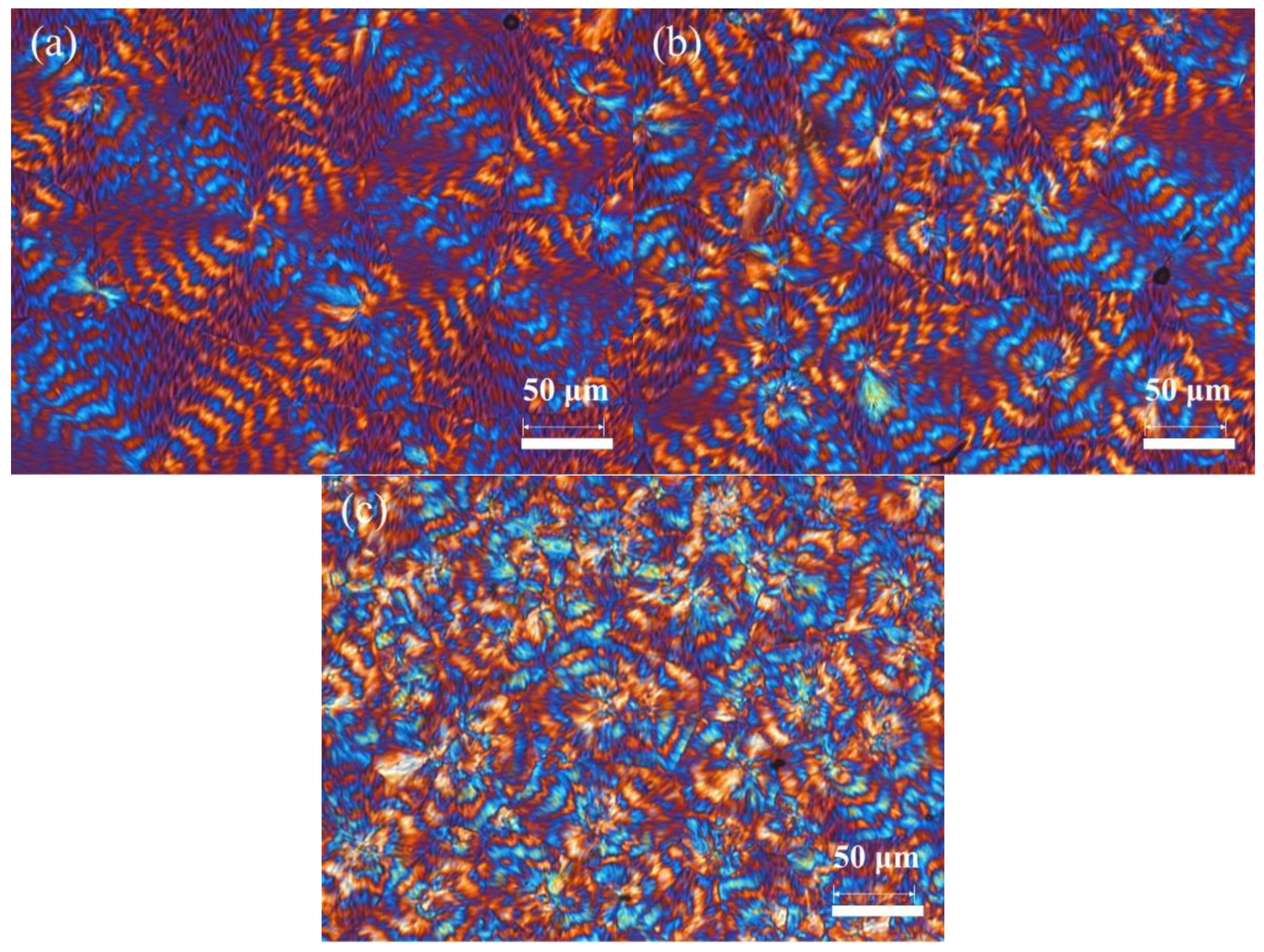
3.5. Crystal Structure and Spherulitic Morphology Studies
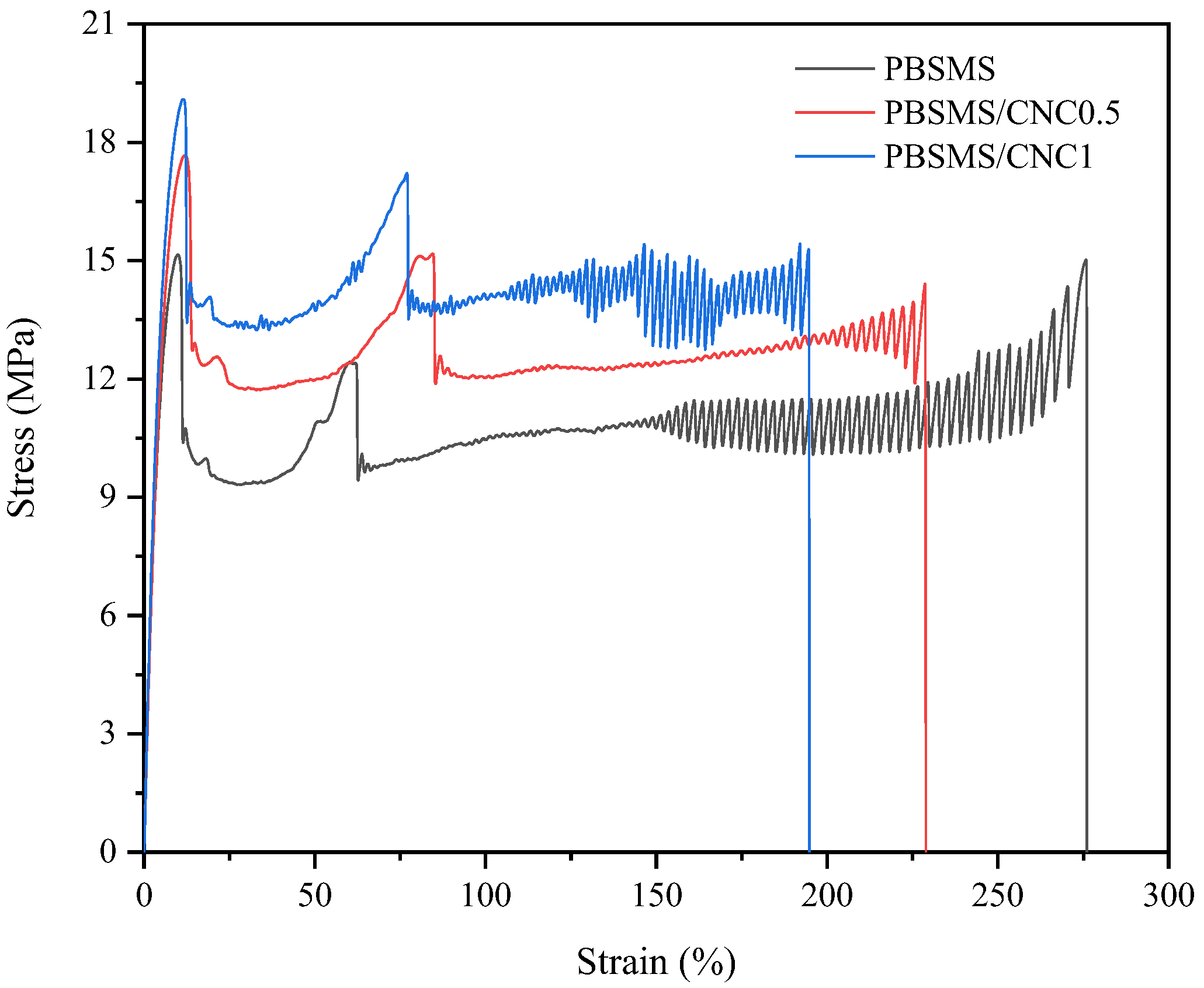
3.6. Mechanical Property Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jambeck, J.; Geyer, R.; Wilcox, C.; Siegler, T.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Wang, Y.; Putten, R.; Tietema, A.; Parsons, J.; Gruter, G. Polyester biodegradability: Importance and potential for optimisation. Green Chem. 2024, 26, 3698–3716. [Google Scholar] [CrossRef]
- Naira, L.; Laurencin, C. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Nonisothermal crystallization kinetics of poly(butylene succinate) and poly(ethylene succinate). Polym. J. 2004, 36, 642–646. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Wang, Z.; Wang, J.; Xiao, Y.; Zhang, D.; Guan, G. Novel biodegradable and double crystalline multiblock copolymers comprising of poly(butylene succinate) and poly(ε-caprolactone):synthesis, characterization, and properties. Ind. Eng. Chem. Res. 2012, 51, 7264–7272. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, H.; Qiu, Z. Thermal properties and crystallization behavior of novel biodegradable poly(hexamethylene succinate-co-6 mol% butylene succinate) and poly(hexamethylene succinate). J. Polym. Environ. 2018, 26, 1320–1327. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Enzymatic synthesis of biobased poly(1,4-butylene succinate-ran-2,3-butylene succinate) copolyesters and characterization. Eur. Polym. J. 2017, 93, 103–115. [Google Scholar] [CrossRef]
- Tsai, Y.; Jheng, L.; Hung, C. Synthesis, properties and enzymatic hydrolysis of biodegradable alicyclic/aliphatic copolyesters based on 1,3/1,4-cyclohexanedimethanol. Polym. Degrad. Stab. 2010, 95, 72–78. [Google Scholar] [CrossRef]
- Ye, H.; Wang, R.; Liu, J.; Xu, J.; Guo, B. Isomorphism in poly(butylene succinate-co-butylene fumarate) and its application as polymeric nucleating agent for poly(butylene succinate). Macromolecules 2012, 45, 5667–5675. [Google Scholar] [CrossRef]
- Chae, H.; Park, S.; Kim, B.; Kim, D. Effect of methyl substitution of the ethylene unit on the physical properties of poly(butylene succinate). J. Polym. Sci. Polym. Phys. 2004, 42, 1759–1766. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Xie, Z.; Xun, J.; Guo, B. Synthesis, Properties of biodegradable poly(butylene succinate-co-butylene 2-methylsuccinate) and application for sustainable release. Materials 2019, 12, 1507. [Google Scholar] [CrossRef]
- Yao, W.; Chen, M.; Qiu, Z. Synthesis, crystallization behavior and mechanical property of biodegradable Poly(butylene succinate-co-2-methyl succinate) copolyesters. Polymer 2024, 303, 127114. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.; Rojas, O. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Julkapli, N.; Bagheri, S. Progress on nanocrystalline cellulose biocomposites. React. Funct. Polym. 2017, 112, 9–21. [Google Scholar] [CrossRef]
- Ferreira, F.; Dufresne, A.; Pinheiro, I.; Souza, D.; Gouveia, R.; Mei, L.; Lona, L. How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: A comprehensive review. Eur. Polym. J. 2018, 108, 274–285. [Google Scholar] [CrossRef]
- Younas, M.; Noreen, A.; Sharif, A.; Majeed, A.; Hassan, A.; Tabasum, S.; Mohammadi, A.; Zia, K. A review on versatile applications of blends and composites of CNC with natural and synthetic polymers with mathematical modeling. Int. J. Biol. Macromol. 2019, 124, 591–626. [Google Scholar] [CrossRef]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S. Development, processing and applications of bio-sourced cellulose nanocrystal composites. Prog. Polym. Sci. 2020, 103, 101221. [Google Scholar] [CrossRef]
- Kaushik, A.; Kaur, R. Thermoplastic starch nanocomposites reinforced with cellulose nanocrystals: Effect of plasticizer on properties. Compos. Interfaces 2016, 23, 701–717. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, C.; Yue, Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical properties and in vitro degradation of electrospun bio-nanocomposite mats from PLA and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Xu, C.; Lv, Q.; Wu, D.; Wang, Z. Polylactide/cellulose nanocrystal composites: A comparative study on cold and melt crystallization. Cellulose 2017, 24, 2163–2175. [Google Scholar] [CrossRef]
- Hegyesi, N.; Zhang, Y.; Kohári, A.; Polyák, P.; Sui, X.; Pukánszky, B. Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind. Crop. Prod. 2019, 141, 111799. [Google Scholar] [CrossRef]
- Eriksson, M.; Goffin, A.; Dubois, P.; Peijs, T.; Goossens, H. The influence of grafting on flow-induced crystallization and rheological properties of poly(ε-caprolactone)/cellulose nanocrystal nanocomposites. Nanocomposites 2018, 4, 87–101. [Google Scholar] [CrossRef]
- Kim, T.; Jeon, H.; Jegal, J.; Kim, J.; Yang, H.; Park, J.; Oh, D.; Hwang, S. Trans crystallization behavior and strong reinforcement effect of cellulose nanocrystals on reinforced poly(butylene succinate) nanocomposites. RSC Adv. 2018, 8, 15389–15398. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z. Surface grafting of cellulose nanocrystal with poly(3-hydrobutyrate-co-3-hydroxy valerate). Carbohydr. Polym. 2014, 101, 471–478. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Influence of two different nanofillers on the crystallization behavior and dynamic mechanical properties of biodegradable poly(ethylene adipate). J. Polym. Environ. 2019, 27, 2674–2681. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Ugarte, L.; Arbelaiz, A.; Gabilondo, N.; Corcuera, M.; Eceiza, A. Two different incorporation routes of cellulose nanocrystals in waterborne polyurethane nanocomposites. Eur. Polym. 2016, 76, 99–109. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Lu, L.; Chen, H.; Gong, T.; Lva, J.; Zhou, S. The improvement of the shape memory function of poly(3-caprolactone)/nano-crystalline cellulose nanocomposites via recrystallization under a high-pressure environment. J. Mater. Chem. 2016, 4, 5984–5992. [Google Scholar] [CrossRef]
- Rader, C.; Fritz, P.; Ashirov, T.; Coskun, A.; Weder, C. One-Component Nanocomposites Made from Diblock Copolymer Grafted Cellulose Nanocrystals. Biomacromolecules 2024, 25, 1637–1648. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Effect of low loadings of cellulose nanocrystals on the significantly enhanced crystallization of biodegradable poly(butylene succinate-co-butylene adipate). Carbohydr. Polym. 2019, 205, 211–216. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Significantly enhanced crystallization of poly(L-lactide) by the synergistic effect of poly(diethylene glycol adipate) and cellulose nanocrystals in their fully biodegradable ternary composite. Ind. Eng. Chem. Res. 2019, 34, 15526–15532. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Isothermal melt crystallization kinetics study of cellulose nanocrystals nucleated biodegradable poly(ethylene succinate). Polymer 2021, 227, 123869. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Thermal and rheological properties of fully biodegradable poly(ethylene succinate)/cellulose nanocrystals composites. Compos. Commun. 2021, 23, 100571. [Google Scholar] [CrossRef]
- Pan, S.; Qiu, Z. Fully biodegradable poly(hexamethylene succinate)/cellulose nanocrystals composites with enhanced crystallization rate and mechanical property. Polymers 2021, 13, 3667. [Google Scholar] [CrossRef]
- Pan, S.; Jiang, Z.; Qiu, Z. Significantly enhanced crystallization of poly(ethylene succinate-co-1,2-propylene succinate) by cellulose nanocrystals as an efficient nucleating agent. Polymers 2022, 14, 224. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Fully biodegradable poly(butylene succinate-co-1,2-decylene succinate)/cellulose nanocrystals composites with significantly enhanced crystallization and mechanical property. Polymer 2022, 252, 124946. [Google Scholar] [CrossRef]
- Pan, S.; Jiang, Z.; Qiu, Z. Crystallization and mechanical property of fully biobased poly(hexamethylene 2,5-furandicarboxylate)/cellulose nanocrystals composites. Polymer 2023, 267, 125689. [Google Scholar] [CrossRef]
- Pan, S.; Jiang, Z.; Qiu, Z. Influence of low content of cellulose nanocrystals on the crystallization behavior of biobased Poly(propylene 2,5-furandicarboxylate). Giant 2024, 17, 100212. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I general theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation—Time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change, III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976; Volume 2. [Google Scholar]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass- forming melts. I. Theory. J. Non-Cryst. Solids 1993, 162, 1–12. [Google Scholar] [CrossRef]
- Song, L.; Qiu, Z. Influence of low multi-walled carbon nanotubes loadings on the crystallization behavior of biodegradable poly(butylene succinate) nanocomposites. Polym. Adv. Technol. 2011, 22, 1642–1649. [Google Scholar] [CrossRef]
- Fillon, B.; Lotz, B.; Thierry, A.; Wittmann, J. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleation additives in isotactic polypropylene(α phase). J. Polym. Sci. Polym. Phys. 1993, 31, 1395–1405. [Google Scholar] [CrossRef]
- Fillon, B.; Thierry, A.; Lotz, B.; Wittmann, J. Efficiency scale for polymer nucleating agents. J. Therm. Anal. 1994, 42, 721–731. [Google Scholar] [CrossRef]
- Sangroniz, L.; Cavallo, D.; Müller, A. Self-nucleation effect on polymer crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Crystallization, melting, and enzymatic degradation of biodegradable poly(butylene succinate-co-14mol% ethylene succinate) copolyester. Biomacromolecules 2001, 2, 313–321. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly (butylene succinate-co-ethylene succinate) copolyesters: Effects of comonomer composition and crystallization temperature. CrystEngComm 2011, 13, 2408–2417. [Google Scholar] [CrossRef]
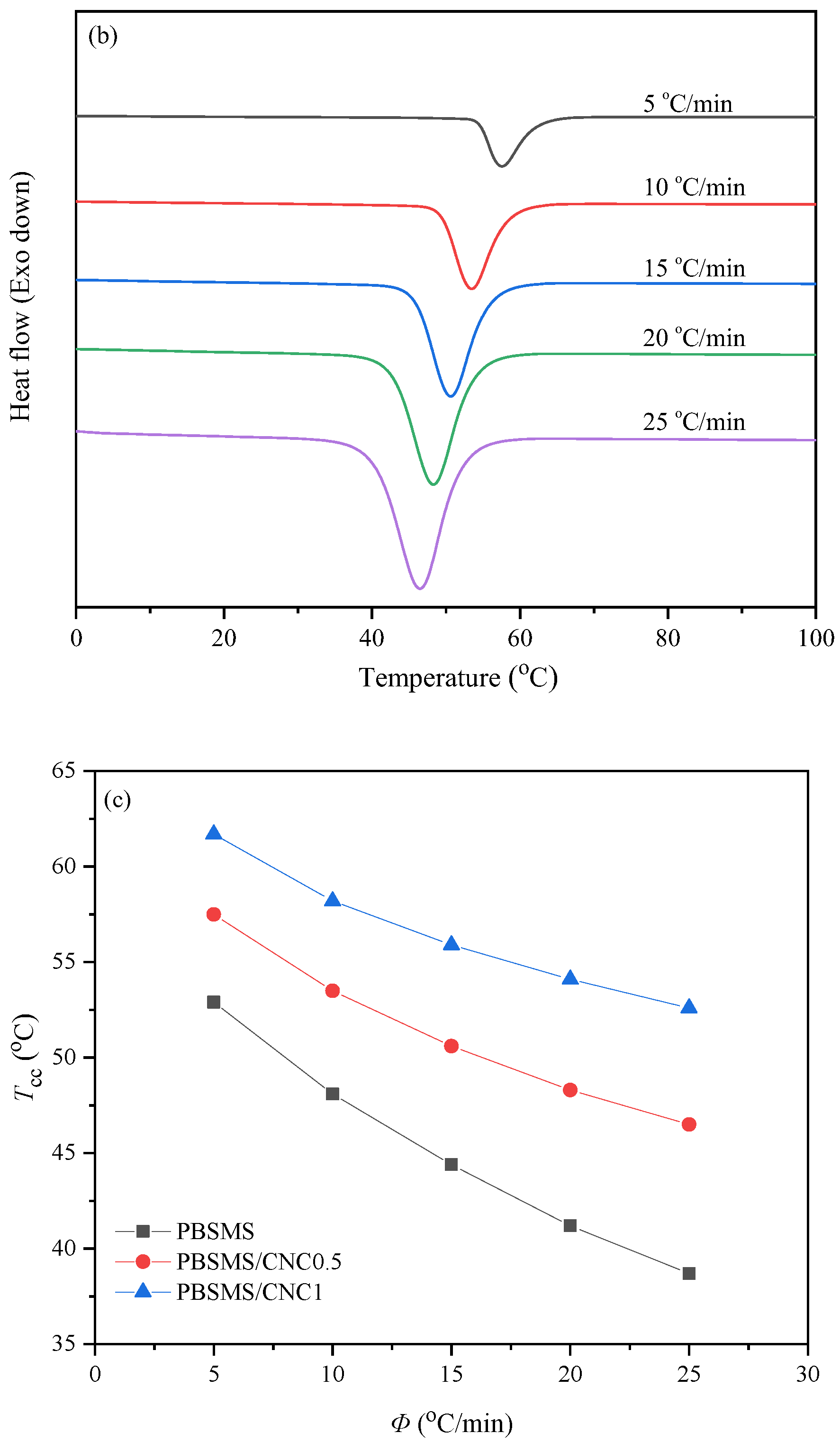
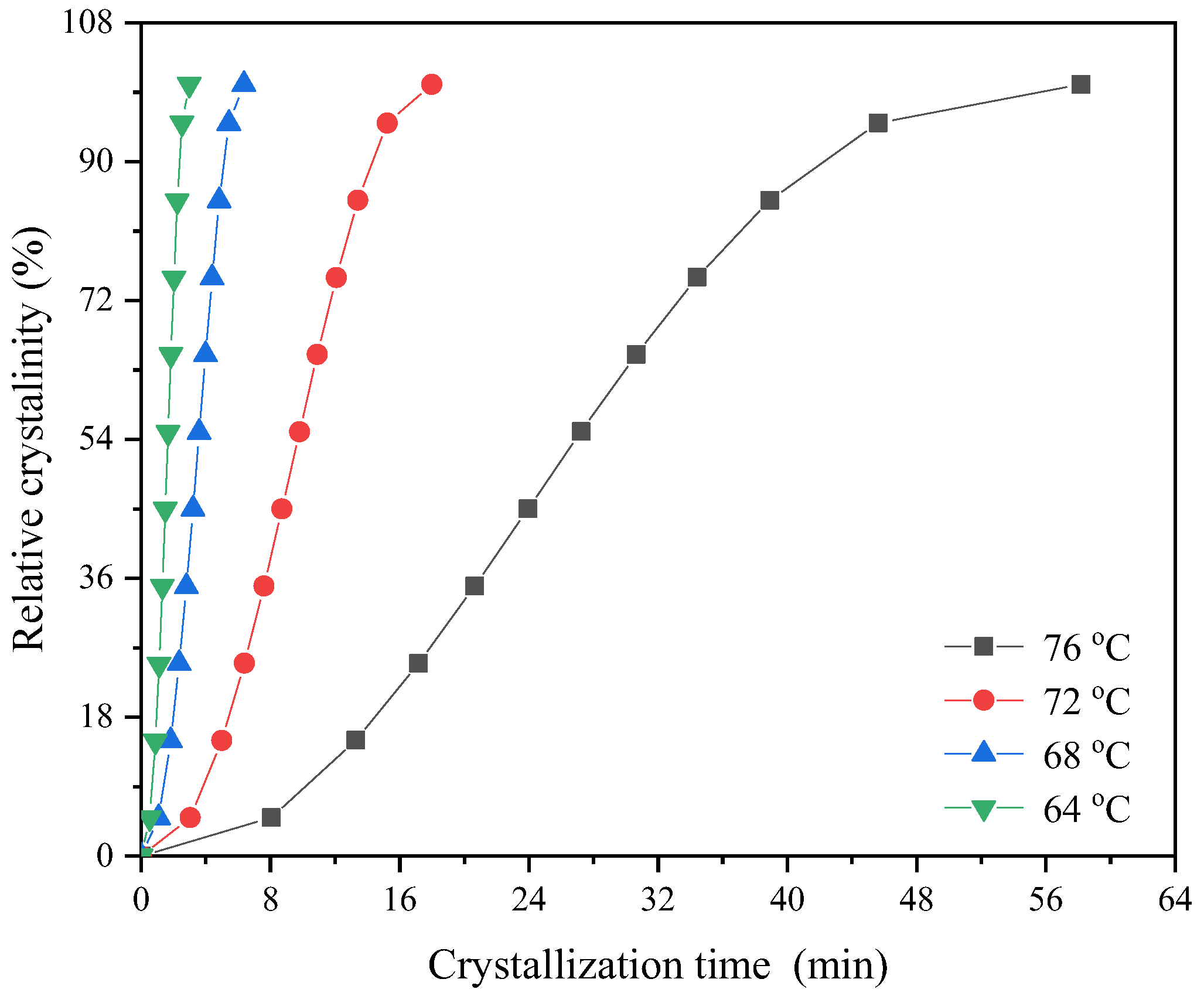
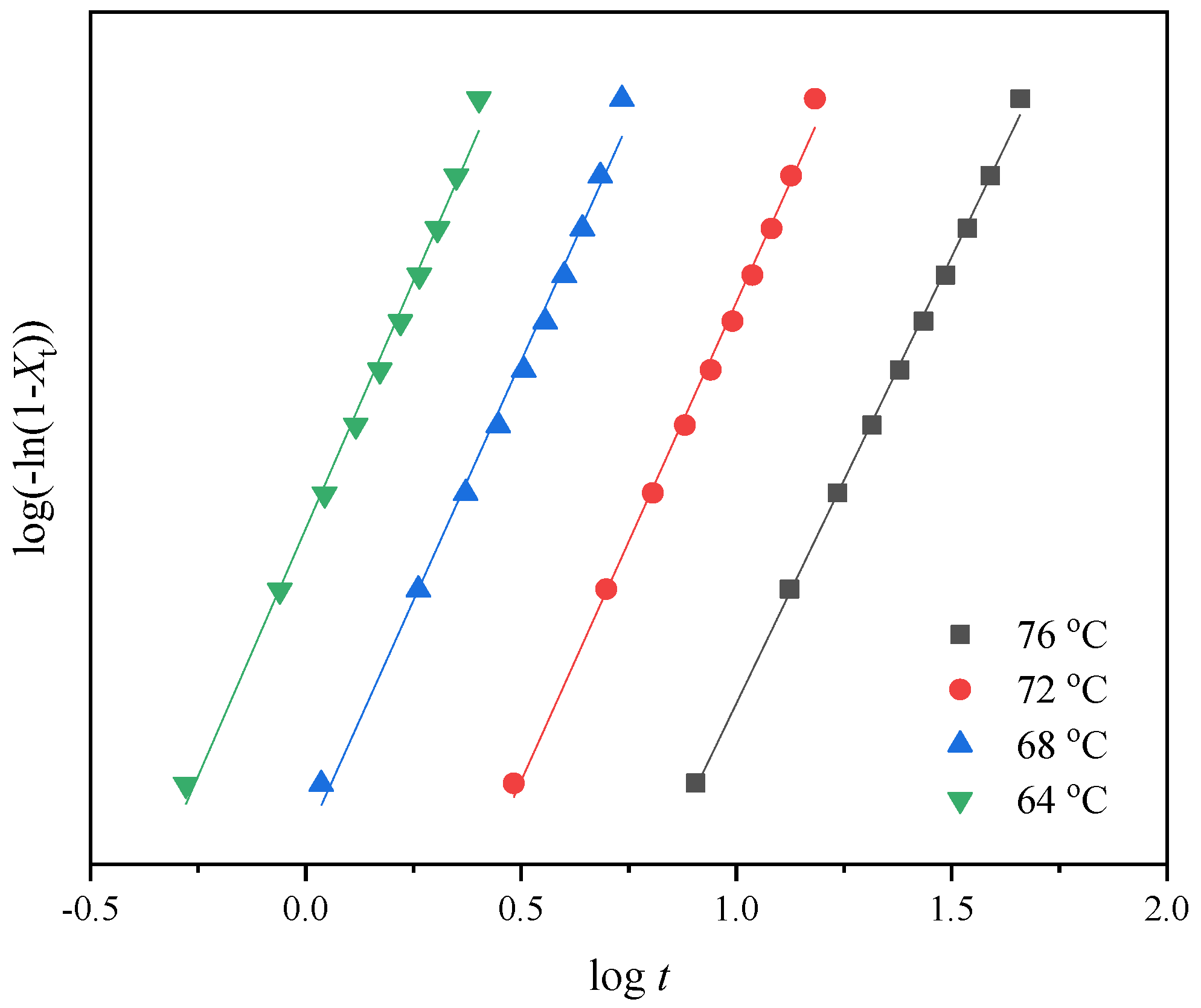

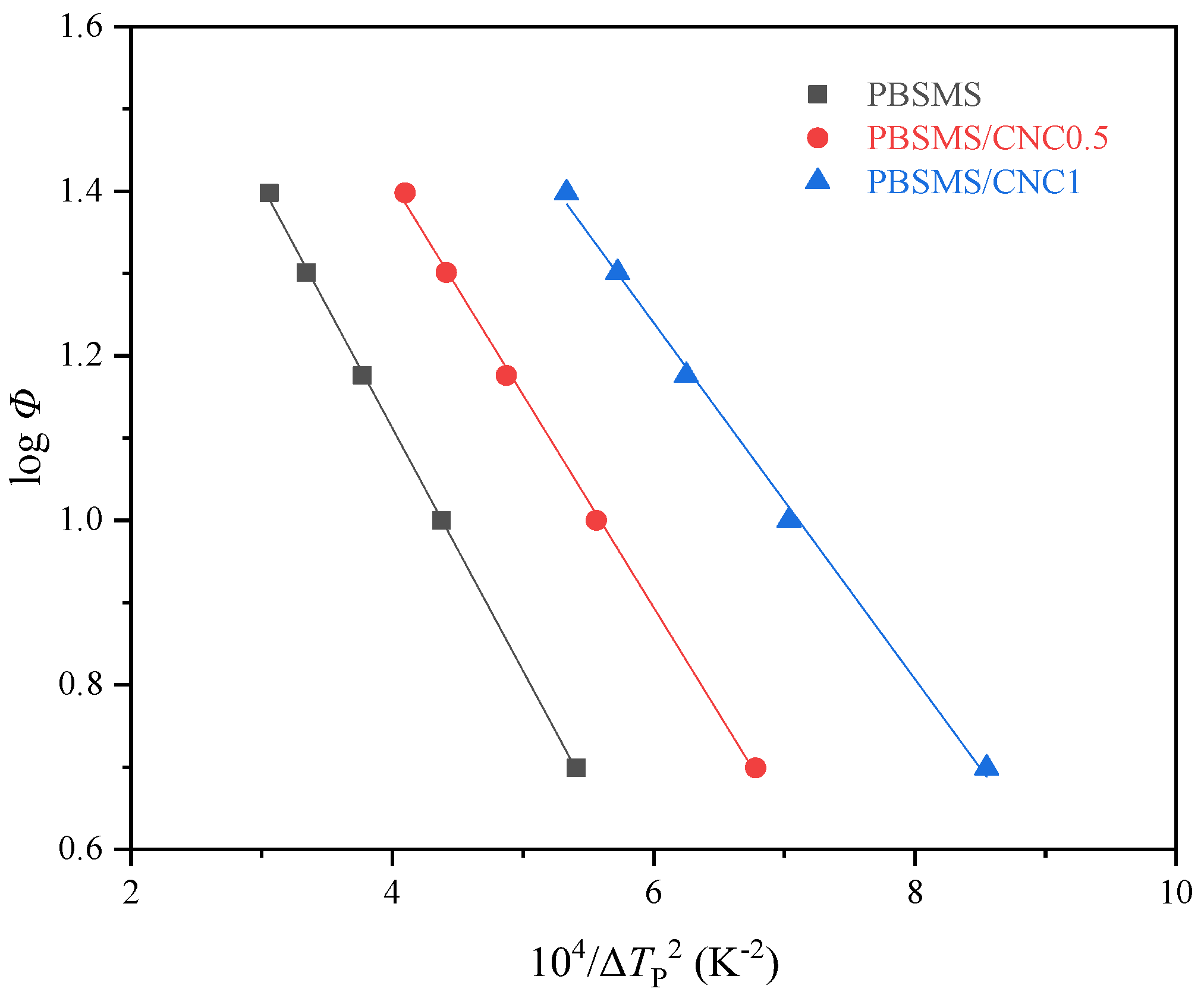

| Samples | Tc (°C) | n | k (min−n) | t0.5 (min) |
|---|---|---|---|---|
| PBSMS20 | 76 | 2.2 | 2.54 × 10−4 | 36.6 |
| 72 | 2.5 | 9.65 × 10−4 | 13.4 | |
| 68 | 2.2 | 6.49 × 10−3 | 8.5 | |
| 64 | 2.2 | 2.63 × 10−2 | 4.2 | |
| PBSMS20/CNC0.5 | 76 | 2.3 | 4.08 × 10−4 | 25.2 |
| 72 | 2.4 | 3.02 × 10−3 | 9.0 | |
| 68 | 2.5 | 3.66 × 10−2 | 3.3 | |
| 64 | 2.5 | 2.32 × 10−1 | 1.5 | |
| PBSMS20/CNC1 | 76 | 2.7 | 7.21 × 10−4 | 17.9 |
| 72 | 2.7 | 6.81 × 10−3 | 5.6 | |
| 68 | 2.6 | 1.40 × 10−1 | 1.8 | |
| 64 | 2.7 | 1.07 | 1.2 |
| Samples | E (MPa) | σy (MPa) | εy (%) | σb (MPa) | εb (%) |
|---|---|---|---|---|---|
| PBSMS | 242.9 ± 12.4 | 10.6 ± 1.3 | 12.1 ± 0.7 | 12.7 ± 1.5 | 333.3 ± 54.6 |
| PBSMS/CNC0.5 | 320.3 ± 14.6 | 16.5 ± 0.9 | 12.1 ± 1.1 | 13.8 ± 0.8 | 231.9 ± 15.6 |
| PBSMS/CNC1 | 321.3 ± 5.14 | 19.1 ± 0.5 | 11.6 ± 1.4 | 13.9 ± 1.2 | 198.6 ± 14.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Pan, S.; Qiu, Z. Crystallization Behavior and Mechanical Property of Biodegradable Poly(butylene succinate-co-2-methyl succinate)/Cellulose Nanocrystals Composites. Polymers 2024, 16, 1735. https://doi.org/10.3390/polym16121735
Yao W, Pan S, Qiu Z. Crystallization Behavior and Mechanical Property of Biodegradable Poly(butylene succinate-co-2-methyl succinate)/Cellulose Nanocrystals Composites. Polymers. 2024; 16(12):1735. https://doi.org/10.3390/polym16121735
Chicago/Turabian StyleYao, Wenxin, Siyu Pan, and Zhaobin Qiu. 2024. "Crystallization Behavior and Mechanical Property of Biodegradable Poly(butylene succinate-co-2-methyl succinate)/Cellulose Nanocrystals Composites" Polymers 16, no. 12: 1735. https://doi.org/10.3390/polym16121735









