Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex
Abstract
:1. Introduction
- chemical modification of fullerene (derivatization),
- direct dispersion in water and the solvent-exchange method,
- non-covalent complexation of fullerene with a number of hydrophilic compounds,
- encapsulation.
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Methods
2.3.1. Synthesis C60/Copolymer Composites
2.3.2. Antimicrobial Activity Tests
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Prato, M. [60]Fullerene chemistry for materials science applications. J. Mater. Chem. 1997, 7, 1097–1109. [Google Scholar] [CrossRef]
- Acquah, S.F.A.; Penkova, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhard, B.E.; Magia, J.M. The Beautiful Molecule: 30 Years of C60 and Its Derivatives. ECS J. Solid State Sci. Technol. 2017, 6, M3155–M3162. [Google Scholar] [CrossRef]
- Chang, X.; Xu, Y.; Delius, M. Recent advances in supramolecular fullerene chemistry. R. Soc. Chem. Chem. Soc. Rev. 2024, 53, 47–83. [Google Scholar] [CrossRef]
- Kramberger, C.; Begichev, I.; Burdanova, M.; Paukov, M.; Kharlamova, M.V. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Troshin, P.A. A Review on Fullerene Derivatives with Reduced Electron Affinity as Acceptor Materials for Organic Solar Cells. Energies 2023, 16, 1924. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H. State-of-the-Art of Polymer/Fullerene C60 Nanocomposite Membranes for Water Treatment: Conceptions, Structural Diversity and Topographies. Membranes 2023, 13, 27. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I. Chapter 11—State-of-the-art of polymer/fullerene nanocomposites in biomedical field. In Micro and Nano Technologies, Polymer/Fullerene Nanocomposites; Kausar, A., Ed.; Elsevier: Cambridge, MA, USA, 2023; pp. 211–250. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Shershakova, N.N.; Andreev, S.M.; Tomchuk, A.A.; Makarova, E.; Nikonova, A.; Turetskiy, E.A.; Petukhova, O.A.; Kamyshnikov, O.Y.; Ivankov, O.; Kyzyma, O.A.; et al. Wound healing activity of aqueous dispersion of fullerene C60 produced by “green technology”. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102619. [Google Scholar] [CrossRef]
- Cataldo, F.; Da Ros, T. (Eds.) Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Dordrecht, The Netherlands, 2008; Volume 1, 408p. [Google Scholar]
- Prylutska, S.V.; Burlaka, A.P.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. Pristine C(60) fullerenes inhibit the rate of tumor growth and metastasis. Exp. Oncol. 2011, 33, 162–164. [Google Scholar]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Burenin, I.S. On medicinal and preventive efficacy of small doses of hydrated C60 fullerenes at cancer pathologies. Chem. Prepr. Arch. 2002, 6, 53–68. [Google Scholar]
- Yang, X.L.; Fan, C.H.; Zhu, H.S. Photo-induced cytotoxicity of malonic acid [C60] fullerene derivatives and its mechanism. Toxicol. Vitr. 2002, 16, 41–46. [Google Scholar] [CrossRef]
- Theriot, C.A.; Casey, R.C.; Moore, V.C.; Mitchell, L.; Reynolds, J.O.; Burgoyne, M.; Partha, R.; Huff, J.L.; Conyers, J.L.; Jeevarajan, A.; et al. Dendro [C60] fullerene DF-1 provides radioprotection to radiosensitive mammalian cells. Radiat. Environ. Biophys. 2010, 49, 437–445. [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Antiinflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J. Nanobiotechnol. 2016, 14, 1483–1493. [Google Scholar] [CrossRef]
- Ma, H.; Liang, X.-J. Fullerenes as unique nanopharmaceuticals for disease treatment. Sci. China Chem. 2010, 53, 2233–2240. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Bruskov, V.I.; Tykhomyrov, A.A.; Gudkov, S.V. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radical. Biol. Med. 2009, 47, 786–793. [Google Scholar] [CrossRef]
- Galvan, Y.P.; Alperovich, I.; Zolotukhin, P.; Prazdnova, E.; Mazanko, M.; Belanova, A.; Chistyakov, V. Fullerenes as Anti-aging Antioxidants. Curr. Aging Sci. 2017, 10, 56–67. [Google Scholar] [CrossRef]
- Tsao, N.; Luh, T.-Y.; Chou, C.-K.; Chang, T.-Y.; Wu, J.-J.; Liu, C.-C.; Lei, H.-Y. In vitro action of carboxyfullerene. J. Antimicrob. Chemother. 2002, 49, 641–649. [Google Scholar] [CrossRef]
- Aoshima, H.; Kokubo, K.; Shirakawa, S.; Ito, M.; Yamana, S.; Oshima, T. Antimicrobial activity of fullerenes and their hydroxylated derivatives. Biocontrol. Sci. 2009, 14, 69–72. [Google Scholar] [CrossRef]
- Mashino, T.; Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M. Inhibition of E. coli growth by fullerene derivatives and inhibition mechanism. Bioorg. Med. Chem. Lett. 1999, 9, 2959–2962. [Google Scholar] [CrossRef]
- Shoji, M.; Takahashi, E.; Hatakeyama, D.; Iwai, Y.; Morita, Y.; Shirayama, R.; Echigo, N.; Kido, H.; Nakamura, S.; Mashino, T.; et al. Anti-influenza Activity of C60 Fullerene Derivatives. PLoS ONE 2013, 8, e66337. [Google Scholar] [CrossRef]
- Klimova, R.; Andreev, S.; Momotyuk, E.; Demidova, N.; Fedorova, N.; Chernoryzh, Y.; Yurlov, K.; Turetskiy, E.; Baraboshkina, E.; Shershakova, N.; et al. Aqueous fullerene C60 solution suppresses herpes simplex virus and cytomegalovirus infections. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 487–499. [Google Scholar] [CrossRef]
- Ryan, J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerenes inhibit IgE-induced anaphylaxis in vivo. J. Immunol. 2007, 179, 665–672. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Tripathy, M.; Karjiban, R.A.; Abdul-Malek, E. Skin intervention of fullerene-integrated nanoemulsion in structural and collagen regeneration against skin aging. Eur. J. Pharm. Sci. 2015, 70, 22–28. [Google Scholar] [CrossRef]
- Kyzyma, E.A.; Tomchuk, A.A.; Bulavin, L.A.; Petrenko, V.I.; Almasy, L.; Korobov, M.V.; Volkov, D.S.; Mikheev, I.V.; Koshlan, I.V.; Koshlan, N.A.; et al. Structure and Toxicity of Aqueous Fullerene C60 Solutions. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 1–5. [Google Scholar] [CrossRef]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Kato, H.; Shinohara, N.; Nakamura, A.; Fujita, K.; Kinugasa, S.; Endoh, S.; Yamamoto, K.; Yamomoto, O.; et al. In vitro evaluation of cellular responses induced by stable fullerene C60 medium dispersion. J. Biochem. 2010, 148, 289–298. [Google Scholar] [CrossRef]
- Bogdanović, V.; Stankov, K.; Icević, I.; Zikic, D.; Nikolić, A.; Solajić, S.; Djordjević, A.; Bogdanović, G. Fullerenol C60(OH)24 effects on antioxidative enzymes activity in irradiated human erythroleukemia cell line. J. Radiat. Res. 2008, 49, 321–327. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef]
- Sitharaman, B.; Asokan, S.; Rusakova, I.; Wong, M.S.; Wilson, L.J. Nanoscale aggregation properties of neuroprotective carboxyfiillerene (C3) in aqueous solutions. Nano Lett. 2004, 4, 1759–1762. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Okamoto, S.; Murata, Y. Synthesis of inter-[60]fullerene conjugates with inherent chirality. Nat. Commun. 2024, 15, 514. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene Polymers: A Brief Review. J. Carbon Res. 2020, 6, 71. [Google Scholar] [CrossRef]
- Bouchard, D.; Ma, X.; Isaacson, C. Colloidal properties of aqueous fullerenes: Isoelectric points and aggregation kinetics of C60 and C70 derivatives. Environ. Sci. Technol. 2009, 43, 6597–6603. [Google Scholar] [CrossRef]
- Labille, J.; Masion, A.; Ziarelli, F.; Rose, J.; Brant, J.; Villieras, F.; Pelletier, M.; Borschneck, D.; Wiesner, M.R.; Bottero, J.-Y. Hydration and Dispersion of C60 in Aqueous Systems: The Nature of Water-Fullerene Interactions. Langmuir 2009, 25, 11232–11235. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Fullerenes in liquid media: An unsettling intrusion into the solution chemistry. Chem. Rev. 2013, 113, 5149–5193. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Kosevich, M.V.; Vovk, O.M.; Shelkovsky, V.S.; Vashcenko, L.A. On the production of an aqueous colloidal solution of fullerenes. J. Chem. Soc. Chem. Comm. 1995, 12, 1281–1282. [Google Scholar] [CrossRef]
- Maples, R.D.; Hilburn, M.E.; Murdianti, B.S.; HikkaduwaKoralege, R.S.; Williams, J.S.; Kuriyavar, S.I.; Ausman, K.D. Optimized solvent-exchange synthesis method for C60 colloidal dispersions. J. Colloid. Interface Sci. 2012, 370, 27–31. [Google Scholar] [CrossRef]
- Konarev, D.V.; Litvinov, A.L.; Kovaltvsky, A.Y.; Drichko, N.V.; Coppens, R.N.; Lubovskaya, R.N. Molecular complexes of fullerene C60 with aromatic hydrocarbons: Crystal structures of (TPE)2C60 and DPA·C60. Synth. Met. 2003, 133–134, 675–677. [Google Scholar] [CrossRef]
- Ikeda, A.; Iizuka, T.; Maekubo, N.; Aono, R.; Kikuchi, J.-I.; Akiyama, M.; Konishi, T.; Ogawa, T.; Ishida-Kitagawa, N.; Tatebe, H.; et al. Cyclodextrin complexed [60]fullerene derivatives with high levels of photodynamic activity by long wavelength excitation. ACS Med. Chem. Lett. 2013, 4, 752–756. [Google Scholar] [CrossRef]
- Murthya, C.N.; Geckeler, K.E. The water-soluble β-cyclodextrin–[60]fullerene complex. Chem. Commun. 2001, 13, 1194–1195. [Google Scholar] [CrossRef]
- Nierengarten, J.F. Supramolecular encapsulation of [60]fullerene with dendritic cyclotriveratrylene derivatives. Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 229–242. [Google Scholar] [CrossRef]
- Hirsch, A. Amphiphilic architectures based on fullerene and calixarene platforms: From buckysomes to shape-persistent micelles. Pure Appl. Chem. 2008, 80, 571–587. [Google Scholar] [CrossRef]
- Komatsu, T.; Nakagawa, A.; Qu, X. Structural and mutagenic approach to create human serum albumin-based oxygen carrier and photosensitizer. Drug Metab. Pharmacokinet. 2009, 24, 287–299. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Y.; Guo, K.; Yin, Z.; Gao, X. Spectroscopic study on the interaction of pristine C60 and serum albumins in solution. Nanoscale Res. Lett. 2012, 7, 433. [Google Scholar] [CrossRef]
- Ikeda, A.; Doi, Y.; Nishiguchi, K.; Kitamura, K.; Hashizume, M.; Kikuchi, J.-I.; Yogo, K.; Ogawa, T.; Takeya, T. Induction of cell death by photodynamic therapy with water-soluble lipid-membrane-incorporated [60]fullerene. Org. Biomol. Chem. 2007, 5, 1158–1160. [Google Scholar] [CrossRef]
- Torres, V.M.; Posa, M.; Srdjenovic, B.; Simplício, A.L. Solubilization of fullerene C60 in micellar solutions of different solubilizers. Colloids Surf. B Biointerfaces 2011, 82, 46–53. [Google Scholar] [CrossRef]
- Ramakanth, I.; Patnaik, A. Characteristics of solubilization and encapsulation of fullerene C60 in non-ionic Triton X-100 micelles. Carbon 2008, 46, 692–698. [Google Scholar] [CrossRef]
- Jeng, U.-S.; Hsu, C.-H.; Lin, T.-L.; Wu, C.-M.; Chen, H.-L.; Tai, L.-A.; Hwang, K.-C. Dispersion of fullerenes in phospholipid bilayers and the subsequent phase changes in the host bilayers. Phys. B Condens. Matter. 2005, 357, 193–198. [Google Scholar] [CrossRef]
- Ikeda, A. Water-soluble fullerenes using solubilizing agents, and their applications. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 49–65. [Google Scholar] [CrossRef]
- Wang, X.S.; Metanawin, T.; Zheng, X.Y.; Wang, P.Y.; Ali, M.; Vernon, D. Structure-defined c60/polymer colloids supramolecular nanocomposites in water. Langmuir 2008, 24, 9230–9232. [Google Scholar] [CrossRef]
- Laiho, A.; Ras, R.H.A.; Valkama, S.; Ruokolainen, J.; Osterbaska, R.; Ikkala, O. Control of Self-Assembly by Charge-Transfer Complexation between C60 Fullerene and Electron Donating Units of Block Copolymers. Macromolecules 2006, 39, 7648–7653. [Google Scholar] [CrossRef]
- Tabata, Y.; Murakami, Y.; Ikada, Y. Photodynamic effect of polyethylene glycol-modified fullereneon tumor. Jpn. J. Cancer Res. 1997, 88, 1108–1116. [Google Scholar] [CrossRef]
- Metanawin, T.; Tang, T.; Chen, R.; Vernon, D.; Wang, X. Cytotoxicity and photocytotoxicity of structure-defined water-soluble C60/micelle supramolecular nanoparticles. Nanotecnology 2011, 22, 235604. [Google Scholar] [CrossRef]
- Pakhomova, V.A.; Gordon, D.A.; Mikhailov, A.I. Synthesis of Water-Soluble Fullerene-Containing Vinyl Polymers by Low-Temperature Radiation-Induced Living Polymerization. Polym. Sci. Ser. A 2006, 48, 689–695. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Solubilization and stabilization of fullerene C60 in presence of poly(vinyl pyrrolidone) molecules in water. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 233–239. [Google Scholar] [CrossRef]
- Ungurenasu, C.; Airinei, A. Highly stable C(60)/poly(vinylpyrrolidone) charge-transfer complexes afford new predictions for biological applications of underivatized fullerenes. J. Med. Chem. 2000, 43, 3186–3188. [Google Scholar] [CrossRef]
- Krasnou, I.; Tarabukina, E.; Melenevskaya, E.; Filippov, A.; Aseyev, V.; Hietala, S.; Tenhu, H. Rheological Behavior of Poly(vinylpyrrolidone)/Fullerene C60 Complexes in Aqueous Medium. J. Macromol. Sci. Ser. B 2008, 47, 500–510. [Google Scholar] [CrossRef]
- Vidanapathirana, A.K.; Thompson, L.C.; Mann, E.E.; Odom, J.T.; Holland, N.A.; Sumner, S.J.; Han, L.; Lewin, A.H.; Fennell, T.R.; Brown, J.M.; et al. PVP formulated fullerene (C60) increases Rho-kinase dependent vascular tissue contractility in pregnant Sprague Dawley rats. Reprod. Toxicol. 2014, 49, 86–100. [Google Scholar] [CrossRef]
- Yamakoshi, Y.N.; Yagami, T.; Fukuhara, K.; Sueyoshia, S.; Miyata, N. Solubilization of Fullerenes into Water with Polyvinylpyrrolidone Applicable to Biological Tests. J. Chem. Soc. Chem. Commun. 1994, 4, 517–518. [Google Scholar] [CrossRef]
- Samoilova, N.; Kurskaya, E.; Krayukhina, M.; Askadsky, A.; Yamskov, I. Copolymers of Maleic Acid and Their Amphiphilic Derivatives as Stabilizers of Silver Nanoparticles. J. Phys. Chem. B 2009, 113, 3395–3403. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Naumkin, A.V.; Korlyukov, A.A.; Anuchina, N.M.; Popov, D.A. Polymer-Stabilized Silver (Gold)-Zinc Oxide Nanoheterodimer Structures as Antimicrobials. Appl. Sci. 2023, 13, 11121. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Korlyukov, A.A.; Klemenkova, Z.S.; Naumkin, A.V.; Mezhuev, Y.O. One-Pot Synthesis of Colloidal Hybrid Au (Ag)/ZnO Nanostructures with the Participation of Maleic Acid Copolymers. Polymers 2023, 15, 1670. [Google Scholar] [CrossRef]
- Andreev, S.; Purgina, D.; Bashkatova, E.; Garshev, A.; Maerle, A.; Andreev, I.; Osipova, N.; Shershakova, N.; Khaitov, M. Study of Fullerene Aqueous Dispersion Prepared by Novel Dialysis Method: Simple Way to Fullerene Aqueous Solution. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 792–800. [Google Scholar] [CrossRef]
- Conix, A.; Smets, G. Ring opening in lactam polymers. J. Polym. Sci. 1955, 15, 221–229. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, M07-A9. [Google Scholar]
- Samoilova, N.; Krayukhina, M.; Naumkin, A.; Anuchina, N.; Popov, D. Silver nanoparticles doped with silver cations and stabilized with maleic acid copolymers: Specific structure and antimicrobial properties. New J. Chem. 2021, 45, 14513–14521. [Google Scholar] [CrossRef]
- Jouyban, A.; Fakhree, M.A.A.; Shayanfar, A. Review of pharmaceutical applications of N-Methyl-2-pyrrolidone N-methyl-2-pyrrolidone. J. Pharm. Pharmaceut. Sci. 2010, 13, 524–535. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Klochkov, V.K.; Bordyuh, A.B.; Dovbeshko, G.I. Comparative analysis of two aqueous-colloidal solutions of C60 fullerene with help of FTIR reflectance and UV-Vis spectroscopy. Chem. Phys. Lett. 2002, 364, 8–17. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Biryulin, Y.F.; Melenevskaja, E.Y.; Zgonnik, V.N.; Rjumtsev, E.I. Aggregation of fullerene C60 in N-methylpyrrolidone. Colloids Surf. A 2002, 209, 167–171. [Google Scholar] [CrossRef]
- Kyzyma, O.A.; Korobov, M.V.; Avdeev, M.V.; Garamus, V.M.; Petrenko, V.I.; Aksenov, V.L.; Bulavin, L.A. Solvatochromism and Fullerene Cluster Formation in C60/N-methyl-2-pyrrolidone. Fuller. Nanotub. Carbon Nanostruct. 2010, 18, 458–461. [Google Scholar] [CrossRef]
- Rivelino, R.; Maniero, A.M.; Prudente, F.V.; Costa, L.S. Theoretical calculations of the structure and UV–vis absorption spectra of hydrated C60 fullerene. Carbon 2006, 44, 2925–2930. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Bienvenue, E.; Dellinger, M.; Leach, S.; Seta, P. C60 in model biological systems. A visible-UV absorption study of solvent-dependent parameters and solute aggregation. J. Phys. Chem. 1994, 98, 3492–3500. [Google Scholar] [CrossRef]
- Vinogradova, L.V.; Melenevskaya, E.Y.; Khachaturov, A.S.; Zgonnik, V.N.; Kever, E.E.; Litvinova, L.S.; Novokreshchenova, A.V.; Sushko, M.L.; Klenin, S.I. Water-soluble complexes of C60 fullerene with poly(N-vinylpyrrolidone). Polym. Sci. Ser. A 1998, 40, 1152–1159. [Google Scholar]
- Ghosh, H.N.; Sapre, A.V.; Mittal, J.P. Aggregation of C70 in solvent mixtures. J. Phys. Chem. 1996, 100, 9439–9443. [Google Scholar] [CrossRef]
- Mrzel, A.; Mertelj, A.; Omerzu, A.; Čopič, M.; Mihailovic, D. Investigation of encapsulation and solvatochromism of fullerenes in binary solvent mixtures. J. Phys. Chem. B 1999, 103, 11256–11260. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Durov, S.S.; Bulavin, L.A.; Adamenko, I.I.; Moroz, K.O.; Geru, I.I.; Dihor, I.N.; Scharff, P.; Eklund, P.C.; Grigorian, L. Structure and Thermophysical Properties of Fullerene C60 Aqueous Solutions. Int. J. Thermophys. 2001, 22, 943–956. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Klimova, T.P.; Babushkina, T.A.; Vyshivannaya, O.V.; Blagodatskikh, I.V.; Yamskov, I.A. Oxidation of glucose to gluconic acid using a colloidal catalyst containing gold nanoparticles and glucose oxidase. Russ. Chem. Bull. 2014, 63, 1009–1016. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Vyshivannaya, O.V.; Blagodatskikh, I.V. Investigation of the Binding of Lectins with Polymer Glycoconjugates and the Glycoconjugates Containing Silver Nanoparticles by Means of Optical Spectroscopy and Light Scattering. Polym. Sci. Ser. A 2022, 64, 267–279. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Setiawan, U.H.; Ihsan, M.R.; Isnaeni, I.; Herbani, Y. The Study of the Optical Properties of C60 Fullerene in Different Organic Solvents. Open Chem. 2019, 17, 1198–1212. [Google Scholar] [CrossRef]
- Krayukhina, M.A.; Samoilova, N.A.; Yamskov, I.A. Complexation of chitosan with maleic acid copolymers. J. Polym. Sci. Ser. A 2010, 52, 243–250. [Google Scholar] [CrossRef]
- Stubenrauch, C. Emulsions, Foams and Suspensions. Fundamentals and Applications. By Schramm, L.L. Chem. Phys. Chem. 2006, 7, 965. [Google Scholar] [CrossRef]
- Fabian, J. Theoretical investigation of the C60 infrared spectrum. Phys. Rev. B 1996, 53, 13864–13870. [Google Scholar] [CrossRef]
- Pikhurov, D.V.; Zuev, V.V. The Effect of Fullerene C60 on the Mechanical and Dielectrical Behavior of Epoxy Resins at Low Loading. AIP Conf. Proc. 2014, 1599, 453–456. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef]

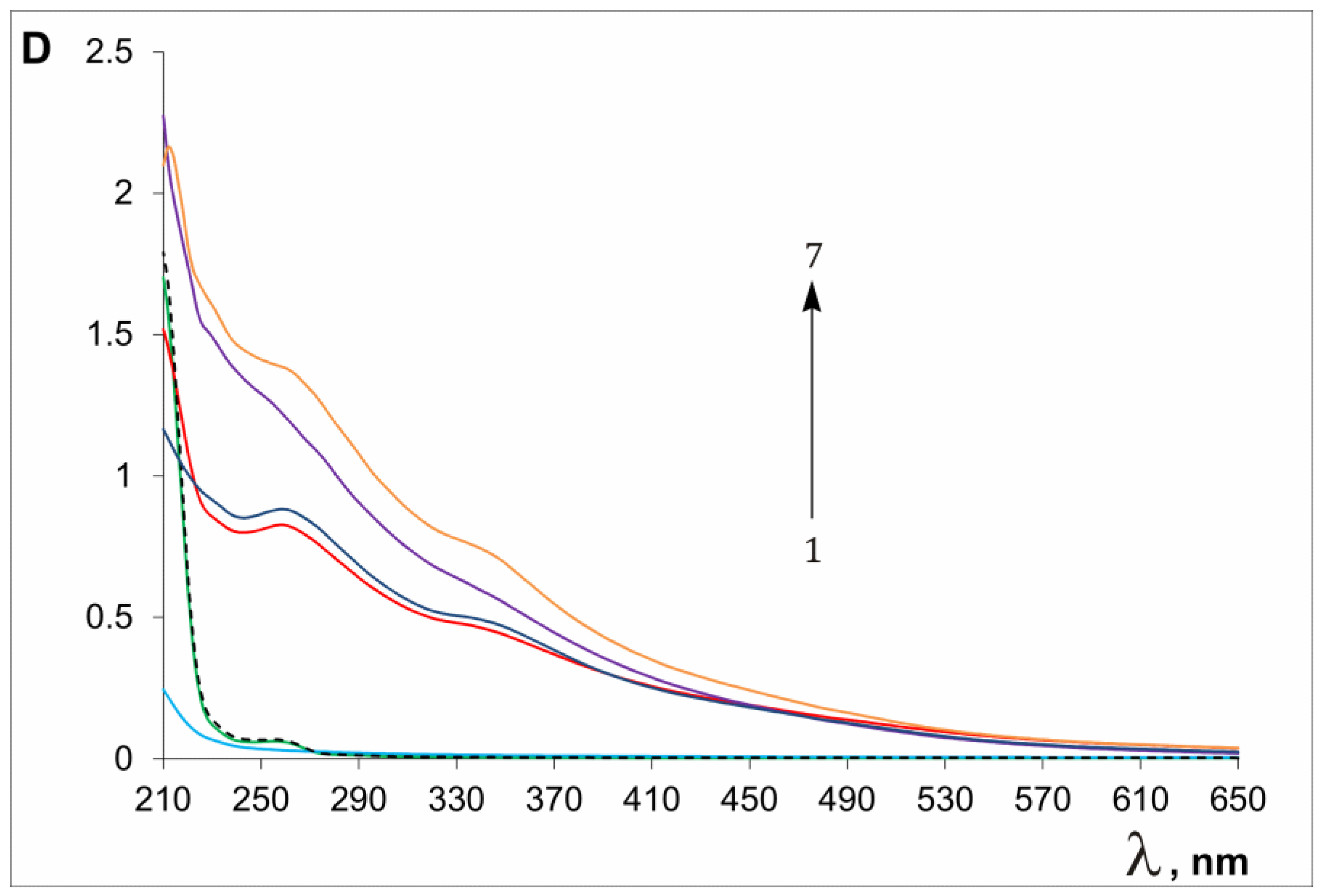
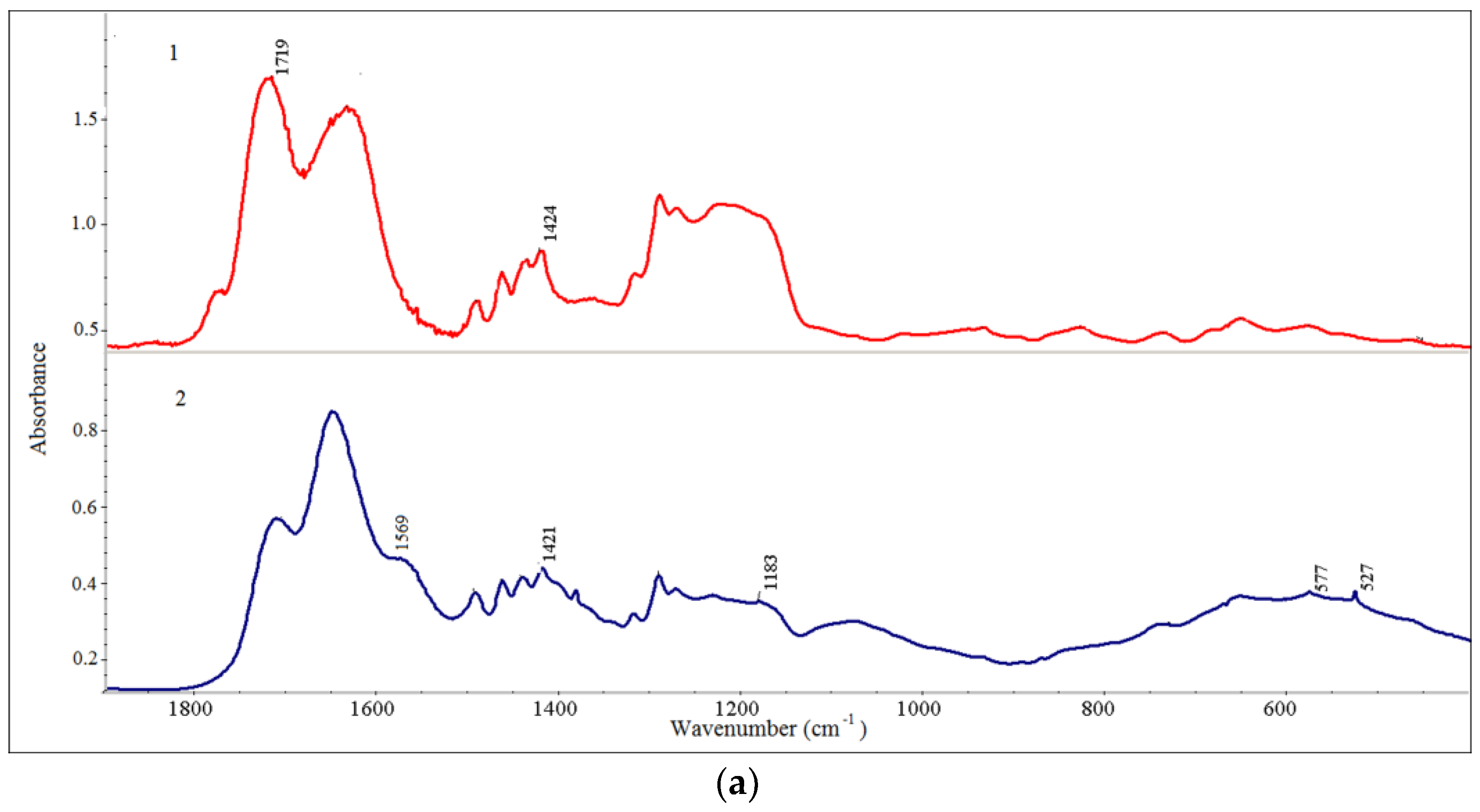
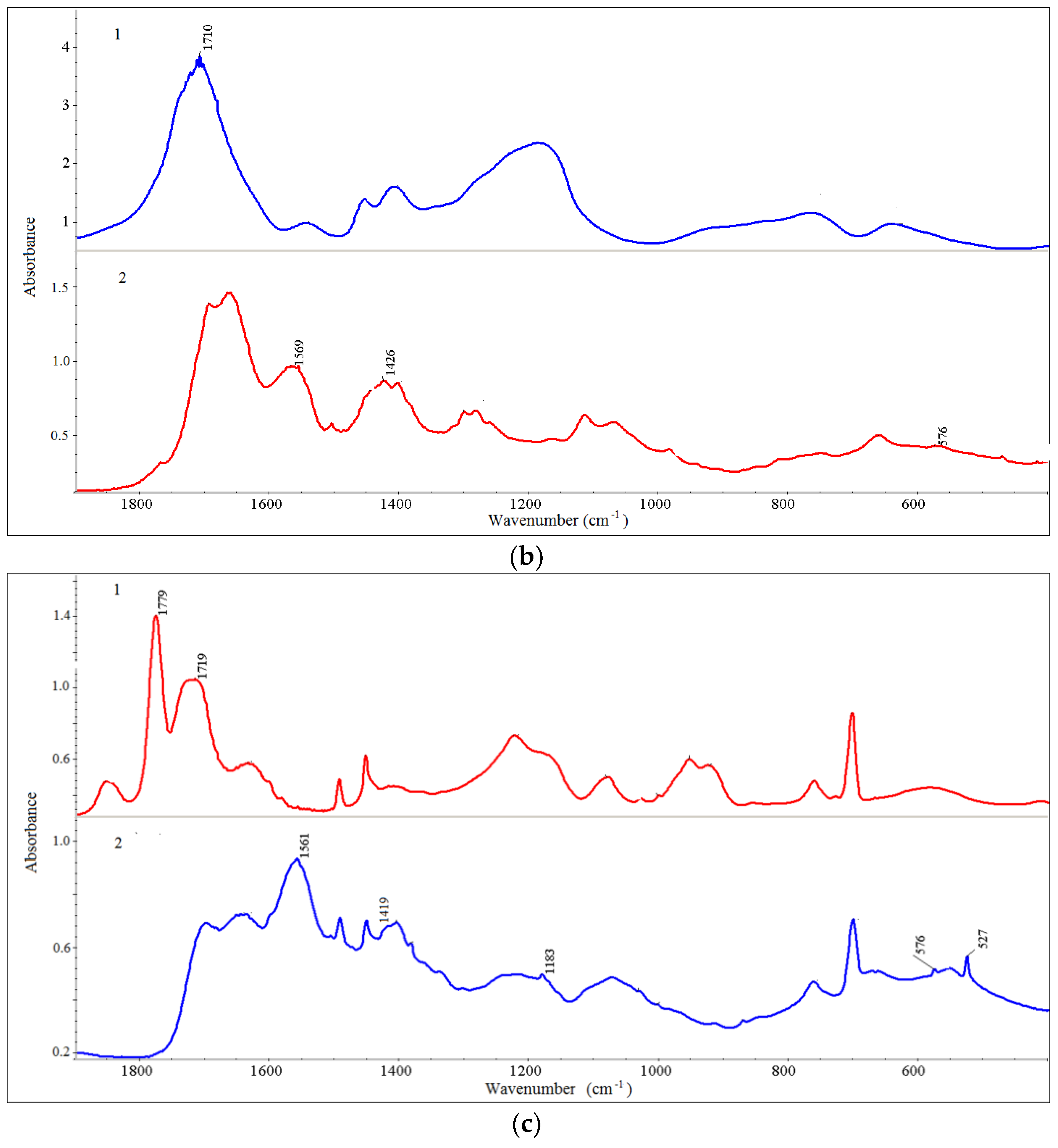


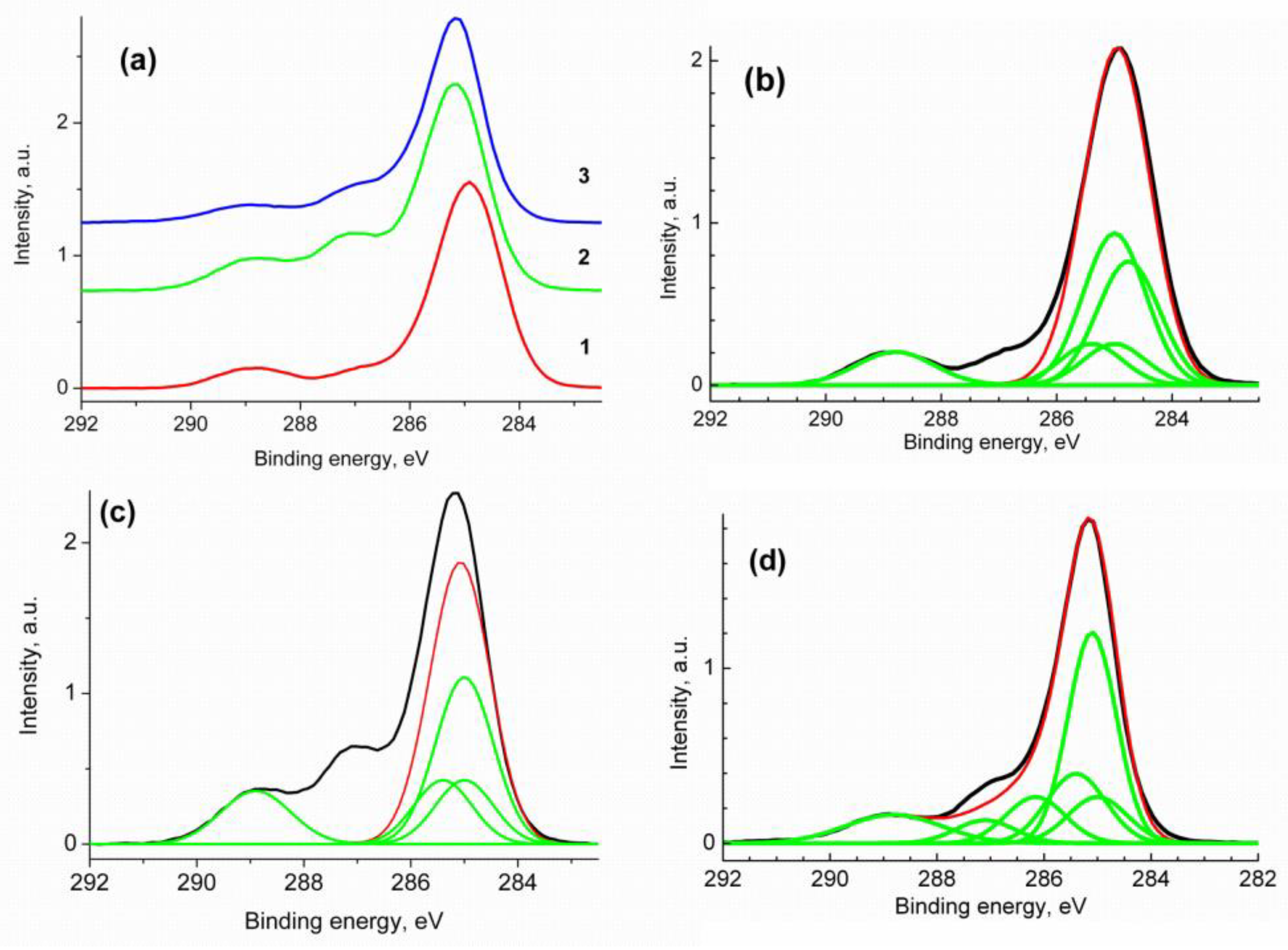
| Sample a | Average Size (nm) b | PDI | ζ (mV) |
|---|---|---|---|
| C60/SM | 202.3 | 0.21 | −19.5 |
| C60/VM | 155.0 | 0.40 | −17.6 |
| C60/EM | 116.1; 1387 | 0.20; 0.40 | −15.7 |
| Sample a | C60 (wt.%) | Residual Weight b (%) | ||
|---|---|---|---|---|
| Calculated | Found a | Found | Calculated | |
| VM | - | - | 27 | - |
| C60/VM complex | 38 | 32 | 55 | 54 |
| C60/VM mixture | 38 | - | 55 | 55 |
| EM | - | - | 22 | - |
| C60/EM complex | 33 | 33 | 53 | 48 |
| C60/EM mixture | 32 | - | 49 | 47 |
| SM | - | - | 14 | - |
| C60/SM complex | 38 | 31 | 60 | 47 |
| C60/SM mixture | 37 | - | 45 | 46 |
| Sample | Group | -C=C- | CH2 | C60 | C*-C(O) | C-N | C(O)N | C(O)O |
|---|---|---|---|---|---|---|---|---|
| C60/SM | Eb | 284.76 | 285.0 | 285.0 | 285.4 | 288.0 | ||
| W | 1.08 | 1.08 | 1.08 | 1.08 | 1.34 | |||
| Irel | 0.31 | 0.10 | 0.38 | 0.10 | 0.10 | |||
| C60/EM | Eb | 285.0 | 285.0 | 285.4 | 288.9 | |||
| W | 1.08 | 1.08 | 1.08 | 1.3 | ||||
| Irel | 0.18 | 0.47 | 0.18 | 0.18 | ||||
| C60/VM | Eb | 285.0 | 285.0 | 285.4 | 288.16 | 287.1 | 288.8 | |
| W | 1.1 | 0.9 | 1.1 | 1.1 | 1.1 | 1.79 | ||
| Irel | 0.11 | 0.43 | 0.17 | 0.11 | 0.06 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilova, N.A.; Krayukhina, M.A.; Klemenkova, Z.S.; Naumkin, A.V.; Buzin, M.I.; Mezhuev, Y.O.; Turetsky, E.A.; Andreev, S.M.; Anuchina, N.M.; Popov, D.A. Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex. Polymers 2024, 16, 1736. https://doi.org/10.3390/polym16121736
Samoilova NA, Krayukhina MA, Klemenkova ZS, Naumkin AV, Buzin MI, Mezhuev YO, Turetsky EA, Andreev SM, Anuchina NM, Popov DA. Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex. Polymers. 2024; 16(12):1736. https://doi.org/10.3390/polym16121736
Chicago/Turabian StyleSamoilova, Nadezhda A., Maria A. Krayukhina, Zinaida S. Klemenkova, Alexander V. Naumkin, Michail I. Buzin, Yaroslav O. Mezhuev, Evgeniy A. Turetsky, Sergey M. Andreev, Nelya M. Anuchina, and Dmitry A. Popov. 2024. "Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex" Polymers 16, no. 12: 1736. https://doi.org/10.3390/polym16121736







