Substrate Neutrality for Obtaining Block Copolymer Vertical Orientation
Abstract
:1. Introduction

2. Early Developments in Obtaining BCP Self-Assembly
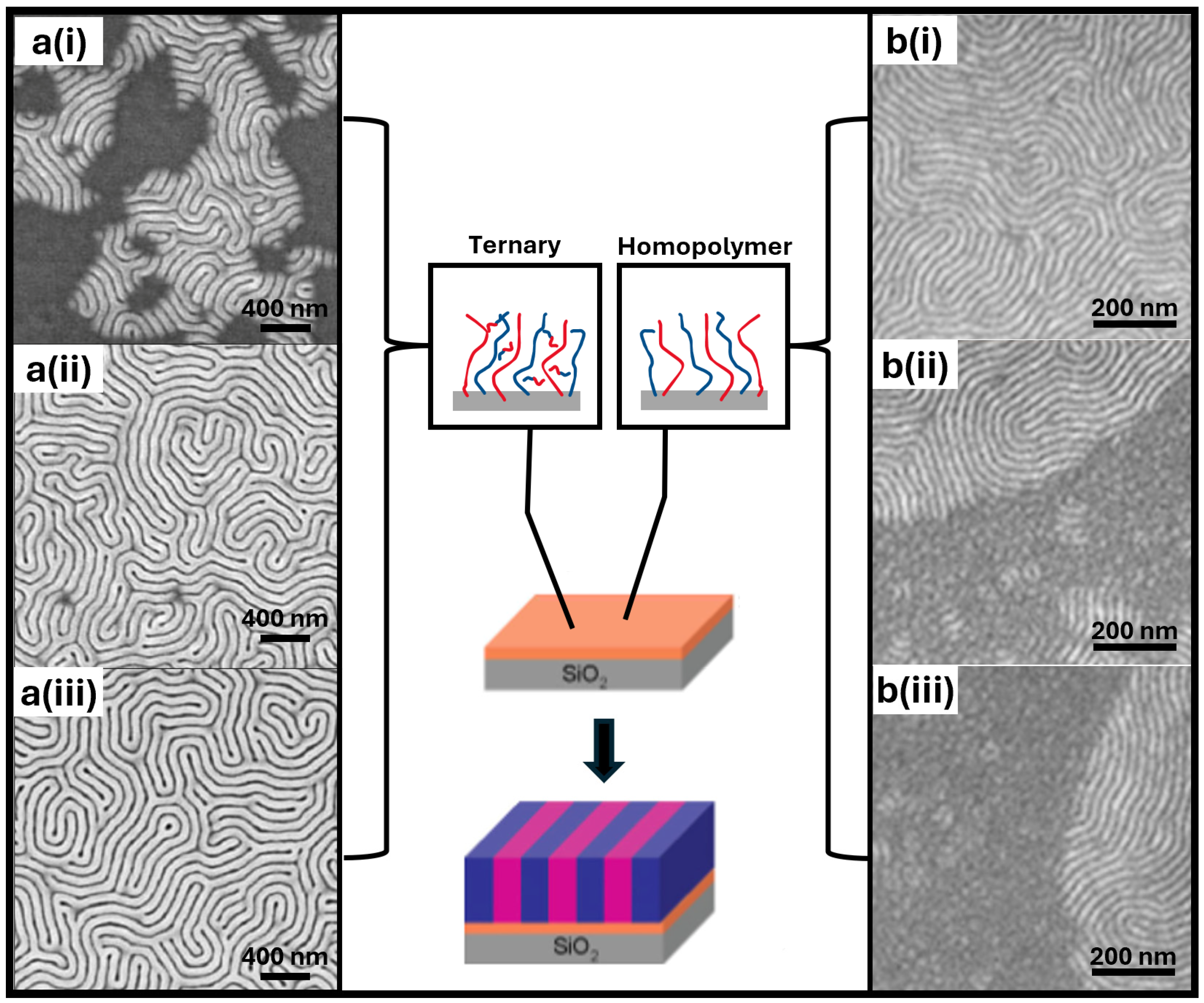
3. Alternative Approaches to Obtaining Neutrality Using Copolymers
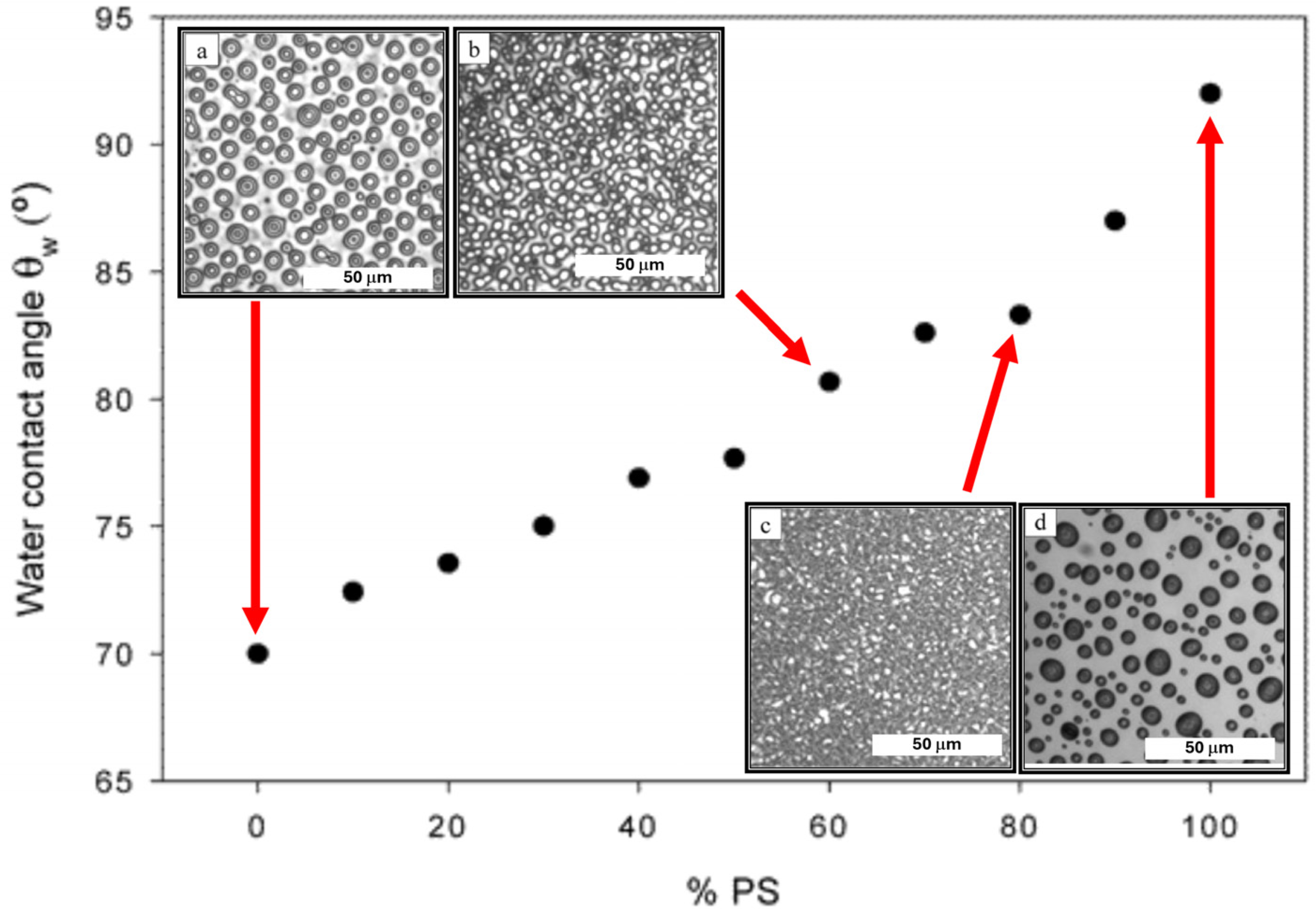
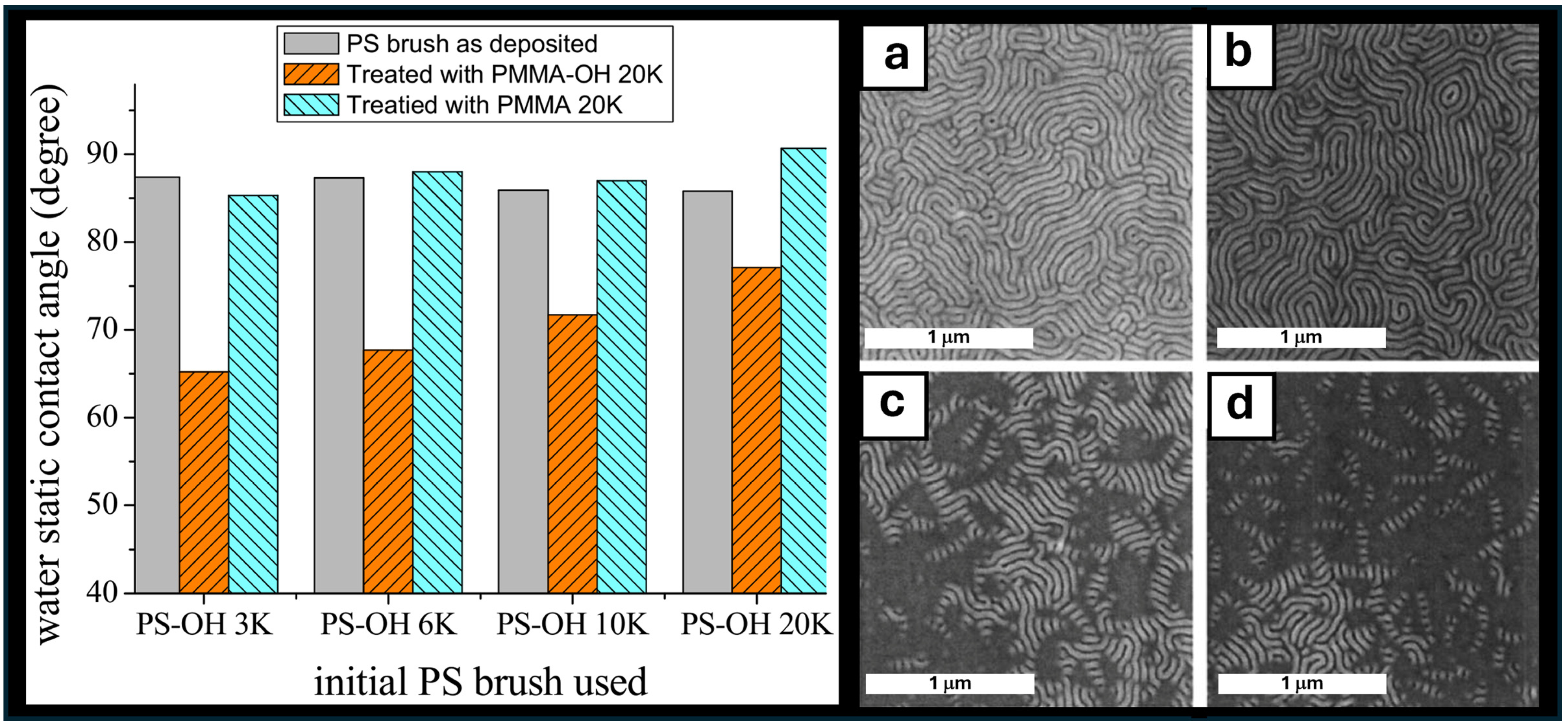
4. A history of Homopolymer Blends
5. Surface Characterization
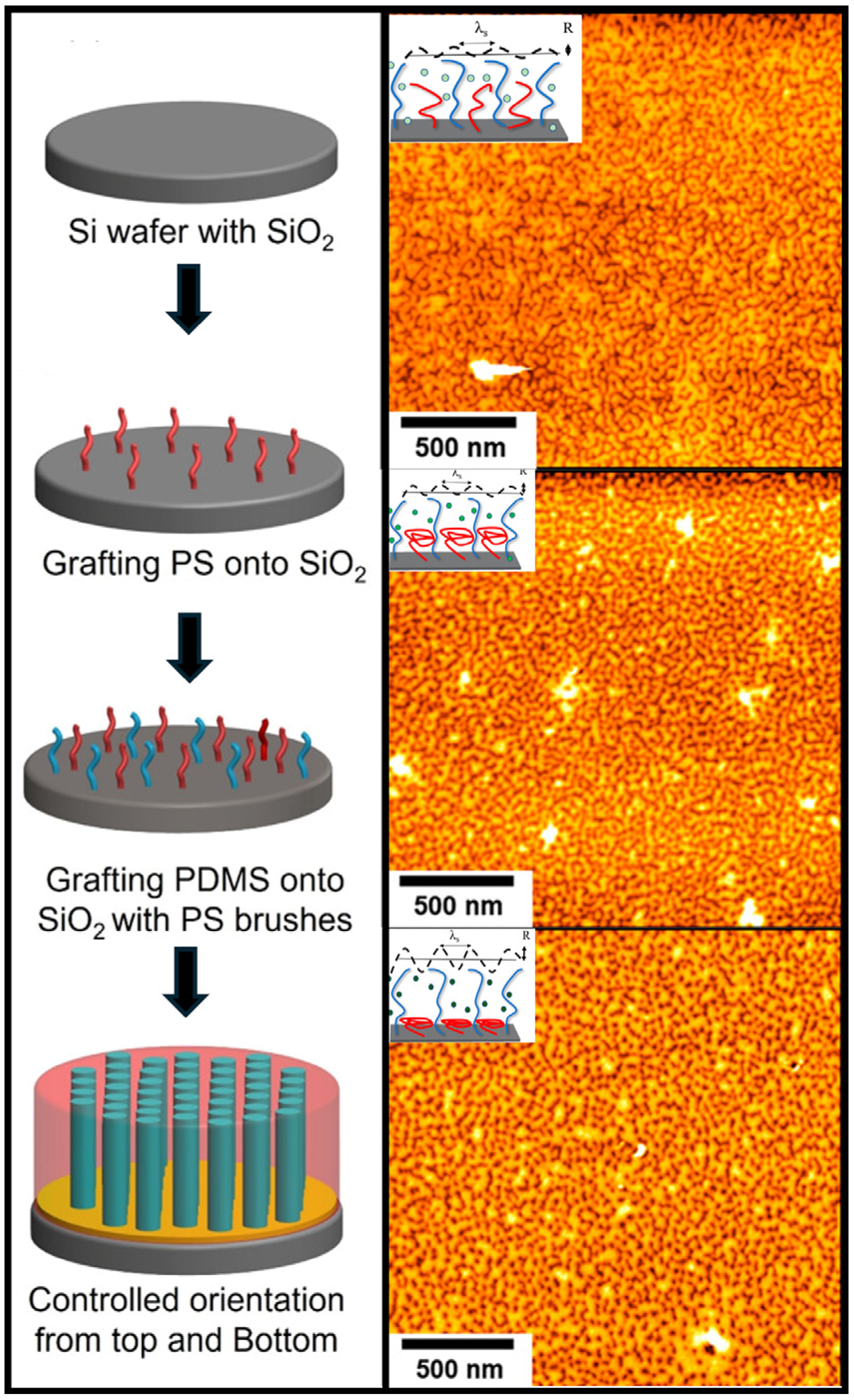
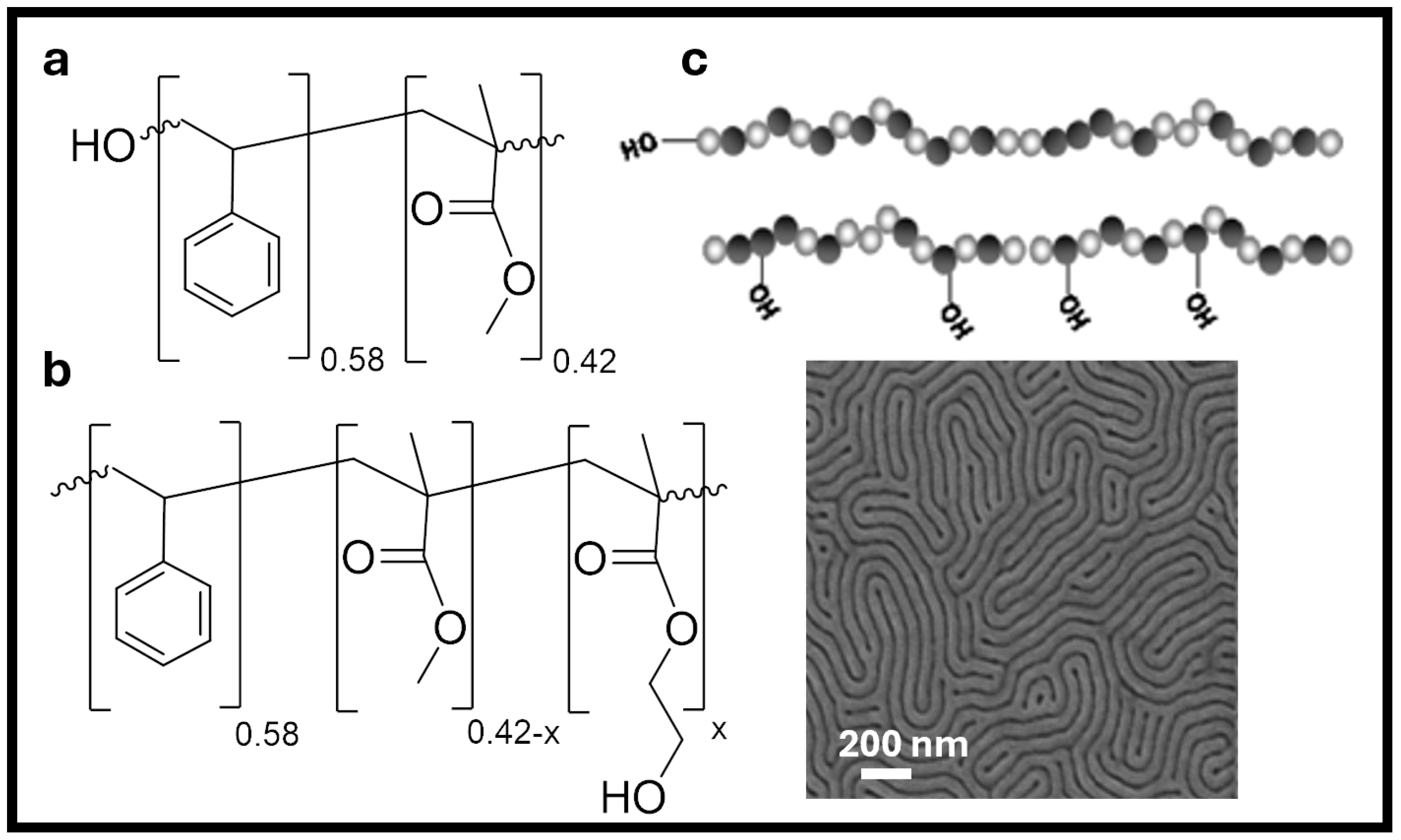
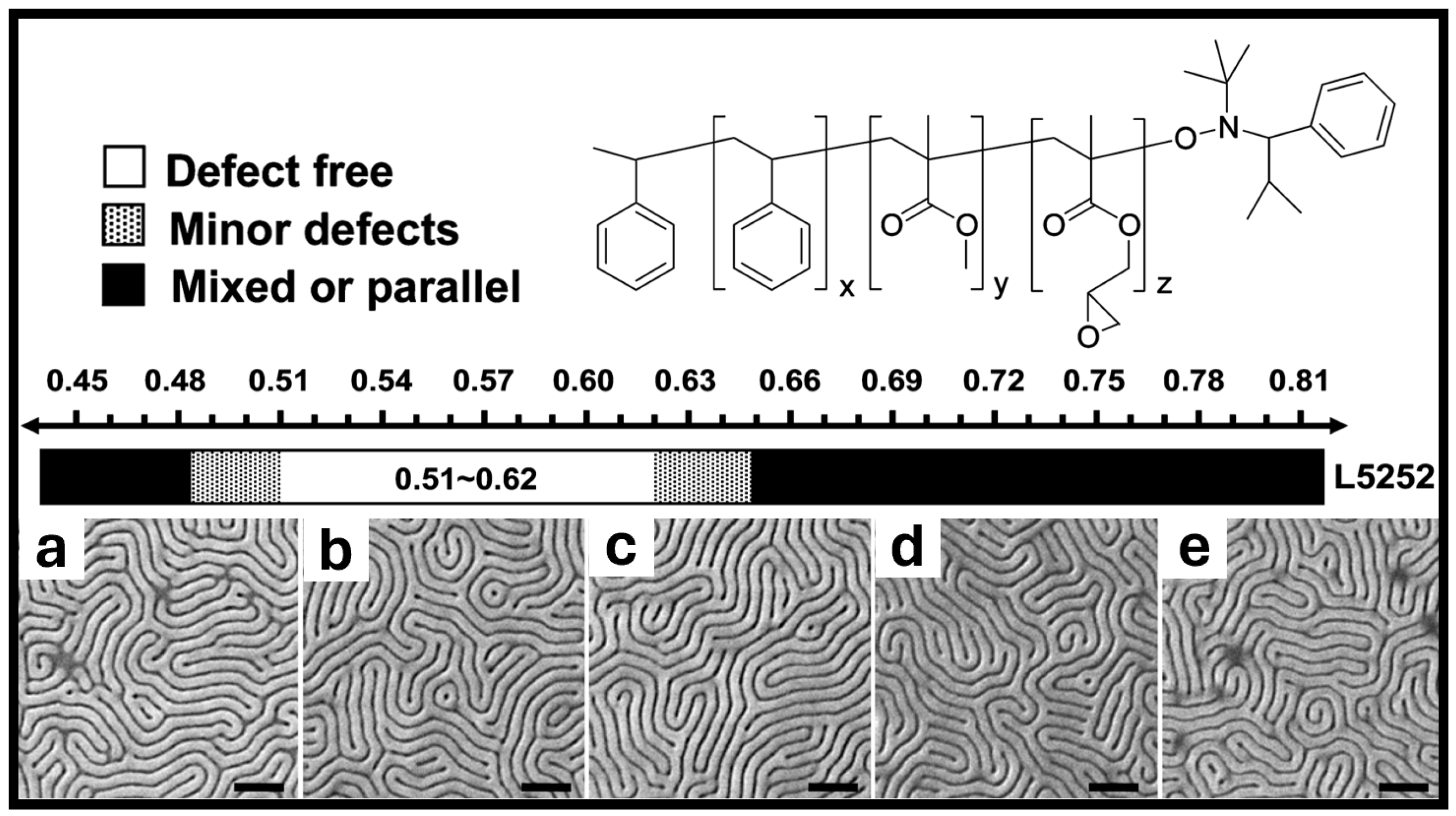
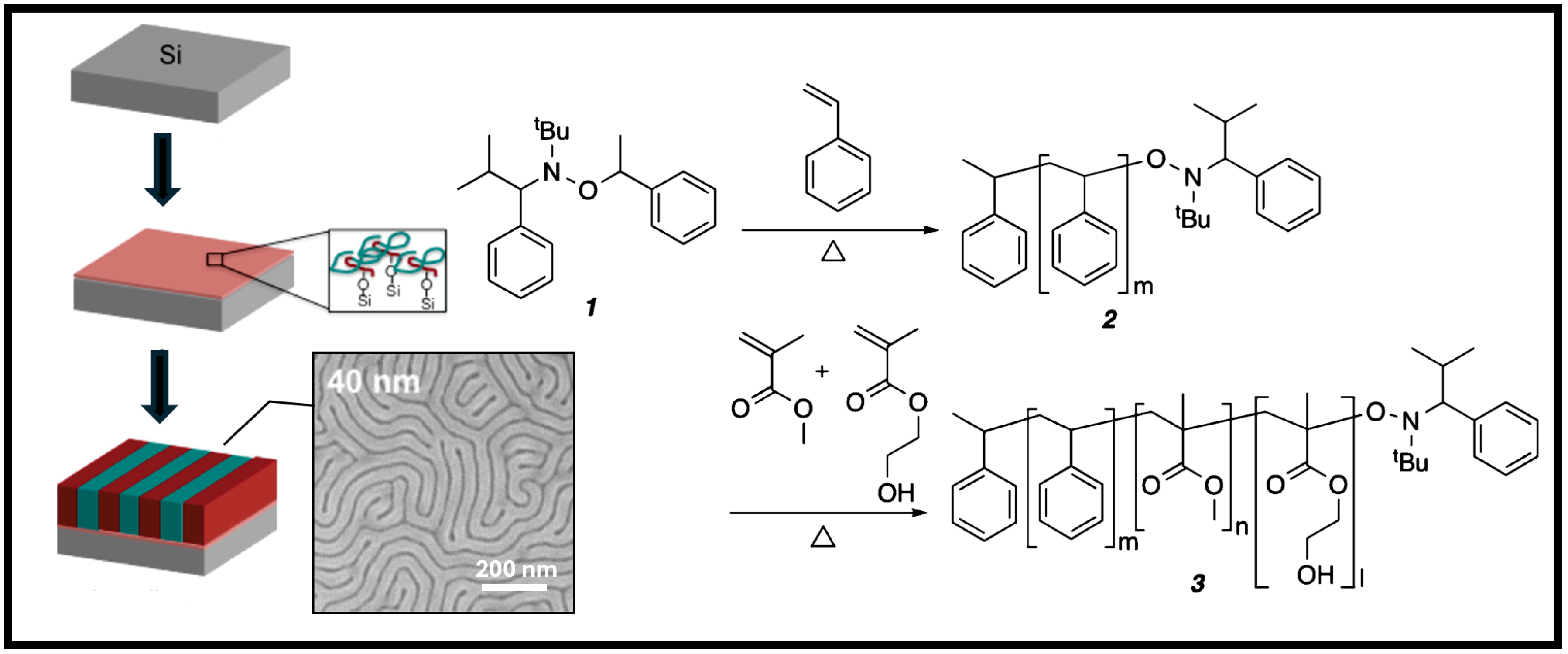
6. Outlook and Future Work
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, B.S.; Loo, W.S.; Yu, B.; Dhuey, S.; Wan, L.; Nealey, P.F.; Ruiz, R. Sequential Brush Grafting for Chemically and Dimensionally Tolerant Directed Self-Assembly of Block Copolymers. ACS Appl. Mater. Interfaces 2022, 15, 2020–2029. [Google Scholar] [CrossRef]
- Ji, S.; Wan, L.; Liu, C.-C.; Nealey, P.F. Directed self-assembly of block copolymers on chemical patterns: A platform for nanofabrication. Prog. Polym. Sci. 2016, 54–55, 76–127. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, X.; Wan, L.; Nealey, P.F.; Ji, S. Fabrication of chemical patterns from graphoepitaxially assembled block copolymer films by molecular transfer printing. Polymer 2014, 55, 3278–3283. [Google Scholar] [CrossRef]
- Liu, G.; Thomas, C.S.; Craig, G.S.W.; Nealey, P.F. Integration of Density Multiplication in the Formation of Device-Oriented Structures by Directed Assembly of Block Copolymer–Homopolymer Blends. Adv. Funct. Mater. 2010, 20, 1251–1257. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, J.M.; Jeong, J.W.; Jung, Y.S. Deep-Nanoscale Pattern Engineering by Immersion-Induced Self-Assembly. ACS Nano 2014, 8, 10009–10018. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, A. Submicrometer Pattern Fabrication by Intensification of Instability in Ultrathin Polymer Films under a Water–Solvent Mix. Macromolecules 2011, 44, 4928–4935. [Google Scholar] [CrossRef]
- Julie Van, B.; Lander, V.; Hyo Seon, S.; Stefan De, G.; Philippe, B.; Jelle, V.; Waikin, L.; Matteo, B.; Amir-Hossein, T.; Christophe, B.; et al. EUV lithography line-space pattern rectification using block copolymer directed self-assembly: A roughness and defectivity study. In Proceedings of the SPIE Advanced Lithography + Patterning, San Jose, CA, USA, 27 February–1 March 2023; Volume 12497, p. 124970K. [Google Scholar] [CrossRef]
- Ruiz, R.; Kang, H.; Detcheverry, F.A.; Dobisz, E.; Kercher, D.S.; Albrecht, T.R.; de Pablo, J.J.; Nealey, P.F. Density Multiplication and Improved Lithography by Directed Block Copolymer Assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef]
- Liu, C.-C.; Han, E.; Onses, M.S.; Thode, C.J.; Ji, S.; Gopalan, P.; Nealey, P.F. Fabrication of Lithographically Defined Chemically Patterned Polymer Brushes and Mats. Macromolecules 2011, 44, 1876–1885. [Google Scholar] [CrossRef]
- Alan, G.J.; Byungki, J.; Christopher, K.O.; Michael, O.T. Control of PS-b-PMMA directed self-assembly registration by laser induced millisecond thermal annealing. In Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 24–27 February 2014. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Bogart, T.D.; Lewis, R.L.; Beers, K.L.; Fasolka, M.J.; Hutchison, J.B.; Vogt, B.D.; Epps, T.H., III. Gradient Solvent Vapor Annealing of Block Copolymer Thin Films Using a Microfluidic Mixing Device. Nano Lett. 2011, 11, 1351–1357. [Google Scholar] [CrossRef]
- Hendeniya, N.; Hillery, K.; Chang, B.S. Processive Pathways to Metastability in Block Copolymer Thin Films. Polymers 2023, 15, 498. [Google Scholar] [CrossRef]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.P.; Hawker, C. Controlling Polymer-Surface Interactions with Random Copolymer Brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Liu, G.; Ji, S.; Stuen, K.O.; Craig, G.S.W.; Nealey, P.F.; Himpsel, F.J. Modification of a polystyrene brush layer by insertion of poly(methyl methacrylate) molecules. J. Vac. Sci. Technol. B 2009, 27, 3038–3042. [Google Scholar] [CrossRef]
- Ceresoli, M.; Palermo, M.; Ferrarese Lupi, F.; Seguini, G.; Perego, M.; Zuccheri, G.; Phadatare, S.D.; Antonioli, D.; Gianotti, V.; Sparnacci, K.; et al. Neutral wetting brush layers for block copolymer thin films using homopolymer blends processed at high temperatures. Nanotechnology 2015, 26, 415603. [Google Scholar] [CrossRef]
- Kim, S.; Wang, H.S.; Choe, Y.; Choi, S.-H.; Bang, J. Controlling the microdomain orientation in block copolymer thin films via cross-linkable random copolymer neutral layer. Polym. J. 2016, 48, 333–340. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; DeRouchey, J.; Russell, T.P.; Jaeger, H.M. Overcoming Interfacial Interactions with Electric Fields. Macromolecules 2000, 33, 3250–3253. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ross, C.A. Solvent-Vapor-Induced Tunability of Self-Assembled Block Copolymer Patterns. Adv. Mater. 2009, 21, 2540–2545. [Google Scholar] [CrossRef]
- Jung, F.A.; Berezkin, A.V.; Tejsner, T.B.; Posselt, D.; Smilgies, D.-M.; Papadakis, C.M. Solvent Vapor Annealing of a Diblock Copolymer Thin Film with a Nonselective and a Selective Solvent: Importance of Pathway for the Morphological Changes. Macromol. Rapid Commun. 2020, 41, 2000150. [Google Scholar] [CrossRef]
- Sivaniah, E.; Hayashi, Y.; Iino, M.; Hashimoto, T.; Fukunaga, K. Observation of Perpendicular Orientation in Symmetric Diblock Copolymer Thin Films on Rough Substrates. Macromolecules 2003, 36, 5894–5896. [Google Scholar] [CrossRef]
- Ji, S.; Liao, W.; Nealey, P.F. Block Cooligomers: A Generalized Approach to Controlling the Wetting Behavior of Block Copolymer Thin Films. Macromolecules 2010, 43, 6919–6922. [Google Scholar] [CrossRef]
- Ji, S.; Liu, C.-C.; Son, J.G.; Gotrik, K.; Craig, G.S.W.; Gopalan, P.; Himpsel, F.J.; Char, K.; Nealey, P.F. Generalization of the Use of Random Copolymers to Control the Wetting Behavior of Block Copolymer Films. Macromolecules 2008, 41, 9098–9103. [Google Scholar] [CrossRef]
- Ji, S.; Liu, G.; Zheng, F.; Craig, G.S.W.; Himpsel, F.J.; Nealey, P.F. Preparation of Neutral Wetting Brushes for Block Copolymer Films from Homopolymer Blends. Adv. Mater. 2008, 20, 3054–3060. [Google Scholar] [CrossRef]
- Huang, E.; Rockford, L.; Russell, T.P.; Hawker, C.J. Nanodomain control in copolymer thin films. Nature 1998, 395, 757–758. [Google Scholar] [CrossRef]
- Huang, E.; Mansky, P.; Russell, T.P.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Hawker, C.J.; Mays, J. Mixed Lamellar Films: Evolution, Commensurability Effects, and Preferential Defect Formation. Macromolecules 2000, 33, 80–88. [Google Scholar] [CrossRef]
- Han, E.; Gopalan, P. Cross-Linked Random Copolymer Mats as Ultrathin Nonpreferential Layers for Block Copolymer Self-Assembly. Langmuir 2010, 26, 1311–1315. [Google Scholar] [CrossRef]
- Suh, H.S.; Kim, D.H.; Moni, P.; Xiong, S.; Ocola, L.E.; Zaluzec, N.J.; Gleason, K.K.; Nealey, P.F. Sub-10-nm patterning via directed self-assembly of block copolymer films with a vapour-phase deposited topcoat. Nat. Nanotechnol. 2017, 12, 575–581. [Google Scholar] [CrossRef]
- Lane, A.P.; Yang, X.; Maher, M.J.; Blachut, G.; Asano, Y.; Someya, Y.; Mallavarapu, A.; Sirard, S.M.; Ellison, C.J.; Willson, C.G. Directed Self-Assembly and Pattern Transfer of Five Nanometer Block Copolymer Lamellae. ACS Nano 2017, 11, 7656–7665. [Google Scholar] [CrossRef]
- Liu, C.-C.; Ramírez-Hernández, A.; Han, E.; Craig, G.S.W.; Tada, Y.; Yoshida, H.; Kang, H.; Ji, S.; Gopalan, P.; de Pablo, J.J.; et al. Chemical Patterns for Directed Self-Assembly of Lamellae-Forming Block Copolymers with Density Multiplication of Features. Macromolecules 2013, 46, 1415–1424. [Google Scholar] [CrossRef]
- Hitesh, A.; Phong, D.; Kwan Wee, T.; Jerome, K.H.; Grazul, J.L.; Huolin, L.X.; David, A.M.; Michael, O.T.; Ulrich, W. Block copolymer self-assembly-directed single-crystal homo- and heteroepitaxial nanostructures. Science 2010, 330, 214–219. [Google Scholar] [CrossRef]
- Kim, S.; Shin, D.O.; Choi, D.-G.; Jeong, J.-R.; Mun, J.H.; Yang, Y.-B.; Kim, J.U.; Kim, S.O.; Jeong, J.-H. Graphoepitaxy of Block-Copolymer Self-Assembly Integrated with Single-Step ZnO Nanoimprinting. Small 2012, 8, 1563–1569. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, X.; Edwards, E.W.; La, Y.-H.; Nealey, P.F. Graphoepitaxy of cylinder-forming block copolymers for use as templates to pattern magnetic metal dot arrays. Nanotechnology 2005, 16, S324. [Google Scholar] [CrossRef]
- Kellogg, G.J.; Walton, D.G.; Mayes, A.M.; Lambooy, P.; Russell, T.P.; Gallagher, P.D.; Satija, S.K. Observed Surface Energy Effects in Confined Diblock Copolymers. Phys. Rev. Lett. 1996, 76, 2503–2506. [Google Scholar] [CrossRef]
- Brown, G.; Chakrabarti, A. Ordering of block copolymer melts in confined geometry. J. Chem. Phys. 1995, 102, 1440–1448. [Google Scholar] [CrossRef]
- Walton, D.G.; Kellogg, G.J.; Mayes, A.M.; Lambooy, P.; Russell, T.P. A Free Energy Model for Confined Diblock Copolymers. Macromolecules 1994, 27, 6225–6228. [Google Scholar] [CrossRef]
- Lambooy, P.; Russell, T.P.; Kellogg, G.J.; Mayes, A.M.; Gallagher, P.D.; Satija, S.K. Observed frustration in confined block copolymers. Phys. Rev. Lett. 1994, 72, 2899–2902. [Google Scholar] [CrossRef]
- Koneripalli, N.; Singh, N.; Levicky, R.; Bates, F.S.; Gallagher, P.D.; Satija, S.K. Confined Block Copolymer Thin Films. Macromolecules 1995, 28, 2897–2904. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Huang, E.; Russell, T.P.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Hawker, C.J.; Mays, J. Using Surface Active Random Copolymers to Control the Domain Orientation in Diblock Copolymer Thin Films. Macromolecules 1998, 31, 7641–7650. [Google Scholar] [CrossRef]
- In, I.; La, Y.-H.; Park, S.-M.; Nealey, P.F.; Gopalan, P. Side-Chain-Grafted Random Copolymer Brushes as Neutral Surfaces for Controlling the Orientation of Block Copolymer Microdomains in Thin Films. Langmuir 2006, 22, 7855–7860. [Google Scholar] [CrossRef]
- Sparnacci, K.; Antonioli, D.; Gianotti, V.; Laus, M.; Ferrarese Lupi, F.; Giammaria, T.J.; Seguini, G.; Perego, M. Ultrathin Random Copolymer-Grafted Layers for Block Copolymer Self-Assembly. ACS Appl. Mater. Interfaces 2015, 7, 10944–10951. [Google Scholar] [CrossRef]
- Ouk Kim, S.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; de Pablo, J.J.; Nealey, P.F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature 2003, 424, 411–414. [Google Scholar] [CrossRef]
- Han, E.; Stuen, K.O.; La, Y.-H.; Nealey, P.F.; Gopalan, P. Effect of Composition of Substrate-Modifying Random Copolymers on the Orientation of Symmetric and Asymmetric Diblock Copolymer Domains. Macromolecules 2008, 41, 9090–9097. [Google Scholar] [CrossRef]
- Winesett, D.A.; Story, S.; Luning, J.; Ade, H. Tuning Substrate Surface Energies for Blends of Polystyrene and Poly(methyl methacrylate). Langmuir 2003, 19, 8526–8535. [Google Scholar] [CrossRef]
- Panda, A.S.; Lee, Y.-C.; Shastry, T.; Manesi, G.-M.; Avgeropoulos, A.; Ho, R.-M. Controlled Orientation of Silicon-Containing Diblock Copolymer Thin Films by Substrate Functionalization Under Vacuum. Macromolecules 2023, 56, 841–849. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, L.; Huang, G.; Zhang, X.; Jin, X.; Xu, P.; Liu, Y.; Han, M.; Wu, G.-P.; Ji, S. Controlling Block Copolymer–Substrate Interactions by Homopolymer Brushes/Mats. Macromolecules 2017, 50, 6733–6741. [Google Scholar] [CrossRef]
- Goodman, D.; Kizhakkedathu, J.N.; Brooks, D.E. Attractive Bridging Interactions in Dense Polymer Brushes in Good Solvent Measured by Atomic Force Microscopy. Langmuir 2004, 20, 2333–2340. [Google Scholar] [CrossRef]
- Goodman, D.; Kizhakkedathu, J.N.; Brooks, D.E. Molecular Weight and Polydispersity Estimation of Adsorbing Polymer Brushes by Atomic Force Microscopy. Langmuir 2004, 20, 3297–3303. [Google Scholar] [CrossRef]
- Kelley, T.W.; Schorr, P.A.; Johnson, K.D.; Tirrell, M.; Frisbie, C.D. Direct Force Measurements at Polymer Brush Surfaces by Atomic Force Microscopy. Macromolecules 1998, 31, 4297–4300. [Google Scholar] [CrossRef]
- Yu, B.; Chang, B.S.; Loo, W.S.; Dhuey, S.; O’Reilly, P.; Ashby, P.D.; Connolly, M.D.; Tikhomirov, G.; Zuckermann, R.N.; Ruiz, R. Nanopatterned Monolayers of Bioinspired, Sequence-Defined Polypeptoid Brushes for Semiconductor/Bio Interfaces. ACS Nano 2024, 18, 7411–7423. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Sui, X.; Zapotoczny, S.; Benetti, E.M.; Schön, P.; Vancso, G.J. Characterization and molecular engineering of surface-grafted polymer brushes across the length scales by atomic force microscopy. J. Mater. Chem. 2010, 20, 4981–4993. [Google Scholar] [CrossRef]
- Cuenot, S.; Gabriel, S.; Jérôme, R.; Jérôme, C.; Fustin, C.-A.; Jonas, A.M.; Duwez, A.-S. First Insights into Electrografted Polymers by AFM-Based Force Spectroscopy. Macromolecules 2006, 39, 8428–8433. [Google Scholar] [CrossRef]
- Benetti, E.M.; Reimhult, E.; de Bruin, J.; Zapotoczny, S.; Textor, M.; Vancso, G.J. Poly(methacrylic acid) Grafts Grown from Designer Surfaces: The Effect of Initiator Coverage on Polymerization Kinetics, Morphology, and Properties. Macromolecules 2009, 42, 1640–1647. [Google Scholar] [CrossRef]
- Wang, X.; Tu, H.; Braun, P.V.; Bohn, P.W. Length Scale Heterogeneity in Lateral Gradients of Poly(N-isopropylacrylamide) Polymer Brushes Prepared by Surface-Initiated Atom Transfer Radical Polymerization Coupled with In-Plane Electrochemical Potential Gradients. Langmuir 2006, 22, 817–823. [Google Scholar] [CrossRef]
- Farhan, T.; Azzaroni, O.; Huck, W.T.S. AFM study of cationically charged polymer brushes: Switching between soft and hard matter. Soft Matter 2005, 1, 66–68. [Google Scholar] [CrossRef]
- Parnell, A.J.; Martin, S.J.; Jones, R.A.L.; Vasilev, C.; Crook, C.J.; Ryan, A.J. Direct visualization of the real time swelling and collapse of a poly(methacrylic acid) brush using atomic force microscopy. Soft Matter 2009, 5, 296–299. [Google Scholar] [CrossRef]
- Nowak, D.; Morrison, W.; Wickramasinghe, H.K.; Jahng, J.; Potma, E.; Wan, L.; Ruiz, R.; Albrecht, T.R.; Schmidt, K.; Frommer, J.; et al. Nanoscale chemical imaging by photoinduced force microscopy. Sci. Adv. 2016, 2, e1501571. [Google Scholar] [CrossRef]
- Dean, M.D.; Eric, K.L.; Daniel, A.F. Organic semiconductor structure and chemistry from near-edge X-ray absorption fine structure (NEXAFS) spectroscopy. In Proceedings of the Optics and Photonics 2005, San Diego, CA, USA, 31 July–4 August 2005; Volume 5940, p. 59400A. [Google Scholar]
- Jablonski, E.L.; Lenhart, J.L.; Sambasivan, S.; Fischer, D.A.; Jones, R.L.; Lin, E.K.; Wu, W.-L.; Goldfarb, D.L.; Temple, K.; Angelopoulos, M.; et al. NEXAFS Measurements of the Surface Chemistry of Chemically Amplified Photoresists. In Proceedings of the Characterization and Metrology for ULSI Technology: 2003 International Conference on Characterization and Metrology for ULSI Technology, Austin, TX, USA, 24–28 March 2003. [Google Scholar]
- Hähner, G. Near edge X-ray absorption fine structure spectroscopy as a tool to probe electronic and structural properties of thin organic films and liquids. Chem. Soc. Rev. 2006, 35, 1244–1255. [Google Scholar] [CrossRef]
- Prabhu, V.M.; Sambasivan, S.; Fischer, D.; Sundberg, L.K.; Allen, R.D. Quantitative depth profiling of photoacid generators in photoresist materials by near-edge X-ray absorption fine structure spectroscopy. Appl. Surf. Sci. 2006, 253, 1010–1014. [Google Scholar] [CrossRef]
- Gesang, T.; Fanter, D.; Höper, R.; Possart, W.; Hennemann, O.D. Comparative film thickness determination by atomic force microscopy and ellipsometry for ultrathin polymer films. Surf. Interface Anal. 1995, 23, 797–808. [Google Scholar] [CrossRef]
- Henck, S.A. In situ real-time ellipsometry for film thickness measurement and control. J. Vac. Sci. Technol. A 1992, 10, 934–938. [Google Scholar] [CrossRef]
- Shelley, P.H.; Booksh, K.S.; Burgess, L.W.; Kowalski, B.R. Polymer Film Thickness Determination with a High-Precision Scanning Reflectometer. Appl. Spectrosc. 1996, 50, 119–125. [Google Scholar] [CrossRef]
- Hirvi, K.; Mäkelä, T.; Pekola, J.; Paalanen, M. Economical device for measuring thickness of a thin polymer film. Rev. Sci. Instrum. 1994, 65, 2735–2736. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.G.; Bradley, R.H. Thickness of Spin-Cast Polymer Thin Films Determined by Angle-Resolved XPS and AFM Tip-Scratch Methods. Langmuir 2000, 16, 2281–2284. [Google Scholar] [CrossRef]
- Shastry, T.; Panda, A.S.; Manesi, G.-M.; Avgeropoulos, A.; Ho, R.-M. Controlled Orientation of Plasma-Treated Diblock Copolymer Films from the Responsive Functionalized Substrate through Solvent Annealing. Macromolecules 2023, 56, 5651–5660. [Google Scholar] [CrossRef]
- Wan, L.; Ruiz, R.; Gao, H.; Patel, K.C.; Albrecht, T.R.; Yin, J.; Kim, J.; Cao, Y.; Lin, G. The Limits of Lamellae-Forming PS-b-PMMA Block Copolymers for Lithography. ACS Nano 2015, 9, 7506–7514. [Google Scholar] [CrossRef]
- Aktas Eken, G.; Ober, C.K. Strong Polyelectrolyte Brushes via Alternating Copolymers of Styrene and Maleimides: Synthesis, Properties, and Stability. Macromolecules 2022, 55, 5291–5300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillery, K.; Hendeniya, N.; Abtahi, S.; Chittick, C.; Chang, B. Substrate Neutrality for Obtaining Block Copolymer Vertical Orientation. Polymers 2024, 16, 1740. https://doi.org/10.3390/polym16121740
Hillery K, Hendeniya N, Abtahi S, Chittick C, Chang B. Substrate Neutrality for Obtaining Block Copolymer Vertical Orientation. Polymers. 2024; 16(12):1740. https://doi.org/10.3390/polym16121740
Chicago/Turabian StyleHillery, Kaitlyn, Nayanathara Hendeniya, Shaghayegh Abtahi, Caden Chittick, and Boyce Chang. 2024. "Substrate Neutrality for Obtaining Block Copolymer Vertical Orientation" Polymers 16, no. 12: 1740. https://doi.org/10.3390/polym16121740
APA StyleHillery, K., Hendeniya, N., Abtahi, S., Chittick, C., & Chang, B. (2024). Substrate Neutrality for Obtaining Block Copolymer Vertical Orientation. Polymers, 16(12), 1740. https://doi.org/10.3390/polym16121740




