The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyamidoamines (PAAs)
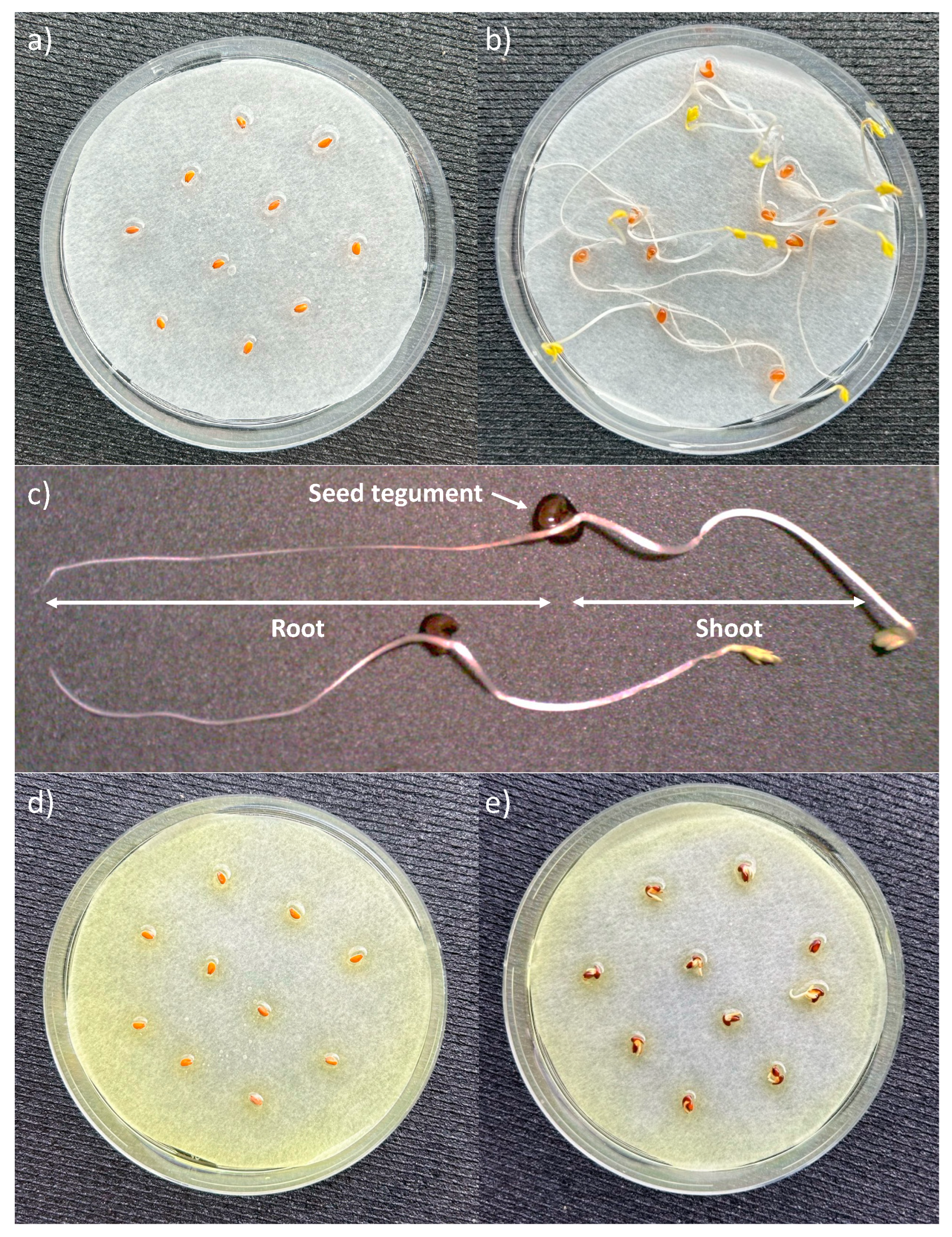
2.3. Seed Germination Test
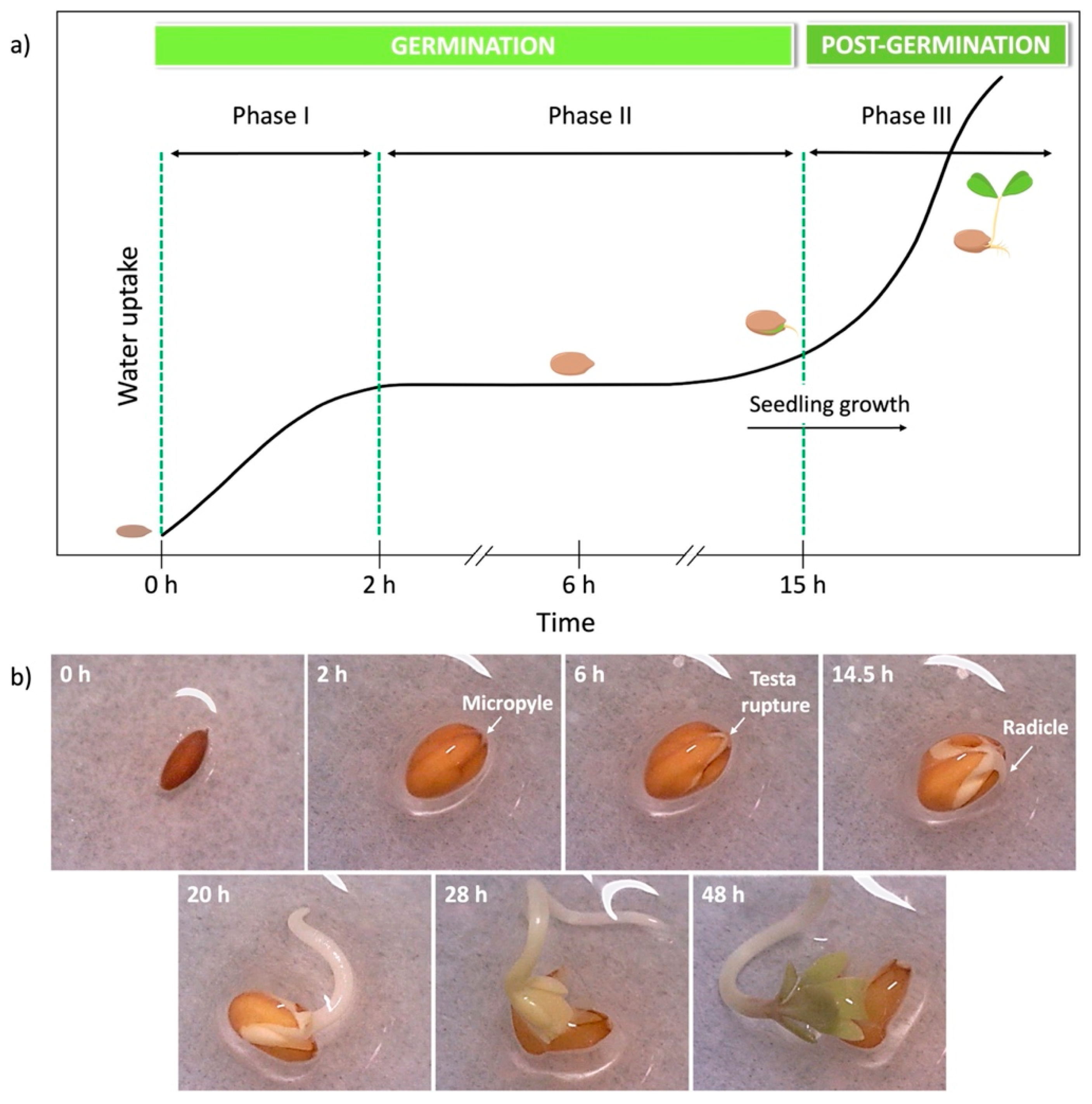
2.4. Seed Germination Kinetics
2.5. Data Analysis
2.5.1. Outlier Data
- For each series, the measurements collected in each experiment were sorted in ascending order.
- Measurements were then divided into quartiles (Q1–Q4), that is, four intervals containing approximately the same number of data points.
- The Q1, Q2, Q3, and Q4 values were identified as the highest values in each group.
- Outliers were identified as the values falling outside the following range:
2.5.2. Experimental Error
2.5.3. T-Test
- The test of variances was performed to define the most suitable equation to determine the t value [44]. In the present study, different variances of measurement were calculated for group 1 (seeds exposed to PAA) and 2 (negative control).
- The unequal variances t-test, also called the Welch’s t-test, was used for calculating the t-statistic (t) value, following Equation (7):
- 3.
- Then, the degrees of freedom (df) were calculated following Equation (8):
- 4.
- Finally, the p-value was calculated as the two-tailed probability of the t-distribution and compared to a chosen significance level of 0.05. If the p-value was less than the significance level, the null hypothesis H0 was rejected, indicating a significant difference in the mean values of groups 1 and 2.
3. Results and Discussion
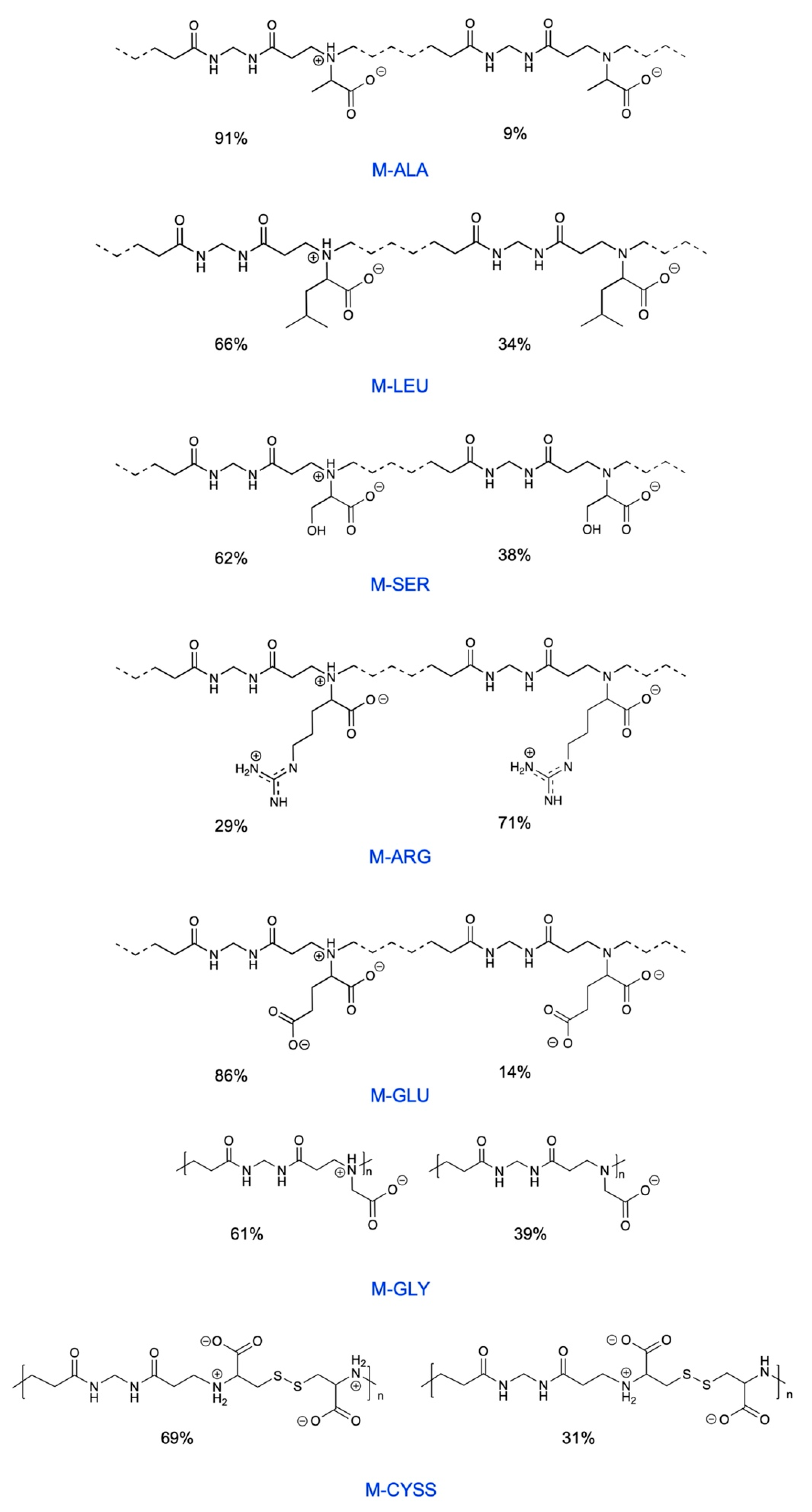
3.1. Water-Soluble Amphoteric Polyamidoamines: Their Synthesis and Ionic Species Distribution
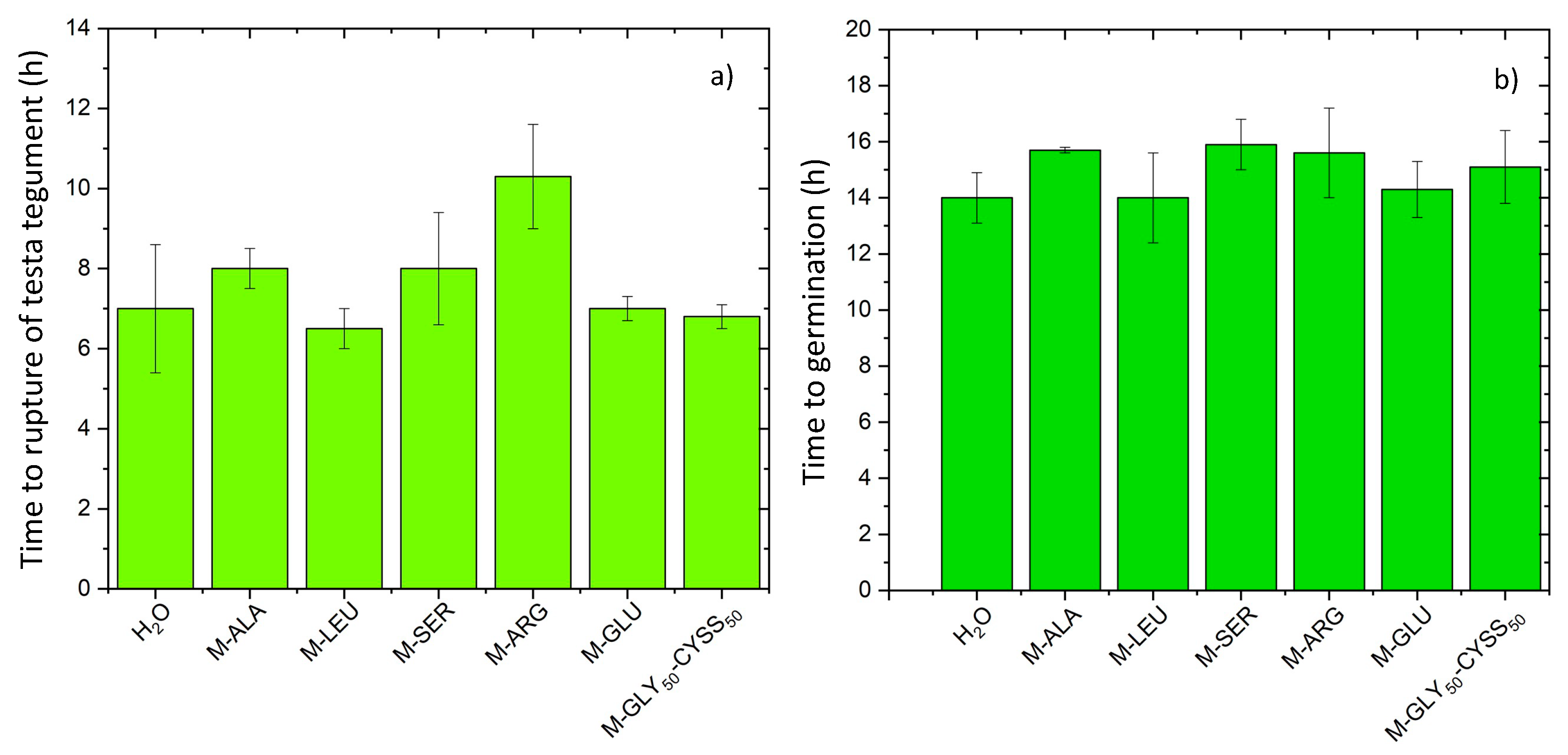
3.2. Effect of PAAs on the Early Stages of Seed Germination
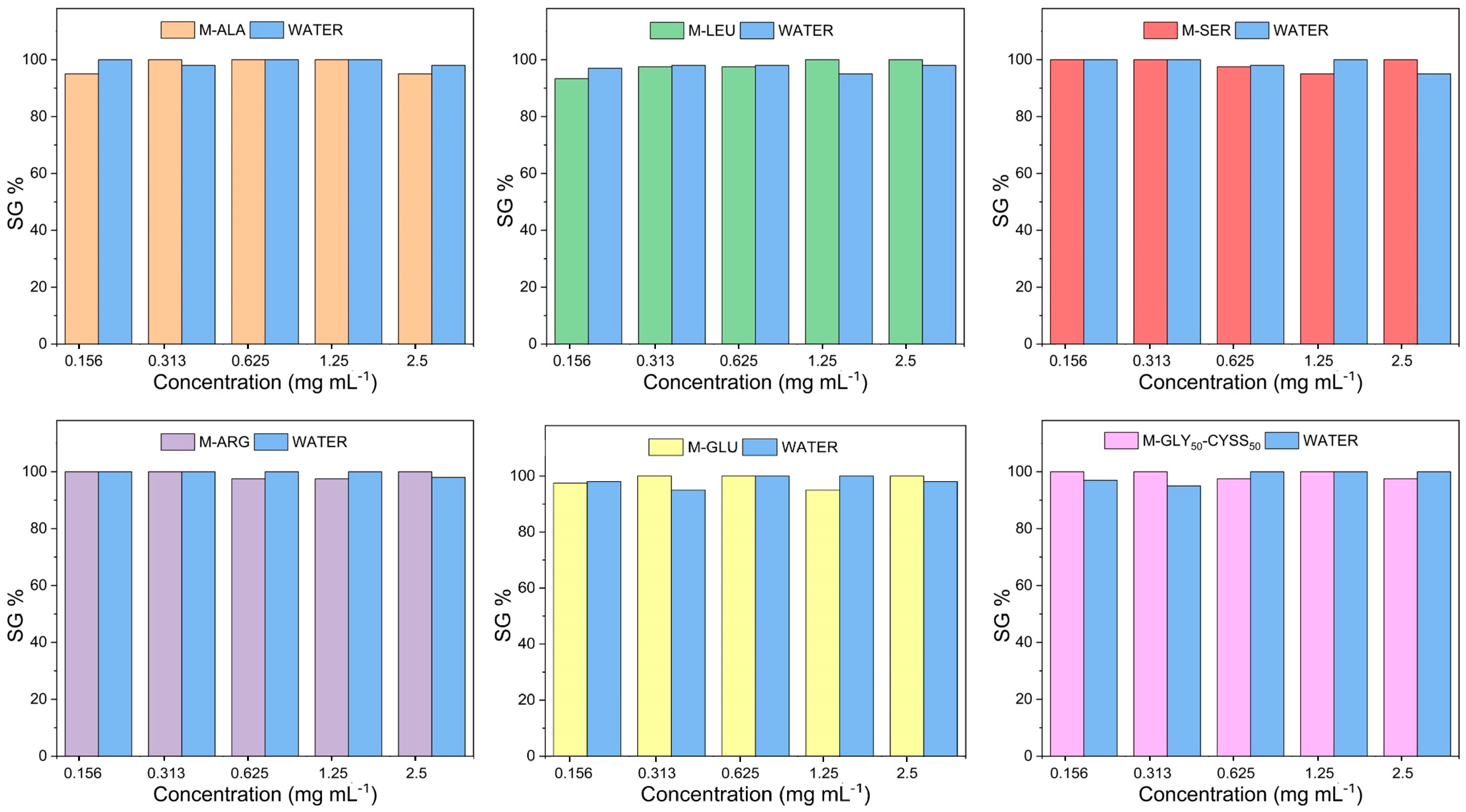
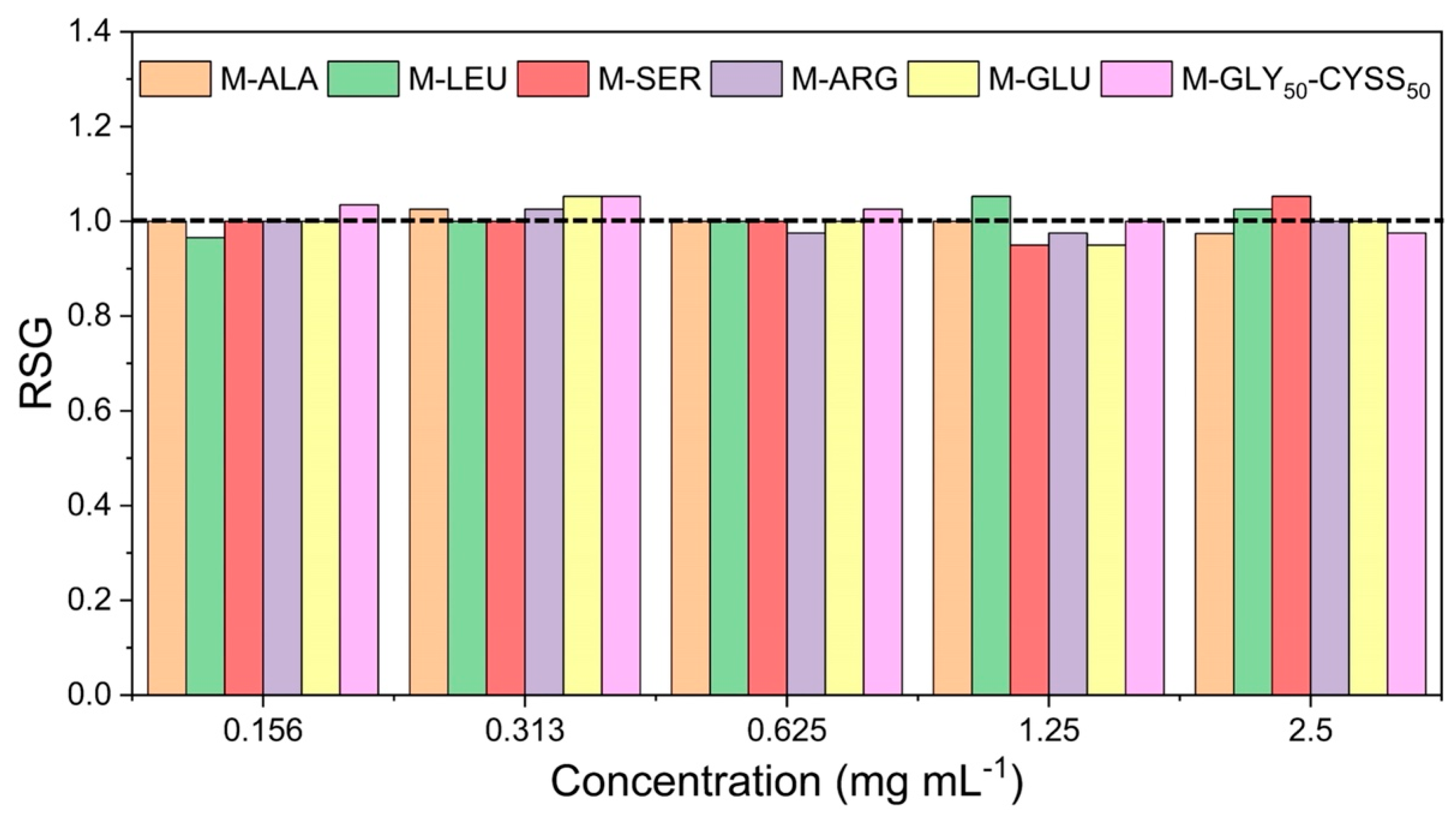
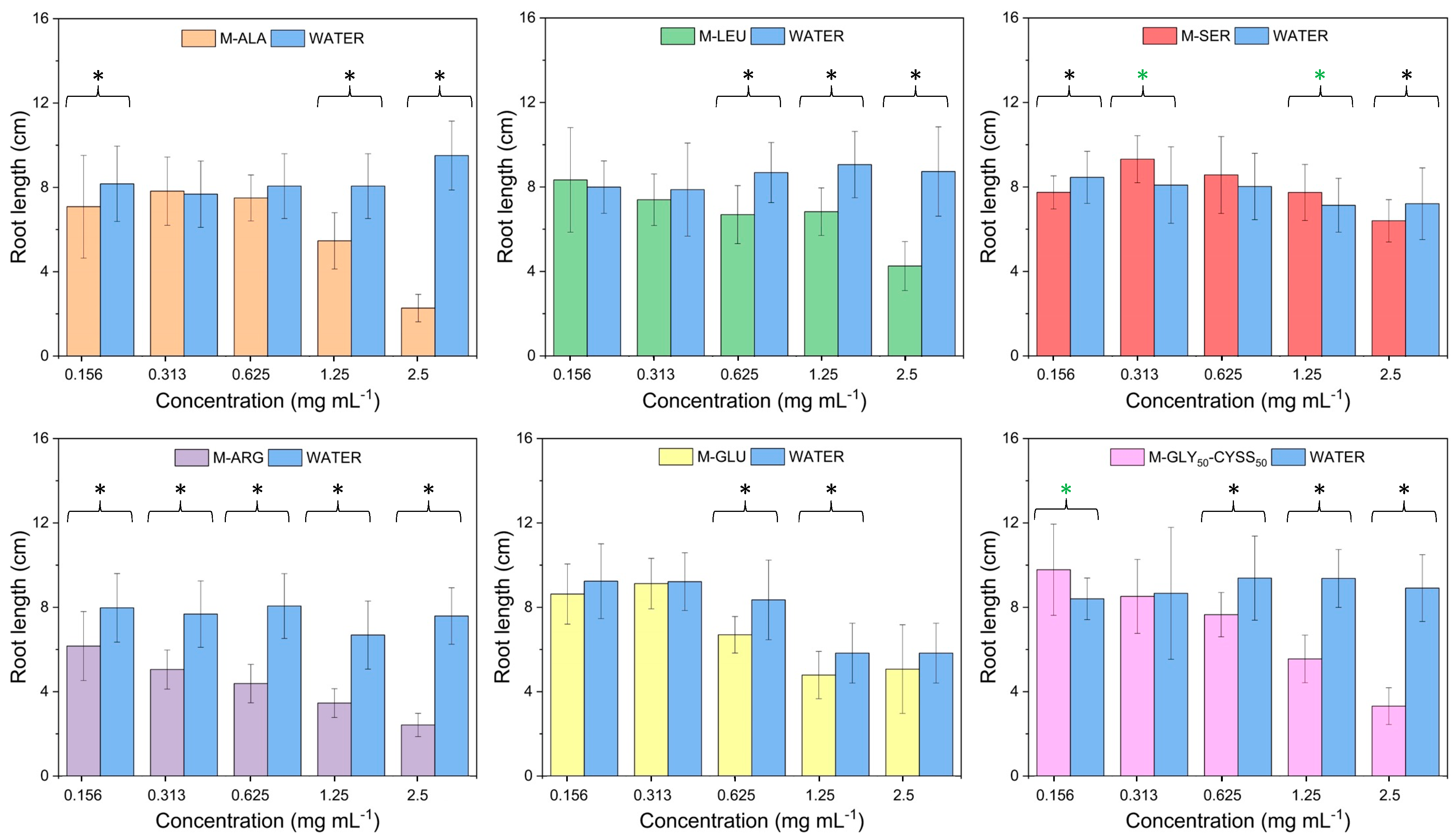
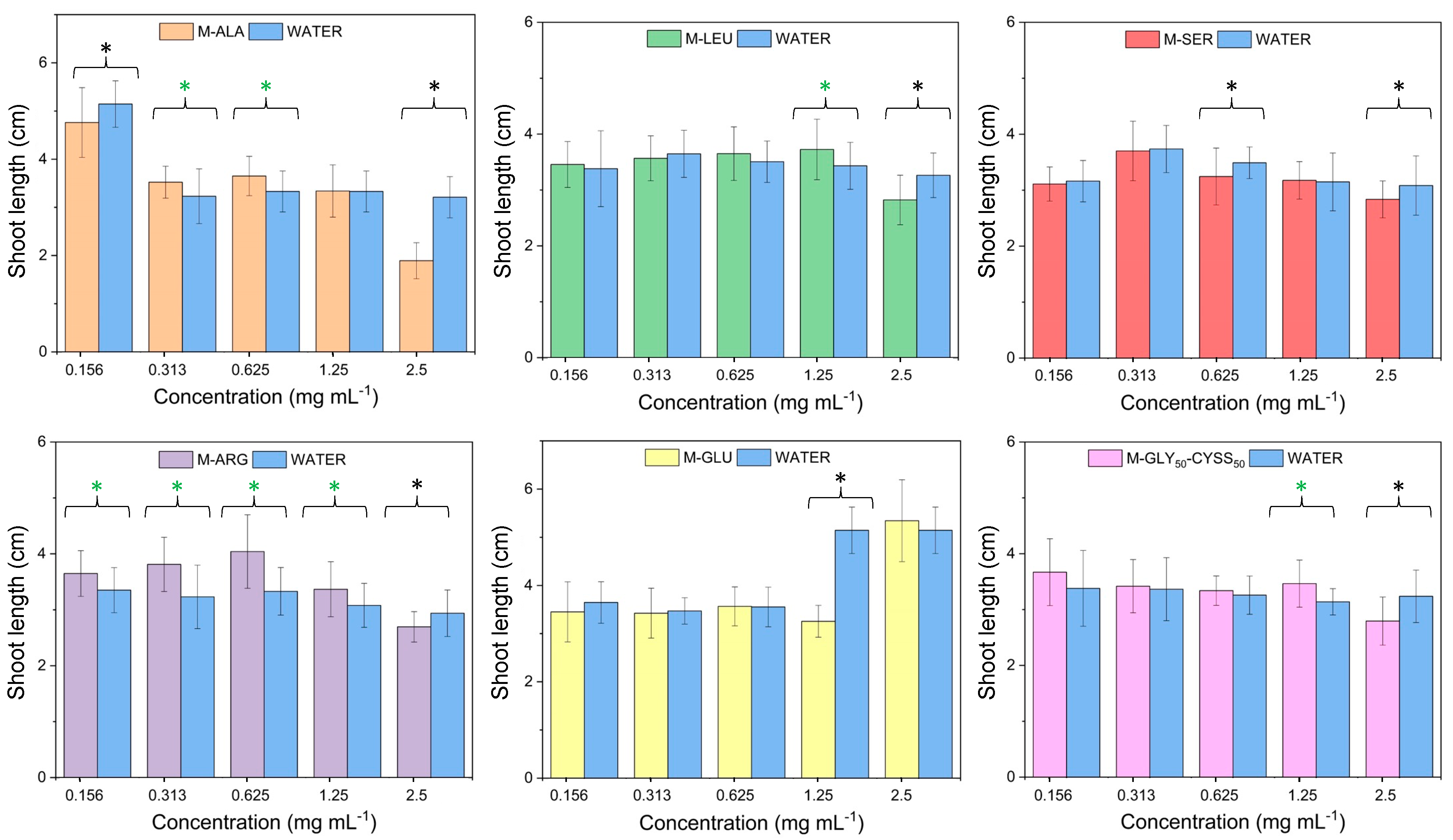
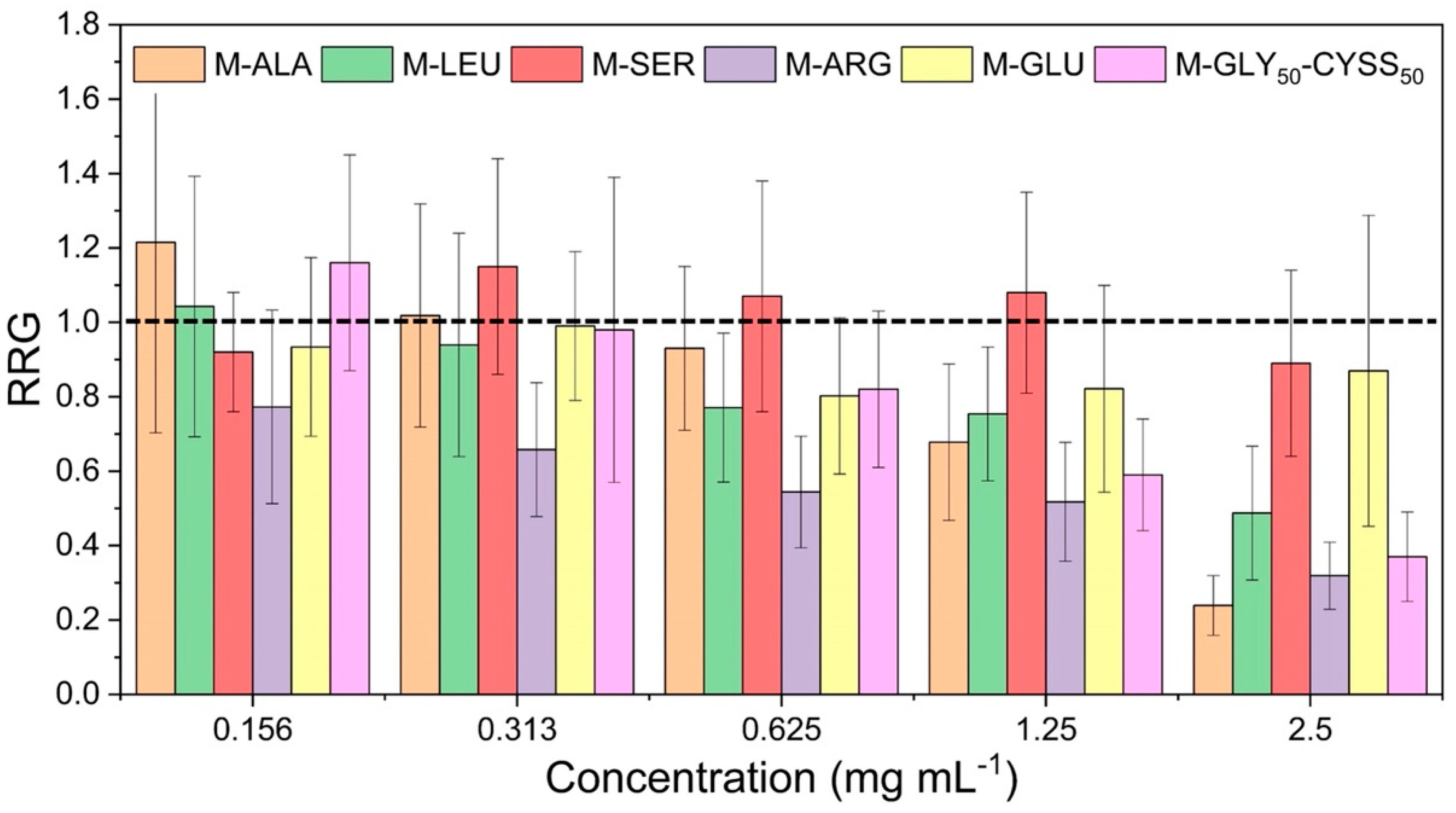
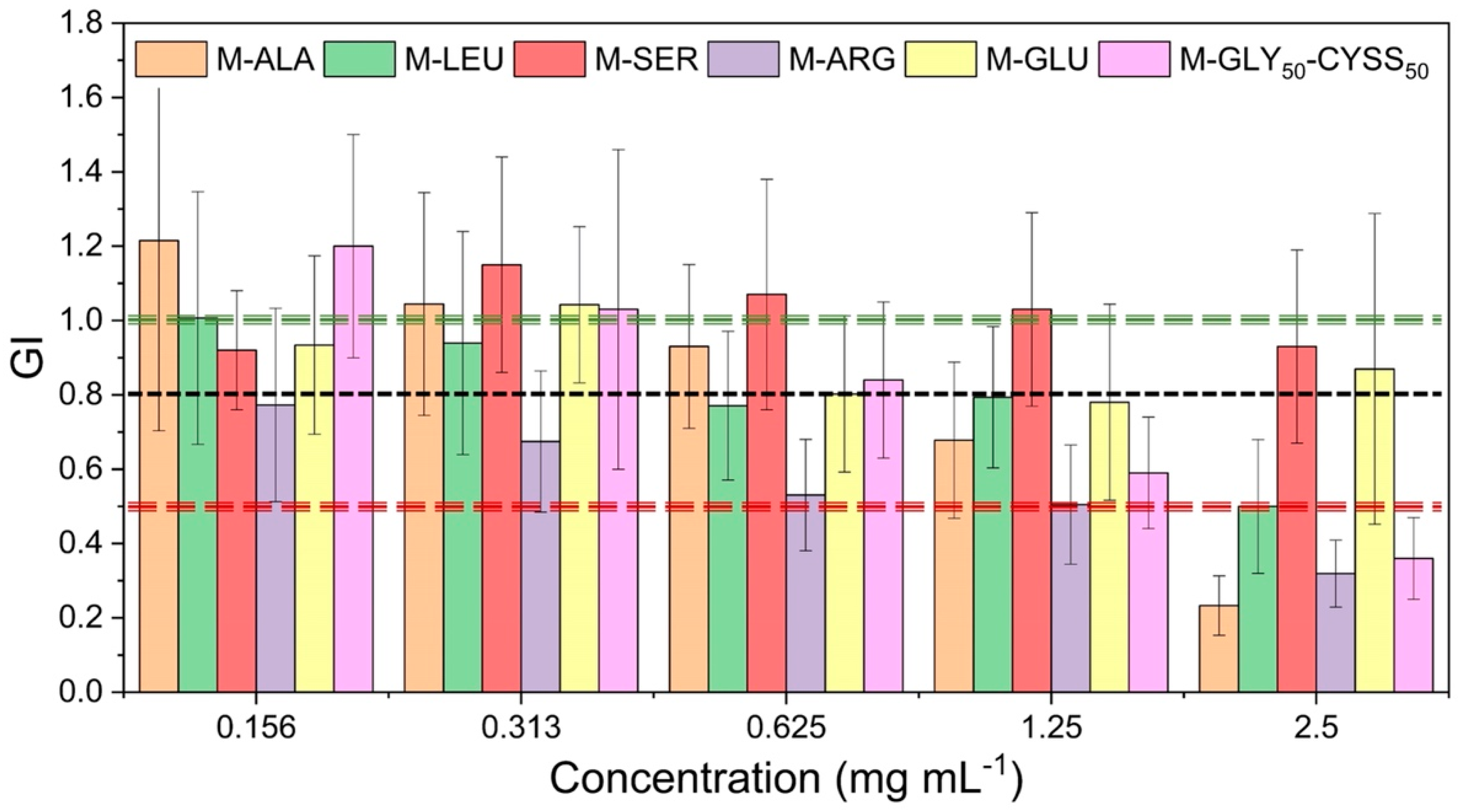
3.3. Effect of PAAs on Seed Germination, Root, and Shoot Elongation of Lepidium sativum L.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Meaning |
| EC50 | 50% effective concentration |
| EPA | Environmental protection agency |
| GI | Germination index |
| M | N,N′-Methylenebisacrylamide |
| M-ALA | M-alanine-derived polyamidoamine |
| M-ARG | M-arginine-derived polyamidoamine |
| M-CYSS | M-cystine-derived polyamidoamine |
| M-GLU | M-glutamic acid-derived polyamidoamine |
| M-GLY | M-glycine-derived polyamidoamine |
| M-GLY50-CYSS50 | M-glycine-cystine-derived polyamidoamine |
| M-LEU | M-leucine-derived polyamidoamine |
| M-SER | M-serine-derived polyamidoamine |
| PAA | Polyamidoamine |
| REACH | Regulation, evaluation, authorization, and restriction of chemicals |
| RRG | Relative radicle growth |
| RSG | Relative seed germination |
| SG% | Seed germination percentage |
References
- Duis, K.; Junker, T.; Coors, A. Environmental fate and effects of water-soluble synthetic organic polymers used in cosmetic products. Environ. Sci. Eur. 2021, 33, 21. [Google Scholar] [CrossRef]
- Krogh, K.A.; Halling-Sørensen, B.; Mogensen, B.B.; Vejrup, K.V. Environmental properties and effects of nonionic surfactant adjuvants in pesticides: A review. Chemosphere 2003, 50, 871–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Chen, Z.-L.; Zhu, J.; Shen, J.-M.; Han, Y. Pilot-scale fluoride-containing wastewater treatment by the ballasted flocculation process. Water Sci. Technol. 2013, 68, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Dąbrowski, T. Impact of cationic polyelectrolytes on activated sludge morphology and biological wastewater treatment in a Sequential Batch Reactor (SBR). J. Water Process Eng. 2023, 52, 103500. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Julinova, M.; Vanharova, L.; Jurca, M. Water-soluble polymeric xenobiotics-polyvinyl alcohol and polyvinylpyrrolidone and potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.J.; Miller, W.P. Polyacrylamide adsorption and aggregate stability. Soil Tillage Res. 1999, 51, 121–128. [Google Scholar] [CrossRef]
- Argillier, J.F.; Audibertm, A.; Lecourtier, J.; Moan, M.; Rousseau, L. Solution and adsorption properties of hydrophobically associating water-soluble polyacrylamides. Colloids Surf. A 1996, 113, 247–257. [Google Scholar] [CrossRef]
- Bougas, K.; Corden, C.; Crookes, M.; Federici, G.; Fisk, P. EU Commission Scientific and Technical Support for the Development of Criteria to Identify and Group Polymers for Registration/Evaluation under REACH and Their Impact Assessment. Final Report. 2020. Available online: http://publications.europa.eu/resource/cellar/1cc811ff-d5fc-11ea-adf7-01aa75ed71a1.0001.01/DOC_1 (accessed on 1 June 2020).
- Groh, K.J.; Peter, H.; Arp, H.; MacLeod, M.; Wang, Z. Assessing and managing environmental hazards of polymers: Historical development, science advances and policy options. Environ. Sci. Process. Impacts 2023, 25, 10–25. [Google Scholar] [CrossRef]
- Nigro, L.; Magni, S.; Ortenzi, M.A.; Gazzotti, S.; Signorini, S.G.; Sbarberi, R.; Della Torre, C.; Binelli, A. Assessment of behavioural effects of three water-soluble polymers in zebrafish embryos. Sci. Total Environ. 2023, 893, 164843. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Protocols for Short Term Toxicity Screening of Hazardous Waste Sites; US Environmental Protection Agency: Washington, DC, USA, 2005.
- Available online: https://www.oecd.org/chemicalsafety/testing/seriesontestingandassessmentecotoxicitytesting.htm (accessed on 1 June 2020).
- Sobanska, M.; Scholz, S.; Nyman, A.-M.; Cesnaitis, R.; Gutierrez Alonso, S.; Klüver, N.; Kühne, R.; Tyle, H.; de Knecht, J.; Dang, I.; et al. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Protocols for Short Term Toxicity Screening of Hazardous Waste Sites; US Environmental Protection Agency: Washington, DC, USA, 1994.
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Li, G.; Ma, R.; Kong, Y.; Yuan, J. Selection of sensitive seeds for evaluation of compost maturity with the seed germination index. Waste Manag. 2021, 136, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Warman, P. Evaluation of seed germination and growth tests for assessing compost maturity compost. Sci. Util. 2013, 7, 33–37. [Google Scholar] [CrossRef]
- Pokorska-Niewiada, K.; Rajkowska-Mysliwiec, M.; Protasowicki, M. Acute lethal toxicity of heavy metals to the seeds of plants of high importance to humans, bulletin of environmental contamination and toxicology. Bull. Environ. Contam. Toxicol. 2018, 101, 222–228. [Google Scholar] [CrossRef]
- Ko, K.-S.; Han, J.; Kong Hum, I.C. Assessment of arsenite, arsenate, and chromate phytotoxicity based on the activity of seed germination and growth (root & shoot) of various plant seeds. Ecol. Risk Assess. 2013, 19, 742–753. [Google Scholar] [CrossRef]
- Di Salvatore, M.; Carafa, A.M.; Carratù, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.E.; García, J.F.; Peinado, F.M. Determination of phytotoxicity of soluble elements in soils, based on a bioassay with lettuce (Lactuca sativa L.). Sci. Total Environ. 2007, 378, 63–66. [Google Scholar] [CrossRef]
- Wieczerzak, M.; Kudłak, B.; Namiesnik, J. Impact of selected drugs and their binary mixtures on the germination of Sorghum bicolor (sorgo) seeds. Env. Sci. Pollut. Res. 2018, 25, 18717–18727. [Google Scholar] [CrossRef]
- Jin, C.; Chen, Q.; Sun, R.; Zhou, Q.; Liu, J. Eco-toxic effects of sulfadiazine sodium, sulfamonomethoxine sodium and enrofloxacin on wheat, Chinese cabbage and tomato. Ecotoxicology 2009, 18, 878–885. [Google Scholar] [CrossRef]
- Banks, M.K.; Schultz, K.E. Comparison of plants for germination toxicity tests in petroleum-contaminated soils. Water Air Soil Pollut. 2005, 167, 211–219. [Google Scholar] [CrossRef]
- Bettiol, C.; De Vettori, S.; Minervini, G.; Zuccon, E.; Marchetto, D.; Volpi Ghirardini, A.; Argese, E. Assessment of phenolic herbicide toxicity and mode of action by different assays. Environ. Sci. Pollut. Res. 2016, 23, 7398–7408. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Caesaria, P.U.; Rillig, M.C. Microplastics of different shapes increase seed germination synchrony while only films and fibers affect seed germination velocity. Front. Environ. Sci. 2022, 10, 1. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Effect of innovative bio-based plastics on early growth of higher plants. Polymers 2023, 15, 438. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Phytotoxicity assessment of biodegradable and non-biodegradable plastics using seed germination and early growth tests. Chemosphere 2022, 289, 133132. [Google Scholar] [CrossRef]
- Ferruti, P. Polyamidoamines: Past, present and perspectives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ferruti, P.; Domini, I.; Barbucci, R.; Beni, M.C. Heparin adsorbing capacities at physiological pH of three (polyamidoamine) resins, and of (polyamidoamine)-surface-grafted glass microspheres. Biomaterials 1983, 4, 218–221. [Google Scholar] [CrossRef]
- Ranucci, E.; Spagnoli, G.; Ferruti, P.; Sgouras, D.; Duncan, R. Poly(amidoamine)s with potential as drug carriers: Degradation and cellular toxicity. J. Biomater. Sci. Polym. Ed. 1991, 2, 303–315. [Google Scholar] [CrossRef]
- Ferruti, P.; Ranucci, E.; Sartore, L.; Bignotti, F.; Marchisio, M.A.; Bianciardi, P.; Veronese, F.M. Recent results on functional polymers and macromonomers of interest as biomaterials or for biomaterial modification. Biomaterials 1994, 15, 1235–1241. [Google Scholar] [CrossRef]
- Alongi, J.; Costantini, A.; Ferruti, P.; Ranucci, E. Evaluation of the eco-compatibility of polyamidoamines by means of seed germination test. Polym. Degrad. Stabil. 2022, 197, 109854. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free surface confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52–64. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Polyamidoamines derived from natural a-amino acids as effective flame retardants for cotton. Polymers 2021, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Lazzari, F.; Manfredi, A.; Alongi, J.; Mendichi, R.; Ganazzoli, F.; Raffaini, G.; Ferruti, P.; Ranucci, E. Self-structuring in water of polyamidoamino acids with hydrophobic side chains deriving from natural α-amino acids. Polymers 2018, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, prevailingly cationic L-arginine polymers of poly(amidoamino acid) structure: Synthesis, acid/base properties and preliminary cytocompatibility and cell-permeating characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Synergism between α-amino acid-derived polyamidoamines and sodium montmorillonite for enhancing the flame retardancy of cotton fabrics. Polym. Degrad. Stabil. 2024, 225, 110764. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Sulfur-based copolymeric polyamidoamines as efficient flame-retardants for cotton. Polymers 2019, 11, 1904. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Seed Germination/Root Elongation Toxicity Test; US Environmental Protection Agency: Washington, DC, USA, 1996.
- Harvey, D. Analytical Chemistry 2.1, 2nd ed.; McGraw-Hill Companies: New York, NY, USA, 2000. [Google Scholar]
- Newbold, P.; Carlson, W.L.; Thorne, B.M. Statistica; Pearson Editor: London, UK, 2007. [Google Scholar]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef]
- Ranucci, E.; Ferruti, P.; Lattanzio, E.; Manfredi, A.; Rossi, M.; Mussini, P.R.; Chiellini, F.; Bartoli, C. Acid-base properties of poly(amidoamine)s. J. Polym. Sci. A Polym. Chem. 2009, 47, 6977–6991. [Google Scholar] [CrossRef]
- Wolny, E.; Betekhtin, A.; Rojek, M.; Braszewska-Zalewska, A.; Lusinska, J.; Hasterok, R. Germination and the early stages of seedling development in brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 2916. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Tintelnot, S.; Leubner-Metzger, G. Endosperm-limited brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of lepidium sativum (cress) and endosperm rupture of cress and arabidopsis thaliana. Plant Cell. Physiol. 2006, 47, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bykova, N.A.; Igamberdiev, A.U. Cell signaling mechanisms and metabolic regulation of germination and dormancyin barley seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Linder, G.; Greene, J.; Ratsch, H.; Nwosu, J.; Smith, S.; Wilborn, D. Seed Germination and Root Elongation Toxicity Tests in Hazardous Waste Site Evaluation: Methods Development and Applications; EPA/600/D-89/109 (NTIS PB90113184); US Environmental Protection Agency: Washington, DC, USA, 1989.
- Alaguprathana, M.; Poonkothai, M.; Al-Ansari, M.M.; Al-Humaid, L.; Kim, W. Cytogenotoxicity assessment in Allium cepa roots exposed to methyl orange treated with Oedogonium subplagiostomum AP1. Environ. Res. 2022, 213, 113612. [Google Scholar] [CrossRef]
- Duncan, R.; Ferruti, P.; Sgouras, D.; Tuboku-Metzger, A.; Ranucci, E.; Bignotti, F. A Polymer-Triton X-100 conjugate capable of pH-dependent red blood cell lysis: A model system illustrating the possibility of drug delivery within acidic intracellular compartments. J. Drug Target. 1994, 2, 341–347. [Google Scholar] [CrossRef]
- Kuboi, T.; Fujii, K. Toxicity of cationic polymer flocculants to higher plants. Soil Sci. Plant Nutr. 1985, 31, 163–173. [Google Scholar] [CrossRef]
- Brun Hansen, A.M.; Brill, J.L.; Connors, K.A.; Belanger, S.E.; Baun, A.; Sanderson, H. Understanding ecotoxicological drivers and responses of freshwater green algae, Raphidocelis subcapitata, to cationic polyquaternium polymers. Environ. Res. 2023, 231, 116282. [Google Scholar] [CrossRef]
- Sobarzo-Bernal, O.; Gómez-Merino, F.C.; Alcántar-González, G.; Saucedo-Veloz, C.; Trejo-Téllez, L.I. Biostimulant effects of cerium on seed germination and initial growth of tomato seedlings. Agronomy 2021, 11, 1525. [Google Scholar] [CrossRef]
- Keerthi Sahasa, R.G.; Dhevagi, P.; Poornima, R.; Ramya, A.; Moorthy, P.S.; Alagirisamy, B.; Karthikeyan, S. Effect of polyethylene microplastics on seed germination of Blackgram (Vigna mungo L.) and Tomato (Solanum lycopersicum L.). Environ. Adv. 2023, 11, 100349. [Google Scholar] [CrossRef]
- Shinde, R.; Kumar Sarkar, P.; Thombare, N. Soil Conditioners. Agric. Food 2019, 1, 1–5. [Google Scholar]

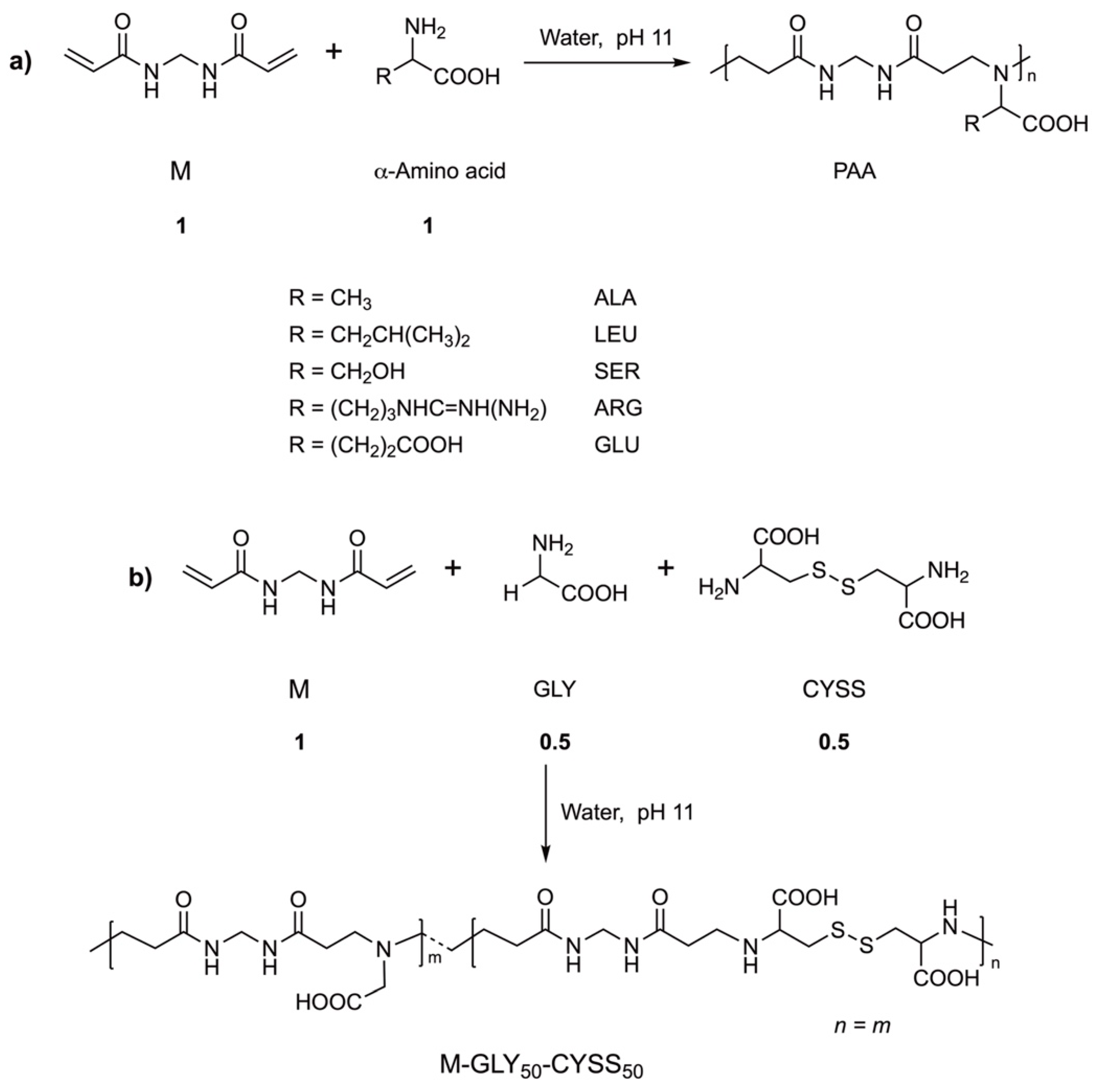

| PAA (a) | α-Amino Acid | α-Amino Acid (g; mmol) | LiOH·H2O (g; mmol) | Time (Days) |
|---|---|---|---|---|
| M-ALA [39] | alanine | 2.90; 32.23 | 1.38; 32.23 | 5 |
| M-LEU | leucine | 4.25; 32.07 | 1.37; 32.00 | 10 |
| M-SER | serine | 3.41; 32.12 | 1.38; 32.23 | 5 |
| M-ARG [40] | arginine | 5.65; 32.11 | - | 9 |
| M-GLU [41] | glutamic acid | 4.83; 32.17 | 2.74; 64.00 | 9 |
| M-GLY50-CYSS50 [42] (b) | glycine | 1.23; 16.06 | 4.12; 96.22 | 2 |
| cystine | 3.89; 16.03 |
| PAA (a) | pKa Values | IP (b) | Net Charge at pH 7 (c) | Positive/Negative Charge (d) |
|---|---|---|---|---|
| M-ALA [39] | pKa-COOH = 2.12 pKa-NR3 = 8.13 | 5.1 | −0.09 | 0.91 |
| M-LEU [39] | pKa-COOH = 2.11 pKa-NR3 = 7.37 | 4.8 | −0.34 | 0.66 |
| M-SER | pKa-COOH = 2.14 pKa-NR3 = 7.08 | 4.6 | −0.38 | 0.62 |
| M-ARG [40] | pKa-COOH = 2.2 pKa-NR3 = 6.4 pKa-guanidine > 10 | 9.7 | +0.29 | 1.29 |
| M-GLU [41] | pKa-COOH,1 = 2.32 pKa-COOH,2 = 4.28 pKa-NR3 = 7.78 | 3.3 | −1.14 | 0.43 |
| M-GLY50-CYSS50 | - | 4.9 | −0.35 | 0.72 |
| M-GLY [36] | pKa-COOH = 1.9 pKa-NR3 = 7.7 | 4.8 | −0.39 | 0.61 |
| M-CYSS [47] | pKa-NR3,1 = 2.4 pKa-NR3,2 = 4.0 pKa-COOH,1 = 8.2 pKa-COOH,2 = 12.7 | 5.0 | −0.31 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranucci, E.; Treccani, S.; Ferruti, P.; Alongi, J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers 2024, 16, 1744. https://doi.org/10.3390/polym16121744
Ranucci E, Treccani S, Ferruti P, Alongi J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers. 2024; 16(12):1744. https://doi.org/10.3390/polym16121744
Chicago/Turabian StyleRanucci, Elisabetta, Sofia Treccani, Paolo Ferruti, and Jenny Alongi. 2024. "The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines" Polymers 16, no. 12: 1744. https://doi.org/10.3390/polym16121744
APA StyleRanucci, E., Treccani, S., Ferruti, P., & Alongi, J. (2024). The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers, 16(12), 1744. https://doi.org/10.3390/polym16121744








