Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Conditions of Immersion
2.2. Design of Experiment
2.3. Accelerated Ageing Tests of GFRP Pipes
2.4. Thermogravimetric Analysis (TGA)
2.5. Fourier Transform Infrared Spectrometer (FTIR)
2.6. X-ray Diffraction (XRD)
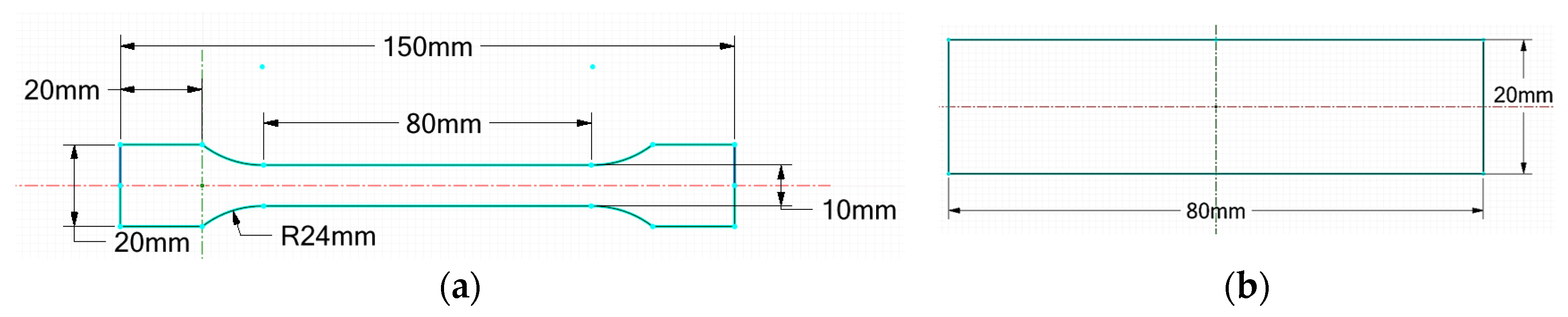
2.7. Mechanical Tests
- (a)
- Tensile test
- (b)
- Bending test
3. Results and Discussion
3.1. Moisture Absortion
3.2. Mechanical Properties
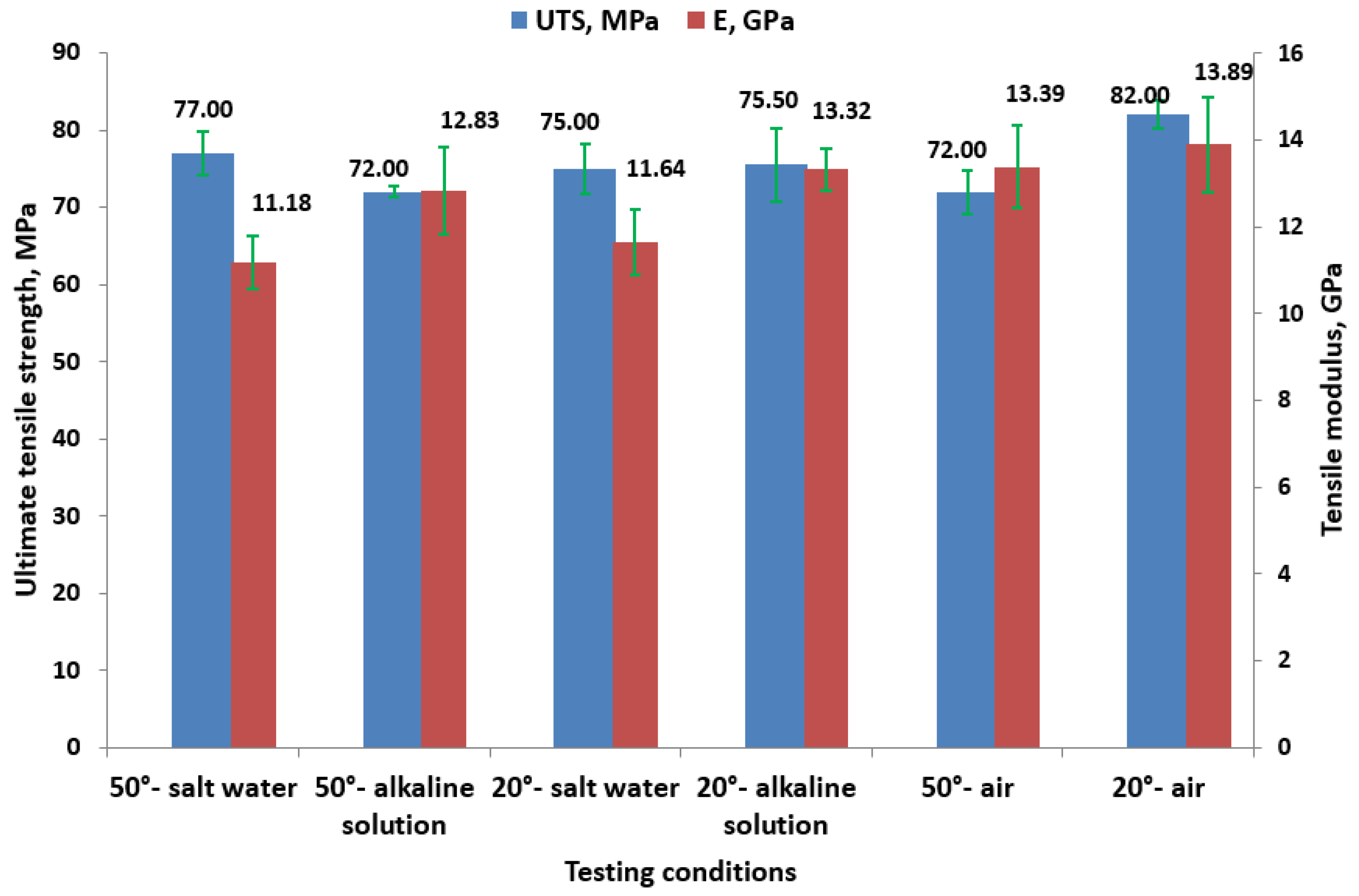
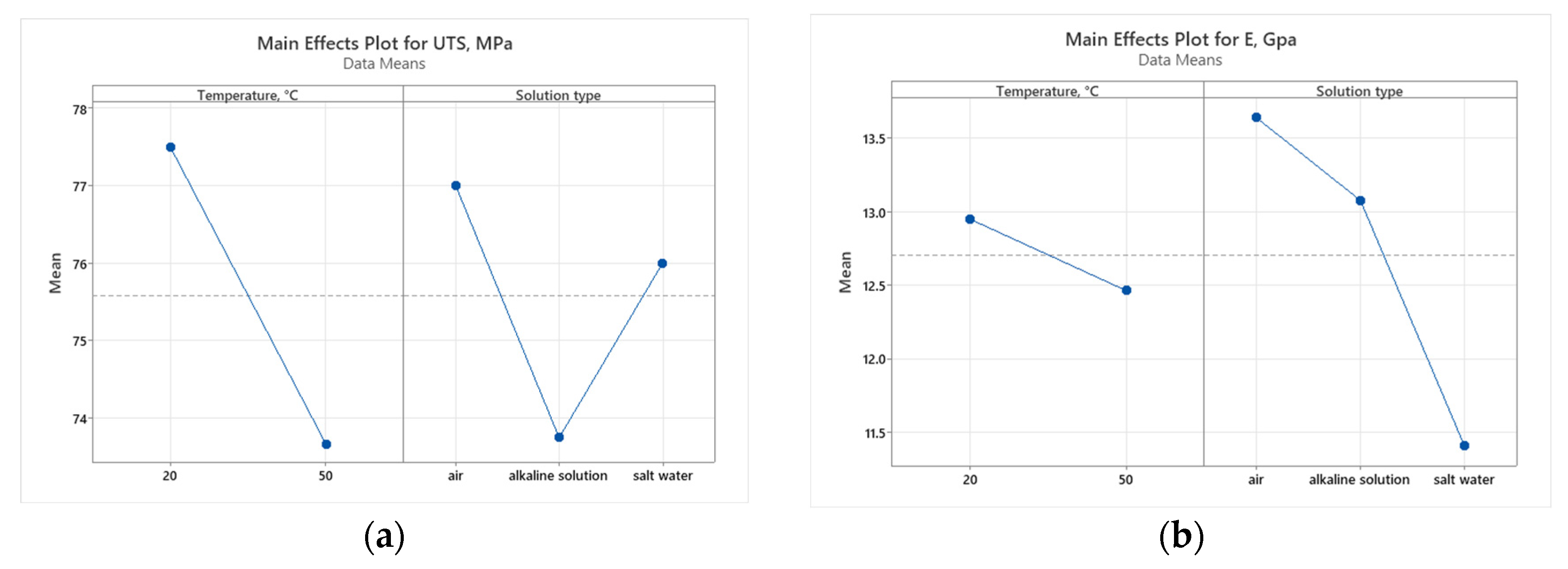
3.2.1. Tensile Results
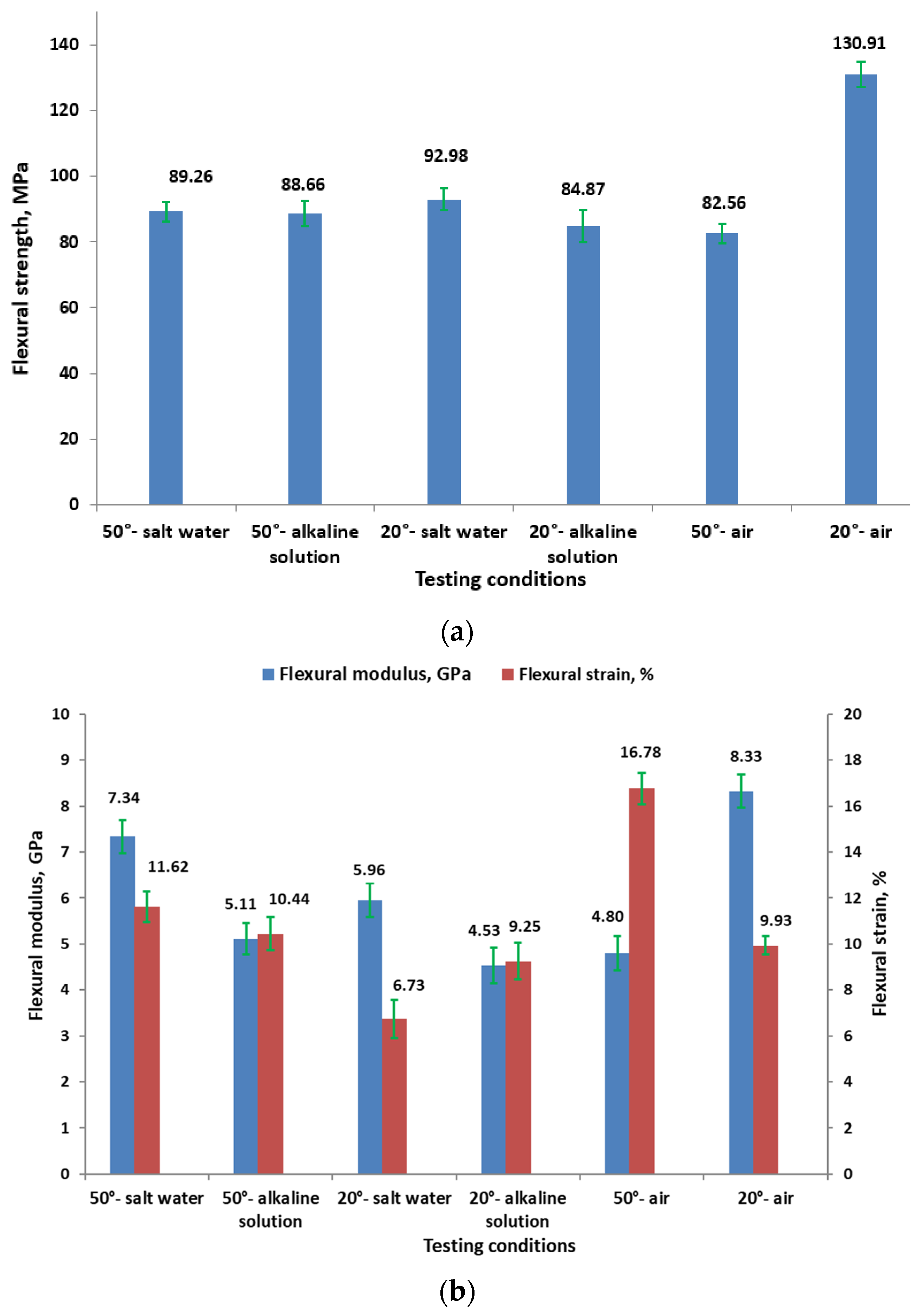
3.2.2. Flexural Results
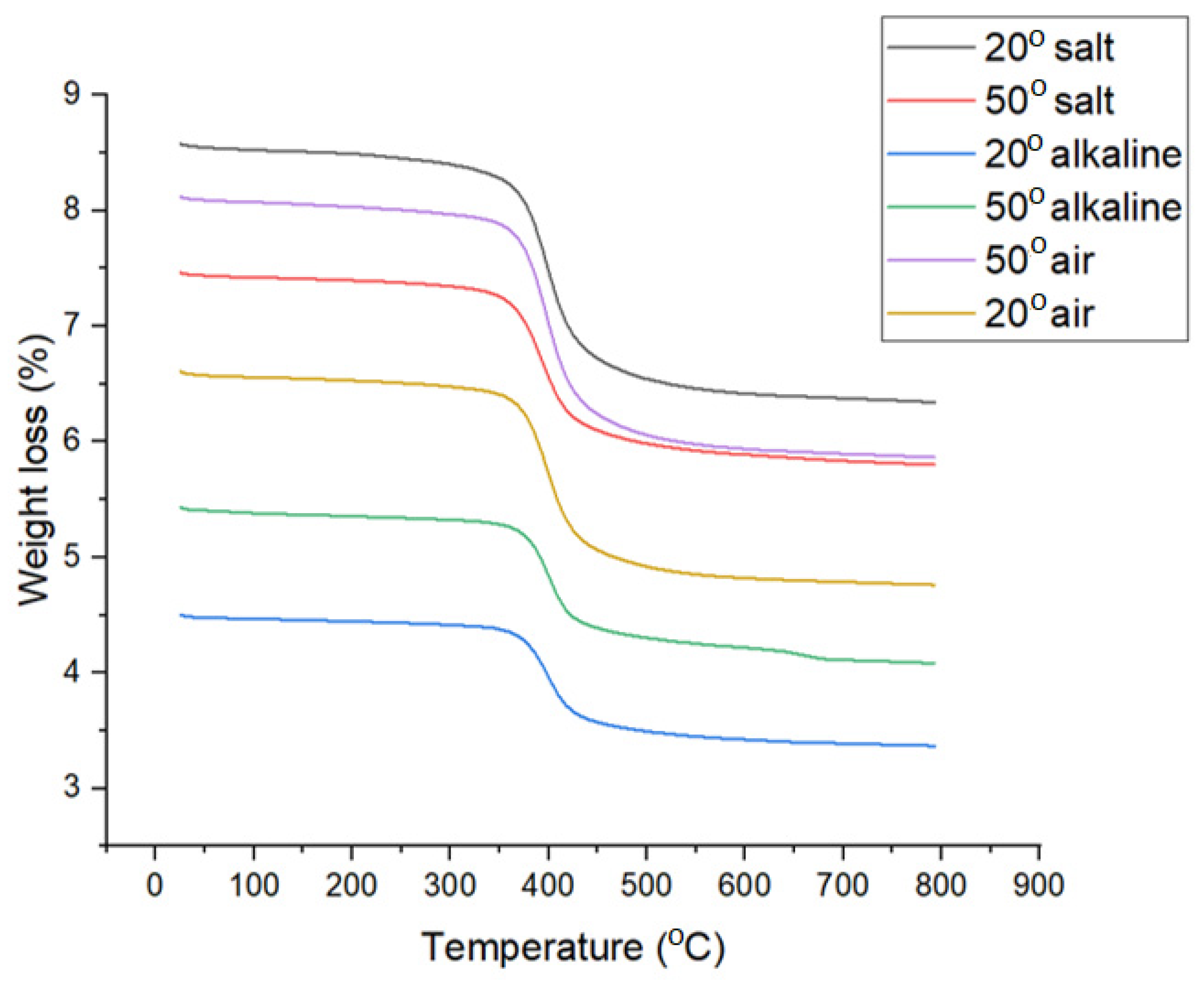
3.3. TGA Analysis
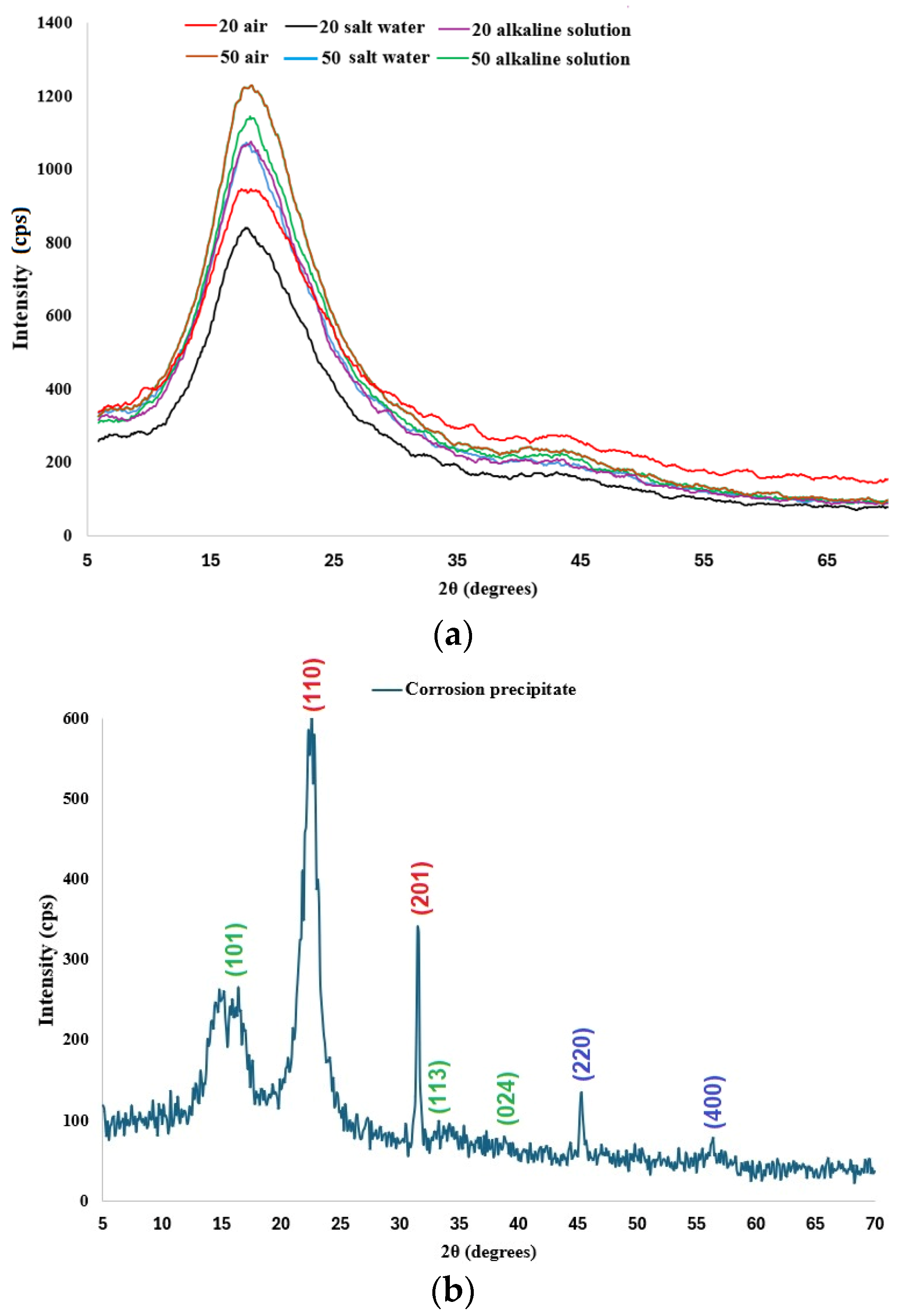
3.4. XRD Analysis
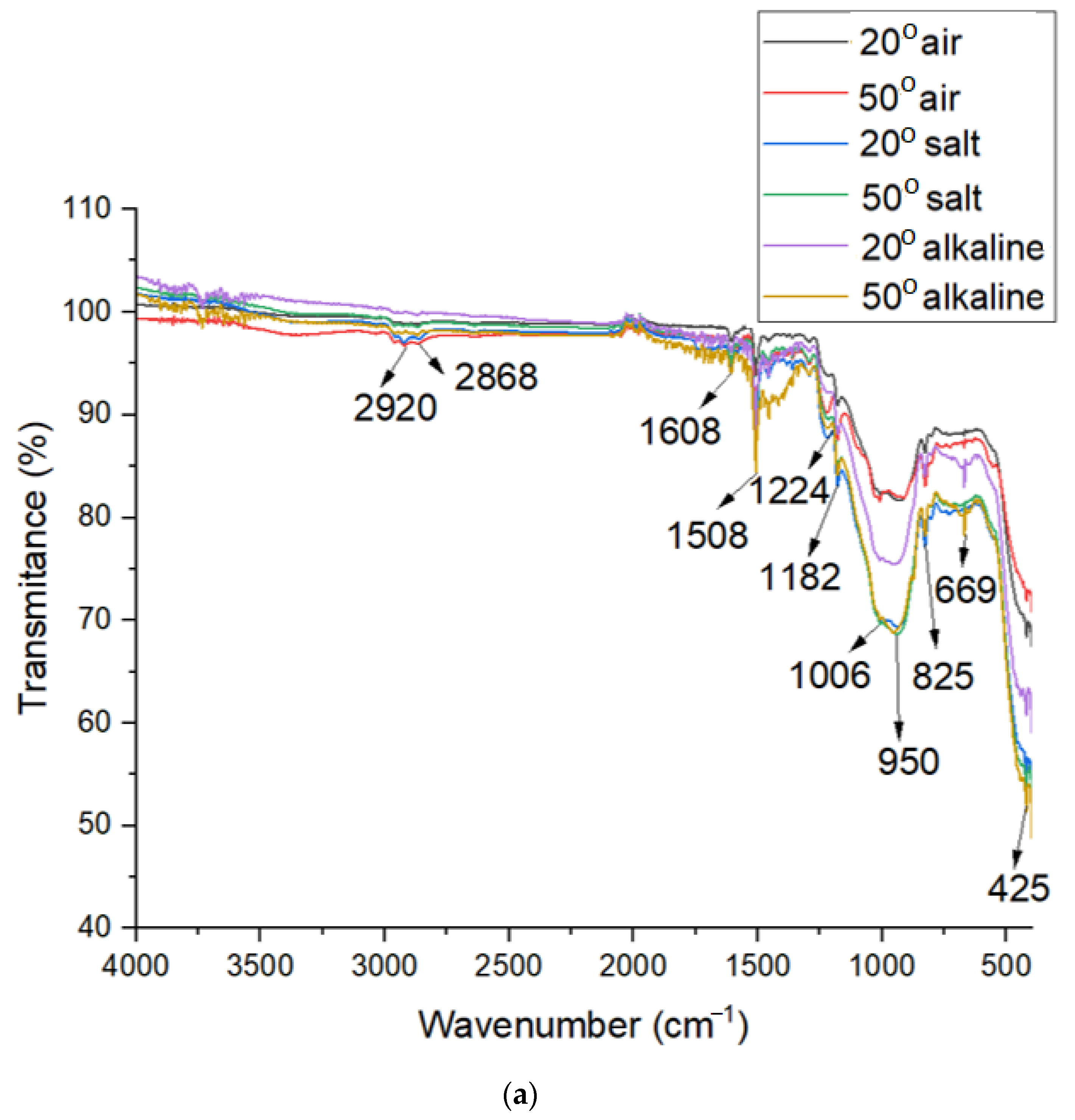
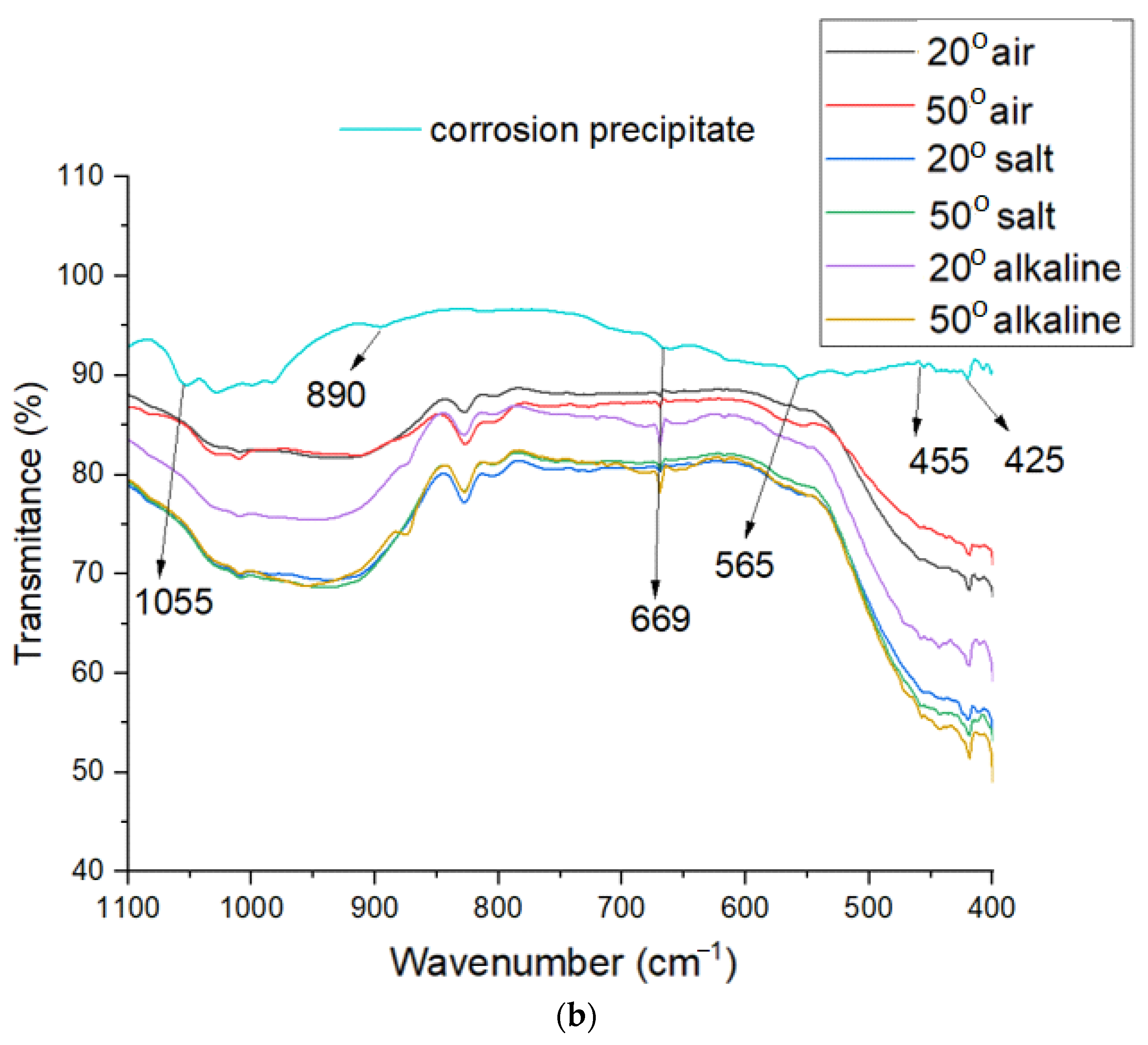
3.5. FTIR Analysis
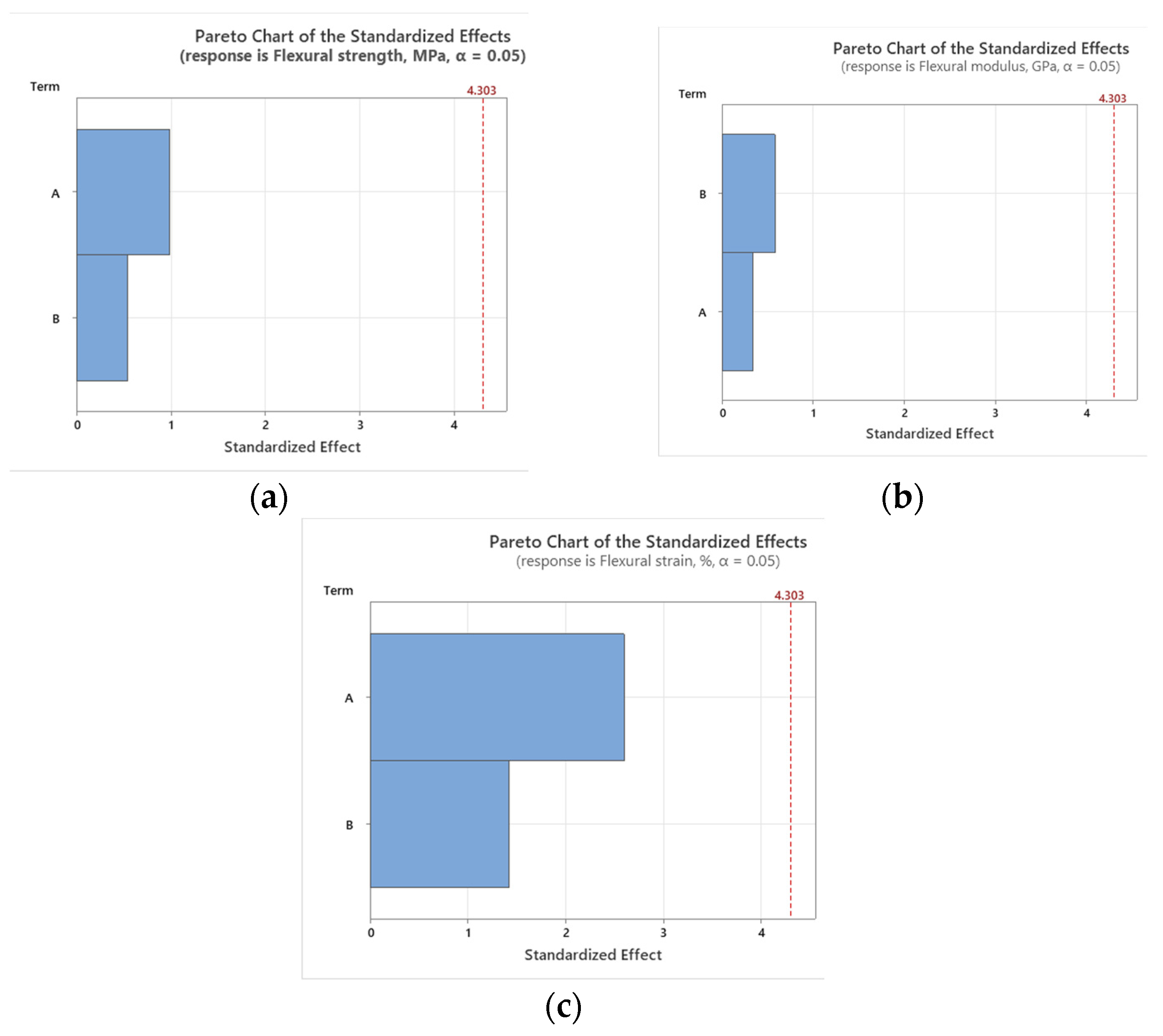
3.6. Statistical Analysis
4. Conclusions
- ➢
- The impact on the diffusion coefficient: High temperatures and alkaline solutions significantly increase the diffusion coefficient in GFRP pipes. This accelerated diffusion can lead to substantial changes in material properties over time, potentially compromising their performance.
- ➢
- The effect on tensile properties: Exposure to higher temperatures (50 °C) and alkaline solutions results in a reduction in UTS, indicating the decreased strength of GFRP pipes under these conditions. Despite this reduction, the tensile modulus remains relatively high, suggesting that the material retains its stiffness, which is crucial for load-bearing applications.
- ➢
- The effect on flexural properties:
- ✓
- Higher temperatures and alkaline solutions significantly reduce the flexural strength of GFRP pipes.
- ✓
- Higher temperatures (50 °C) reduce the flexural modulus, indicating decreased stiffness, but also increase the flexural strain, suggesting increased flexibility.
- ✓
- Alkaline solutions degrade the flexural properties more significantly than salt water.
- ➢
- The primary influencing factors:
- ✓
- Temperature is the primary factor affecting UTS, flexural strength, and flexural strain, highlighting the critical role of thermal conditions in determining the mechanical performance of GFRP composites.
- ✓
- The solution type, particularly in alkaline environments, mainly influences the tensile and flexural modulus, underscoring the importance of chemical resistance for the long-term performance of GFRP materials.
- ➢
- Behavior in salt water: For samples immersed in salt water at 50 °C, the flexural modulus is higher (7.34 GPa) compared to other 50 °C conditions, with moderate strain (11.62%). This behavior could be explained by the formation of a corrosive precipitate, as shown by XRD and FT-IR analyses.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morampudi, P.; Namala, K.K.; Gajjela, Y.K.; Barath, M.; Prudhvi, G. Review on Glass Fiber Reinforced Polymer Composites. Mater. Today Proc. 2021, 43, 314–319. [Google Scholar] [CrossRef]
- Rao, P.S. Effect of Hydrothermal Ageing on Glass Fibre Reinforced Plastic (GFRP) Composite Laminates Exposed to Water and Salt Water. Int. J. Curr. Engg. Technol 2013, 2, 47–53. [Google Scholar] [CrossRef]
- Mouallif, I.; Latrach, A.; Chergui, M.; Benali, A.; Elghorba, M.; Mouallif, Z.; Hangouët, J.-P.; Barbe, N. Degradation of Dynamic Mechanical Properties of Glass Fiber Reinforced Polyester Composite Pipes after Immersion in Various Temperatures. J. Compos. Mater. 2014, 48, 3025–3034. [Google Scholar] [CrossRef]
- Mayandi, K.; Rajini, N.; Ayrilmis, N.; Indira Devi, M.P.; Siengchin, S.; Mohammad, F.; Al-Lohedan, H.A. An Overview of Endurance and Ageing Performance under Various Environmental Conditions of Hybrid Polymer Composites. J. Mater. Res. Technol. 2020, 9, 15962–15988. [Google Scholar] [CrossRef]
- Stamenović, M.; Putić, S.; Rakin, M.; Medjo, B.; Čikara, D. Effect of Alkaline and Acidic Solutions on the Tensile Properties of Glass–Polyester Pipes. Mater. Des. 2011, 32, 2456–2461. [Google Scholar] [CrossRef]
- Fitriah, S.N.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Daud, R.; Gibson, A.G.; Assaleh, T.A. Influence of Hydrothermal Ageing on the Compressive Behaviour of Glass Fibre/Epoxy Composite Pipes. Compos. Struct. 2017, 159, 350–360. [Google Scholar] [CrossRef]
- Carra, G.; Carvelli, V. Ageing of Pultruded Glass Fibre Reinforced Polymer Composites Exposed to Combined Environmental Agents. Compos. Struct. 2014, 108, 1019–1026. [Google Scholar] [CrossRef]
- Shreepannaga; Vijaya Kini, M.; Pai, D. The Ageing Effect on Static and Dynamic Mechanical Properties of Fibre Reinforced Polymer Composites under Marine Environment- A Review. Mater. Today Proc. 2022, 52, 689–696. [Google Scholar] [CrossRef]
- Sepetcioglu, H.; Gunoz, A.; Kara, M. Effect of Hydrothermal Ageing on the Mechanical Behaviour of Graphene Nanoplatelets Reinforced Basalt Fibre Epoxy Composite Pipes. Polym. Polym. Compos. 2021, 29, S166–S177. [Google Scholar] [CrossRef]
- Muralidharan, M.; Sathishkumar, T.P.; Rajini, N.; Navaneethakrishnan, P.; Arun Kumar, S.; Ismail, S.O.; Senthilkumar, K.; Siengchin, S. Evaluation of Tensile Strength Retention and Service Life Prediction of Hydrothermal Aged Balanced Orthotropic Carbon/Glass and Kevlar/Glass Fabric Reinforced Polymer Hybrid Composites. J. Appl. Polym. Sci. 2022, 139, 51602. [Google Scholar] [CrossRef]
- Silva, M.A.G.; Da Fonseca, B.S.; Biscaia, H. On Estimates of Durability of FRP Based on Accelerated Tests. Compos. Struct. 2014, 116, 377–387. [Google Scholar] [CrossRef]
- Liao, D.; Gu, T.; Liu, J.; Chen, S.; Zhao, F.; Len, S.; Dou, J.; Qian, X.; Wang, J. Degradation Behavior and Ageing Mechanism of E-Glass Fiber Reinforced Epoxy Resin Composite Pipes under Accelerated Thermal Ageing Conditions. Compos. Part B Eng. 2024, 270, 111131. [Google Scholar] [CrossRef]
- Mahmoud, M.K.; Tantawi, S.H. Effect of Strong Acids on Mechanical Properties of Glass/Polyester GRP Pipe at Normal and High Temperatures. Polym.-Plast. Technol. Eng. 2003, 42, 677–688. [Google Scholar] [CrossRef]
- Calabrese, L.; Fiore, V. A Simplified Predictive Approach to Assess the Mechanical Behavior of Pinned Hybrid Composites Aged in Salt-Fog Environment. Compos. Struct. 2020, 249, 112589. [Google Scholar] [CrossRef]
- Tu, J.; Xie, H.; Gao, K.; Li, Z.; Zhang, J. Durability Prediction of GFRP Rebar Based on Elastic Modulus Degradation. Front. Mater. 2019, 6, 258. [Google Scholar] [CrossRef]
- Miyano, Y.; Nakada, M.; Sekine, N. Accelerated Testing for Long-Term Durability of FRP Laminates for Marine Use. J. Compos. Mater. 2005, 39, 5–20. [Google Scholar] [CrossRef]
- Sunny, J.; Nazaripoor, H.; Palacios Moreno, J.; Mertiny, P. Accelerated Zero-Stress Hydrothermal Aging of Dry E-Glass Fibers and Service Life Prediction Using Arrhenius Model. Fibers 2023, 11, 70. [Google Scholar] [CrossRef]
- Nakayama, M.; Hosokawa, Y.; Muraoka, Y.; Katayama, T. Life Prediction under Sulfuric Acid Environment of FRP Using X-Ray Analysis Microscope. J. Mater. Process. Technol. 2004, 155–156, 1558–1563. [Google Scholar] [CrossRef]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Castilho, E. Influence of Elevated Temperatures on the Mechanical Properties of Glass Fibre Reinforced Polymer Laminates Produced by Vacuum Infusion. Constr. Build. Mater. 2022, 345, 128340. [Google Scholar] [CrossRef]
- ASTM D3039; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D790-10; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- CSA S806-12 (R2017); Design and Construction of Building Components with Fiber-Reinforced Polymers. Canadian Standards Association: Toronto, ON, Canada, 2017.
- ASTM D5229; Standard Test Method for Moisture Absorption Properties and Equilibrium Conditioning of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- Portoacă, A.-I.; Diniță, A.; Tănase, M.; Săvulescu, A.; Sirbu, E.-E.; Călin, C.; Brănoiu, G. Analyzing Sustainable 3D Printing Processes: Mechanical, Thermal, and Crystallographic Insights. Polymers 2024, 16, 1364. [Google Scholar] [CrossRef]
- Guermazi, N.; Ben Tarjem, A.; Ksouri, I.; Ayedi, H.F. On the Durability of FRP Composites for Aircraft Structures in Hygrothermal Conditioning. Compos. Part B Eng. 2016, 85, 294–304. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; reprint; Clarendon Press: Oxford, UK, 1976; ISBN 978-0-19-853344-3. [Google Scholar]
- Hajmoosa, A.; Mahmoudi, M.; Ebrahimzadeh, M.; Shakiba, M.; Bazli, M. Tensile Strength Retention of Glass Fibre-Reinforced Stirrups Subjected to Aggressive Solutions: Effect of Environmental Condition, Stirrup Shape and Stirrup Diameter. Mater. Struct. 2024, 57, 31. [Google Scholar] [CrossRef]
- Qiao, Y.; Das, O.; Zhao, S.-N.; Sun, T.-S.; Xu, Q.; Jiang, L. Pyrolysis Kinetic Study and Reaction Mechanism of Epoxy Glass Fiber Reinforced Plastic by Thermogravimetric Analyzer (TG) and TG–FTIR (Fourier-Transform Infrared) Techniques. Polymers 2020, 12, 2739. [Google Scholar] [CrossRef] [PubMed]
- Azhary, T.; Kusmono; Wildan, M.W. Herianto Mechanical, Morphological, and Thermal Characteristics of Epoxy/Glass Fiber/Cellulose Nanofiber Hybrid Composites. Polym. Test. 2022, 110, 107560. [Google Scholar] [CrossRef]
- Guo, F.; Al-Saadi, S.; Singh Raman, R.K.; Zhao, X. Durability of Fibre Reinforced Polymers in Exposure to Dual Environment of Seawater Sea Sand Concrete and Seawater. Materials 2022, 15, 4967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, L.; Cui, X.; Guo, W. Crystallization Behavior and Properties of Glass Fiber Reinforced Polypropylene Composites. Polymers 2019, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, F.; Shi, H.; Han, E.; Wang, Z. The Influence of Ultra-Fine Glass Fibers on the Mechanical and Anticorrosion Properties of Epoxy Coatings. Prog. Org. Coat. 2011, 71, 188–197. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.I.; García-Díaz, I.; Rodríguez, O.; Alguacil, F.J.; Romero, M. Recycling of Glass Fibers from Fiberglass Polyester Waste Composite for the Manufacture of Glass-Ceramic Materials. JEP 2012, 03, 740–747. [Google Scholar] [CrossRef]
- Kanny, K.; Mohan, T.P. Resin Infusion Analysis of Nanoclay Filled Glass Fiber Laminates. Compos. Part B Eng. 2014, 58, 328–334. [Google Scholar] [CrossRef]
- Quaresimin, M.; Salviato, M.; Zappalorto, M. Fracture and Interlaminar Properties of Clay-Modified Epoxies and Their Glass Reinforced Laminates. Eng. Fract. Mech. 2012, 81, 80–93. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and Mechanical Properties of Polymer Nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Adekomaya, O.; Adediran, A.A.; Adama, K. Characterization and Morphological Properties of Glass Fiber Reinforced Epoxy Composites Fabricated under Varying Degrees of Hand Lay-up Techniques. J. Appl. Sci. Environ. Manag. 2018, 22, 110. [Google Scholar] [CrossRef][Green Version]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; International Union of Crystallography: Chester, UK, 1995; ISBN 0-19-855912-7. [Google Scholar]
- Rubio Arias, J.J.; Thielemans, W. Efficient Depolymerization of Glass Fiber Reinforced PET Composites. Polymers 2022, 14, 5171. [Google Scholar] [CrossRef] [PubMed]
- Frihi, D.; Layachi, A.; Gherib, S.; Stoclet, G.; Masenelli-Varlot, K.; Satha, H.; Seguela, R. Crystallization of Glass-Fiber-Reinforced Polyamide 66 Composites: Influence of Glass-Fiber Content and Cooling Rate. Compos. Sci. Technol. 2016, 130, 70–77. [Google Scholar] [CrossRef]
- Badogu, K.; Kumar, R.; Kumar, R. 3D Printing of Glass Fiber-Reinforced Polymeric Composites: A Review. J. Inst. Eng. India Ser. C 2022, 103, 1285–1301. [Google Scholar] [CrossRef]
- Arvanitopoulos, C.D.; Koenig, J.L. FT-IR Microspectroscopic Investigation of the Interphase of Epoxy Resin-Glass Fiber-Reinforced Composites. Appl. Spectrosc. 1996, 50, 1–10. [Google Scholar] [CrossRef]
- Demircan, G. Structural Integrity of Glass Fiber Reinforced Nanocomposites under Hydrothermal Aging for Offshore Structure Applications. Appl. Ocean Res. 2024, 146, 103959. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface Modification of Fe3O4@SiO2 Microsphere by Silane Coupling Agent. Int Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Jafari, A.; Zandipak, R.; Meidanchi, A. Removal of Heavy Metal (Hg(II) and Cr(VI)) Ions from Aqueous Solutions Using Fe2O3@SiO2 Thin Films as a Novel Adsorbent. Process Saf. Environ. Prot. 2018, 120, 348–357. [Google Scholar] [CrossRef]
- Tefera, G.; Adali, S.; Bright, G. Mechanical Behavior of GFRP Laminates Exposed to Thermal and Moist Environmental Conditions: Experimental and Model Assessment. Polymers 2022, 14, 1523. [Google Scholar] [CrossRef] [PubMed]







| Test No. | Temperature, °C | Environment |
|---|---|---|
| 1 | 50 | salt water |
| 2 | 50 | alkaline solution |
| 3 | 20 | salt water |
| 4 | 20 | alkaline solution |
| 5 | 50 | air |
| 6 | 20 | air |
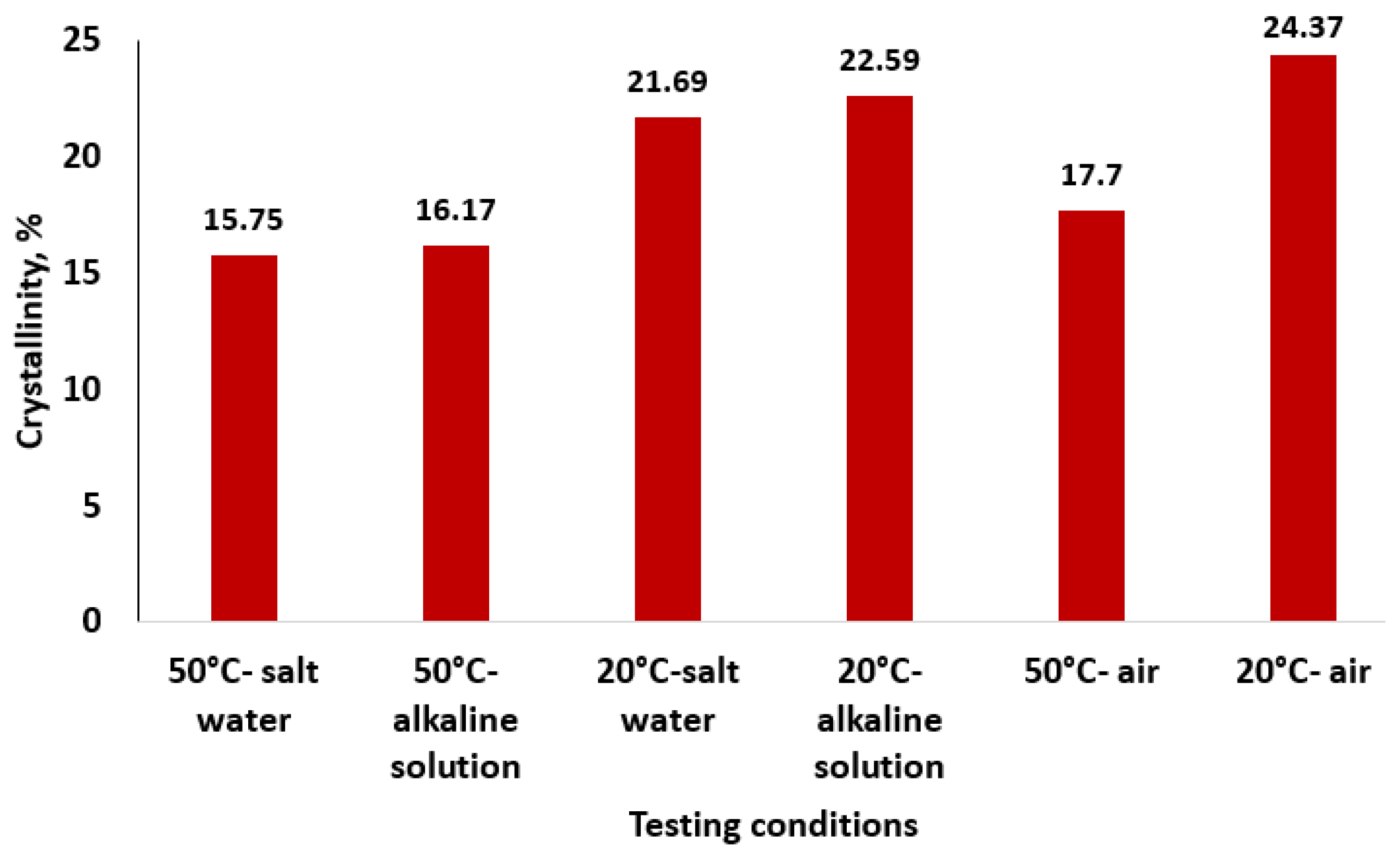
| Testing Conditions (Temperature/Immersion Solution) | Ac | Aam | Xc, % |
|---|---|---|---|
| 50 °C/salt water | 82.017 | 438.568 | 15.75 |
| 50 °C/alkaline solution | 111.738 | 579.122 | 16.17 |
| 20 °C/salt water | 66.004 | 238.198 | 21.69 |
| 20 °C/alkaline solution | 98.663 | 338.129 | 22.59 |
| 50 °C/air | 144.852 | 673.379 | 17.70 |
| 20 °C/air | 106.134 | 329.407 | 24.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călin, C.; Diniță, A.; Brănoiu, G.; Popovici, D.R.; Tănase, M.; Sirbu, E.-E.; Portoacă, A.-I.; Mihai, S. Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties. Polymers 2024, 16, 1779. https://doi.org/10.3390/polym16131779
Călin C, Diniță A, Brănoiu G, Popovici DR, Tănase M, Sirbu E-E, Portoacă A-I, Mihai S. Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties. Polymers. 2024; 16(13):1779. https://doi.org/10.3390/polym16131779
Chicago/Turabian StyleCălin, Cătălina, Alin Diniță, Gheorghe Brănoiu, Daniela Roxana Popovici, Maria Tănase, Elena-Emilia Sirbu, Alexandra-Ileana Portoacă, and Sonia Mihai. 2024. "Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties" Polymers 16, no. 13: 1779. https://doi.org/10.3390/polym16131779
APA StyleCălin, C., Diniță, A., Brănoiu, G., Popovici, D. R., Tănase, M., Sirbu, E.-E., Portoacă, A.-I., & Mihai, S. (2024). Assessment of Environmental Impact on Glass-Fiber-Reinforced Polymer Pipes Mechanical and Thermal Properties. Polymers, 16(13), 1779. https://doi.org/10.3390/polym16131779








