Prolonged Lifespan of Superhydrophobic Thin Films and Coatings Using Recycled Polyethylene
Abstract
:1. Introduction
2. Experimental
3. Result and Discussion
3.1. CA

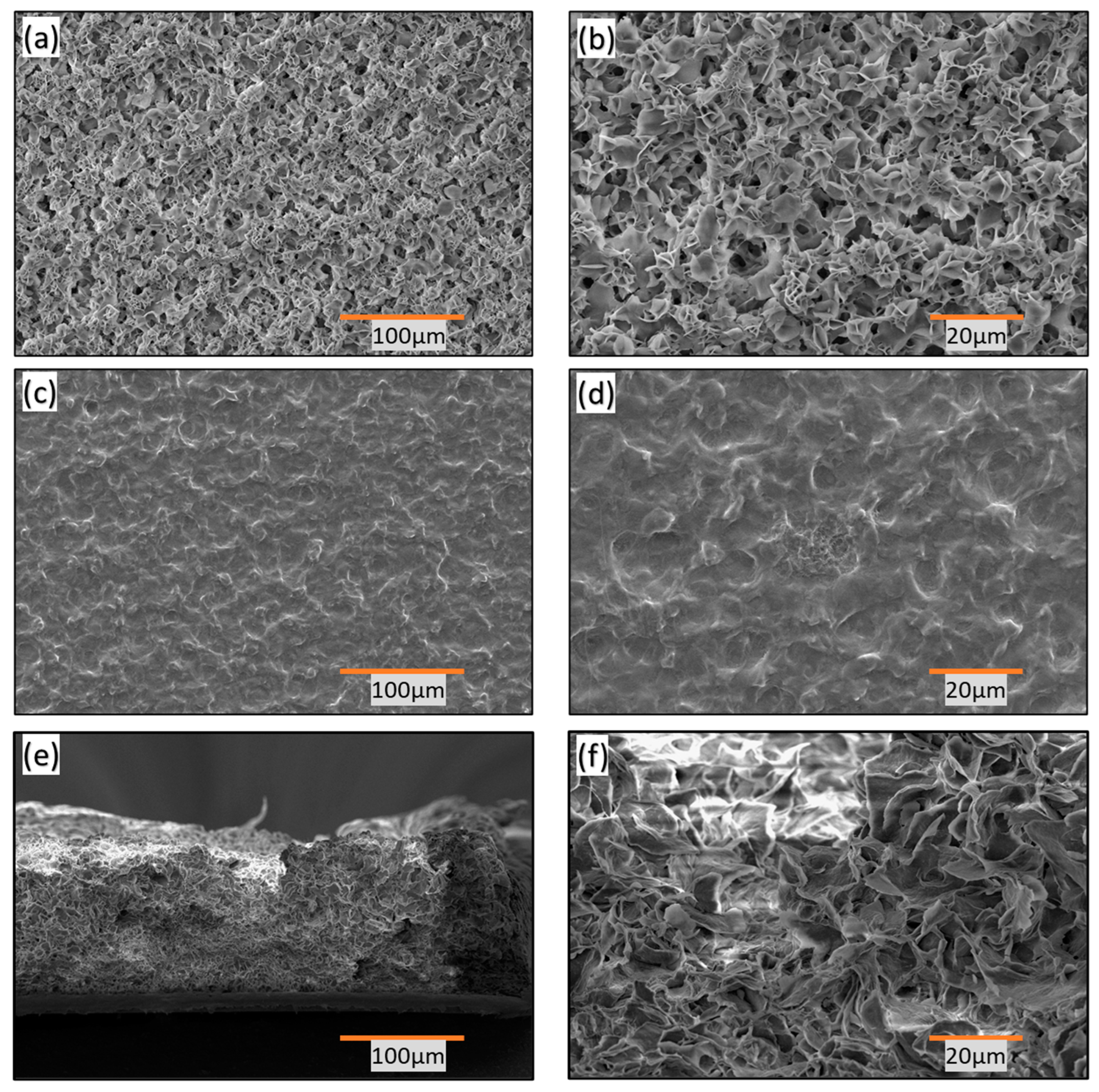
3.2. Surface Morphology
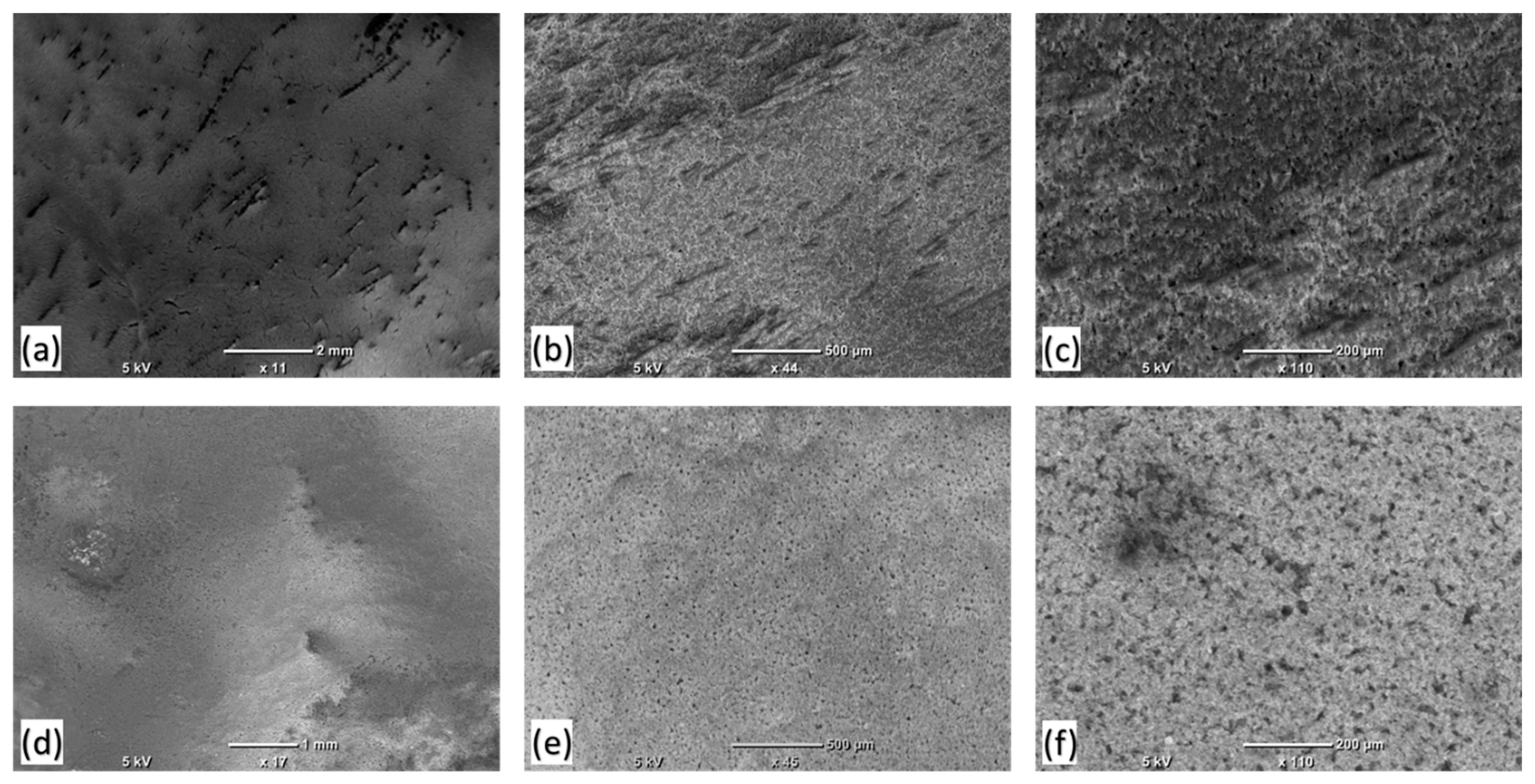
3.3. Weathering Test
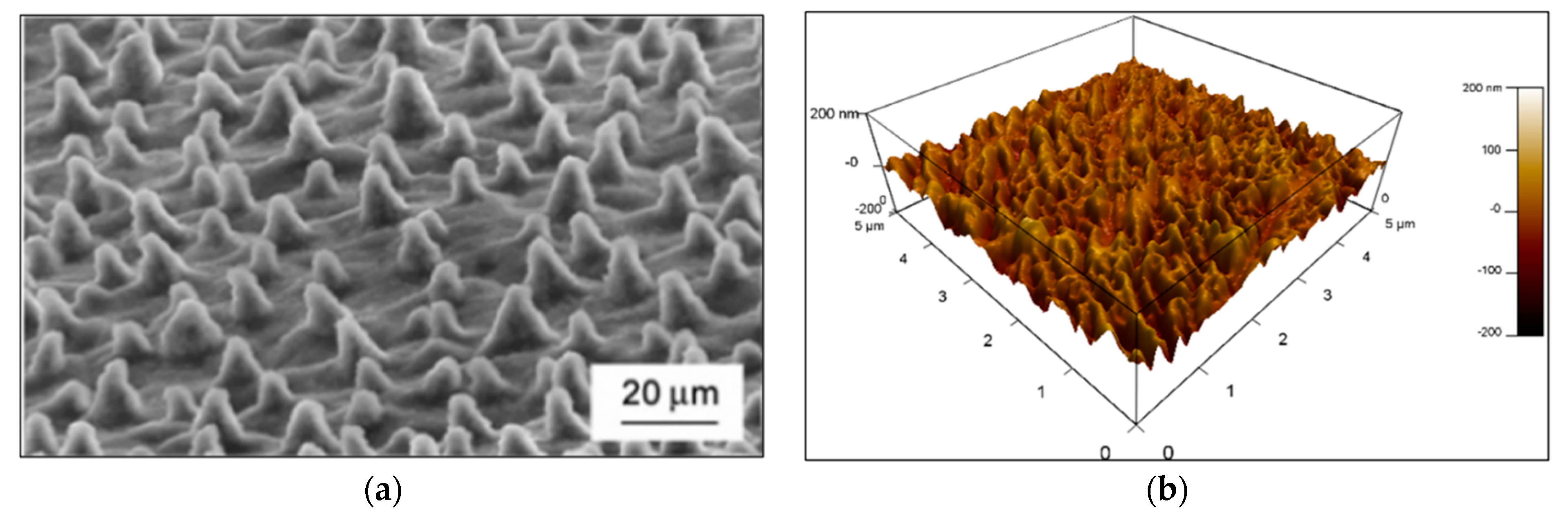
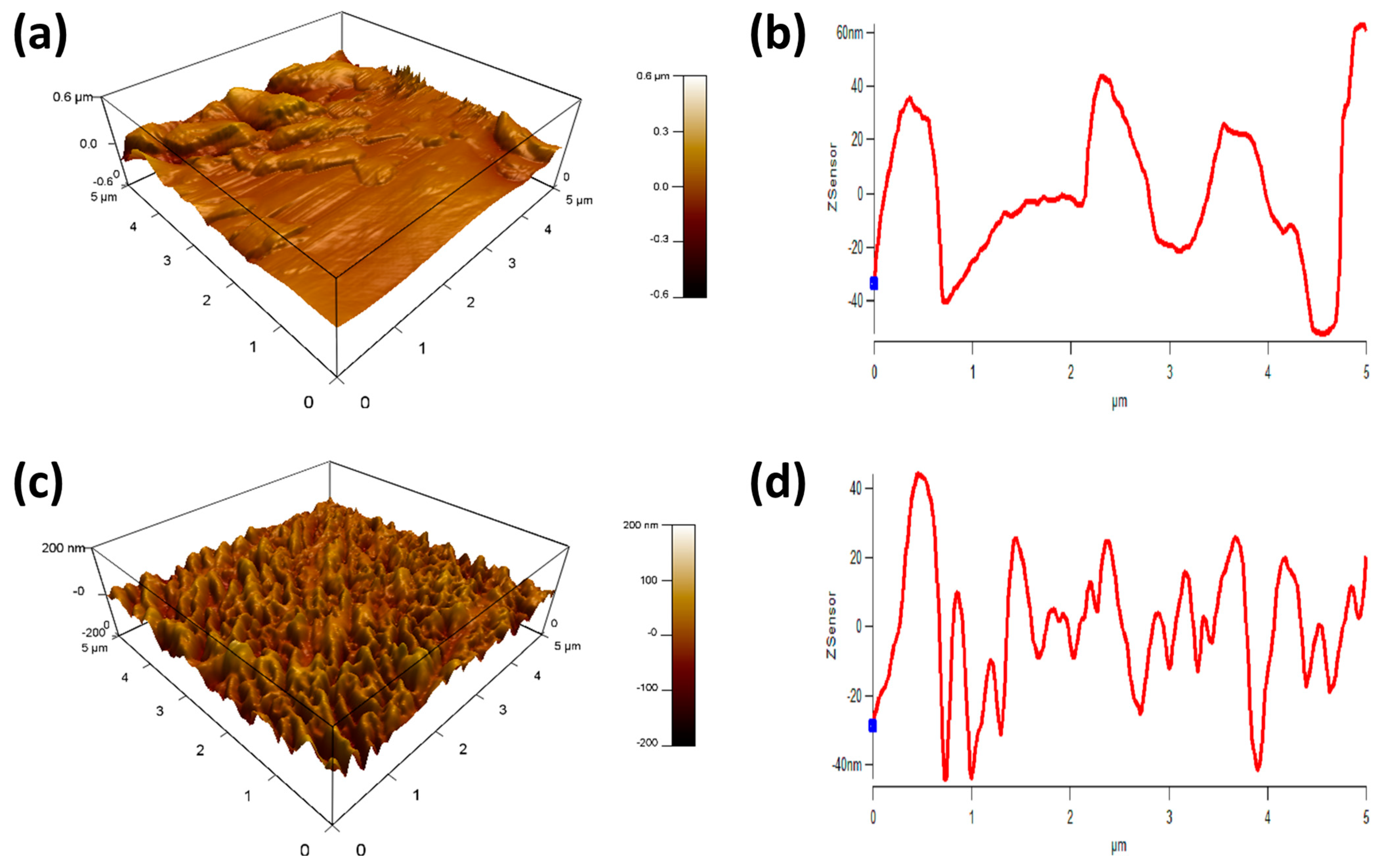
3.4. Surface Roughness
3.5. XPS
3.6. FTIR
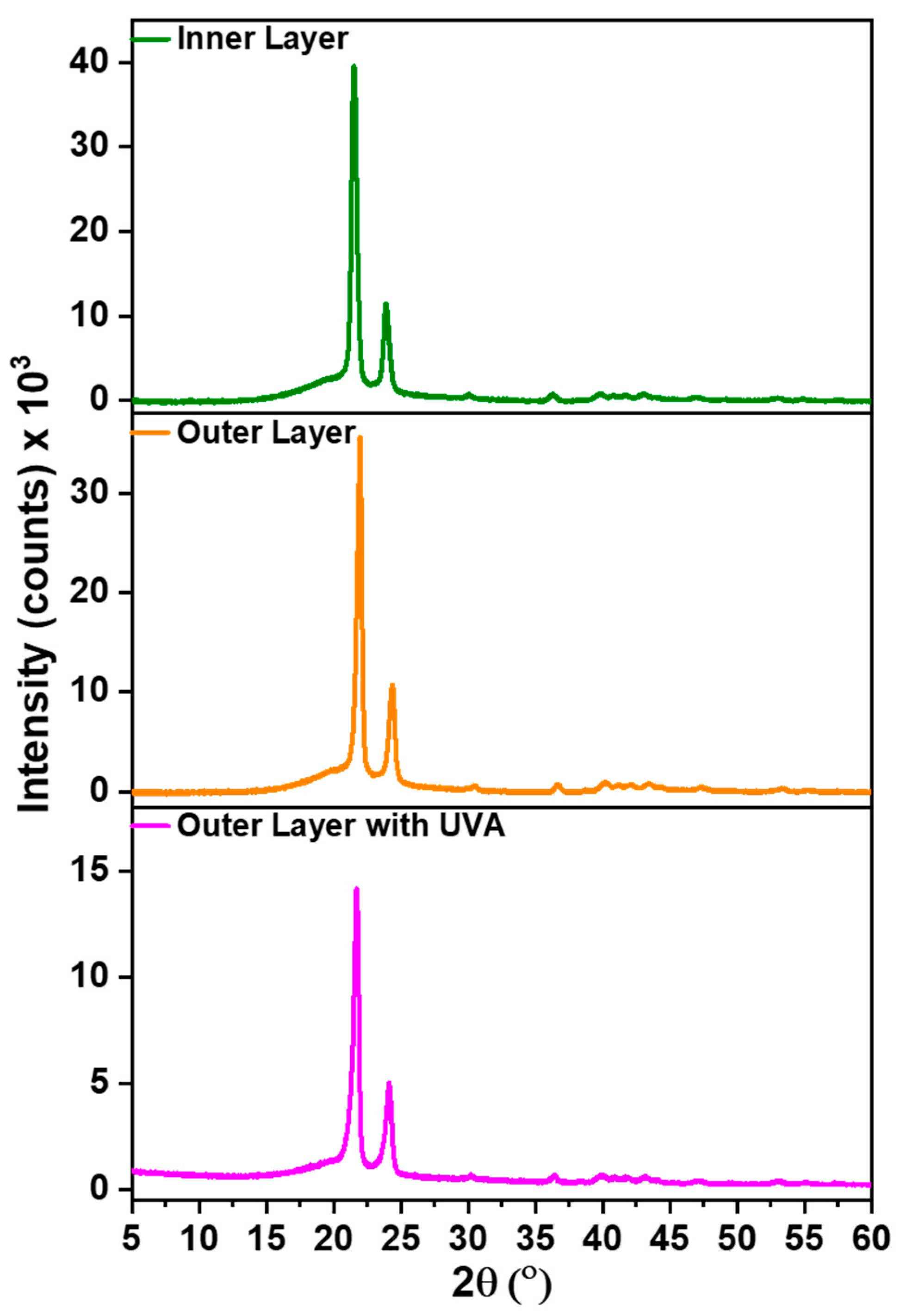
3.7. XRD
3.8. DSC
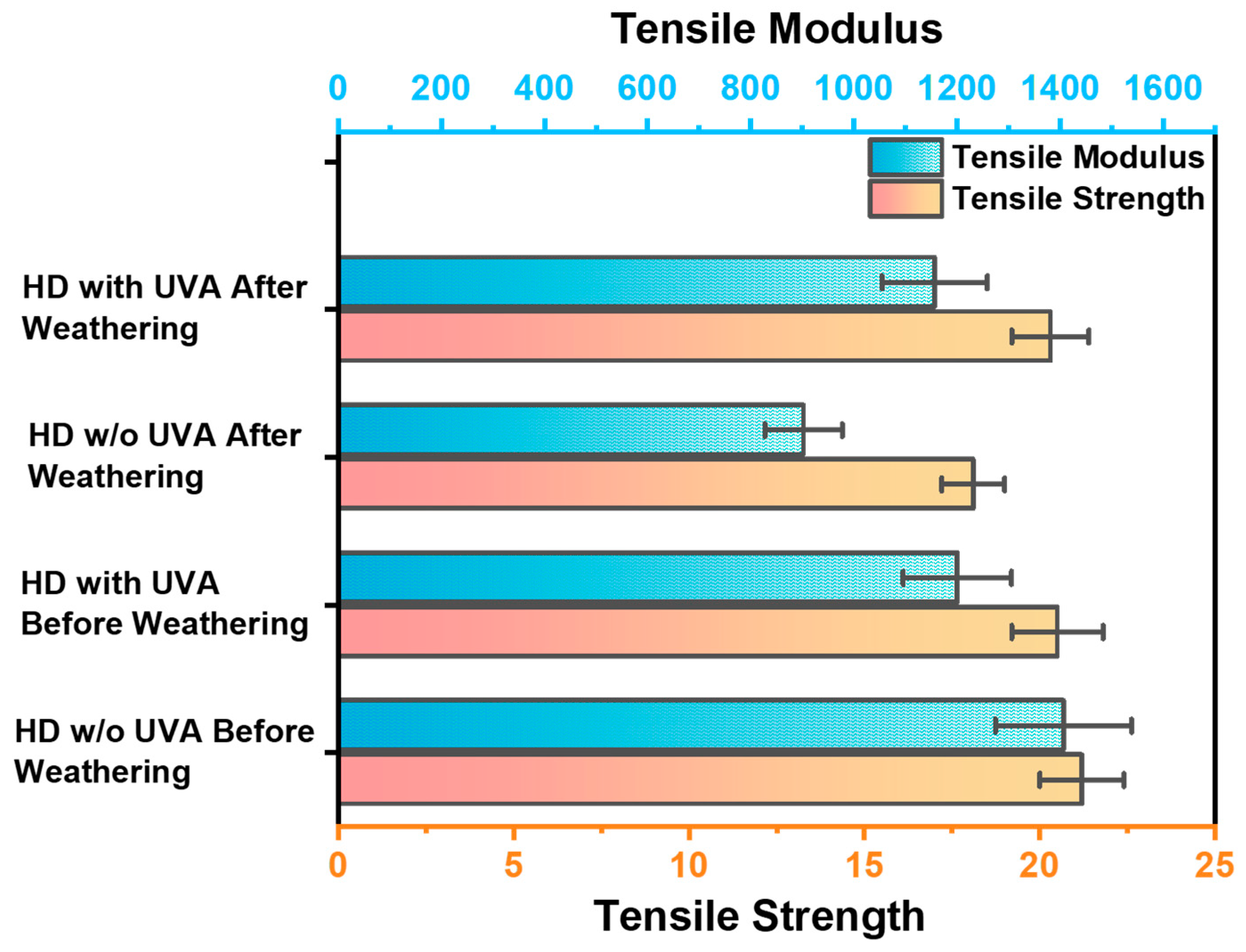
3.9. Tensile Strength and Modulus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Mishra, S.; Rath, C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, D.E. Rethinking the Future of Plastics Rethinking the Future of Plastics the New Plastics Economy; Ellen MacArthur Foundation: Cowes, UK, 2014; pp. 1–120. [Google Scholar]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Life-Cycle Assessment of Polypropylene Production in the Gulf Cooperation Council (GCC) Region. Polymers 2021, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a Simple Plastic into a Superhydrophobic Surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, J.; Li, F.; Jia, W.; Liang, D.; Chen, H.; Li, R.; Hu, J.; Ni, J.; Wu, T.; et al. Nitrogen-doped graphene supported palladium-nickel nanoparticles with enhanced catalytic performance for formic acid oxidation. Electrochim. Acta 2016, 220, 83–90. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Lase, I.S.; Tonini, D.; Caro, D.; Albizzati, P.F.; Cristóbal, J.; Roosen, M.; Kusenberg, M.; Ragaert, K.; Van Geem, K.M.; Dewulf, J.; et al. How much can chemical recycling contribute to plastic waste recycling in Europe? An assessment using material flow analysis modeling. Resour. Conserv. Recycl. 2023, 192, 106916. [Google Scholar] [CrossRef]
- Pan, D.; Su, F.; Liu, C.; Guo, Z. Research progress for plastic waste management and manufacture of value-added products. Adv. Compos. Hybrid Mater. 2020, 3, 443–461. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar] [CrossRef]
- Saleem, J.; Tahir, F.; Baig, M.Z.K.; Al-Ansari, T.; McKay, G. Assessing the environmental footprint of recycled plastic pellets: A life-cycle assessment perspective. Environ. Technol. Innov. 2023, 32, 103289. [Google Scholar] [CrossRef]
- Bazargan, A.; Hui, C.W.; McKay, G. Porous carbons from plastic waste. Adv. Polym. Sci. 2013, 266, 1–26. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, D.; Yue, X.; Xu, J.; Qiu, F.; Zhang, T. A recyclable and regenerated aerogel membrane derived from waste plastic for emulsion separation. J. Environ. Chem. Eng. 2022, 10, 108221. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; McKay, G. Up-cycling plastic waste into swellable super-sorbents. J. Hazard. Mater. 2023, 453, 131356. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Ravekar, V.; Rama Rao, P.; Waigokar, S.; Hingankar, S. Recycling of waste HDPE and PP plastic in preparation of plastic brick and its mechanical properties. Clean. Mater. 2022, 5, 100113. [Google Scholar] [CrossRef]
- Lock, E.; Walton, S.; Fernsler, R. Preparation of Ultra Thin Polystyrene, Polypropylene and Polyethylene Films on Si Substrate Using Spin Coating Technology; NRL/MR/6750--08-9092; Naval Research Lab Washington DC Plasma Physics Division: Washington, DC, USA, 2008. [Google Scholar]
- Mellbring, O.; Kihlman Øiseth, S.; Krozer, A.; Lausmaa, J.; Hjertberg, T. Spin Coating and Characterization of Thin High-Density Polyethylene Films. Macromolecules 2001, 34, 7496–7503. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Luyt, A.S.; Shakoor, R.A.; McKay, G. Free-Standing Porous and Nonporous Polyethylene Thin Films Using Spin Coating: An Alternate to the Extrusion–Stretching Process. ACS Appl. Polym. Mater. 2023, 5, 2177–2184. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; McKay, G. Transforming Polypropylene Waste into Transparent Anti-corrosion Weather-resistant and Anti-bacterial Superhydrophobic Films. J. Hazard. Mater. 2024, 466, 133597. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Sun, L.; McKay, G. Valorization of mixed plastics waste for the synthesis of flexible superhydrophobic films. Adv. Compos. Hybrid Mater. 2024, 7, 11. [Google Scholar] [CrossRef]
- Berger, T.; Sundhararajan, S.; Nagaosa, T. Influence of Temperature on the Hydrophobicity of Polymers. In Proceedings of the IEEE 1997 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Minneapolis, MN, USA, 19–22 October 1997; pp. 1–4. [Google Scholar]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- McHale, G.; Newton, M.I.; Shirtcliffe, N.J. Water-repellent soil and its relationship to granularity, surface roughness and hydrophobicity: A materials science view. Eur. J. Soil Sci. 2005, 56, 445–452. [Google Scholar] [CrossRef]
- Burton, Z.; Bhushan, B. Hydrophobicity, adhesion, and friction properties of nanopatterned polymers and scale dependence for micro- and nanoelectromechanical systems. Nano Lett. 2005, 5, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of surface roughness on superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.D.; Kittikorn, T.; Strömberg, E.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A.; Ek, M.; Karlsson, S. Water absorption and hydrothermal performance of PHBV/sisal biocomposites. Polym. Degrad. Stab. 2014, 108, 166–174. [Google Scholar] [CrossRef]
- Sadi, R.K.; Fechine, G.J.M.; Demarquette, N.R. Photodegradation of poly (3-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2318–2327. [Google Scholar] [CrossRef]
- Van Ngo, H.; Nguyen, P.K.; Van Vo, T.; Duan, W.; Tran, V.-T.; Tran, P.H.-L.; Tran, T.T.-D. Hydrophilic-hydrophobic polymer blend for modulation of crystalline changes and molecular interactions in solid dispersion. Int. J. Pharm. 2016, 513, 148–152. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; McKay, G. Environmental Nanotechnology. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–32. [Google Scholar]





| Temperature (°C) | Heating Time (min) | Water CA Top Layer |
|---|---|---|
| 25 | 25 | 148° ± 2° |
| 45 | 25 | 145° ± 1.3° |
| 65 | 25 | 141° ± 1.2° |
| 85 | 25 | 138° ± 1.3° |
| 105 | 25 | 114° ± 2.5° |
| 130 | 25 | 103° ± 0.8° |
| Parameters | Base Layer (nm) | Top Layer (nm) |
|---|---|---|
| RMS | 20 | 50 |
| Max | 86 | 222 |
| Min | −74 | −264 |
| Avg Deviation | 16 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, J.; Moghal, Z.K.B.; McKay, G. Prolonged Lifespan of Superhydrophobic Thin Films and Coatings Using Recycled Polyethylene. Polymers 2024, 16, 1791. https://doi.org/10.3390/polym16131791
Saleem J, Moghal ZKB, McKay G. Prolonged Lifespan of Superhydrophobic Thin Films and Coatings Using Recycled Polyethylene. Polymers. 2024; 16(13):1791. https://doi.org/10.3390/polym16131791
Chicago/Turabian StyleSaleem, Junaid, Zubair Khalid Baig Moghal, and Gordon McKay. 2024. "Prolonged Lifespan of Superhydrophobic Thin Films and Coatings Using Recycled Polyethylene" Polymers 16, no. 13: 1791. https://doi.org/10.3390/polym16131791
APA StyleSaleem, J., Moghal, Z. K. B., & McKay, G. (2024). Prolonged Lifespan of Superhydrophobic Thin Films and Coatings Using Recycled Polyethylene. Polymers, 16(13), 1791. https://doi.org/10.3390/polym16131791






