Biodegradable Polyurethane Derived from Hydroxylated Polylactide with Superior Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
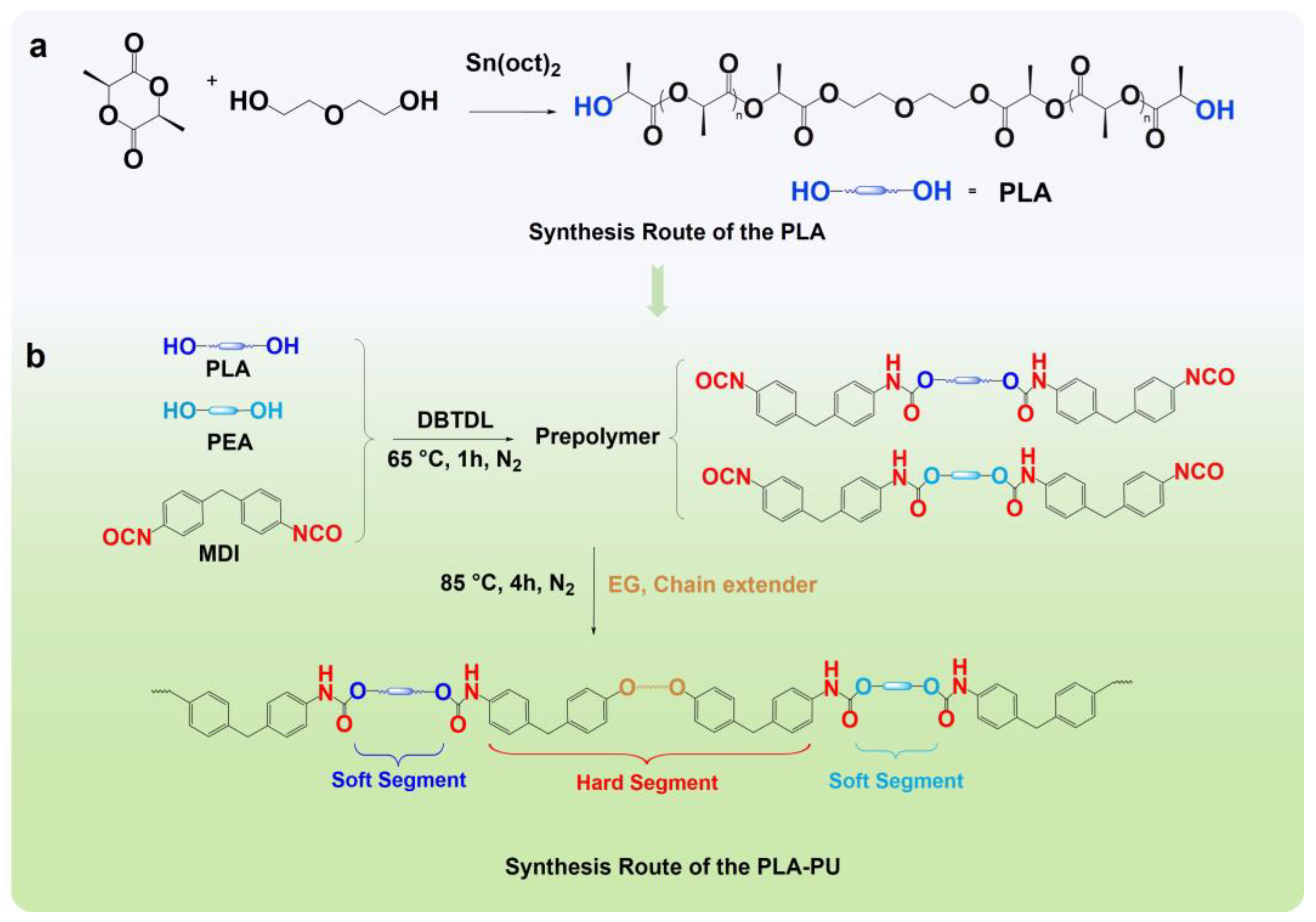
2.2. Synthesis of PLA-OH and PLA-PUs
2.3. Characterizations
2.4. Degradation Performance Test
3. Results and Discussion
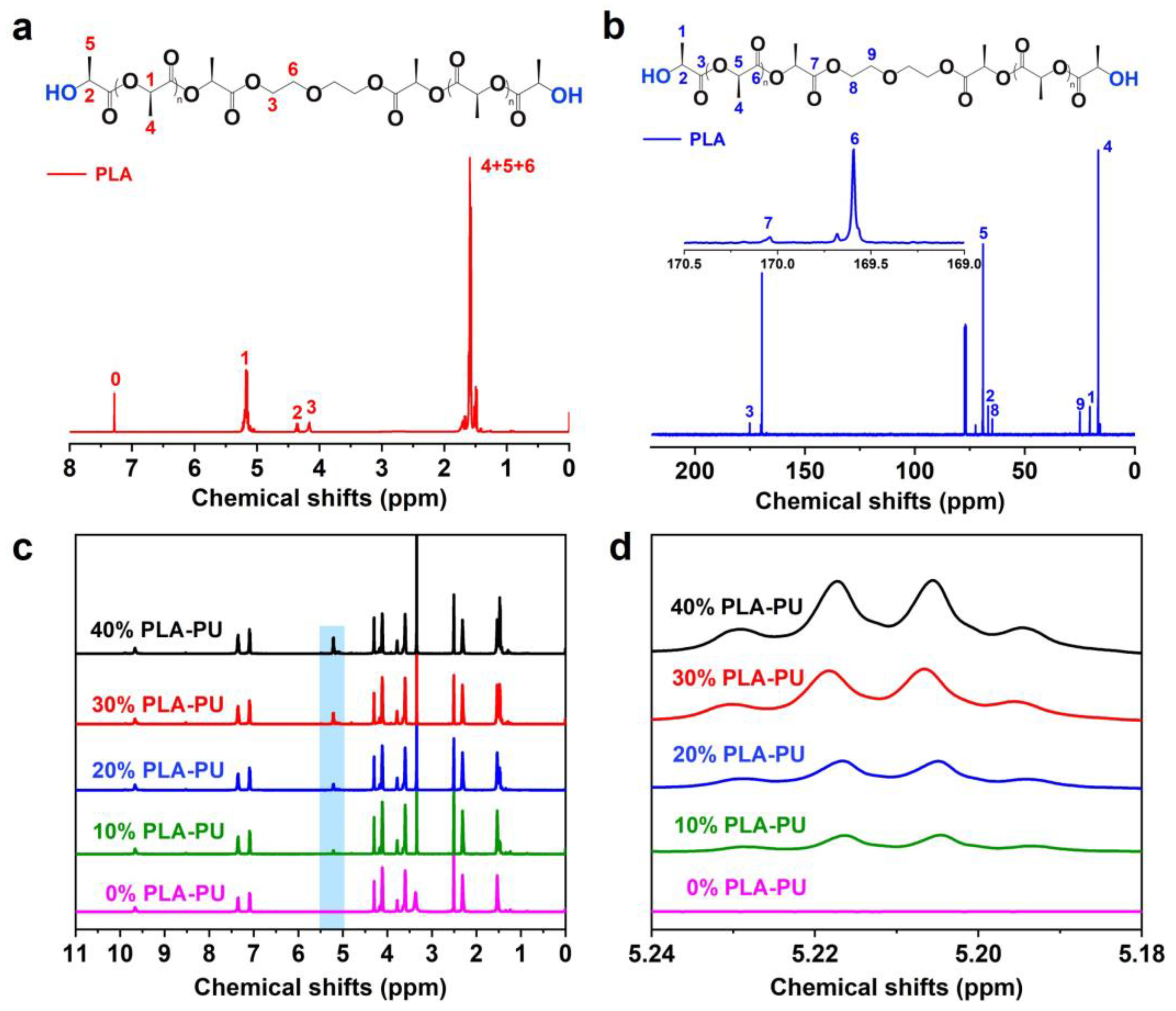
3.1. Chemical Structure of PLA-OH and PLA-PUs
3.2. FTIR Analysis of PLA-OH and PLA-PUs
3.3. Thermal, Mechanical and Optical Properties of PLA-PUs
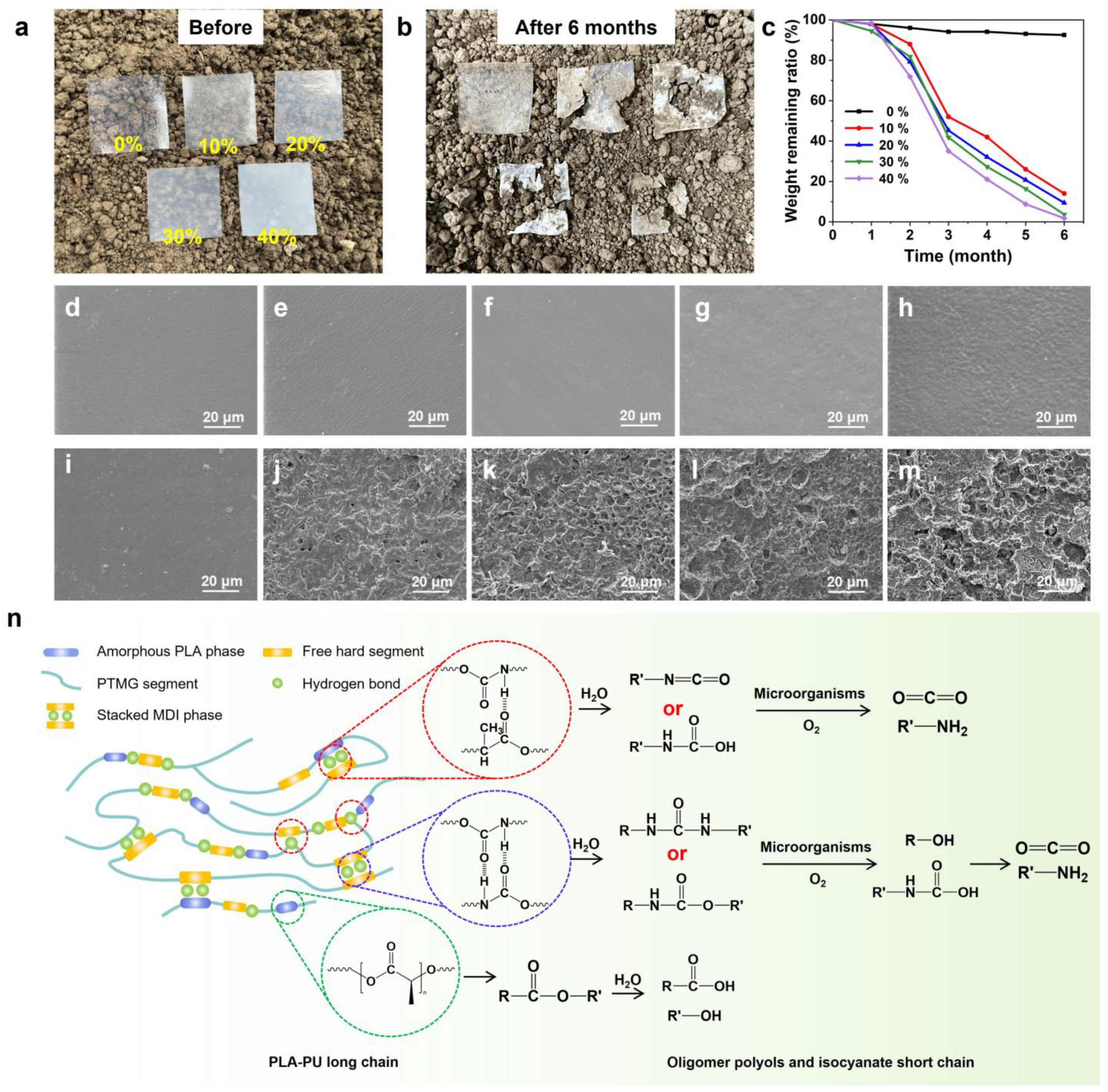
3.4. Biodegradation Performance of PLA-PUs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Niesiobędzka, J.; Datta, J. Challenges and Recent Advances in Bio-Based Isocyanate Production. Green Chem. 2023, 25, 2482–2504. [Google Scholar] [CrossRef]
- Wang, L.; Guo, L.; Zhang, K.; Xia, Y.; Hao, J.; Wang, X. Development of Tough Thermoplastic Elastomers by Leveraging Rigid–Flexible Supramolecular Segment Interplays. Angew. Chem. Int. Ed. 2023, 62, e202301762. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, B.; Xu, Z.; Yang, J.; Liu, W. An Unparalleled H-Bonding and Ion-Bonding Crosslinked Waterborne Polyurethane with Super Toughness and Unprecedented Fracture Energy. Mater. Horiz. 2021, 8, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; García, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The Rise of Plastic Bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Záleská, M.; Pavlíková, M.; Pokorný, J.; Jankovský, O.; Pavlík, Z.; Černý, R. Structural, Mechanical and Hygrothermal Properties of Lightweight Concrete Based on the Application of Waste Plastics. Constr. Build. Mater. 2018, 180, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Shou, T.; Liang, R.; Hu, S.; Yu, P.; Zhang, L. Bio-Based Thermoplastic Polyurethane Derived from Polylactic Acid with High-Damping Performance. Ind. Crops Prod. 2020, 154, 112619. [Google Scholar] [CrossRef]
- Boisaubert, P.; Kébir, N.; Schuller, A.S.; Burel, F. Polyurethane Coatings from Formulations with Low Isocyanate Content Using a Transurethane Polycondensation Route. Polymer 2022, 240, 124522. [Google Scholar] [CrossRef]
- Petersen, S.R.; Prydderch, H.; Worch, J.C.; Stubbs, C.J.; Wang, Z.; Yu, J.; Arno, M.C.; Dobrynin, A.V.; Becker, M.L.; Dove, A.P. Ultra-Tough Elastomers from Stereochemistry-Directed Hydrogen Bonding in Isosorbide-Based Polymers. Angew. Chem. Int. Ed. 2022, 61, e202115904. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.d.N.; Jiang, Y. 3D Printable Sustainable Composites with Thermally Tunable Properties Entirely from Corn-Based Products. ACS Sustain. Chem. Eng. 2022, 10, 7818–7824. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Barnes, S.H.; Bowland, C.C.; Meek, K.M.; Littrell, K.C.; Keum, J.K.; Naskar, A.K. A Path for Lignin Valorization via Additive Manufacturing of High-Performance Sustainable Composites with Enhanced 3D Printability. Sci. Adv. 2018, 4, eaat4967. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Kim, K.-H. Functionalization and Customization of Polyurethanes for Biosensing Applications: A State-of-the-Art Review. TrAC Trend Anal. Chem. 2020, 126, 115881. [Google Scholar] [CrossRef]
- Li, J.-W.; Tsen, W.-C.; Tsou, C.-H.; Suen, M.-C.; Chiu, C.-W. Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, K.; Potzmann, P.; Dworak, C.; Bergmeister, H.; Eilenberg, M.; Grasl, C.; Koch, T.; Schima, H.; Liska, R.; Baudis, S. Hard Block Degradable Polycarbonate Urethanes: Promising Biomaterials for Electrospun Vascular Prostheses. Biomacromolecules 2020, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Karkan, S.; Banimohamad-Shotorbani, B.; Saghati, S.; Rahbarghazi, R.; Davaran, S. A Critical Review of Fibrous Polyurethane-Based Vascular Tissue Engineering Scaffolds. J. Biol. Eng. 2022, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.D.; Kim, S.; Wagner, W.R. Biodegradable Polyurethane Scaffolds in Regenerative Medicine: Clinical Translation Review. J. Biomed. Mater. Res. A 2022, 110, 1460–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, R.; Wang, X.; Zhang, J.; Wang, J.; Cao, B.; Zhao, Y.; Xu, L.; Chen, Y.; Zou, G. How Do Controlled-Release Fertilizer Coated Microplastics Dynamically Affect Cd Availability by Regulating Fe Species and DOC Content in Soil? Sci. Total Environ. 2022, 850, 157886. [Google Scholar] [CrossRef]
- Vishtal, A.; Khakalo, A.; Retulainen, E. Extensible Cellulosic Fibre-Polyurethane Composites Prepared via the Papermaking Pathway. BioResources 2018, 13, 5360–5376. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, T.; Zhang, W.; Wang, G.; Zhang, Z.; Zhao, P.; Liu, X.; Li, H. Antibacterial Castor Oil-Based Waterborne Polyurethane/Gelatin Films for Packaging of Strawberries. Food Packag. Shelf Life 2023, 36, 101055. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Li, L.; Deng, W.; Liu, M.; Hu, J. Biodegradable Starch-Based Packaging Films Incorporated with Polyurethane-Encapsulated Essential-Oil Microcapsules for Sustained Food Preservation. Int. J. Biol. Macromol. 2023, 235, 123889. [Google Scholar] [CrossRef]
- Mort, R.; Olson, E.; Thurber, H.; Jiang, S.; Vorst, K.; Curtzwiler, G. Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria Fendleri Oil for Food Packaging Applications. Sustainability 2022, 14, 8657. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Kang, B.; Chen, L.-S.; Lu, X. Enhancing Toughness of Poly (Lactic Acid)/Thermoplastic Polyurethane Blends via Increasing Interface Compatibility by Polyurethane Elastomer Prepolymer and Its Toughening Mechanism. Polym. Test. 2020, 87, 106521. [Google Scholar] [CrossRef]
- Qin, C.; Chen, K.; Xu, R.; Wang, Y. Nucleating Effect of Boron Nitride Nanotubes on Poly(Lactic Acid) Crystallization. Colloid Polym. Sci. 2022, 300, 775–784. [Google Scholar] [CrossRef]
- Chai, J.; Wang, G.; Li, B.; Wan, G.; Zhang, L.; Zhao, G. Strong and Ductile Poly (Lactic Acid) Achieved by Carbon Dioxide Treatment at Room Temperature. J. CO2 Util. 2019, 33, 292–302. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Fluorine-Free Waterborne Coating for Environmentally Friendly, Robustly Water-Resistant, and Highly Breathable Fibrous Textiles. ACS Nano 2020, 14, 1045–1054. [Google Scholar] [CrossRef]
- Sethi, J.; Illikainen, M.; Sain, M.; Oksman, K. Polylactic Acid/Polyurethane Blend Reinforced with Cellulose Nanocrystals with Semi-Interpenetrating Polymer Network (S-IPN) Structure. Eur. Polym. J. 2017, 86, 188–199. [Google Scholar] [CrossRef]
- Wang, H.; Yu, J.; Fang, H.; Wei, H.; Wang, X.; Ding, Y. Largely Improved Mechanical Properties of a Biodegradable Polyurethane Elastomer via Polylactide Stereocomplexation. Polymer 2018, 137, 1–12. [Google Scholar] [CrossRef]
- Storey, R.F.; Wiggins, J.S.; Mauritz, K.A.; Puckett, A.D. Bioabsorbable Composites. II: Nontoxic, L-Lysine-Based Poly(Ester-Urethane) Matrix Composites. Polym. Compos. 1993, 14, 17–25. [Google Scholar] [CrossRef]
- Lovinčić Milovanović, V.; Hajdinjak, I.; Lovriša, I.; Vrsaljko, D. The Influence of the Dispersed Phase on the Morphology, Mechanical and Thermal Properties of PLA/PE-LD and PLA/PE-HD Polymer Blends and Their Nanocomposites with TiO2 and CaCO3. Polym. Eng. Sci. 2019, 59, 1395–1408. [Google Scholar] [CrossRef]
- Gu, S.; Yang, M.; Yu, T.; Ren, T.; Ren, J. Synthesis and Characterization of Biodegradable Lactic Acid-based Polymers by Chain Extension. Polym. Int. 2008, 57, 982–986. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, R.-Y.; Ying, W.-B.; Hu, H.; Wang, Y.-B.; Guo, Y.-Q.; Wang, W.-Q.; Tang, Z.-B.; Zhu, J. Polyether-Polyester and HMDI Based Polyurethanes: Effect of PLLA Content on Structure and Property. Chin. J. Polym. Sci. 2019, 37, 1152–1161. [Google Scholar] [CrossRef]
- Kim, M.-N.; Kim, K.-H.; Jin, H.-J.; Park, J.-K.; Yoon, J.-S. Biodegradability of Ethyl and N-Octyl Branched Poly(Ethylene Adipate) and Poly(Butylene Succinate). Eur. Polym. J. 2001, 37, 1843–1847. [Google Scholar] [CrossRef]
- Jin, H.-J.; Lee, B.-Y.; Kim, M.-N.; Yoon, J.-S. Properties and Biodegradation of Poly(Ethylene Adipate) and Poly(Butylene Succinate) Containing Styrene Glycol Units. Eur. Polym. J. 2000, 36, 2693–2698. [Google Scholar] [CrossRef]
- Pan, J.; Xie, Q.; Chiang, H.; Peng, Q.; Qian, P.-Y.; Ma, C.; Zhang, G. “From the Nature for the Nature”: An Eco-Friendly Antifouling Coating Consisting of Poly(Lactic Acid)-Based Polyurethane and Natural Antifoulant. ACS Sustain. Chem. Eng. 2020, 8, 1671–1678. [Google Scholar] [CrossRef]
- Hu, H.; Tian, Y.; Kong, Z.; Ying, W.B.; Chen, C.; Li, F.; Zhang, R.; Zhu, J. A High Performance Copolyester with “Locked” Biodegradability: Solid Stability and Controlled Degradation Enabled by Acid-Labile Acetal. ACS Sustain. Chem. Eng. 2021, 9, 2280–2290. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Sessini, V.; Peponi, L. Biodegradable Poly(Ester-Urethane) Incorporated with Catechin with Shape Memory and Antioxidant Activity for Food Packaging. Eur. Polym. J. 2017, 94, 111–124. [Google Scholar] [CrossRef]
- Hu, J.; Mo, R.; Sheng, X.; Zhang, X. A Self-Healing Polyurethane Elastomer with Excellent Mechanical Properties Based on Phase-Locked Dynamic Imine Bonds. Polym. Chem. 2020, 11, 2585–2594. [Google Scholar] [CrossRef]
- Wang, W.; Ping, P.; Chen, X.; Jing, X. Shape Memory Effect of Poly(L-lactide)-Based Polyurethanes with Different Hard Segments. Polym. Int. 2007, 56, 840–846. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, 2005759. [Google Scholar] [CrossRef] [PubMed]
- Gregorí Valdés, B.S.; Gomes, C.S.B.; Gomes, P.T.; Ascenso, J.R.; Diogo, H.P.; Gonçalves, L.M.; Galhano dos Santos, R.; Ribeiro, H.M.; Bordado, J.C. Synthesis and Characterization of Isosorbide-Based Polyurethanes Exhibiting Low Cytotoxicity Towards HaCaT Human Skin Cells. Polymers 2018, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Nakagawa, S.; Seshimo, M.; Ejima, H.; Houjou, H.; Yoshie, N. Tough Supramolecular Elastomer via Entropy-Driven Hydrogen Bonds between Vicinal Diols. Macromolecules 2020, 53, 4121–4125. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.-L.; Li, R.-W.; Zhang, Q. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nakagawa, S.; Houjou, H.; Yoshie, N. Insights into the Role of Hydrogen Bonds on the Mechanical Properties of Polymer Networks. Macromolecules 2021, 54, 4070–4080. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Ding, M.; Tan, H.; Li, J.; Fu, Q. Preparation and Rapid Degradation of Nontoxic Biodegradable Polyurethanes Based on Poly(Lactic Acid)-Poly(Ethylene Glycol)-Poly(Lactic Acid) and l-Lysine Diisocyanate. Polym. Chem. 2011, 2, 601–607. [Google Scholar] [CrossRef]
- Huang, Y.; Müller, M.T.; Boldt, R.; Zschech, C.; Gohs, U.; Wießner, S. A New Strategy to Improve Viscoelasticity, Crystallization and Mechanical Properties of Polylactide. Polym. Test. 2021, 97, 107160. [Google Scholar] [CrossRef]
- Song, P.; Wang, H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mater. 2020, 32, 1901244. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ping, P.; Chen, X.; Jing, X. Polylactide-Based Polyurethane and Its Shape-Memory Behavior. Eur. Polym. J. 2006, 42, 1240–1249. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Liu, S.; Wang, X. Bio-Based Upcycling of Poly(Ethylene Terephthalate) Waste to UV-Curable Polyurethane Acrylate. Polym. Chem. 2023, 14, 1110–1116. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Ying, W.B.; Kong, Z.; Wang, K.; Wang, J.; Zhu, J. Biodegradable Elastomer from 2,5-Furandicarboxylic Acid and ε-Caprolactone: Effect of Crystallization on Elasticity. ACS Sustain. Chem. Eng. 2019, 7, 17778–17788. [Google Scholar] [CrossRef]
- Ma, Z.; Hong, Y.; Nelson, D.M.; Pichamuthu, J.E.; Leeson, C.E.; Wagner, W.R. Biodegradable Polyurethane Ureas with Variable Polyester or Polycarbonate Soft Segments: Effects of Crystallinity, Molecular Weight, and Composition on Mechanical Properties. Biomacromolecules 2011, 12, 3265–3274. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guan, J.; Wang, X.; Yu, J.; Ding, B. Polylactic Acid (PLA) Melt-Blown Nonwovens with Superior Mechanical Properties. ACS Sustain. Chem. Eng. 2023, 11, 4279–4288. [Google Scholar] [CrossRef]
- Wang, C.; Meng, N.; Babar, A.A.; Gong, X.; Liu, G.; Wang, X.; Yu, J.; Ding, B. Highly Transparent Nanofibrous Membranes Used as Transparent Masks for Efficient PM0.3 Removal. ACS Nano 2022, 16, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, Q.; Wu, Y.; Chen, H.; Liu, Y.; Wang, J.; Duan, X.; Chen, Q.; Ge, Z.; Zhang, Y. Extremely Strong and Tough Biodegradable Poly(Urethane) Elastomers with Unprecedented Crack Tolerance via Hierarchical Hydrogen-Bonding Interactions. Adv. Mater. 2023, 35, 2212130. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liang, J.; Wang, Z.; Qin, J.; Zhang, Q.; Zhu, S.; Zhang, K.; Zhu, H. Tough, Recyclable, and Degradable Elastomers for Potential Biomedical Applications. Adv. Mater. 2023, 35, 2210092. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-Q.; Fu, T.; Li, X.-L.; Li, Y.; Deng, C.; Wang, X.-L.; Wang, Y.-Z. High-Performance Biobased Polyesters with High Gas Barrier, Glass Transition Temperature, and Tensile Strength Enabled by Hydrogen Bonds and Flexible Segments. ACS Sustain. Chem. Eng. 2022, 10, 14240–14247. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Zhang, X.; Xu, H.; Zhou, Y.; Tian, M.; Chen, S.; Jerrams, S.; Zhou, F.-L.; Jiang, L. Synthesis of Silicone Blocked Bio-Polyurethane and Its Application in Highly Stretchable Fiber-Shaped Strain Sensor. Nano Res. 2023, 16, 7982–7990. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, Y.; Zhao, C.; Meng, N.; Bai, Y.; Wang, X.; Yu, J.; Ding, B. Biodegradable Polyurethane Derived from Hydroxylated Polylactide with Superior Mechanical Properties. Polymers 2024, 16, 1809. https://doi.org/10.3390/polym16131809
Li X, Lin Y, Zhao C, Meng N, Bai Y, Wang X, Yu J, Ding B. Biodegradable Polyurethane Derived from Hydroxylated Polylactide with Superior Mechanical Properties. Polymers. 2024; 16(13):1809. https://doi.org/10.3390/polym16131809
Chicago/Turabian StyleLi, Xueqin, Yanyan Lin, Cengceng Zhao, Na Meng, Ying Bai, Xianfeng Wang, Jianyong Yu, and Bin Ding. 2024. "Biodegradable Polyurethane Derived from Hydroxylated Polylactide with Superior Mechanical Properties" Polymers 16, no. 13: 1809. https://doi.org/10.3390/polym16131809
APA StyleLi, X., Lin, Y., Zhao, C., Meng, N., Bai, Y., Wang, X., Yu, J., & Ding, B. (2024). Biodegradable Polyurethane Derived from Hydroxylated Polylactide with Superior Mechanical Properties. Polymers, 16(13), 1809. https://doi.org/10.3390/polym16131809









