Diverse Approaches in Wet-Spun Alginate Filament Production from the Textile Industry Perspective: From Process Optimization to Composite Filament Production
Abstract
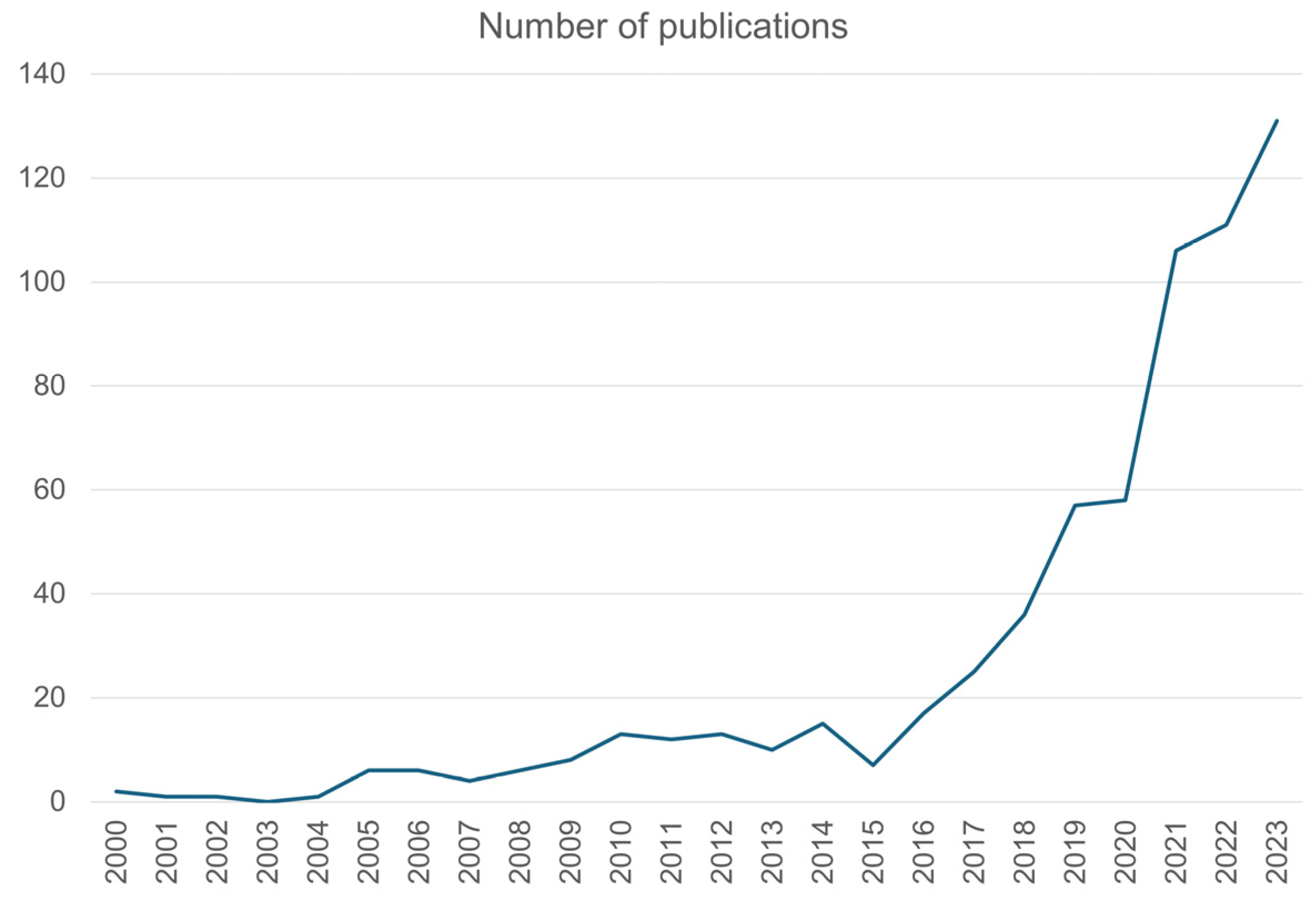
:1. Introduction
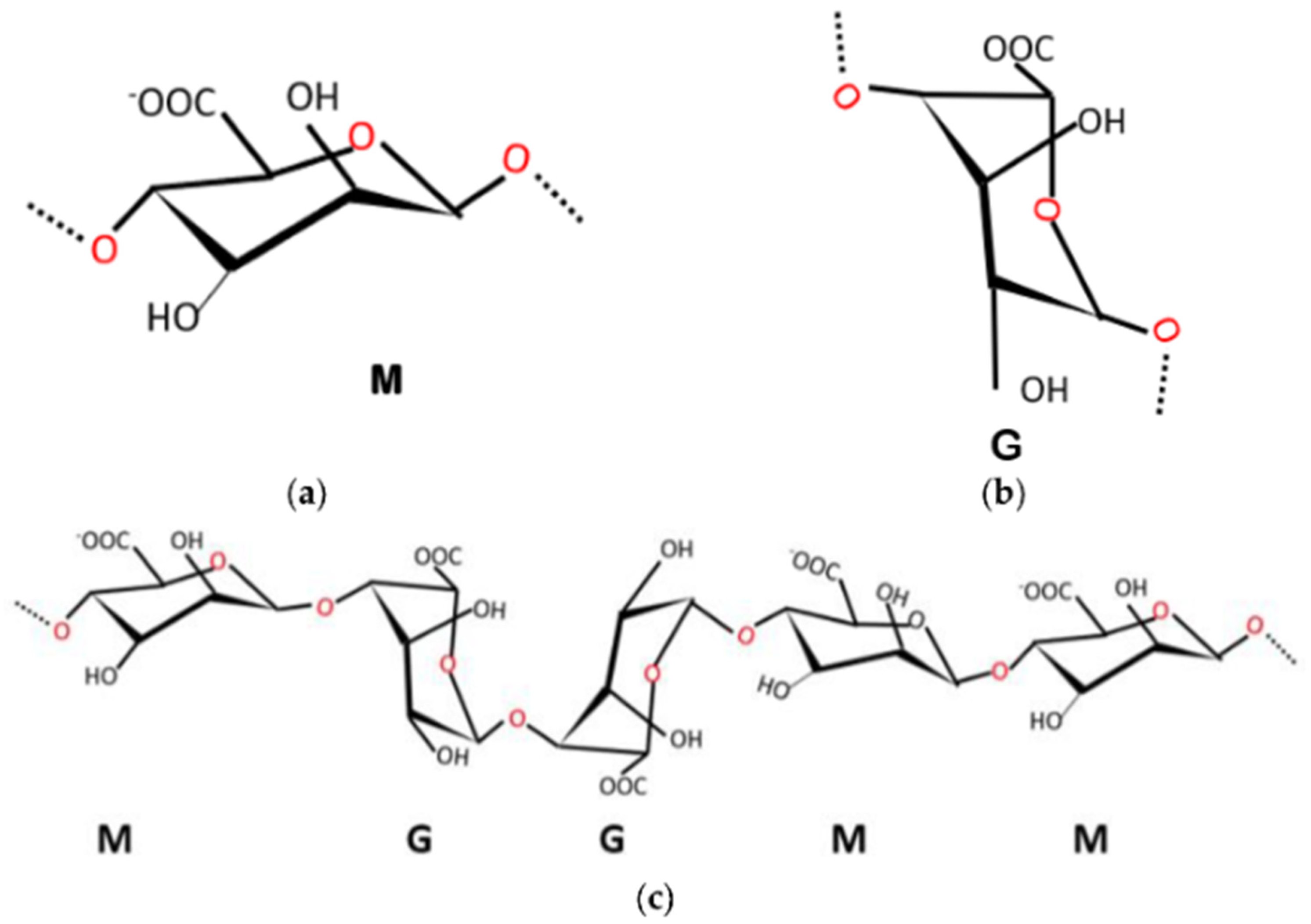
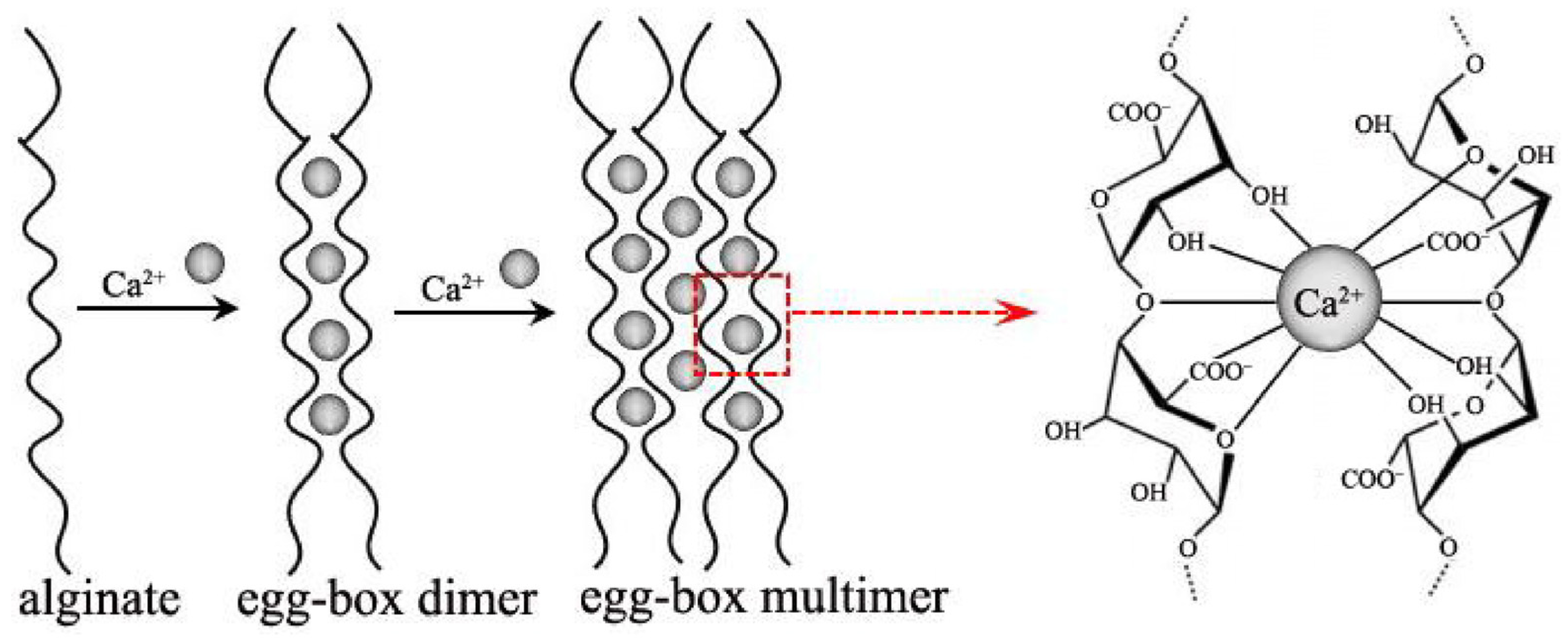
2. Gel Forming Mechanism of Alginate
2.1. Cross-Linking with Divalent Cations
2.1.1. Calcium, Ca2+
2.1.2. Barium, Ba2+
2.1.3. Strontium, Sr2+
2.1.4. Copper, Cu2+
2.1.5. Zinc, Zn2+
2.1.6. Ferrous, Fe2+
2.1.7. Mangane, Mn2+
2.2. Cross-Linking with Trivalent Cations
Aluminum, Al3+
2.3. Cross-Linking with Chemical Bonding
3. Principles of Textile Filament Manufacturing Methods
3.1. Melt Spinning
3.2. Wet Spinning
4. Mechanical Properties of Wet-Spun Alginate Filaments
4.1. Elasticity Modulus
4.2. Yield Strength
4.3. Tenacity
4.4. Knot Strength
4.5. Elongation
4.6. Toughness
| Filament Type | Elasticity Modulus | Yield Strength | Tensile Strength | Knot Strength (cN/dtex) | Elongation (%) | Toughness (MJ·m−3) | Reference |
|---|---|---|---|---|---|---|---|
| Alginate 1 | 3.62 GPa | 200 MPa | 16 | [35] | |||
| Alginate 2 | 9.01 cN/tex | 17.96 cN/tex | 6.63 | [67] | |||
| Alginate 3 | 8.32 cN/dtex | 6.97 | [68] | ||||
| Alginate 4 | 10.21 cN/tex | 18.2 | [69] | ||||
| Alginate | 4.3 cN/tex | 20.4 | [70] | ||||
| Alginate 5 | 0.8–2.2 cN/dtex | 5.8–20.4 | [71] | ||||
| Alginate 6 | 15.80 cN/tex | 4.39 | [72] | ||||
| Alginate | 3.1 gpd | [73] | |||||
| Alginate 7 | 13.6 cN/tex | 8 | [74] | ||||
| Alginate 7 | 10.2 cN/tex | 5 | [74] | ||||
| Alginate 7 | 8.69 cN/tex | 4 | [74] | ||||
| 5% Alginate−25 G needle | 116 MPa | 173 MPa | 18 | 16.16 | [75] | ||
| 5% Alginate−21 G needle | 135 MPa | 35 | 37.47 | [75] | |||
| Alginate−10% Aluminum 5 | 20.7 cN/tex | [76] | |||||
| Alginate−20% Aluminum 5 | 19.7 | [76] | |||||
| Alginate treated with silver nitrate 4 | 10.14 cN/tex | 19.4 | [69] | ||||
| Alginate−Ca-DMSO | 88 MPa | 1.82 MPa | 43 | [37] | |||
| Alginate−Ba-DMSO | 34 MPa | 1.4 MPa | 32 | [37] | |||
| Alginate/chitosan 5 | 0.6- 2 cN/dtex | 4.8–29.1 | [71] | ||||
| Alginate/chitosan 8 | 5.6–7.3 GPa | 105.5–119.5 MPa | 202.4–225.6 MPa | 13.7–26.8 | [77] | ||
| Alginate/chitosan with molecular weight 4.0 × 104 9 | 1.0–1.5 cN/dtex | 0.5 | [78] | ||||
| Alginate/chitosan with molecular weight 1.6 × 105 9 | 1.0–1.6 cN/dtex | 0.5–0.6 | [78] | ||||
| Alginate/5.11% hydrolyzed chitosan content 10 | 11.42 cN/dtex | 13.60 | [68] | ||||
| Alginate/4.92% hydrolyzed chitosan content 10 | 4.52 cN/dtex | 11.69 | [68] | ||||
| Alginate/4.51%−hydrolyzed chitosan content 10 | 7.88 cN/dtex | 9.67 | [68] | ||||
| Alginate/hydrolyzed chitosan 5 | 0.7–2.7 cN/dtex | 5.6–29.3 | [71] | ||||
| Alginate/CM−chitosan treated with HTCC 4 | 8.02–12.64 cN/tex | 15.8–23.2 | [79] | ||||
| Alginate/CM−chitosan treated with Ag 4 | 8.12–14.50 cN/tex | 14.5–21.6 | [79] | ||||
| Alginate/N−Succinyl-chitosan 4 | 10.32–14.32 cN/tex | 20.4–43.5 | [69] | ||||
| Alginate/N−Succinyl-chitosan treated with silver nitrate 4 | 10.40–14.43 cN/tex | 20.7–45.7 | [69] | ||||
| CNC/alginate (97/3) 11 | 1040.5 MPa | 10.5 MPa | 2.9 | [80] | |||
| CNC/alginate (95/5) 11 | 769.8 MPa | 11.9 MPa | 6.3 | [80] | |||
| CNC/alginate (90/10) 11 | 317.4 MPa | 6.2 MPa | 3.3 | [80] | |||
| 2% CNC-added alginate 4 | 2.05 cN/dtex | 15.05 | [56] | ||||
| 2–50% CNC−added alginate | 2.5–6.8 gpd | [73] | |||||
| MWCNT−added alginate 2 | 8.22 cN/tex | 16.54 cN/tex | 5.71 | [67] | |||
| SWNCT−added alginate 1 | 4.01–6.97 GPa | 208–250 MPa | 13–18 | [35] | |||
| GO−added alginate 2 | 8.50 cN/tex | 20.42 cN/tex | 8.71 | [67] | |||
| 7% Nano TiO2−added alginate 6 | 16.88 cN/tex | 3.15 | [72] | ||||
| 2% Nano ZnO−added alginate 6 | 18.38 cN/tex | 3.62 | [72] | ||||
| Hydroxyapatite−added alginate 6 | 20.63–26.71 cN/tex | 8.38–9.45 | [81] | ||||
| 75/25 Gelatine/alginate 12 | 3.8 | [45] | |||||
| 75/25 Gelatine/alginate treated with TGA enzyme 12 | 10 042 N | 6.75 N | 11.98 | [45] | |||
| Alginate/HA by dip coating | 6.13–6.58 cN/tex | [82] | |||||
| Alginate/HA by dope mixing | 4.20–9.059 cN/tex | [82] | |||||
| Alginate/AKP 4 | 2.28–2.68 cN/dtex | [83] |
5. Process Parameters Affecting the Mechanical Properties of Wet-Spun Alginate Filaments
5.1. Effect of Ion Source and Alginate Spinning Dope Concentration
5.2. Effect of Needle Diameter
5.3. Effect of Temperature
5.4. Effect of Coagulants
6. Different Approaches in the Literature to Producing Composite Alginate Filaments for Textile Applications
6.1. Use of Additives
6.2. Preparation of Blended Filaments
6.3. New Approaches
7. Use of Different Cations in the Coagulation Phase
8. Functionalism of Wet-Spun Alginate Filaments
9. Conclusions
10. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Role of Alginate in Bone Tissue Engineering. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Busan, Republic of Korea, 2014; Volume 73, pp. 45–57. [Google Scholar]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 240–250. [Google Scholar]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Dudun, A.A.; Akoulina, E.A.; Zhuikov, V.A.; Makhina, T.K.; Voinova, V.V.; Belishev, N.V.; Khaydapova, D.D.; Shaitan, K.V.; Bonartseva, G.A.; Bonartsev, A.P. Competitive Biosynthesis of Bacterial Alginate Using Azotobacter vinelandii 12 for Tissue Engineering Applications. Polymers 2022, 14, 131. [Google Scholar] [CrossRef]
- Dudun, A.A.; Akoulina, E.A.; Voinova, V.V.; Makhina, T.K.; Myshkina, V.L.; Zhuikov, V.A.; Bonartsev, A.P.; Bonartseva, G.A. Biosynthesis of Alginate and Poly(3-Hydroxybutyrate) by the Bacterial Strain Azotobacter agile 12. Appl. Biochem. Microbiol. 2019, 55, 654–659. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Jankowska, A.M.; Michalak, I.; Detyna, J.; Kulbacka, J. Applications of Alginates in the Biomedical Field. In Advances in Biomedical Research—From COVID to Medical Humanities; Biały, Ł., Młynarczuk-Biały, I., Eds.; Tygiel: Lublin, Poland, 2020; pp. 97–121. [Google Scholar]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Sun, L.; Shen, Y.; Li, M.; Wang, Q.; Li, R.; Gong, S. Preparation and Modification of Collagen/Sodium Alginate-Based Biomedical Materials and Their Characteristics. Polymers 2024, 16, 171. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Stevelić, N.; Rezić, I. Development and Characterization of Sustainable Coatings on Cellulose Fabric and Nonwoven for Medical Applications. Sustainability 2024, 16, 857. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wan, P.; Liu, Q.; Xu, S.; Jiang, J.; Fan, L.; Tu, L. Healing Evaluation of Asphalt Mixtures with Polymer Capsules Containing Rejuvenator under Different Water Solutions. Sustainability 2023, 15, 15258. [Google Scholar] [CrossRef]
- Zhang, L.; Hoff, I.; Zhang, X.; Liu, J.; Yang, C.; Wang, F.A. Methodological Review on Development of Crack Healing Technologies of Asphalt Pavement. Sustainability 2023, 15, 9659. [Google Scholar] [CrossRef]
- Ribeiro, T.; Freire, A.C.; Sá-da-Costa, M.; Canejo, J.; Cordeiro, V.; Micaelo, R. Investigating Asphalt Self-Healing with Colorless Binder and Pigmented Rejuvenator. Sustainability 2023, 15, 4556. [Google Scholar] [CrossRef]
- Omar, A.; Almomani, F.; Qiblawey, H.; Rasool, K. Advances in Nitrogen-Rich Wastewater Treatment: A Comprehensive Review of Modern Technologies. Sustainability 2024, 16, 2112. [Google Scholar] [CrossRef]
- Osman, M.; Xiaohou, S.; Zhao, D.; Basheer, A.; Jin, H.; Zhang, Y. Methane Production from Alginate-Extracted and Non-Extracted Waste of Laminaria japonica: Anaerobic Mono- and Synergetic Co-Digestion Effects on Yield. Sustainability 2019, 11, 1269. [Google Scholar] [CrossRef]
- Sardroudi, N.P.; Sorolla, S.; Casas, C.; Bacardit, A. A Study of the Composting Capacity of Different Kinds of Leathers, Leatherette and Alternative Materials. Sustainability 2024, 16, 2324. [Google Scholar] [CrossRef]
- Nawaz, M.; Shakoor, R.A.; Al-Qahtani, N.; Bhadra, J.; Al-Thani, N.J.; Kahraman, R. Polyolefin-Based Smart Self-Healing Composite Coatings Modified with Calcium Carbonate and Sodium Alginate. Polymers 2024, 16, 636. [Google Scholar] [CrossRef]
- Ho, B.K.X.; Azahari, B.; Yhaya, M.F.B.; Talebi, A.; Ng, C.W.C.; Tajarudin, H.A.; Ismail, N. Green Technology Approach for Reinforcement of Calcium Chloride Cured Sodium Alginate Films by Isolated Bacteria from Palm Oil Mill Effluent (POME). Sustainability 2020, 12, 9468. [Google Scholar] [CrossRef]
- El Hammadi, N.; Almajano, M.P.; Pastor, M.V.; Codina-Torrella, I. Evaluating the Incorporation of Myrtus communis L. Leaves Infusion in Alginate-Based Films and Spheres to Enhance the Oxidative Stability of Oil-in-Water Emulsions. Polymers 2024, 16, 649. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Galimov, D. Development of Biodegradable Alginate-Based Films with Bioactive Properties and Optimal Structural Characteristics with Incorporation of Protein Hydrolysates. Sustainability 2023, 15, 15086. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Mazur, A.; Ermakov, S.; Penkova, A. Novel Pervaporation Membranes Based on Biopolymer Sodium Alginate Modified by FeBTC for Isopropanol Dehydration. Sustainability 2021, 13, 6092. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Q.L.; Hu, S.R.; Ni, J.C.; He, Y.S. Adsorption Mechanism of Cu(II) Ions from Aqueous Solution by Glutaraldehyde Crosslinked Humic Acid-Immobilized Sodium Alginate Porous Membrane Adsorbent. Chem. Eng. J. 2011, 173, 511–519. [Google Scholar] [CrossRef]
- Musa, M.T.; Shaari, N.; Raduwan, N.F.; Kamarudin, S.K.; Wong, W.Y. Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application. Polymers 2023, 15, 2590. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, K.; Bao, Q.; Pan, P.; Zhao, Y.; Yang, Z.; Wang, H.; Wei, J. Adsorption and Electrochemical Detection of Bovine Serum Albumin Imprinted Calcium Alginate Hydrogel Membrane. Polymers 2019, 11, 622. [Google Scholar] [CrossRef]
- Available online: https://www.scopus.com/results/results.uri?sort=plf-f&src=s&st1=alginate+wet+spinning+filament&sid=dac5028265ca65fb879c0bf9328a3928&sot=b&sdt=b&sl=45&s=ALL%28alginate+AND+wet+AND+spinning+AND+filament%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present&sessionSearchId=dac5028265ca65fb879c0bf9328a3928&limit=10 (accessed on 24 May 2024).
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical Properties of Alginate Hydrogels Manufactured Using External Gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. [Google Scholar] [CrossRef]
- Templeman, J.R.; Rogers, M.A.; Cant, J.P.; McBride, B.W.; Osborne, V.R. Effects of a Wax Organogel and Alginate Gel Complex on Holy Basil (Ocimum sanctum) In Vitro Ruminal Dry Matter Disappearance and Gas Production. J. Sci. Food Agric. 2018, 98, 4488–4494. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium Ions and Alginate Do Form Hydrogels: A Rheological Study. Soft Matter 2012, 8, 4877. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Sa, V.; Kornev, K.G.A. Method for Wet Spinning of Alginate Fibers with a High Concentration of Single-Walled Carbon Nanotubes. Carbon 2011, 49, 1859–1868. [Google Scholar] [CrossRef]
- Qin, Y. Alginate Fibres: An Overview of the Production Processes and Applications in Wound Management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Aneem, T.H.; Wong, S.Y.; Afrin, H.; Nurunnabi, M.; Li, X.; Arafat, M.T. Investigation of Coagulation Process of Wet-Spun Sodium Alginate Polymannuronate Fibers with Varied Functionality Using Organic Coagulants and Cross-Linkers. Mater. Today Chem. 2021, 22, 100580. [Google Scholar] [CrossRef]
- Cattelan, G.; Guerrero Gerbolés, A.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Caffarra Malvezzi, C. Alginate Formulations: Current Developments in the Race for Hydrogel-Based Cardiac Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The Tensile Properties of Alginate Hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous Polysaccharide Ionic Gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Eriningsih, R.; Marlina, R. Pre-Clinical Research of Gelatin/Alginate Yarn for Medical Textile. Sci. Res. J. 2014, 2, 26–32. [Google Scholar]
- Kim, Y.J.; Yoon, K.J.; Ko, S.W. Preparation and Properties of Alginate Superabsorbent Filament Fibers Crosslinked with Glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of Glutaraldehyde Exposure on Human Health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef]
- Var, C.; Palamutcu, S. Man-Made Bio-Based and Biodegradable Fibers for Textile Applications. In Sustainable Manufacturing Practices in the Textiles and Fashion Sector. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Ed.; Springer: Cham, Switzerland, 2024; pp. 229–280. [Google Scholar]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation Behavior and Mechanism of Alginate with Calcium: Dependence on Monovalent Counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef]
- Meyer, M.; Baltzer, H.; Schwikal, K. Collagen Fibres by Thermoplastic and Wet Spinning. Mater. Sci. Eng. C 2010, 30, 1266–1271. [Google Scholar] [CrossRef]
- Tonndorf, R.; Gossla, E.; Aibibu, D.; Lindner, M.; Gelinsky, M.; Cherif, C. Wet Spinning and Riboflavin Crosslinking of Collagen Type I/III Filaments. Biomed. Mater. 2018, 14, 015007. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, D.; Lee, J.Y.; Zhai, L.; Kim, J. Effect of Wet Spinning and Stretching to Enhance Mechanical Properties of Cellulose Nanofiber Filament. Int. J. Precis. Eng. Manuf. Technol. 2019, 6, 567–575. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Du, X.; Cheng, B. Novel Collagen/GO-MWNT Hybrid Fibers with Improved Strength and Toughness by Dry-Jet Wet Spinning. Compos. Interfaces 2022, 29, 413–429. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Ci, M.; Sui, S.; Zhu, P. Sodium Alginate/Cellulose Nanocrystal Fibers with Enhanced Mechanical Strength Prepared by Wet Spinning. J. Eng. Fiber. Fabr. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Remadevi, R.; Razal, J.M.; Naebe, M. Impact of the Wet Spinning Parameters on the Alpaca-Based Polyacrylonitrile Composite Fibers: Morphology and Enhanced Mechanical Properties Study. J. Appl. Polym. Sci. 2020, 137, 49264. [Google Scholar] [CrossRef]
- Lal Regar, M.; Ram Meena, C.; Singh Hada, J. Fiber Testing. In Textile Calculation Fibre to Finished Garment; Chattopadhyay, R., Sinha, S.K., Regar, L., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 301–324. [Google Scholar] [CrossRef]
- Farag, R.; Elmogahzy, Y. Tensile Properties of Cotton Fibers. In Handbook of Properties of Textile and Technical Fibres; Bunsell, A.R., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 223–273. [Google Scholar]
- Wang, X.; Liu, X.; Deakin, C.H. Physical and Mechanical Testing of Textiles. In Fabric Testing; Woodhead Publishing: Cambridge, UK, 2008; pp. 90–124. [Google Scholar]
- Jafferson, J.M.; Chatterjee, D. A Review on Polymeric Materials in Additive Manufacturing. Mater. Today Proc. 2021, 46, 1349–1365. [Google Scholar] [CrossRef]
- Savile, B.P. Physical Testing of Textiles, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 1999; pp. 184–207. [Google Scholar]
- Meredith, R. Properties of Textile Materials. I—Tensile Strength, Breaking Extension and Stress-Strain Relations of Fibres. J. Text. Inst. Proc. 1952, 43, 755–764. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.K.; Lim, B.S.; Rhee, S.H.; Yang, H.C. Comparison of Tensile and Knot Security Properties of Surgical Sutures. J. Mater. Sci. Mater. Med. 2007, 18, 2363–2369. [Google Scholar] [CrossRef]
- Mathangadeera, R.W.; Hequet, E.F.; Kelly, B.; Dever, J.K.; Kelly, C.M. Importance of Cotton Fiber Elongation in Fiber Processing. Ind. Crops Prod. 2020, 147, 112217. [Google Scholar] [CrossRef]
- Moriam, K.; Sawada, D.; Nieminen, K.; Hummel, M.; Ma, Y.; Rissanen, M.; Sixta, H. Towards Regenerated Cellulose Fibers with High Toughness. Cellulose 2021, 28, 9547–9566. [Google Scholar] [CrossRef]
- Szparaga, G.; Brzezińska, M.; Pabjańczyk-Wlazło, E.; Puchalski, M.; Sztajnowski, S.; Krucińska, I. Structure–Property of Wet-Spun Alginate-Based Precursor Fibers Modified with Nanocarbons. Autex Res. J. 2020, 20, 32–42. [Google Scholar] [CrossRef]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. Absorbent Alginate Fibres Modified with Hydrolysed Chitosan for Wound Care Dressings—II. Pilot Scale Development. Carbohydr. Polym. 2014, 102, 920–927. [Google Scholar] [CrossRef]
- Fan, L.; Yu, L.; Xu, Y.; Yi, C.; Cai, J.; Li, M.; Huang, J. The Novel Alginate/N-Succinyl-Chitosan Antibacterial Blend Fibers. J. Appl. Polym. Sci. 2010, 116, 2151–2156. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Supaphol, P.; Furuike, T.; Tokura, S.; Tamura, H.; Rujiravanit, R. Novel Chitosan-Spotted Alginate Fibers from Wet-Spinning of Alginate Solutions Containing Emulsified Chitosan−Citrate Complex and Their Characterization. Biomacromolecules 2009, 10, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Knill, C.J.; Kennedy, J.F.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.R.; Williams, H.J. Alginate Fibres Modified with Unhydrolysed and Hydrolysed Chitosans for Wound Dressings. Carbohydr. Polym. 2004, 55, 65–76. [Google Scholar] [CrossRef]
- Borkowski, D.; Krucińska, I.; Draczyński, Z. Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide. Polymers 2020, 12, 1040. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Kitchens, C.L. Wide-Angle X-ray Diffraction of Cellulose Nanocrystal−Alginate Nanocomposite Fibers. Macromolecules 2011, 44, 3478–3484. [Google Scholar] [CrossRef]
- Xu, G.K.; Liu, L.; Yao, J.M. Fabrication and Characterization of Alginate Fibers by Wet-Spinning. Adv. Mater. Res. 2013, 796, 87–91. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Xia, Y.; Jiang, Y.; Murillo, L.L.; Tsigkou, O.; Wang, T.; Li, Y. High Strength and Strain Alginate Fibers by a Novel Wheel Spinning Technique for Knitting Stretchable and Biocompatible Wound-Care Materials. Mater. Sci. Eng. C 2021, 127, 112204. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and Functional Assessment of Alginate Fibers Prepared by Metal-Calcium Ion Complex Coagulation Bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef] [PubMed]
- Sibaja, B.; Culbertson, E.; Marshall, P.; Boy, R.; Broughton, R.M.; Solano, A.A.; Esquivel, M.; Parker, J.; De La Fuente, L.; Auad, M.L. Preparation of Alginate–Chitosan Fibers with Potential Biomedical Applications. Carbohydr. Polym. 2015, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tsuruta, Y.; Tokura, S. Preparation of Chitosan-Coated Alginate Filament. Mater. Sci. Eng. C 2002, 20, 143–147. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Zhang, B.; Yang, J.; Zhou, J.; Kennedy, J.F. Preparation and Properties of Alginate/Carboxymethyl Chitosan Blend Fibers. Carbohydr. Polym. 2006, 65, 447–452. [Google Scholar] [CrossRef]
- Park, J.S.; Park, C.W.; Han, S.Y.; Lee, E.A.; Azelia Wulan, C.; Kim, J.K.; Kwon, G.J.; Seo, Y.H.; Youe, W.J.; Gwon, J.; et al. Preparation and Properties of Wet-Spun Microcomposite Filaments from Cellulose Nanocrystals and Alginate Using a Microfluidic Device. BioResources 2021, 16, 5780–5793. [Google Scholar] [CrossRef]
- Boguń, M.; Mikołajczyk, T.; Rabiej, S. Effect of Formation Conditions on the Structure and Properties of Nanocomposite Alginate Fibers. J. Appl. Polym. Sci. 2009, 114, 70–82. [Google Scholar] [CrossRef]
- Umar, M.; Ullah, A.; Nawaz, H.; Areeb, T.; Hashmi, M.; Kharaghani, D.; Kim, K.O.; Kim, I.S. Wet-Spun Bi-Component Alginate Based Hydrogel Fibers: Development and In-Vitro Evaluation as a Potential Moist Wound Care Dressing. Int. J. Biol. Macromol. 2021, 168, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, J.; Liu, Y.; Chen, S.; Zhang, S.; Yu, Y. Effects of Sodium Salt Types on the Intermolecular Interaction of Sodium Alginate/Antarctic Krill Protein Composite Fibers. Carbohydr. Polym. 2018, 189, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Benavides, E.E.; Brown, P.J.; Kitchens, C.L. Effect of Jet Stretch and Particle Load on Cellulose Nanocrystal−Alginate Nanocomposite Fibers. Langmuir 2010, 26, 14263–14270. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Masood, R.; Umar, M.; Areeb, T.; Ullah, A. Development and Characterization of Alginate-Chitosan-Hyaluronic Acid (ACH) Composite Fibers for Medical Applications. Fibers Polym. 2016, 17, 1749–1756. [Google Scholar] [CrossRef]
- Cui, B.; Mao, Y.; Liu, J.; Liang, X.; Wu, D.; Chen, X.; Wang, X.; Liang, H.; Li, J.; Zhou, B.; et al. Effect of Salt on Solution Behavior of Spinning Medium and Properties of Meat Analogue Fibers. Food Hydrocoll. 2023, 139, 108540. [Google Scholar] [CrossRef]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and Shear Properties of Alginate Gel: Effects of Sodium Ions and Alginate Concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Zhang, J.; Daubert, C.R.; Foegeding, E.A. Fracture Analysis of Alginate Gels. J. Food Sci. 2005, 70, e425–e431. [Google Scholar] [CrossRef]
- Zhou, T.; NajafiKhoshnoo, S.; Esfandyarpour, R.; Kulinsky, L. Dissolvable Calcium Alginate Microfibers Produced via Immersed Microfluidic Spinning. Micromachines 2023, 14, 318. [Google Scholar] [CrossRef]
- Lin, H.Y.; Wang, H.W. The Influence of Operating Parameters on the Drug Release and Antibacterial Performances of Alginate Fibrous Dressings Prepared by Wet Spinning. Biomatter 2012, 2, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Zhang, W.; Zhou, Z.; Fu, H.; Tan, Y.; Sui, K.; Xia, Y. Functionalized Alginate with Liquid-Like Behaviors and Its Application in Wet-Spinning. Carbohydr. Polym. 2017, 174, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Watthanaphanit, A.; Supaphol, P.; Tamura, H.; Tokura, S.; Rujiravanit, R. Wet-Spun Alginate/Chitosan Whiskers Nanocomposite Fibers: Preparation, Characterization and Release Characteristic of the Whiskers. Carbohydr. Polym. 2010, 79, 738–746. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.; Li, D.; Zhu, G.; Lin, N. Flexible Conductive Fibers from Alginate, Cellulose Nanocrystals, and Polyaniline by Wet Spinning. ACS Sustain. Chem. Eng. 2023, 11, 10895–10905. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Cui, L.; Tu, C.; Yan, C.; Guo, Y. Toughen and Strengthen Alginate Fiber by Incorporation of Polyethylene Glycol Grafted Cellulose Nanocrystals. Cellulose 2022, 29, 5021–5035. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Kitchens, C.L. Cellulose Nanocrystal Reinforced Alginate Fibers—Biomimicry Meets Polymer Processing. Mol. Cryst. Liq. Cryst. 2012, 556, 275–287. [Google Scholar] [CrossRef]
- Ma, X.; Li, R.; Zhao, X.; Ji, Q.; Xing, Y.; Sunarso, J.; Xia, Y. Biopolymer Composite Fibres Composed of Calcium Alginate Reinforced with Nanocrystalline Cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- Park, J.S.; Park, C.W.; Han, S.Y.; Lee, E.A.; Cindradewi, A.W.; Kim, J.K.; Kwon, G.J.; Seo, Y.H.; Yoo, W.J.; Gwon, J.; et al. Preparation and Properties of Wet-Spun Microcomposite Filaments from Various CNFs and Alginate. Polymers 2021, 13, 1709. [Google Scholar] [CrossRef]
- Shen, X.J.; Huang, P.L.; Chen, J.H.; Wu, Y.Y.; Liu, Q.Y.; Sun, R.C. Comparison of Acid-Hydrolyzed and TEMPO-Oxidized Nanocellulose for Reinforcing Alginate Fibers. BioResources 2017, 12, 8180–8198. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Supaphol, P.; Tamura, H.; Tokura, S.; Rujiravanit, R. Fabrication, Structure, and Properties of Chitin Whisker-Reinforced Alginate Nanocomposite Fibers. J. Appl. Polym. Sci. 2008, 110, 890–899. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Innovative Biofibers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ren, N.; Qiao, A.; Cui, M.; Huang, R.; Qi, W.; Su, R. Design and Fabrication of Nanocellulose-Based Microfibers by Wet Spinning. Chem. Eng. Sci. 2023, 282, 119320. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate Fibers as Photocatalyst Immobilizing Agents Applied in Hybrid Photocatalytic/Ultrafiltration Water Treatment Processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Neibert, K.; Gopishetty, V.; Grigoryev, A.; Tokarev, I.; Al-Hajaj, N.; Vorstenbosch, J.; Philip, A.; Minko, S.; Maysinger, D. Wound-Healing with Mechanically Robust and Biodegradable Hydrogel Fibers Loaded with Silver Nanoparticles. Adv. Healthc. Mater. 2012, 1, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, T.; Boguń, M.; Kurzak, A.; Szparaga, G. Zinc Alginate Fibres with a Tricalcium Phosphate (TCP) Nanoadditive. FIBRES Text. East. Eur. 2009, 17, 12–18. [Google Scholar]
- Mikołajczyk, T.; Boguń, M.; Rabiej, S.; Król, P. Zinc Alginate Fibres with a Silica (SiO2) Nanoadditive. FIBRES Text. East. Eur. 2010, 18, 83. [Google Scholar]
- Fahma, F.; Febiyanti, I.; Lisdayana, N.; Sari, Y.W.; Noviana, D.; Yunus, M.; Kadja, G.T.M.; Kusumaatmaja, A. Production of Polyvinyl Alcohol–Alginate–Nanocellulose Fibers. Starch Stärke 2022, 74, 2100032. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Wang, X.; Huang, R.; Zhang, L.; Hu, L. Preparation and Characterization of Alginate/Poly(Vinyl Alcohol) Blend Fibers. J. Macromol. Sci. Part A 2005, 42, 41–50. [Google Scholar] [CrossRef]
- Davydova, G.A.; Chaikov, L.L.; Melnik, N.N.; Gainutdinov, R.V.; Selezneva, I.I.; Perevedentseva, E.V.; Mahamadiev, M.T.; Proskurin, V.A.; Yakovsky, D.S.; Mohan, A.G.; et al. Polysaccharide Composite Alginate–Pectin Hydrogels as a Basis for Developing Wound Healing Materials. Polymers 2024, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhuikova, Y.V.; Zhuikov, V.A.; Khaydapova, D.D.; Lunkov, A.P.; Bonartseva, G.A.; Varlamov, V.P. Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan. Polymers 2024, 16, 1124. [Google Scholar] [CrossRef]
- Azevedo, F.F.; Cantarutti, T.A.; Remiro, P.D.F.R.; Barbieri, B.; Azoubel, R.A.; Nagahara, M.H.T.; Moraes, A.M.; Lima, M.H.M. Histological and Molecular Evidence of the Positive Performance of Glycerol-Plasticized Chitosan-Alginate Membranes on Skin Lesions of Hyperglycemic Mice. Polymers 2022, 14, 4754. [Google Scholar] [CrossRef]
- Zhuikova, Y.; Zhuikov, V.; Varlamov, V. Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review. Polymers 2022, 14, 5549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tsuruta, Y.; Itoyama, K.; Worakitkanchanakul, W.; Rujiravanit, R.; Tokura, S. Preparation of Chitosan Filament Applying New Coagulation System. Carbohydr. Polym. 2004, 56, 205–211. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, H.; Zheng, H.; Xu, Y.; Zhang, C. Structure and Properties of Blend Fibers Prepared from Alginate and Konjac Glucomannan. J. Appl. Polym. Sci. 2007, 106, 3903–3907. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Hou, F.; Zhu, K.; Xu, C.; Wang, C.; Wang, H. Preparation and Mechanism of Bio-Based Sodium Alginate Fibers with Flame Retardant and Antibacterial Properties. Polymers 2022, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Sun, Y.; Cui, Y.; Yang, W.; Lu, Z.; Shen, S.; Xia, Y.; Xiong, Z. Superhydrophobic and Flame-Retardant Alginate Fabrics Prepared through a One-Step Dip-Coating Surface-Treatment. Cellulose 2021, 28, 5973–5984. [Google Scholar] [CrossRef]
- Tian, G.; Ji, Q.; Xu, D.; Tan, L.; Quan, F.; Xia, Y. The Effect of Zinc Ion Content on Flame Retardance and Thermal Degradation of Alginate Fibers. Fibers Polym. 2013, 14, 767–771. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.; Sun, F.; Sun, L.; Wang, T.; Liu, Y.; Li, M. Flexible Wearable Graphene/Alginate Composite Non-Woven Fabric Temperature Sensor with High Sensitivity and Anti-Interference. Cellulose 2020, 27, 2369–2380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Var, C.; Palamutcu, S. Diverse Approaches in Wet-Spun Alginate Filament Production from the Textile Industry Perspective: From Process Optimization to Composite Filament Production. Polymers 2024, 16, 1817. https://doi.org/10.3390/polym16131817
Var C, Palamutcu S. Diverse Approaches in Wet-Spun Alginate Filament Production from the Textile Industry Perspective: From Process Optimization to Composite Filament Production. Polymers. 2024; 16(13):1817. https://doi.org/10.3390/polym16131817
Chicago/Turabian StyleVar, Cansu, and Sema Palamutcu. 2024. "Diverse Approaches in Wet-Spun Alginate Filament Production from the Textile Industry Perspective: From Process Optimization to Composite Filament Production" Polymers 16, no. 13: 1817. https://doi.org/10.3390/polym16131817





