A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
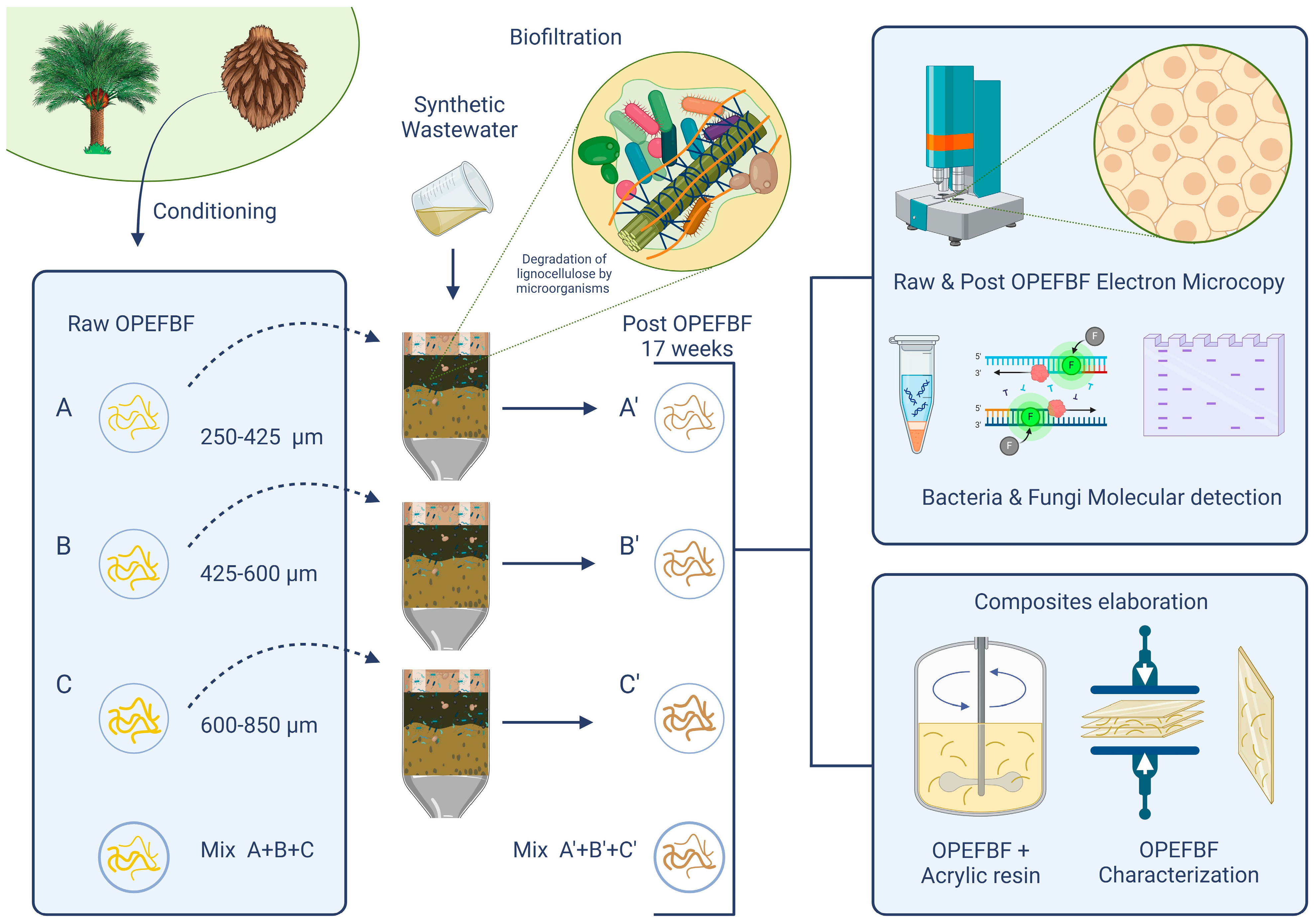
2.2. Oil Palm Empty Fruit Bunches Conditioning
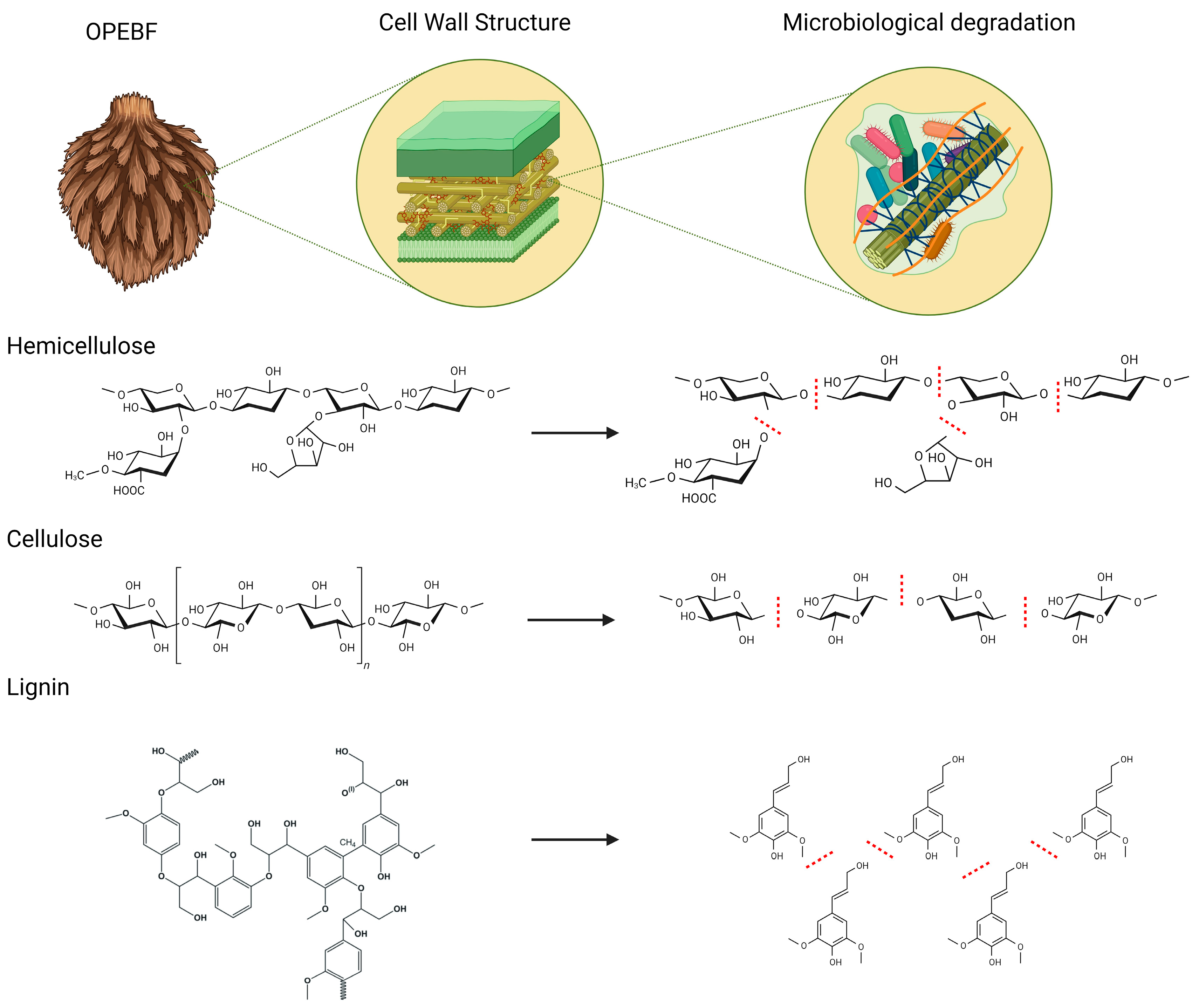
2.3. Utilization of Oil Palm Empty Fruit Bunches in Biofiltration Systems
2.4. Fabrication of the Composites
2.5. OPEFBF and Composites Characterization
2.5.1. Instrumental Characterization of R/P-OPEFBFs
2.5.2. Molecular Detection of Microorganisms in OPEFBF Composites
2.5.3. Composite Characterization
3. Results and Discussion
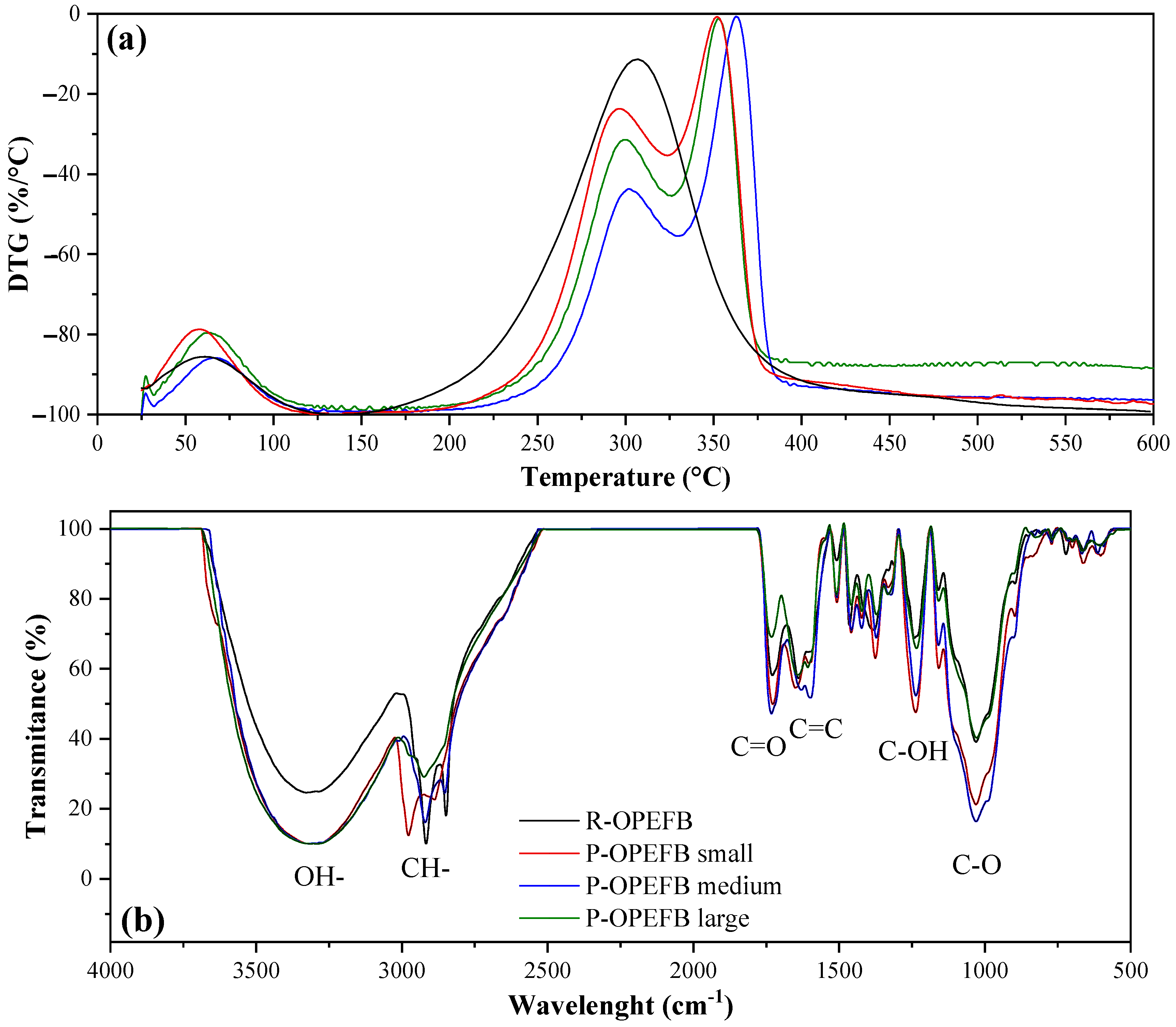
3.1. Raw and Post-OPEFBF Characterization
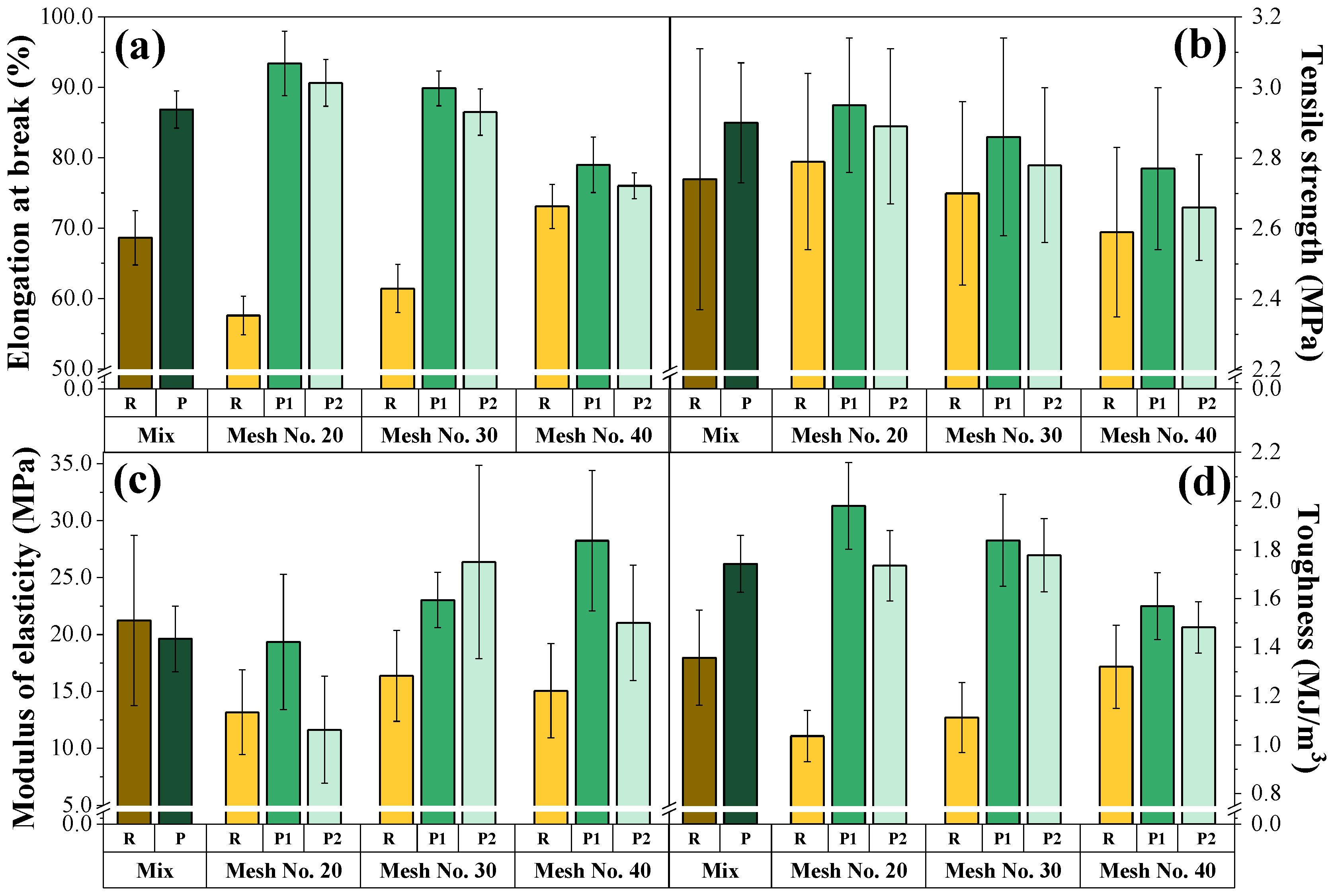
3.2. Composite Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kundu, D.; Dutta, D.; Samanta, P.; Dey, S.; Chhunji, K. Valorization of Wastewater: A Paradigm Shift towards Circular Bioeconomy and Sustainability. Sci. Total Environ. 2022, 848, 157709. [Google Scholar] [CrossRef]
- Qadir, M.; Cisneros, B.J.; Kim, Y.; Pramanik, A.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Frutos, M.; Tejedor, J.; Cuestas, J.; Valenzuela, F.; Rivadeneira, M.I.; Villamar, C.A.; Guerrero, V.H. Caffeine Adsorptive Performance and Compatibility Characteristics (Eisenia foetida Savigny) of Agro-Industrial Residues Potentially Suitable for Vermifilter Beds. Sci. Total Environ. 2021, 801, 149666. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean. Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Rial, J.B.; Ferreira, M.L. Potential Applications of Spent Adsorbents and Catalysts: Re-Valorization of Waste. Sci. Total Environ. 2022, 823, 153370. [Google Scholar] [CrossRef]
- Valle, V.; Aguilar, A.; Kreiker, J.; Raggiotti, B.; Cadena, F. Oil Palm Empty Fruit Bunch (OPEFB) Fiber-Reinforced Acrylic Thermoplastic Composites: Effect of Salt Fog Aging on Tensile, Spectrophotometric, and Thermogravimetric Properties. Int. J. Polym. Sci. 2022, 2022, 6372264. [Google Scholar] [CrossRef]
- Ching, K.S.; Ealid, M.; Ching, Y.C.; Haniff, M.; Khalid, M.; Beg, M.T.H. Preparation and Characterisation of Polyvinyl Alcohol/Oil Palm Empty Fruit Bunch Fibre Composite. Mat. Res. Innov. 2014, 18, S6–S364. [Google Scholar] [CrossRef]
- Binhussain, M.A.; El-Tonsy, M.M. Palm Leave and Plastic Waste Wood Composite for Out-Door Structures. Constr. Build. Mater. 2013, 47, 1431–1435. [Google Scholar] [CrossRef]
- Ravi, M.; Dubey, R.R.; Shome, A.; Guha, S.; Anil Kumar, C. Effect of Surface Treatment on Natural Fibers Composite. IOP Conf. Ser. Mater. Sci. Eng. 2016, 376, 012053. [Google Scholar] [CrossRef]
- Boey, J.Y.; Yusoff, S.B.; Tay, G.S. A Review on the Enhancement of Composite’s Interface Properties through Biological Treatment of Natural Fibre/Lignocellulosic Material. Polym. Polym. Compos. 2022, 30, 09673911221103600. [Google Scholar] [CrossRef]
- Kenned, J.J.; Sankaranarayanasamy, K.; Kumar, C.S. Chemical, Biological, and Nanoclay Treatments for Natural Plant Fiber-Reinforced Polymer Composites: A Review. Polym. Polym. Compos. 2021, 29, 1011–1038. [Google Scholar] [CrossRef]
- Kinvi-Dossou, G.; Matadi Boumbimba, R.; Bonfoh, N.; Garzon-Hernandez, S.; Garcia-Gonzalez, D.; Gerard, P.; Arias, A. Innovative Acrylic Thermoplastic Composites versus Conventional Composites: Improving the Impact Performances. Compos. Struct. 2019, 217, 1–13. [Google Scholar] [CrossRef]
- Obande, W.; Conchúr, M.O.; Ray, D. Continuous Fibre-Reinforced Thermoplastic Acrylic-Matrix Composites Prepared by Liquid Resin Infusion—A Review. Compos. B Eng. 2021, 215, 108771. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Ozkan, C.K.; Yilmaz, O. Synthesis and Application of Reactive Acrylic Latexes: Effect of Particle Morphology. Polymers 2022, 14, 2187. [Google Scholar] [CrossRef]
- Aguilar, A.D.; Tenemaza, K.; Valle, V.; Bastidas-Caldes, C.; Almeida-Naranjo, C.E.; Gutiérrez, P. A Two-Stage Analysis for the Role of Fiber Size in Terms of Length Distribution on the Performance under Accelerated Weathering Tests of Oil Palm Empty Fruit Bunch Fiber-Reinforced Vinyl Acrylic Composites. Polym. Compos. 2023, 45, 2232–2252. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Espinoza-Montero, P.J.; Muñoz-Rodríguez, M.I.; Villamar-Ayala, C.A. Hydraulic Retention Time Influence on Improving Flocculation in the Activated Sludge Processes Through Polyelectrolytes. Water Air Soil. Pollut. 2017, 228, 253. [Google Scholar] [CrossRef]
- Tejedor, J.; Cóndor, V.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar, C.A. Performance of Wood Chips/Peanut Shells Biofilters Used to Remove Organic Matter from Domestic Wastewater. Sci. Total Environ. 2020, 738, 139589. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing Materials (ASTM D4442-2020) Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2020.
- American Society for Testing Materials (ASTM D 1107-2021) Standard Test Method for Ethanol-Toluene Solubility of Wood. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2021.
- American Society for Testing Materials (ASTM D1110-21)—Standard Test Methods for Water Solubility of Wood. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2021.
- American Society for Testing Materials (ASTM D 1106-2021) Standard Test Method for Acid-Insoluble Lignin in Wood. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2021.
- American Society for Testing Materials (ASTM D1109-84(2021)) Standard Test Method for 1% Sodium Hydroxide Solubility of Wood. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2021.
- American Society for Testing Materials (ASTM E 872-19) Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2019.
- Calvopiña, M.; Toro, M.; Bastidas-Caldes, C.; Vasco-Julio, D.; Muñoz, G. A Fatal Case of Disseminated Histoplasmosis by Histoplasma Capsulatum Var. Capsulatum Misdiagnosed as Visceral Leishmaniasis—Molecular Diagnosis and Identification. Pathogens 2023, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lee, C.C.; Lin, Y.L.; Yin, K.M.; Ho, C.L.; Liu, T. Obtaining Long 16S RDNA Sequences Using Multiple Primers and Its Application on Dioxin-Containing Samples. BMC Bioinform. 2015, 16, S13. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Julio, D.; Huilca-Ibarra, M.; Ledesma, Y.; Echeverria, G.; Guerrero-Freire, S.; Jagielski, T.; Bastidas-Caldes, C.; de Waard, J.H. The Development of a Multiplex PCR Assay for Fast and Cost-Effective Identification of the Five Most Significant Pathogenic Prototheca Species. Pathogens 2023, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing Materials (ASTM D 638-2022 Standard Test Method for Tensile Properties of Plastics. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2022.
- Almeida-Naranjo, C.E.; Valle, V.; Aguilar, A.; Cadena, F.; Kreiker, J.; Raggiotti, B. Water Absorption Behavior of Oil Palm Empty Fruit Bunch. Materials 2022, 15, 5015. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Article Biological Characterization and Instrumental Analytical Comparison of Two Biorefining Pretreatments for Water Hyacinth (Eicchornia crassipes) Biomass Hydrolysis. Sustainability 2021, 13, 245. [Google Scholar] [CrossRef]
- Yi, W.; Nadeem, F.; Xu, G.; Zhang, Q.; Joshee, N.; Tahir, N. Modifying Crystallinity, and Thermo-Optical Characteristics of Paulownia Biomass through Ultrafine Grinding and Evaluation of Biohydrogen Production Potential. J. Clean. Prod. 2020, 269, 122386. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, E.; Ntaikou, I.; Lyberatos, G. Fungal Pretreatment of Willow Sawdust and Its Combination with Alkaline Treatment for Enhancing Biogas Production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gurovic, M.S.V.; Viceconte, F.R.; Bidegain, M.A.; Dietrich, J. Regulation of Lignocellulose Degradation in Microorganisms. J. Appl. Microbiol. 2023, 134, lxac002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, M.H.; Lin, Y.; Fu, Z. Physicochemical Properties of Arenga pinnata (Wurmb.) Merr Starch: Effect of High-Speed Jet Treatment. Int. J. Food Prop. 2019, 22, 477–486. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, F.; Shi, F.; Zhang, N.; Zhang, H. Pyrolysis Characteristics and Kinetics of Prunus avium L. Leaves Using Thermogravimetric Analysis. Bioresourse 2023, 18, 7320–7332. [Google Scholar] [CrossRef]
- Lubbers, R.J.M.; Dilokpimol, A.; Visser, J.; De Vries, R.P. Aspergillus Niger Uses the Peroxisomal CoA-Dependent β-Oxidative Genes to Degrade the Hydroxycinnamic Acids Caffeic Acid, Ferulic Acid, and p-Coumaric Acid. Appl. Genet. Mol. Biotechnol. 2021, 105, 4199–4211. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent Developments on Solid-State Fermentation for Production of Microbial Secondary Metabolites: Challenges and Solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.D.; Valle, V.; Almeida-Naranjo, C.E.; Naranjo, Á.; Cadena, F.; Kreiker, J.; Raggiotti, B. Characterization Dataset of Oil Palm Empty Fruit Bunch (OPEFB) Fibers—Natural Reinforcement/Filler for Materials Development. Data Brief. 2022, 45, 108618. [Google Scholar] [CrossRef] [PubMed]
- Šuchová, K.; Fehér, C.; Ravn, J.L.; Bedő, S.; Biely, P.; Geijer, C. Cellulose-and Xylan-Degrading Yeasts: Enzymes, Applications and Biotechnological Potential. Biotechnol. Adv. 2022, 59, 107981. [Google Scholar] [CrossRef] [PubMed]
- Tanasă, F.; Zănoagă, M.; Teacă, C.A.; Nechifor, M.; Shahzad, A. Modified Hemp Fibers Intended for Fiber-Reinforced Polymer Composites Used in Structural Applications—A Review. I. Methods of Modification. Polym. Compos. 2020, 41, 5–31. [Google Scholar] [CrossRef]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of Biofilms on the Conversion of Cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Baramee, S.; Siriatcharanon, A.; Ketbot, P.; Teeravivattanakit, T.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Phitsuwan, P. Biological Pretreatment of Rice Straw with Cellulase-Free Xylanolytic Enzyme-Producing Bacillus Firmus K-1: Structural Modification and Biomass Digestibility. Renew. Energy 2020, 160, 555–563. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, S.; Singh, S.; Arora, A.; Kaushik, R.; Agrawal, V.V.; Saxena, A.K.; Nain, L. Biological Delignification of Paddy Straw and Parthenium Sp. Using a Novel Micromycete Myrothecium Roridum LG7 for Enhanced Saccharification. Bioresour. Technol. 2013, 135, 7–11. [Google Scholar] [CrossRef]
- Li, G.; Chen, H. Synergistic Mechanism of Steam Explosion Combined with Fungal Treatment by Phellinus baumii for the Pretreatment of Corn Stalk. Biomass Bioenergy 2014, 67, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Li, T. New Insights Into Microbial Induced Calcium Carbonate Precipitation Using Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 904095. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of Potassium Solubilizing Microbes in Sustainable Food Production System: A Review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; Cole, B.J.W.; Howell, C.; Gardner, D.J.; Wang, J. Fungal and Enzymatic Pretreatments in Hot-Pressed Lignocellulosic Bio-Composites: A Critical Review. J. Clean. Prod. 2022, 353, 131659. [Google Scholar] [CrossRef]
- Sze, Y.S.; Aris, A.; Zaidi, N.S.; Bahrodin, M.B. Performance of Sand Filtration System with Different Sand Bed Depth for Polishing Wastewater Treatment. J. Environ. Treat. Tech. 2021, 9, 451–456. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Naranjo, C.E.; Aguilar, A.D.; Valle, V.; Bastidas-Caldes, C.; Debut, A.; Sinchiguano, B. A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements. Polymers 2024, 16, 1822. https://doi.org/10.3390/polym16131822
Almeida-Naranjo CE, Aguilar AD, Valle V, Bastidas-Caldes C, Debut A, Sinchiguano B. A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements. Polymers. 2024; 16(13):1822. https://doi.org/10.3390/polym16131822
Chicago/Turabian StyleAlmeida-Naranjo, Cristina E., Alex Darío Aguilar, Vladimir Valle, Carlos Bastidas-Caldes, Alexis Debut, and Britanny Sinchiguano. 2024. "A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements" Polymers 16, no. 13: 1822. https://doi.org/10.3390/polym16131822
APA StyleAlmeida-Naranjo, C. E., Aguilar, A. D., Valle, V., Bastidas-Caldes, C., Debut, A., & Sinchiguano, B. (2024). A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements. Polymers, 16(13), 1822. https://doi.org/10.3390/polym16131822








