Dispersion Characteristics, the Mechanical, Thermal Stability, and Durability Properties of Epoxy Nanocomposites Reinforced with Carbon Nanotubes, Graphene, or Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GO Sheet Synthesis
2.3. Freeze-Dried Graphene Oxide
2.4. Nanofiller Dispersion
2.5. Fabrication of Epoxy Nanocomposites
2.6. Characterization
3. Results and Discussion
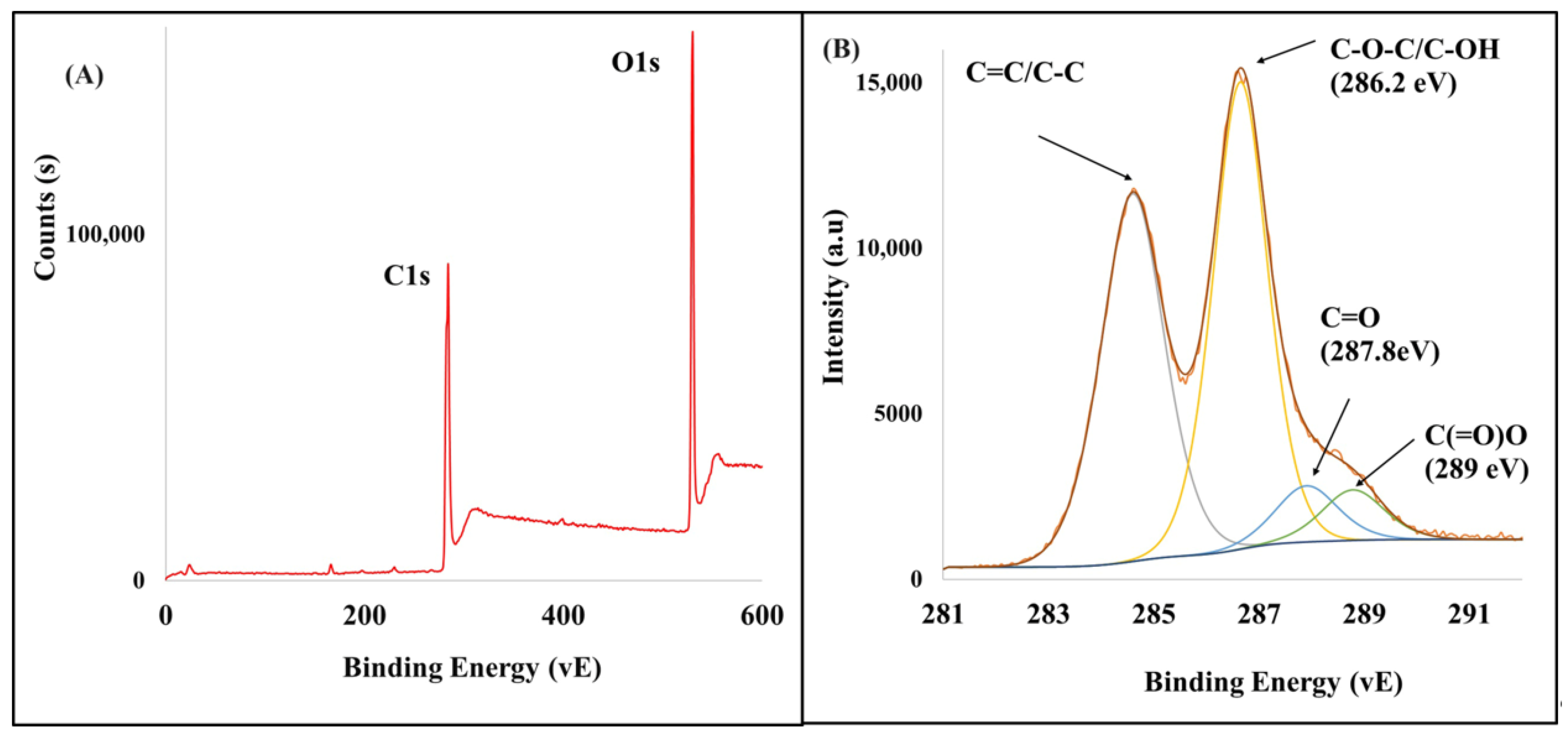
3.1. Characterization of As-Synthesized GO
3.2. Dispersion Validation
3.3. Tensile Properties
| Samples | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) | Toughness a (MJ m−3) |
|---|---|---|---|---|
| Pure EP | 37.2 (±3.1) | 2.1 (±0.2) | 1.81(±0.24) | 0.66 ± 0.05 |
| EP/CNT 0.2 | 41.5 (±1.9) | 2.7 (±0.1) | 2.65 (±0.13) | 1.10 ± 0.03 |
| EP/CNT 0.4 | 47.8 (±2.4) | 2.8 (±0.1) | 2.71 (±0.11) | 1.30 ± 0.04 |
| EP/CNT 0.6 | 46.2 (±2.6) | 2.9 (±0.2) | 2.67 (±0.31) | 1.23 ± 0.05 |
| EP/GNP 0.2 | 47.3 (±1.1) | 2.5 (±0.1) | 2.71 (±0.23) | 1.28 ± 0.02 |
| EP/GNP 0.4 | 49.2 (±2.3) | 2.6 (±0.2) | 2.83 (±0.17) | 1.39 ± 0.04 |
| EP/GNP 0.6 | 53.6 (±1.4) | 2.7 (±0.1) | 2.90 (±0.15) | 1.55 ± 0.05 |
| EP/GO 0.4 | 55.4 (±2.7) | 2.6 (±0.1) | 3.11 (±0.23) | 1.72 ± 0.03 |
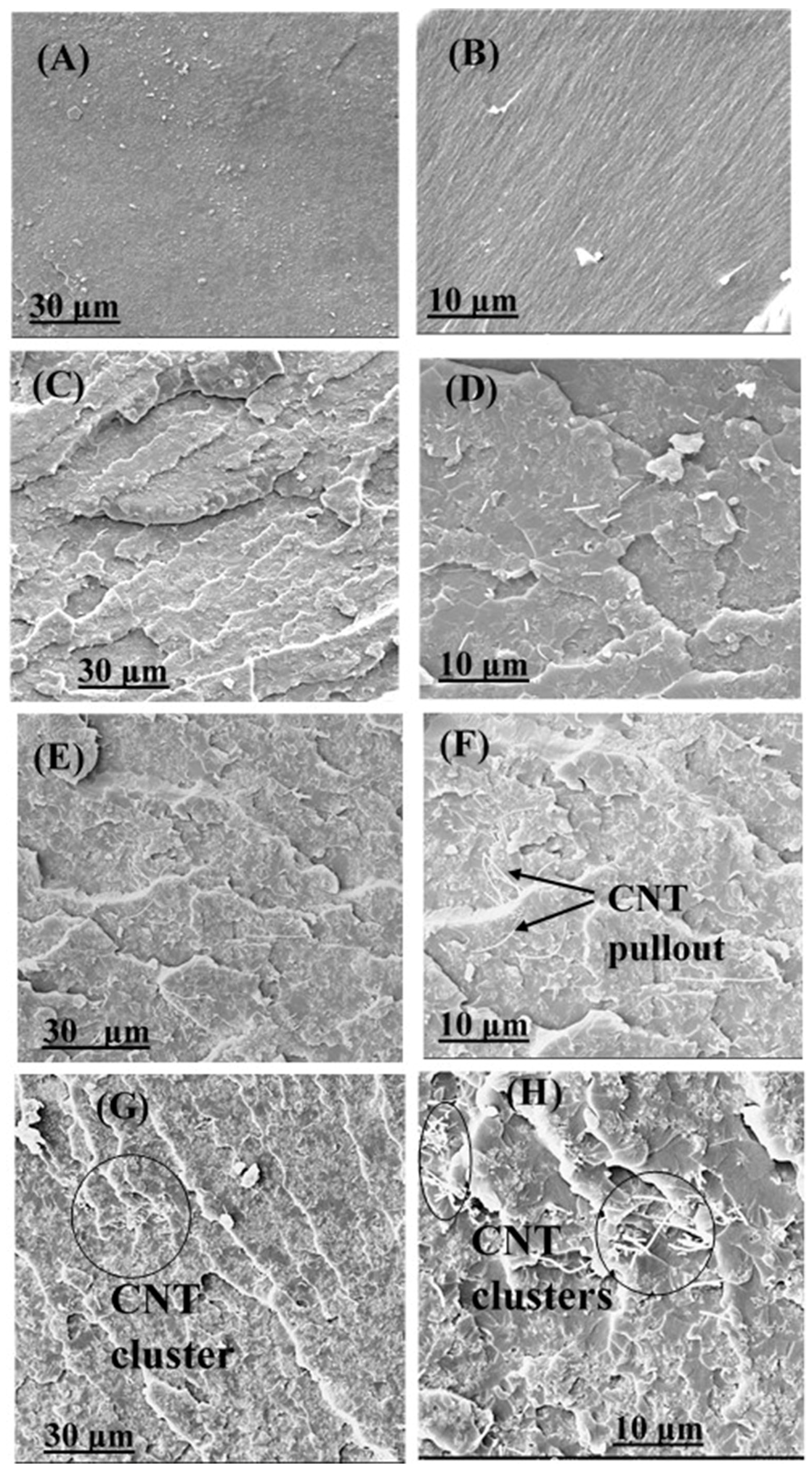
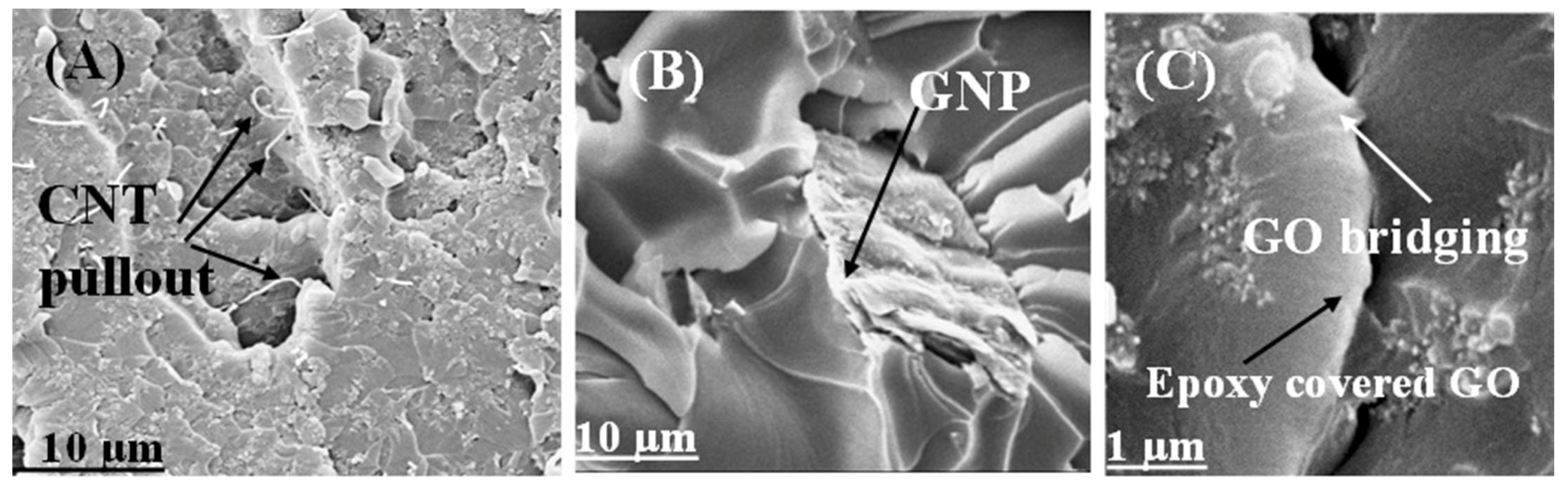
3.4. Fractography and Microstructure Analysis
3.5. Secondary Mechanisms to Improve the Mechanical Characteristics of the Nanocomposites
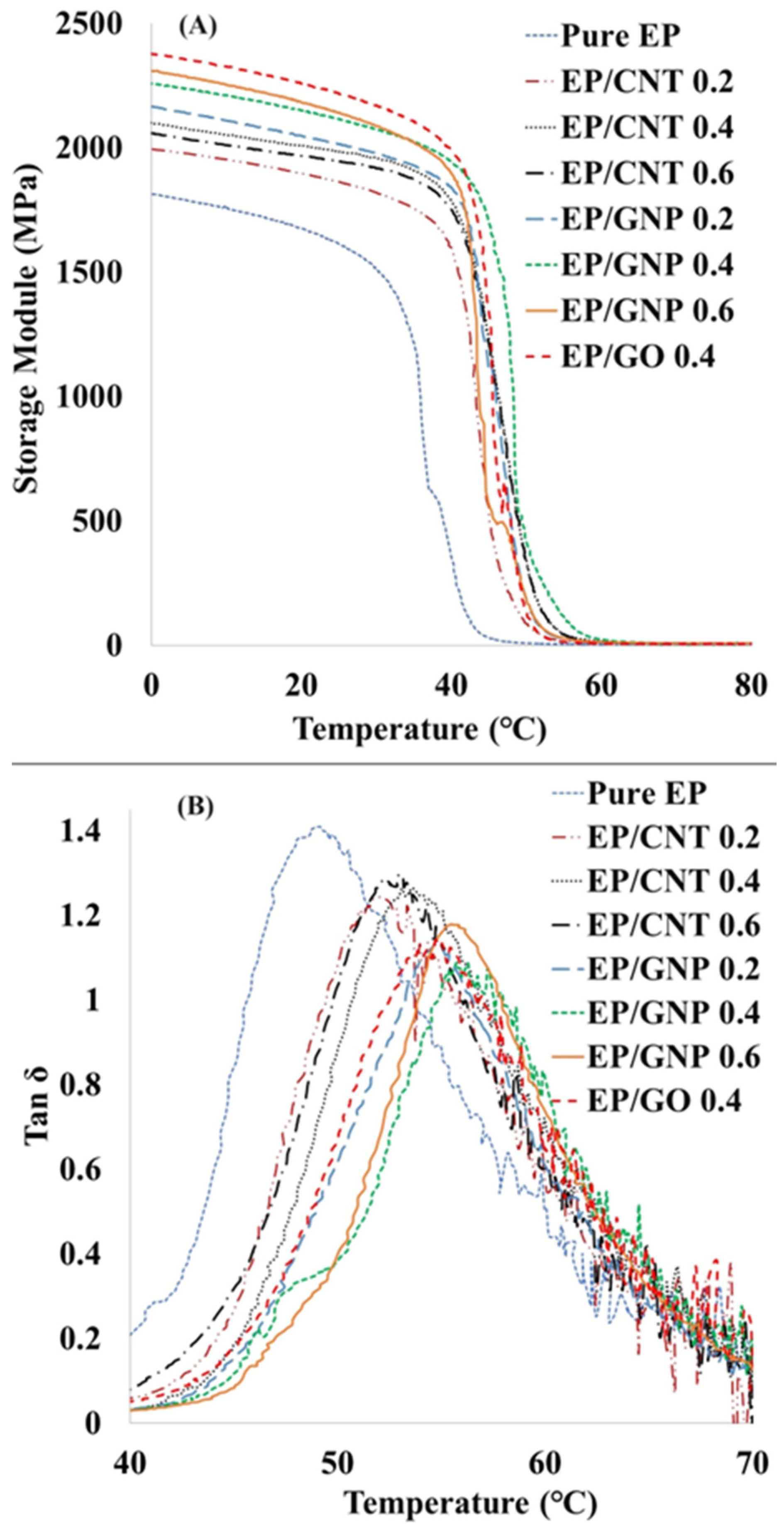
3.6. Thermomechanical Properties
| Sample | Pure EP | EP/CNT 0.2 | EP/CNT 0.4 | EP/CNT 0.6 | EP/GNP 0.2 | EP/GNP 0.4 | EP/GNP 0.6 | EP/GO 0.4 |
|---|---|---|---|---|---|---|---|---|
| Storage modulus at 0 °C (MPa) | 1810 | 1990 | 2100 | 2055 | 2160 | 2247 | 2300 | 2353 |
| Tg (°C) | 49 | 52 | 53 | 54 | 54 | 55 | 56 | 54 |
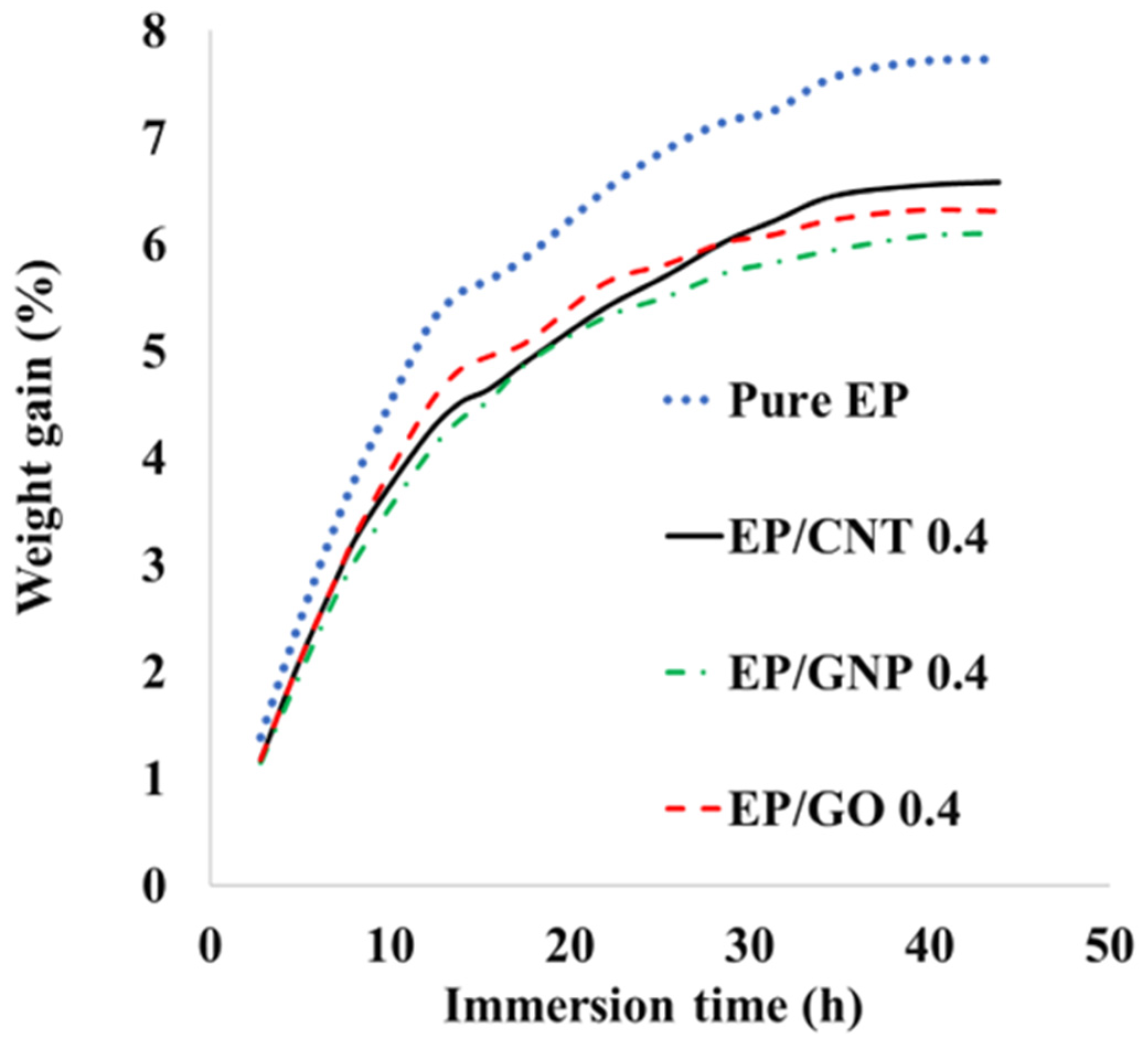
3.7. Moisture Uptake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, A.; Budholiya, S.; Raj, S.A.; Sultan, M.T.H.; Hui, D.; Shah, A.U.M.; Safri, S.N.A. Review on nanocomposites based on aerospace applications. Nanotechnol. Rev. 2021, 10, 237–253. [Google Scholar] [CrossRef]
- Shukla, P.; Saxena, P. Polymer Nanocomposites in Sensor Applications: A Review on Present Trends and Future Scope. Chin. J. Polym. Sci. 2021, 39, 665–691. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021, 16, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pour, Z.S.; Ghaemy, M. Polymer grafted graphene oxide: For improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Compos. Sci. Technol. 2016, 136, 145–157. [Google Scholar] [CrossRef]
- Kiersnowska, A.; Fabianowski, W.; Koda, E. The Influence of the Accelerated Aging Conditions on the Properties of Polyolefin Geogrids Used for Landfill Slope Reinforcement. Polymers 2020, 12, 1874. [Google Scholar] [CrossRef] [PubMed]
- Chalot, A.; Michel, L.; Ferrier, E. Experimental study of external bonded CFRP-concrete interface under low cycle fatigue loading. Compos. Part B Eng. 2019, 177, 107255. [Google Scholar] [CrossRef]
- Benmokrane, B.; Chaallal, O.; Masmoudi, R. Glass fibre reinforced plastic (GFRP) rebars for concrete structures. Constr. Build. Mater. 1995, 9, 353–364. [Google Scholar] [CrossRef]
- Benmokrane, B.; Mehany, S.; Shield, C.; Nanni, A.; Brown, V. Physical Properties, Longitudinal Tensile Properties, and Bond Strength of the New Generation of GFRP Bars. J. Compos. Constr. 2023, 27. [Google Scholar] [CrossRef]
- ACI 440.1R-15; Guide for the Design and Construction of Structural Concrete Reinforced with Fiber-Reinforced Polymer (FRP) Bars. American Concrete Institute: Farmington Hills, MI, USA, 2015.
- Mosallam, A.S. Polymer Composites in Construction: An Overview. SOJ Mater. Sci. Eng. 2014, 2, 1–25. [Google Scholar] [CrossRef]
- ACI 440.2 R-17; Guide for the Design and Construction of Externally Bonded FRP Systems for Strengthening Concrete Structures. American Concrete Institute: Farmington Hills, MI, USA, 2017.
- Mirzapour, A.; Asadollahi, M.H.; Baghshaei, S.; Akbari, M. Effect of nanosilica on the microstructure, thermal properties and bending strength of nanosilica modified carbon fiber/phenolic nanocomposite. Compos. Part A Appl. Sci. Manuf. 2014, 63, 159–167. [Google Scholar] [CrossRef]
- Gauvin, F.; Cousin, P.; Robert, M. Effect of modified graphene oxide on the mechanical, thermal, and barrier properties of vinylester. J. Compos. Mater. 2018, 52, 3853–3864. [Google Scholar] [CrossRef]
- Elsayed, T.A.; Eldaly, A.M.; El-Hefnawy, A.A.; Ghanem, G.M. Behaviour of Concrete Beams Reinforced with Hybrid Fiber Reinforced Bars. Adv. Compos. Mater. 2011, 20, 245–259. [Google Scholar] [CrossRef]
- You, Y.-J.; Park, Y.-H.; Kim, H.-Y.; Park, J.-S. Hybrid effect on tensile properties of FRP rods with various material compositions. Compos. Struct. 2007, 80, 117–122. [Google Scholar] [CrossRef]
- Rudenko, A.; Biryukov, A.; Kerzhentsev, O.; Fediuk, R.; Vatin, N.; Vasilev, Y.; Klyuev, S.; Amran, M.; Szelag, M. Nano- and Micro-Modification of Building Reinforcing Bars of Various Types. Crystals 2021, 11, 323. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rhee, K.Y. Recent advances in basalt-fiber-reinforced composites: Tailoring the fiber-matrix interface. Compos. Part B Eng. 2020, 192, 108011. [Google Scholar] [CrossRef]
- Gauvin, F.; Robert, M. Durability study of vinylester/silicate nanocomposites for civil engineering applications. Polym. Degrad. Stab. 2015, 121, 359–368. [Google Scholar] [CrossRef]
- Ji, J.-H.; Lee, G.; Koh, J.-H. Synthesis of a nitrogen doped reduced graphene oxide based ceramic polymer composite nanofiber film for wearable device applications. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Seidel, C.; Schulte, K. Preparation and characterization of graphite nano-platelet (GNP)/epoxy nano-composite: Mechanical, electrical and thermal properties. Eur. Polym. J. 2013, 49, 3878–3888. [Google Scholar] [CrossRef]
- Manoli, A.; Ghadge, R.; Kumar, P. Effect of Surface Roughness on the Fatigue Strength of E-Glass Composite Single Lap Joint Bonded with Modified Graphene Oxide-Epoxy Adhesive. Mater. Phys. Mech. 2023, 51, 65–80. [Google Scholar] [CrossRef]
- Eslami, Z.; Yazdani, F.; Mirzapour, M.A. Thermal and mechanical properties of phenolic-based composites reinforced by carbon fibres and multiwall carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2015, 72, 22–31. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of Graphene by Exfoliation of Graphite Us-ing Wet Ball Milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Johnsen, B.; Kinloch, A.; Mohammed, R.; Taylor, A.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2006, 48, 530–541. [Google Scholar] [CrossRef]
- Eslami, Z.; Mirzapour, M. Compatibilizing effect and reinforcing efficiency of nanosilica on ethylene-propylene diene monomer/chloroprene rubber blends. Polym. Compos. 2021, 42, 1809–1817. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Enhanced mechanical properties of silanized silica nanoparticle attached graphene oxide/epoxy composites. Compos. Sci. Technol. 2013, 79, 115–125. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Yavari, F.; Rafiee, M.A.; Rafiee, J.; Yu, Z.-Z.; Koratkar, N. Dramatic Increase in Fatigue Life in Hierarchical Graphene Composites. ACS Appl. Mater. Interfaces 2010, 2, 2738–2743. [Google Scholar] [CrossRef]
- Oun, A.; Manalo, A.; Alajarmeh, O.; Abousnina, R.; Gerdes, A. Long-Term Water Absorption of Hybrid Flax Fibre-Reinforced Epoxy Composites with Graphene and Its Influence on Mechanical Properties. Polymers 2022, 14, 3679. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Schulte, K. Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites—A comparative study. Compos. Sci. Technol. 2005, 65, 2300–2313. [Google Scholar] [CrossRef]
- Shukla, M.K.; Sharma, K. Improvement in mechanical and thermal properties of epoxy hybrid composites by functionalized graphene and carbon-nanotubes. Mater. Res. Express 2019, 6, 125323. [Google Scholar] [CrossRef]
- Jen, Y.-M.; Chang, H.-H.; Lu, C.-M.; Liang, S.-Y. Temperature-Dependent Synergistic Effect of Multi-Walled Carbon Nanotubes and Graphene Nanoplatelets on the Tensile Quasi-Static and Fatigue Properties of Epoxy Nanocomposites. Polymers 2020, 13, 84. [Google Scholar] [CrossRef]
- Maldonado-Magnere, S.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Thermally Reduced Graphene Oxide/Thermoplastic Polyurethane Nanocomposites: Mechanical and Barrier Properties. Polymers 2020, 13, 85. [Google Scholar] [CrossRef]
- Moriche, R.; Prolongo, S.; Sánchez, M.; Jiménez-Suárez, A.; Sayagués, M.; Ureña, A. Morphological changes on graphene nanoplatelets induced during dispersion into an epoxy resin by different methods. Compos. Part B Eng. 2015, 72, 199–205. [Google Scholar] [CrossRef]
- Naeem, M.; Kuan, H.-C.; Michelmore, A.; Meng, Q.; Qui, A.; Aakyiir, M.; Losic, D.; Zhu, S.; Ma, J. A New Method for Preparation of Functionalized Graphene and Its Epoxy Nanocomposites. Composites Part B Engineering 2020, 196, 108096. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, H.; Jing, H.; Ren, Q.; Liu, Z.; Gao, Z.; Ban, Q. Graphene dispersed by pyrene-terminated polyethylene glycol for reinforced epoxy composites. J. Appl. Polym. Sci. 2022, 139, 52110. [Google Scholar] [CrossRef]
- Xue, G.; Xing, J.; Sun, M.; Zhang, X.; Liu, C.; Xue, S.; Yuan, Z.; Zhang, B. In situ exfoliation and surface functionalization of graphene oxide for epoxy composites with improved thermal and mechanical properties. Polym. Compos. 2023, 45, 1826–1838. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Mann, J.A.; Dichtel, W.R. Noncovalent Functionalization of Graphene by Molecular and Polymeric Adsorbates. J. Phys. Chem. Lett. 2013, 4, 2649–2657. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Kamal, A.; Otabil, A.; Mamoun, O.; Liao, K. Graphene/Epoxy Nanocomposites for Improved Fracture Toughness: A Focused Review on Toughening Mechanism. Chem. Eng. J. Adv. 2023, 16, 100537. [Google Scholar] [CrossRef]
- Nadler, M.; Werner, J.; Mahrolz, T.; Riedel, U.; Hufenbach, W. Effect of CNT surface functionalization on the mechanical properties of multi walled carbon nanotube/epoxy-composites. Compos. A 2009, 40, 932–937. [Google Scholar] [CrossRef]
- La, L.B.T.; Nguyen, H.; Tran, L.C.; Su, X.; Meng, Q.; Kuan, H.-C.; Ma, J. Exfoliation and dispersion of graphene nanoplatelets for epoxy nanocomposites. Adv. Nanocomposites 2024, 1, 39–51. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Y.; Xia, W.; Xu, L.; Wang, X. Dispersion characteristics and mechanical properties of epoxy nanocomposites reinforced with carboxymethyl cellulose functionalized nanodiamond, carbon nanotube, and graphene. Polym. Compos. 2023, 45, 398–412. [Google Scholar] [CrossRef]
- Varghese, A.S.; Sreekanth, M.S. Investigation on the effect of multiwalled carbon nanotubes in carbon fiber-reinforced epoxy composites manufactured using vacuum infusion process and hand layup process followed by vacuum bagging. Polym. Compos. 2023, 45, 1600–1618. [Google Scholar] [CrossRef]
- Mirabedini, A.; Anderson, L.; Antiohos, D.; Ang, A.; Nikzad, M.; Fuss, F.K.; Hameed, N. Scalable Production and Thermoelectrical Modeling of Infusible Functional Graphene/Epoxy Nanomaterials for Engineering Applications. Ind. Eng. Chem. Res. 2022, 61, 5141–5157. [Google Scholar] [CrossRef]
- Naeem, M.; Kuan, H.-C.; Michelmore, A.; Yu, S.; Mouritz, A.P.; Chelliah, S.S.; Ma, J. Epoxy/graphene nanocomposites prepared by in-situ microwaving. Carbon 2021, 177, 271–281. [Google Scholar] [CrossRef]
- Mirzapour, M.; Robert, M.; Benmokrane, B. Vinyl-ester nanocomposites based on a binary-solvent system for well-exfoliated graphene and enhanced performance. J. Reinf. Plast. Compos. 2024. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D-638; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. Annual Book of ASTM Standards: West Conshohocken, PA, USA, 2014; pp. 1–13.
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Kotsyubynsky, V.O.; Boychuk, V.M.; Budzulyak, I.M.; Rachiy, B.I.; Hodlevska, M.A.; Kachmar, A.I. Graphene oxide synthesis using modified Tour method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035006. [Google Scholar] [CrossRef]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive Fatigue Life and Fracture Toughness Improvements in Graphene Oxide/Epoxy Composites. Macromolecules 2011, 45, 238–245. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; George, G.; Motta, N.; Waclawik, E.; Yan, C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, D.S. Effects of an aminosilane and a tetra-functional epoxy on the physical properties of di-functional epoxy/graphene nanoplatelets nanocomposites. Polym. Eng. Sci. 2013, 54, 969–976. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Tiwari, A.; Tiwari, A. Spectroscopic And Morphological Analysis Of Graphene Vinylester Nanocomposites. Adv. Mater. Lett. 2013, 4, 656–661. [Google Scholar] [CrossRef]
- Zegeye, E.; Ghamsari, A.K.; Woldesenbet, E. Mechanical properties of graphene platelets reinforced syntactic foams. Compos. Part B Eng. 2014, 60, 268–273. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2012, 62, 33–40. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Y. Dispersion characterizations and adhesion properties of epoxy composites reinforced by carboxymethyl cellulose surface treated carbon nanotubes. Powder Technol. 2022, 404. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, Conductive, and Processable Multifunctional Graphene Nanosheets Coated with High-Density Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef]
- Guimont, A.; Beyou, E.; Martin, G.; Sonntag, P.; Cassagnau, P. Viscoelasticity of Graphite Oxide-Based Suspensions in PDMS. Macromolecules 2011, 44, 3893–3900. [Google Scholar] [CrossRef]
- Corezzi, S.; De Michele, C.; Zaccarelli, E.; Tartaglia, P.; Sciortino, F. Connecting Irreversible to Reversible Aggregation: Time and Temperature. J. Phys. Chem. B 2009, 113, 1233–1236. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites – fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, S. Enhancing polymer performance through graphene sheets. J. Appl. Polym. Sci. 2010, 119, 3670–3674. [Google Scholar] [CrossRef]
- Mirzapour, M.; Robert, M.; Benmokrane, B. In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading. C 2024, 10, 52. [Google Scholar] [CrossRef]
- Zhao, Q.; Hoa, S.V. Toughening Mechanism of Epoxy Resins with Micro/Nano Particles. J. Compos. Mater. 2006, 41, 201–219. [Google Scholar] [CrossRef]
- Razavi, N.; Ayatollahi, M.; Giv, A.N.; Khoramishad, H. Single lap joints bonded with structural adhesives reinforced with a mixture of silica nanoparticles and multi walled carbon nanotubes. Int. J. Adhes. Adhes. 2018, 80, 76–86. [Google Scholar] [CrossRef]
- Shah, W.A.; Luo, X.; Yang, Y.Q. Microstructure, mechanical, and thermal properties of graphene and carbon nanotube-reinforced Al2O3 nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 13656–13672. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Kotaki, M.; He, C. Preparation, microstructure and thermal mechanical properties of epoxy/crude clay nanocomposites. Compos. Part A: Appl. Sci. Manuf. 2007, 38, 192–197. [Google Scholar] [CrossRef]
- Bhandakkar, A.; Kumar, N.; Prasad, R.C.; Sastry, S.M.L. Interlaminar Fracture Toughness of Epoxy Glass Fiber Fly Ash Laminate Composite. Mater. Sci. Appl. 2014, 05, 231–244. [Google Scholar] [CrossRef]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/epoxy composites. Mater. Des. 2014, 66, 142–149. [Google Scholar] [CrossRef]
- Li, W.; Dichiara, A.; Bai, J. Carbon nanotube–graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Jen, Y.-M.; Huang, J.-C.; Zheng, K.-Y. Synergistic Effect of Multi-Walled Carbon Nanotubes and Graphene Nanoplatelets on the Monotonic and Fatigue Properties of Uncracked and Cracked Epoxy Composites. Polymers 2020, 12, 1895. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Park, Y.T.; Qian, Y.; Chan, C.; Suh, T.; Nejhad, M.G.; Macosko, C.W.; Stein, A. Epoxy Toughening with Low Graphene Loading. Adv. Funct. Mater. 2014, 25, 575–585. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Cui, J.; Yan, Y.; Liu, J.; Wu, Q. Phenolic Resin-MWNT Nanocomposites Prepared through an in situ Polymerization Method. Polym. J. 2008, 40, 1067–1073. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Fracture and Fatigue in Graphene Nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.A.; Ridzuan, M.J.M.; Majid, M.S.A.; Sapuan, S.M.; Shahriman, A.B.; Mat, F. Effect of Water Absorption on Graphene Nanoplatelet and Multiwalled Carbon Nanotubes-impregnated Glass Fibre-Reinforced Epoxy Composites. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1802–1816. [Google Scholar] [CrossRef]
- Zulfli, N.M.; Abu Bakar, A.; Chow, W. Mechanical and water absorption behaviors of carbon nanotube reinforced epoxy/glass fiber laminates. J. Reinf. Plast. Compos. 2013, 32, 1715–1721. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of enhanced hydrophilic and hydrophobic graphene oxide nanosheets by a solvothermal method. Carbon 2008, 47, 68–72. [Google Scholar] [CrossRef]





| Nanoparticle | Multi-Wall CNT | Graphene Nanoplate |
|---|---|---|
| Morphology | Cylindrical | Planar |
| Purity (%) | >95 | >99 |
| Dimensions |
Diameter: 50~100 nm Length: 5~20 μm |
Diameter: 5~15 μm Thickness: 6~8 nm |
| Density (g/cm3) | 1.9 | 2.1 |
| Surface area (m2/g) | >70 | 80 |
| Specimen | Nanofiller (wt%) | Epoxy/Hardener (g) |
|---|---|---|
| Pure epoxy (EP) | 0 | 100 |
| EP/CNT 0.2 | 0.2 | 100 |
| EP/CNT 0.4 | 0.4 | 100 |
| EP/CNT 0.6 | 0.6 | 100 |
| EP/GNP 0.2 | 0.2 | 100 |
| EP/GNP 0.4 | 0.4 | 100 |
| EP/GNP 0.6 | 0.6 | 100 |
| EP/GO 0.4 | 0.4 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzapour, M.; Cousin, P.; Robert, M.; Benmokrane, B. Dispersion Characteristics, the Mechanical, Thermal Stability, and Durability Properties of Epoxy Nanocomposites Reinforced with Carbon Nanotubes, Graphene, or Graphene Oxide. Polymers 2024, 16, 1836. https://doi.org/10.3390/polym16131836
Mirzapour M, Cousin P, Robert M, Benmokrane B. Dispersion Characteristics, the Mechanical, Thermal Stability, and Durability Properties of Epoxy Nanocomposites Reinforced with Carbon Nanotubes, Graphene, or Graphene Oxide. Polymers. 2024; 16(13):1836. https://doi.org/10.3390/polym16131836
Chicago/Turabian StyleMirzapour, Miraidin, Patrice Cousin, Mathieu Robert, and Brahim Benmokrane. 2024. "Dispersion Characteristics, the Mechanical, Thermal Stability, and Durability Properties of Epoxy Nanocomposites Reinforced with Carbon Nanotubes, Graphene, or Graphene Oxide" Polymers 16, no. 13: 1836. https://doi.org/10.3390/polym16131836






