Study on the Performance of Aniline Electrodeposited on MnO2 Nanowire as an Anode for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of MnO2 Nanowires on Carbon Cloth
2.2. Preparation of MnO2/PANI Nanowires
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
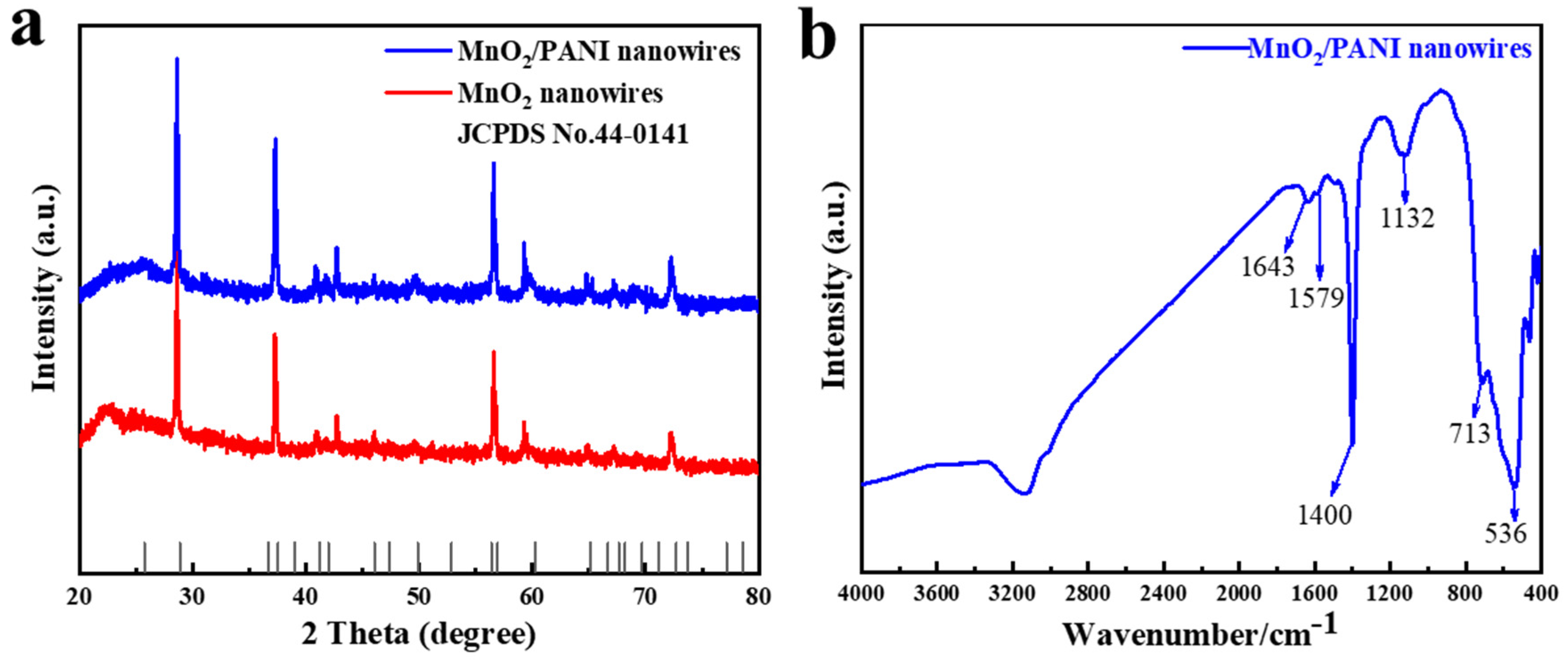
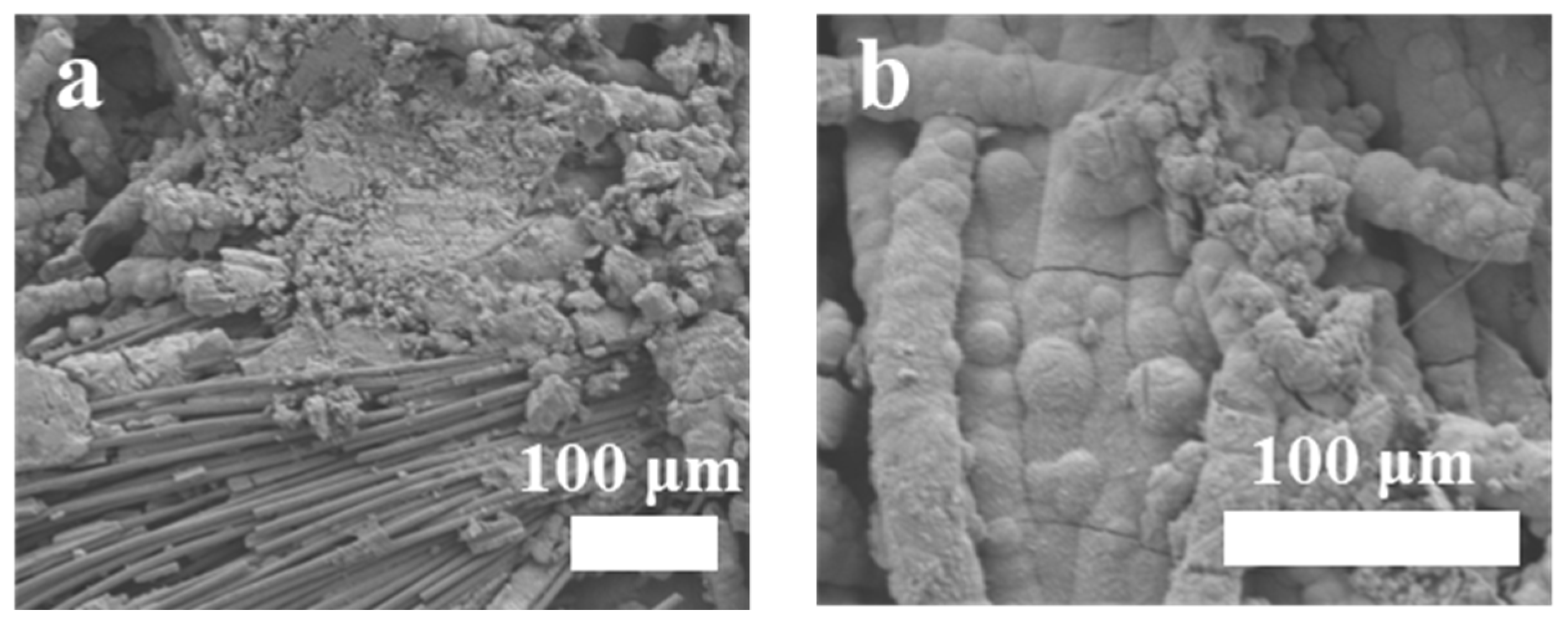
3.1. Morphology and Structure
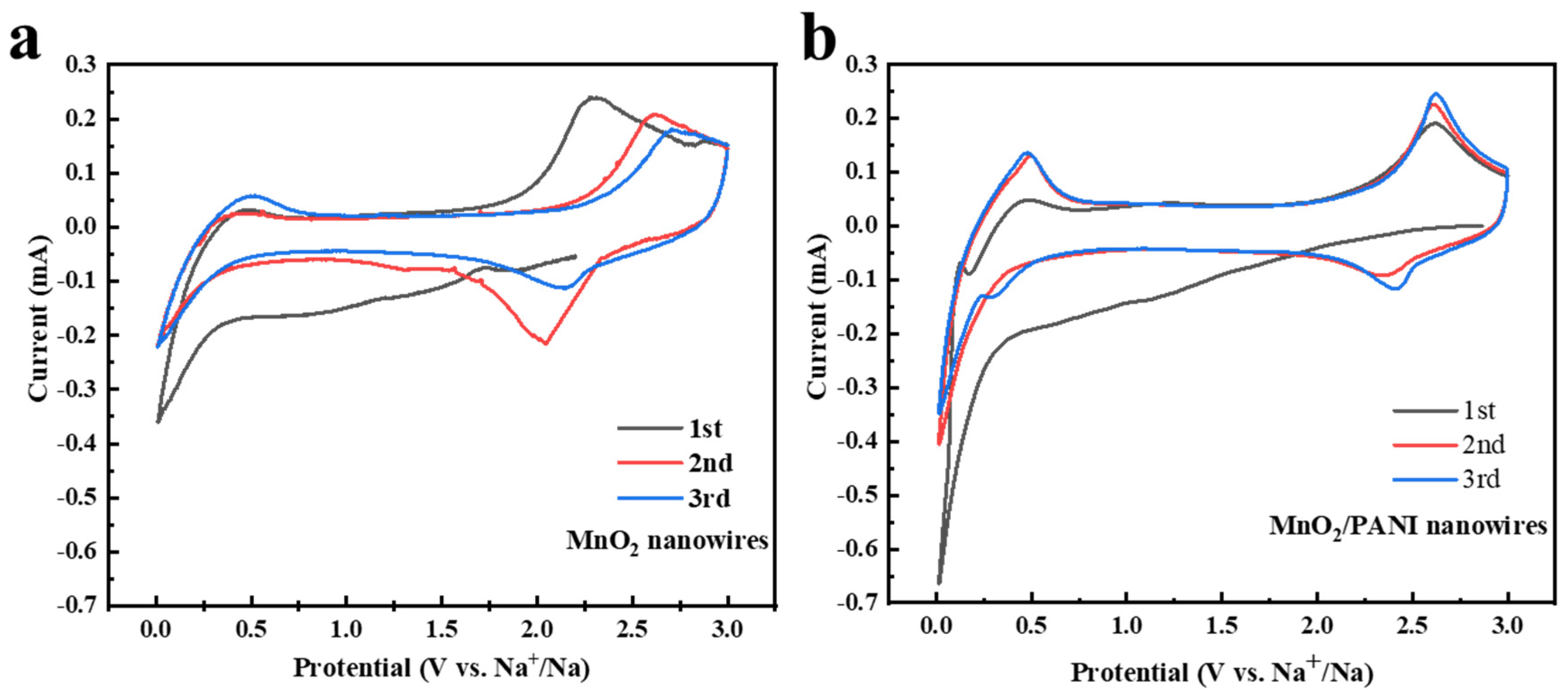
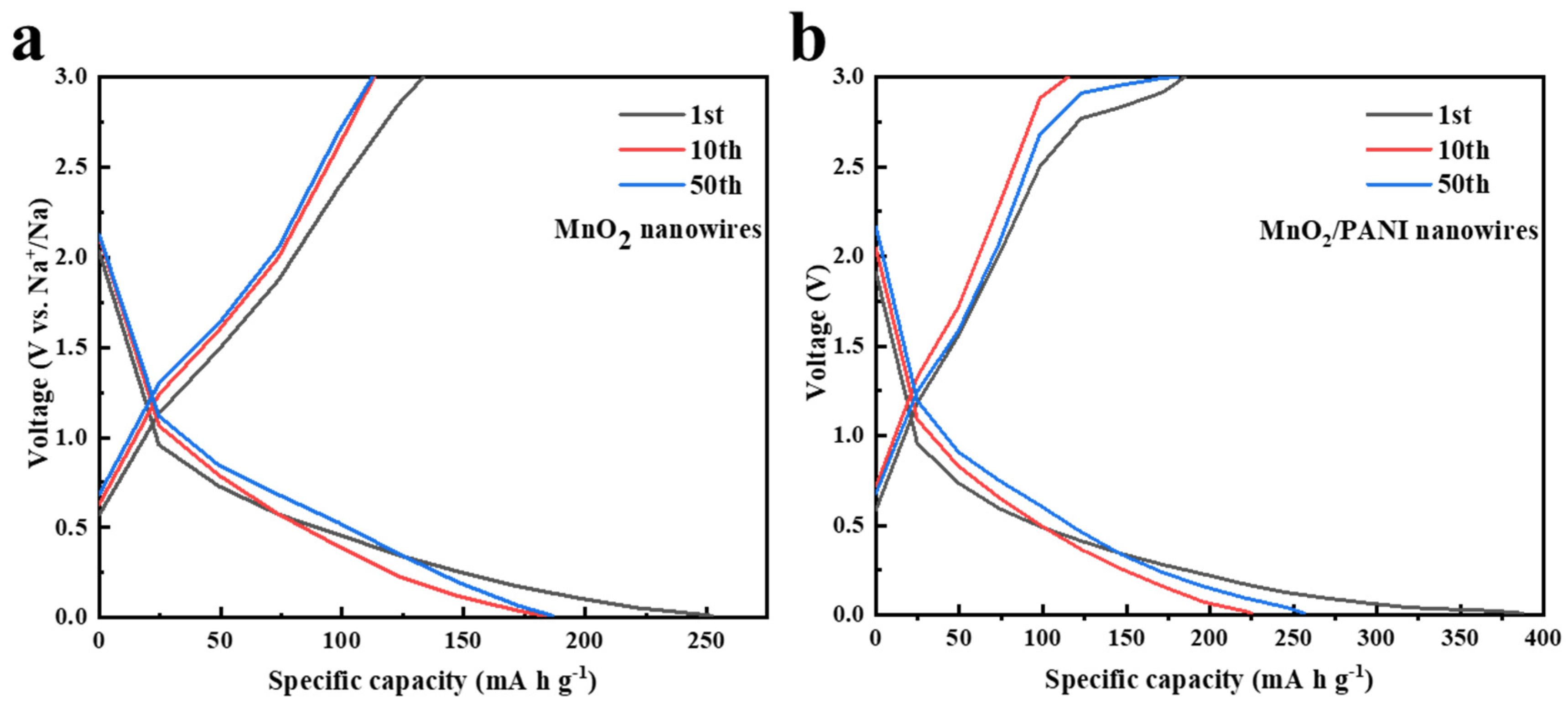
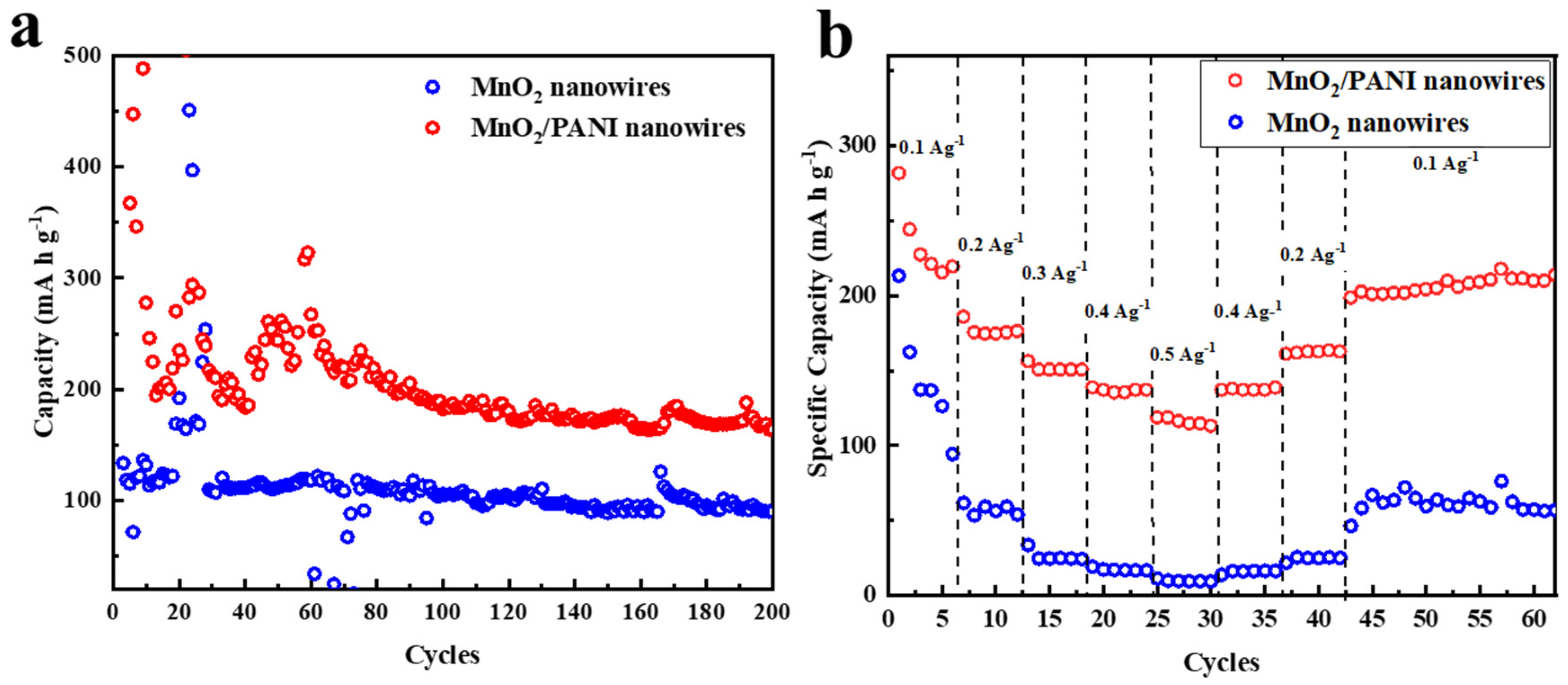
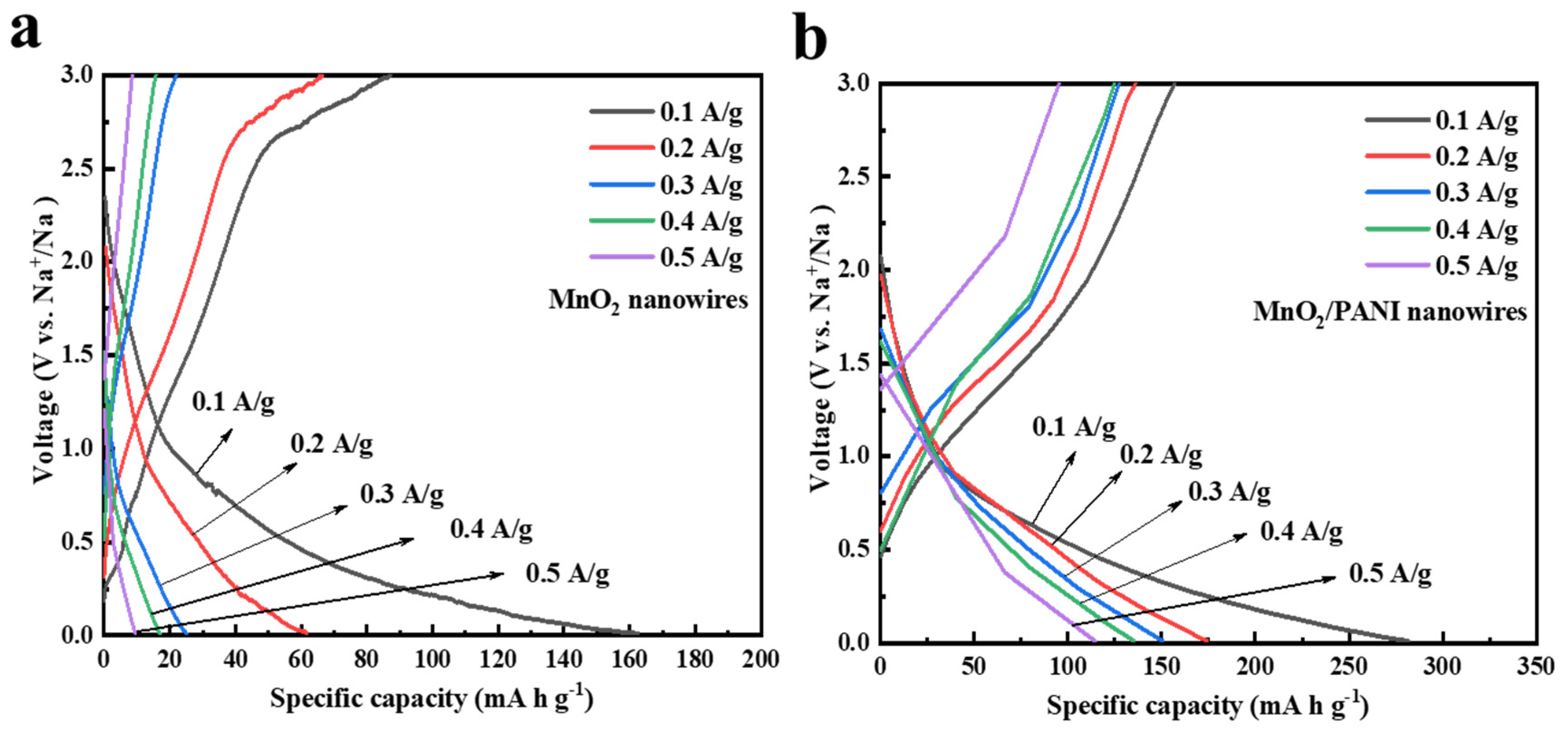
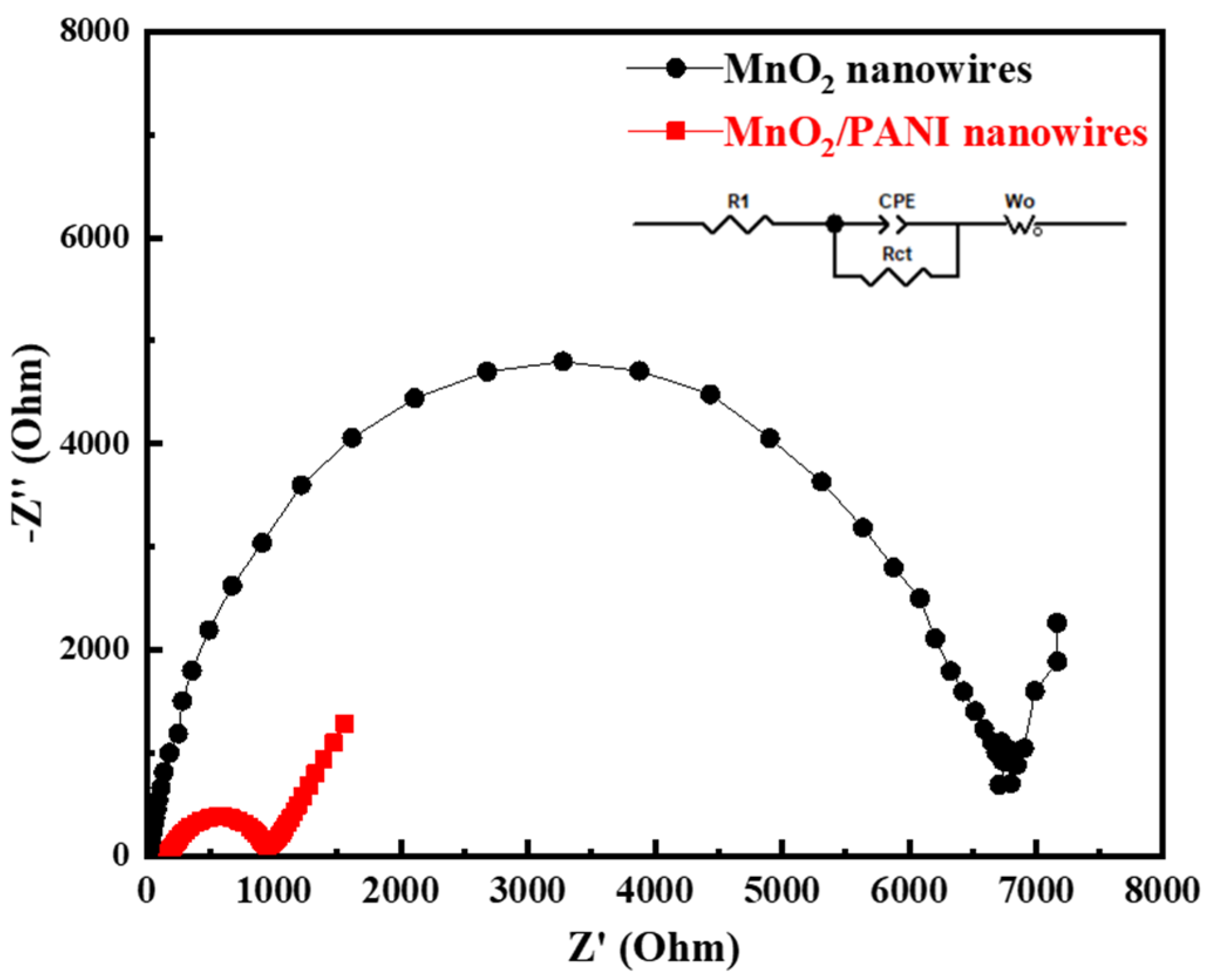
3.2. Electrochemical Performance
3.3. Analysis of Electrode Structure after Circulation
3.4. Comparison of MnO2/PANI Nanowires with Different Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.T.A.; Soram, B.S.; Tran, D.T. Enhanced electrochromic capacity performances of hierarchical MnO2-polyaniline/PEDOT:PSS/Ag@Ni nanowires cathode for flexible and rechargeable electrochromic Zn-Ion battery. Chem. Eng. J. 2023, 452, 139555. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. Facile Synthesis of Nanostructured MnO2 as Anode Materials for Sodium-Ion Batteries. ChemNanoMat 2016, 2, 196–200. [Google Scholar] [CrossRef]
- Li, H.; Liu, A.; Zhao, S.; Guo, Z.; Wang, N.; Ma, T. In Situ Growth of a Feather-like MnO2 Nanostructure on Carbon Paper for High-Performance Rechargeable Sodium-Ion Batteries. Chemelectrochem 2018, 5, 3266–3272. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Dong, Z.; Shang, Y. 1D nanostructured sodium vanadium oxide as a novel anode material for aqueous sodium ion batteries. Nano Energy 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Yang, C.; Xin, S.; Mai, L.; You, Y. Materials Design for High-Safety Sodium-Ion Battery. Adv. Energy Mater. 2021, 2, 11. [Google Scholar] [CrossRef]
- Or, T. Development of Electrodes and Coatings for Sodium Ion Energy Storage. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2020. [Google Scholar]
- Peng, B.; Gao, J.; Sun, Z.; Li, J.; Zhang, G. High performance sodium-ion full battery based on one-dimensional nanostructures: The case of Na 0.44 MnO2 cathode and MoS2 anode. J. Phys. D Appl. Phys. 2021, 54, 014001. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.J.; Li, Y.Q.; Zhong, Y.; Wang, X.L.; Xie, D.; Xia, X.H.; Gu, C.D.; Tu, J.P. Sodium-rich manganese oxide porous microcubes with polypyrrole coating as a superior cathode for sodium ion full batteries. J. Colloid Interface Sci. 2020, 565, 218–226. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Chao, D.; Li, W.; Zhao, D. Recent advances in hard carbon anodes with high initial Coulombic efficiency for sodium-ion batteries. Nano Mater. Sci. 2023, 5, 189–201. [Google Scholar] [CrossRef]
- Sun, J.N.B. NiP nanoparticles encapsulated in lamellar carbon as high-performance anode materials for sodium-ion batteries. Electrochem. Commun. 2022, 141, 107344. [Google Scholar] [CrossRef]
- Guo, S.Y.X.; Wang, D.; Chen, A.; Wang, Y.; Li, P.; Liang, G.; Zhi, C. The energy storage mechanisms of MnO2 in batteries. Curr. Opin. Electrochem. 2021, 30, 100769. [Google Scholar] [CrossRef]
- Ma, T.Z.Z. Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle Lithium ion batteries. Electrochim. Acta 2016, 201, 165–171. [Google Scholar] [CrossRef]
- Pandit, B.; Rondiya, S.R.; Dzade, N.Y.; Shaikh, S.F.; Kumar, N.; Goda, E.S.; Al-Kahtani, A.; Mane, R.S.; Mathur, S.; Salunkhe, R.R. High Stability and Long Cycle Life of Rechargeable Sodium-Ion Battery Using Manganese Oxide Cathode: A Combined Density Functional Theory (DFT) and Experimental Study. ACS Appl. Mater. Interfaces 2021, 13, 11433–11441. [Google Scholar] [CrossRef]
- Ahn, C.; Cavalleri, A.; Georges, A.; Ismail-Beigi, S.; Millis, A.J.; Triscone, J.M. Designing and controlling the properties of transition metal oxide quantum materials. Nat. Mater. 2021, 20, 1462–1468. [Google Scholar] [CrossRef]
- Yusoff, N.F.M.; Idris, N.H.; Din, M.F.M.; Majid, S.R.; Harun, N.A.; Rahman, M.M. Investigation on the Electrochemical Performances of Mn2O3 as a Potential Anode for Na-Ion Batteries. Sci. Rep. 2020, 10, 9207. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Huynh, L.T.N.; Nguyen, T.H.; Vu, T.P.; Le, M.L.P.; Grag, A.; Tran, V.M. Promising electrode material using Ni-doped layered manganese dioxide for sodium-ion batteries. Appl. Electrochem. 2018, 48, 793–800. [Google Scholar] [CrossRef]
- Li, W.; Xu, K.; Li, B.; Sun, J.; Jiang, F.; Yu, Z.; Zou, R.; Chen, Z.; Hu, J. MnO2 Nanoflower Arrays with High Rate Capability for Flexible Supercapacitors. ChemElectroChem 2014, 1, 1003–1008. [Google Scholar] [CrossRef]
- Zhai, T.; Lu, X.; Wang, F.; Xia, H.; Tong, Y. MnO2 Nanomaterials for Flexible Supercapacitors: Performance Enhancement via Intrinsically and Extrinsically Modification. Nanoscale Horizons 2015, 1, 109–124. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Nanostructured Electrode Materials for Advanced Sodium-Ion Batteries. Matter 2019, 1, 90–114. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, K.G.; Kim, H.K.; Hwang, S.J. MnO2-based nanostructured materials for various energy applications. Mater. Chem. Front. 2021, 5, 3549–3575. [Google Scholar] [CrossRef]
- Ghosh, T.; Bansal, L.; Kandpal, S.; Rani, C.; Rath, D.K.; Sahu, B.; Chhoker, S.; Kumar, R. Multifunctional electrochromic hybrid PANI@WO3 core-shell for energy generation and storage. J. Energy Storage 2023, 72, 108640. [Google Scholar] [CrossRef]
- Tian, M.; Liu, X.; Diao, X.; Zhong, X. High performance PANI/MnO2 coral-like nanocomposite anode for flexible and robust electrochromic energy storage device. Sol. Energy Mater. Sol. Cells 2023, 253, 112239. [Google Scholar] [CrossRef]
- Tikhonov, A.A.; Filippov, D.A.; Arendateleva, S.I.; Perepelitsa, T.I. Technology of electrodepositing and properties of indium-containing chromium alloys. IOP Conf. Ser. Mater. Sci. Eng. 2019, 656, 012051. [Google Scholar] [CrossRef]
- Rahayu, E.I.P.; Putri, N.P. The Effect of Solution Concentration and Deposition Time on Viscoelasticity and Morphology of Polyaniline Coating. J. Phys. Conf. Ser. 2020, 1491, 012050. [Google Scholar] [CrossRef]
- Dadashi, R.; Bahram, M.; Faraji, M. Fabrication of a solid-state symmetrical supercapacitor based on polyaniline grafted multiwalled carbon nanotube deposit onto Created Vertically Oriented Graphene Nanosheets on graphite sheet. J. Energy Storage 2022, 52, 104775. [Google Scholar] [CrossRef]
- Qi, M.; Xie, D.; Zhong, Y.; Chen, M.; Xia, X. Smart construction of polyaniline shell on cobalt oxides as integrated core-shell arrays for enhanced lithium ion batteries. Electrochim. Acta 2017, 247, 701–707. [Google Scholar] [CrossRef]
- Patil, S.S.; Mane, S.M.; Nimbalkar, N.A.; Khilare, C.J.; Kulkarni, S.B.; Dhasade, S.S.; Kamat, R.K.; Lee, J.; Chavan, S.G. A simple facile synthesis for phase transforming of δ-MnO2 into α-MnO2 and thereby enhancing Na-ion supercapacitive performance. Ionics 2024, 30, 3055–3068. [Google Scholar] [CrossRef]
- Smolin, Y.Y.; Masoud, S.; Lau, K.K.S. Oxidative chemical vapor deposition of polyaniline thin films. Beilstein J. Nanotechnol. 2017, 8, 1266–1276. [Google Scholar] [CrossRef]
- Mahamad Yusoff, N.F.; Idris, N.H.; Noerochim, L. Review on recent progress in Manganese-based anode materials for sodium-ion batteries. Int. J. Energy Res. 2022, 46, 667–683. [Google Scholar] [CrossRef]




| Mn-Based Anode Materials | Voltage Range (V) | Capacity (mA h g−1) | Current Density/Cycles |
|---|---|---|---|
| MnO2/PANI (nanowires) | 0.00–3.00 | 182 | 100 mA g−1/100th |
| MnO/graphene [29] | 0.50–3.00 | 191 | 50 mA g−1/100th |
| MnO2 (nanorods) [2] | 0.00–3.00 | 129.2 | 50 mA g−1/100th |
| MnO2 (nanoflowers) [2] | 0.00–3.00 | 177.1 | 50 mA g−1/100th |
| Mn2O3 (powders) [15] | 0.00–3.00 | 130 | 100 mA g−1/200th |
| Mn3O4 thin film [29] | 0.005-3.00 | 70 | 100 mA g−1/200th |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Yin, X.; Li, X.; Qin, X.; Qi, M. Study on the Performance of Aniline Electrodeposited on MnO2 Nanowire as an Anode for Sodium-Ion Batteries. Polymers 2024, 16, 1856. https://doi.org/10.3390/polym16131856
Ma D, Yin X, Li X, Qin X, Qi M. Study on the Performance of Aniline Electrodeposited on MnO2 Nanowire as an Anode for Sodium-Ion Batteries. Polymers. 2024; 16(13):1856. https://doi.org/10.3390/polym16131856
Chicago/Turabian StyleMa, Dandan, Xiangyu Yin, Xinyi Li, Xiangge Qin, and Meili Qi. 2024. "Study on the Performance of Aniline Electrodeposited on MnO2 Nanowire as an Anode for Sodium-Ion Batteries" Polymers 16, no. 13: 1856. https://doi.org/10.3390/polym16131856






