Recent Progress in Polyion Complex Nanoparticles with Enhanced Stability for Drug Delivery
Abstract
:1. Introduction
2. Strategies to Improve PIC Stability for Protein Delivery
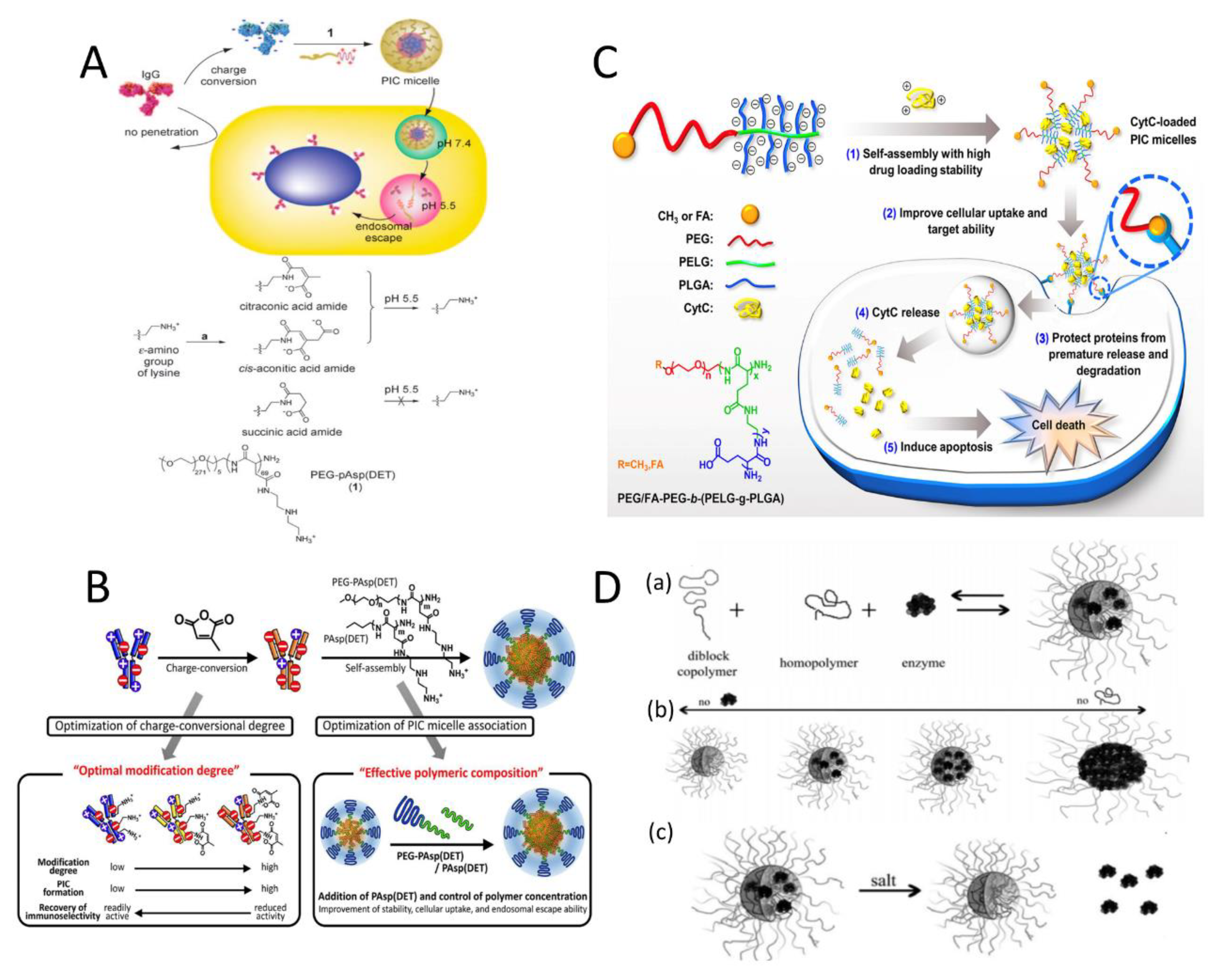
2.1. Improving the Charge Density of PIC Components
2.1.1. Enhancing the Charge Density of the Protein Drugs
2.1.2. Increasing the Charge Density of the Charged Segment of the Block Copolymers
2.1.3. Incorporation of Charged Homopolymers
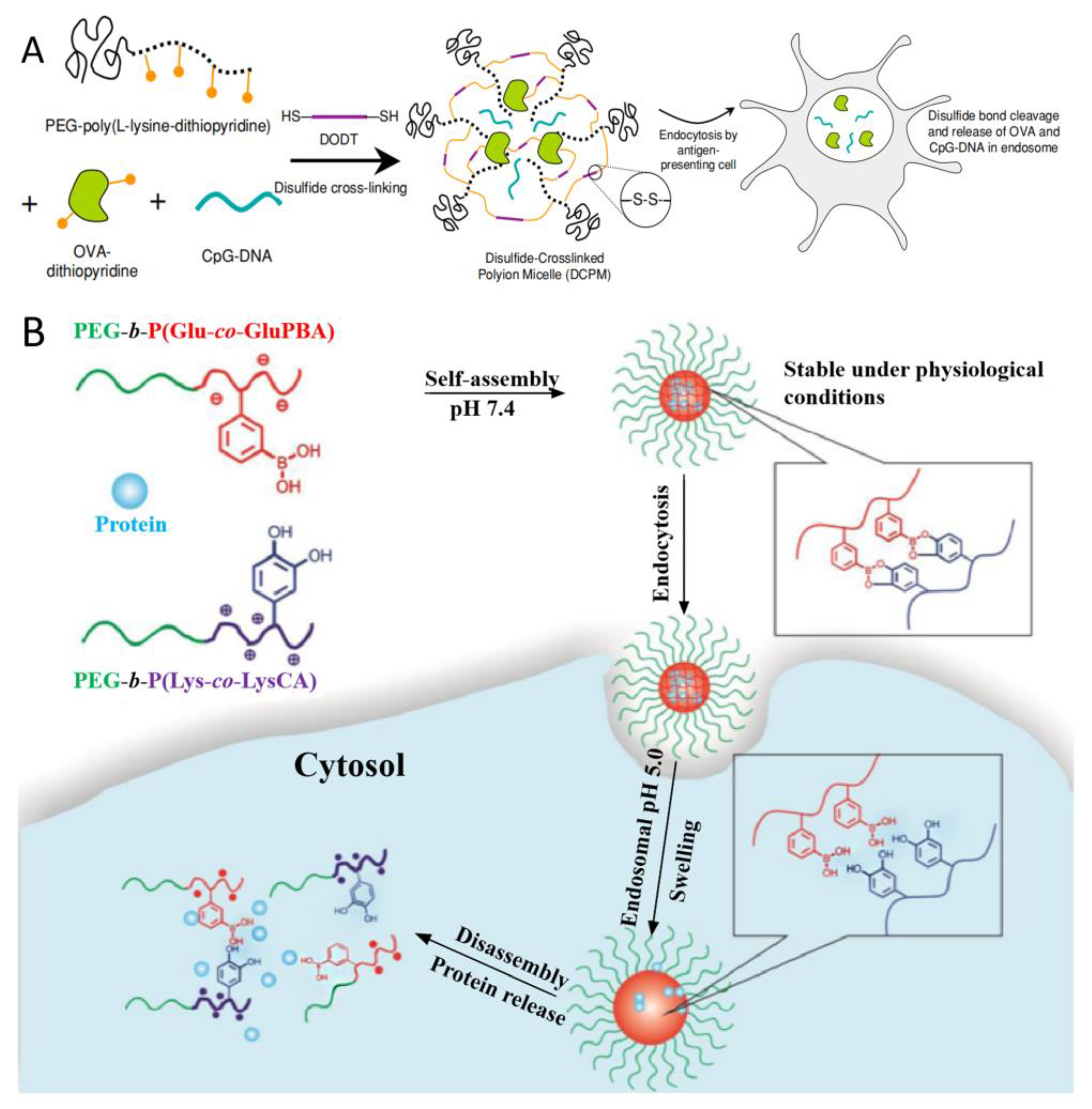
2.2. Introducing the Crosslinked Structure in PIC Micelles
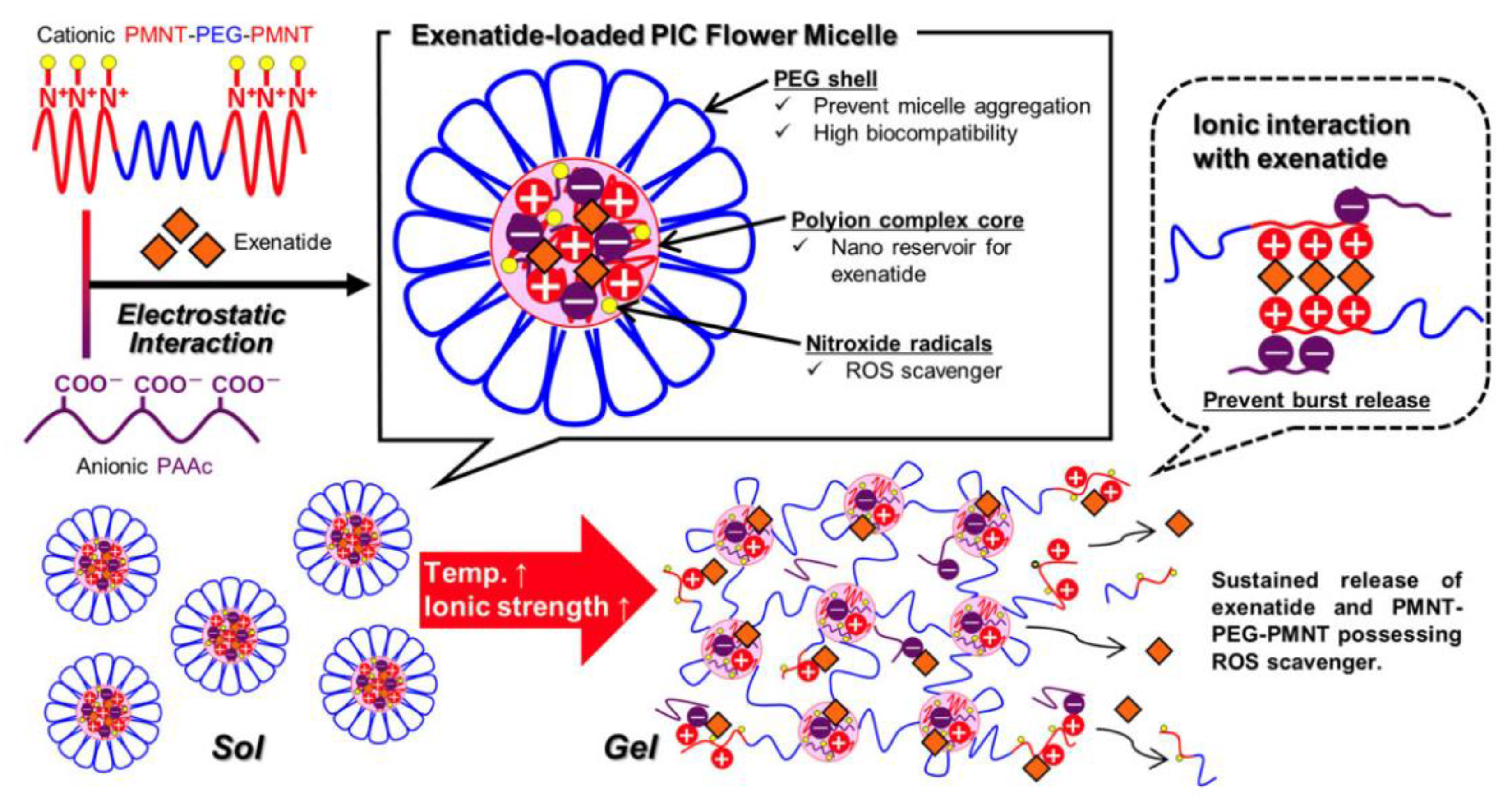
2.3. PIC Reinforced by Hydrophobic Interactions
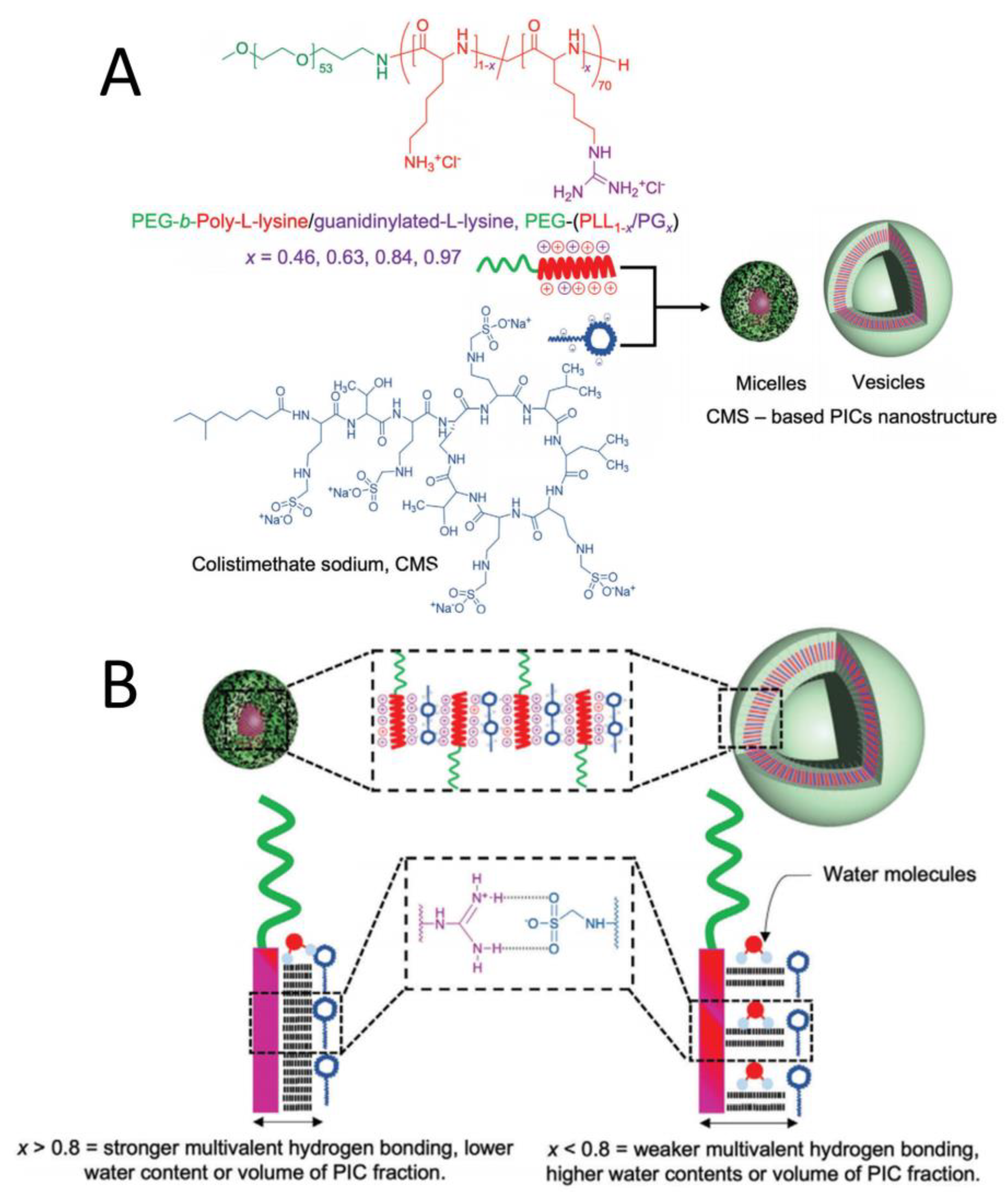
2.4. PIC Reinforced by Hydrogen Bonds
2.5. Other Methods to Enhance Stability of PIC
3. PIC Nanoparticles for Anticancer Drug Delivery
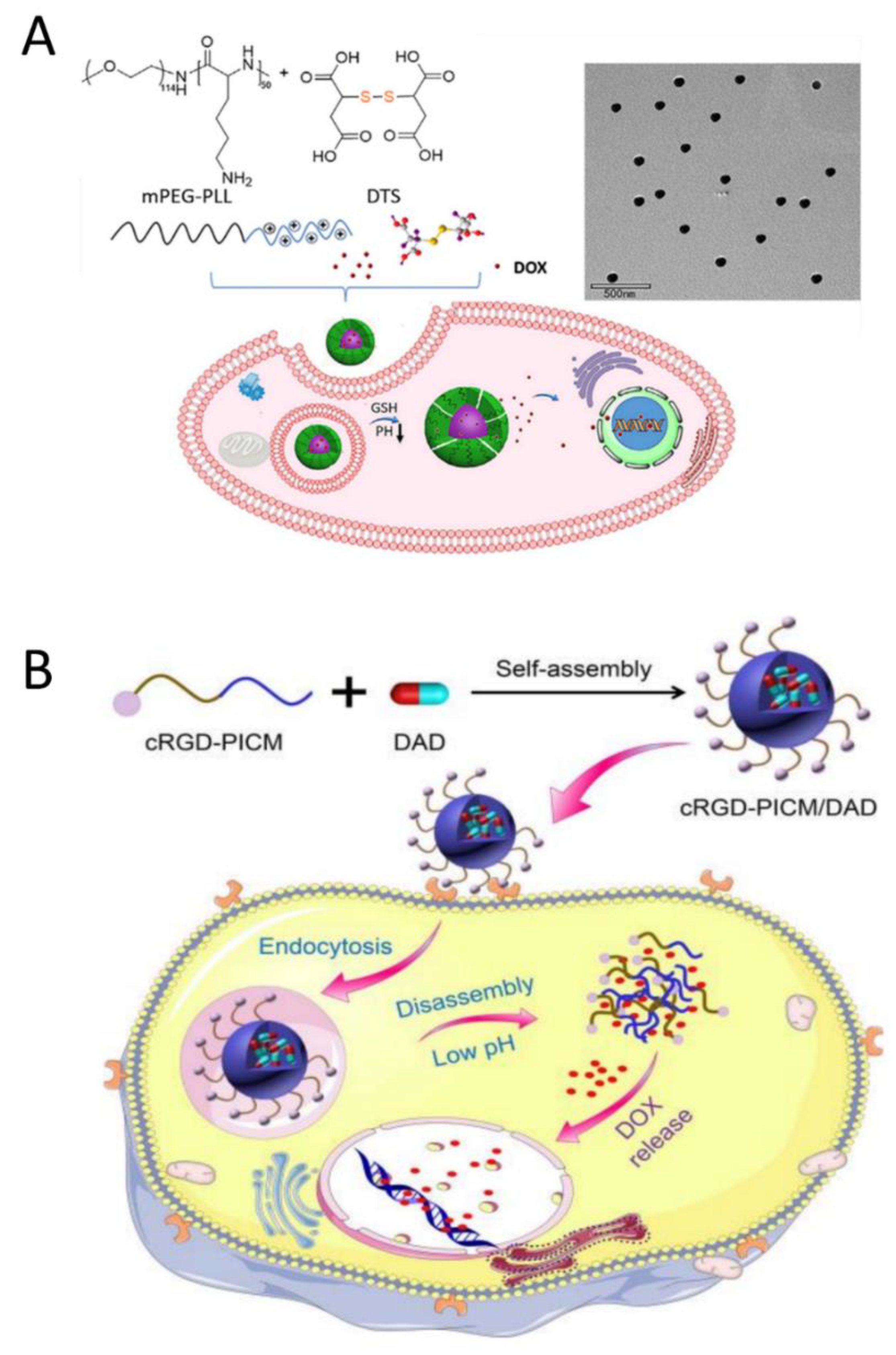
3.1. PIC Delivery of Chemotherapy Drugs
3.2. PIC Delivery of Gaseous Donors for Cancer Therapy
3.3. PIC Nanoparticles Containing Other Drugs for Cancer Therapy
4. PIC Nanoparticles for Delivery of Nucleic-Acid Drugs
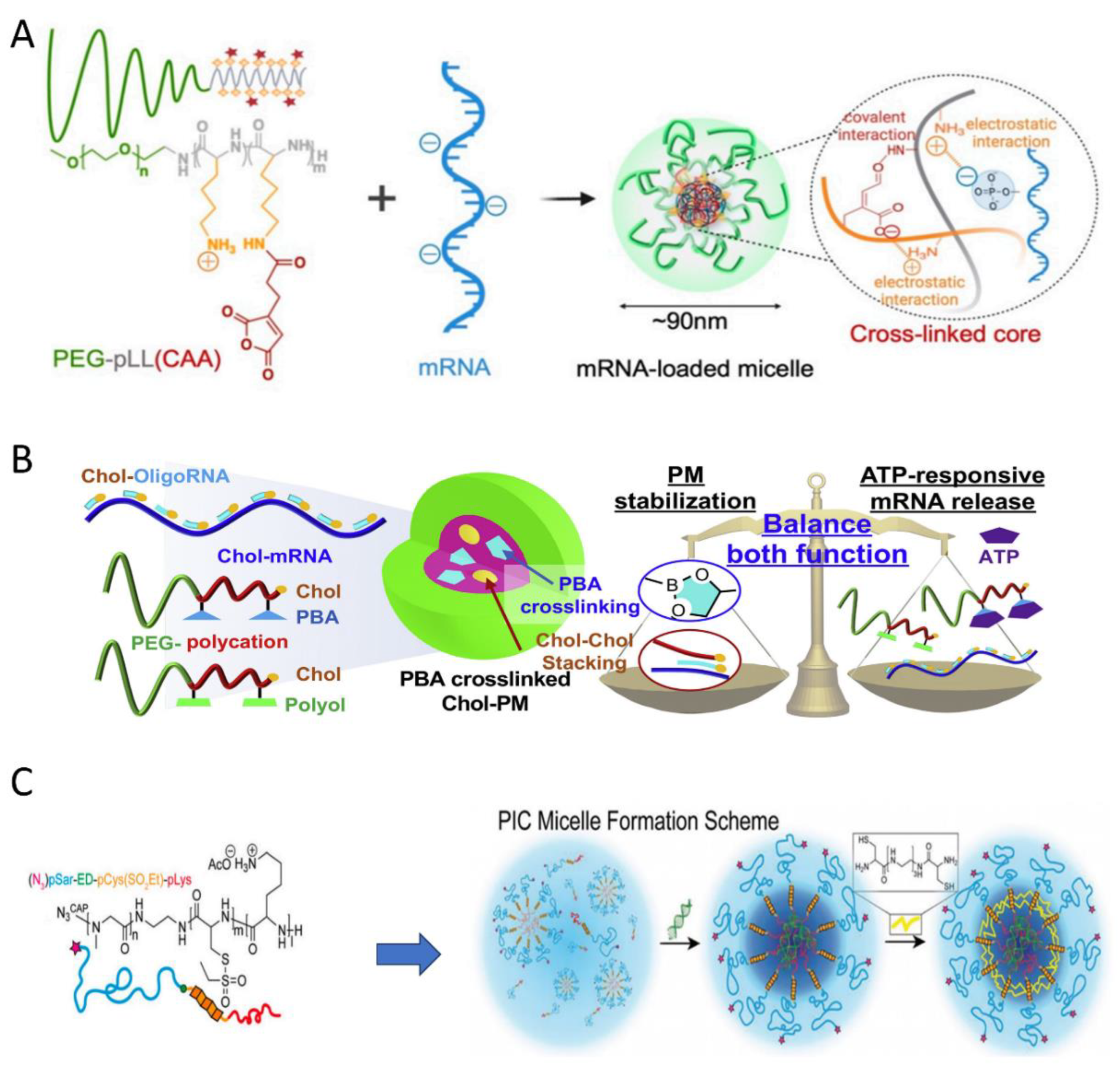
4.1. PIC Nanoparticles Stabilized by Crosslinking for siRNA Delivery
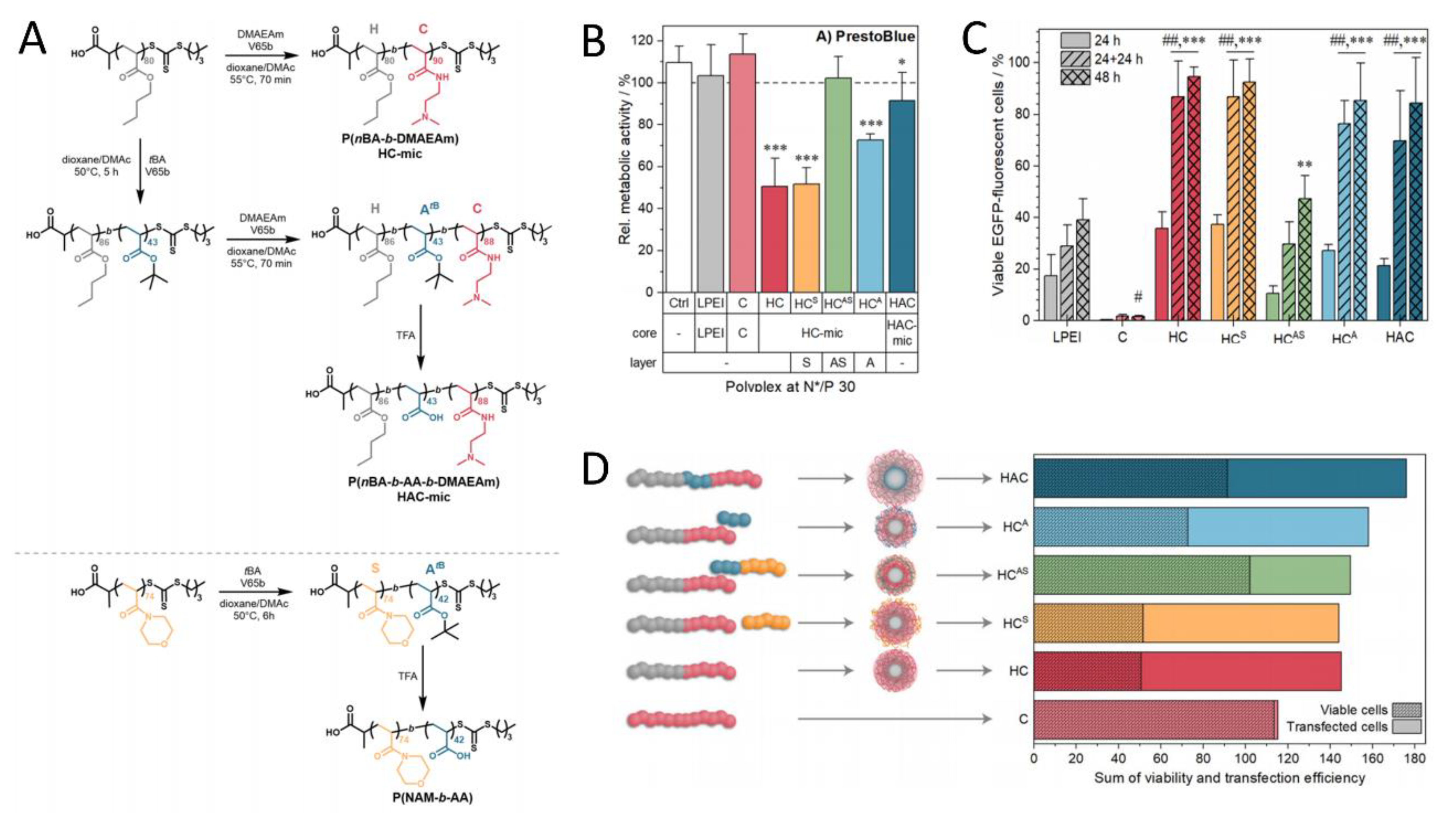
4.2. PIC Nanoparticles for Gene Delivery
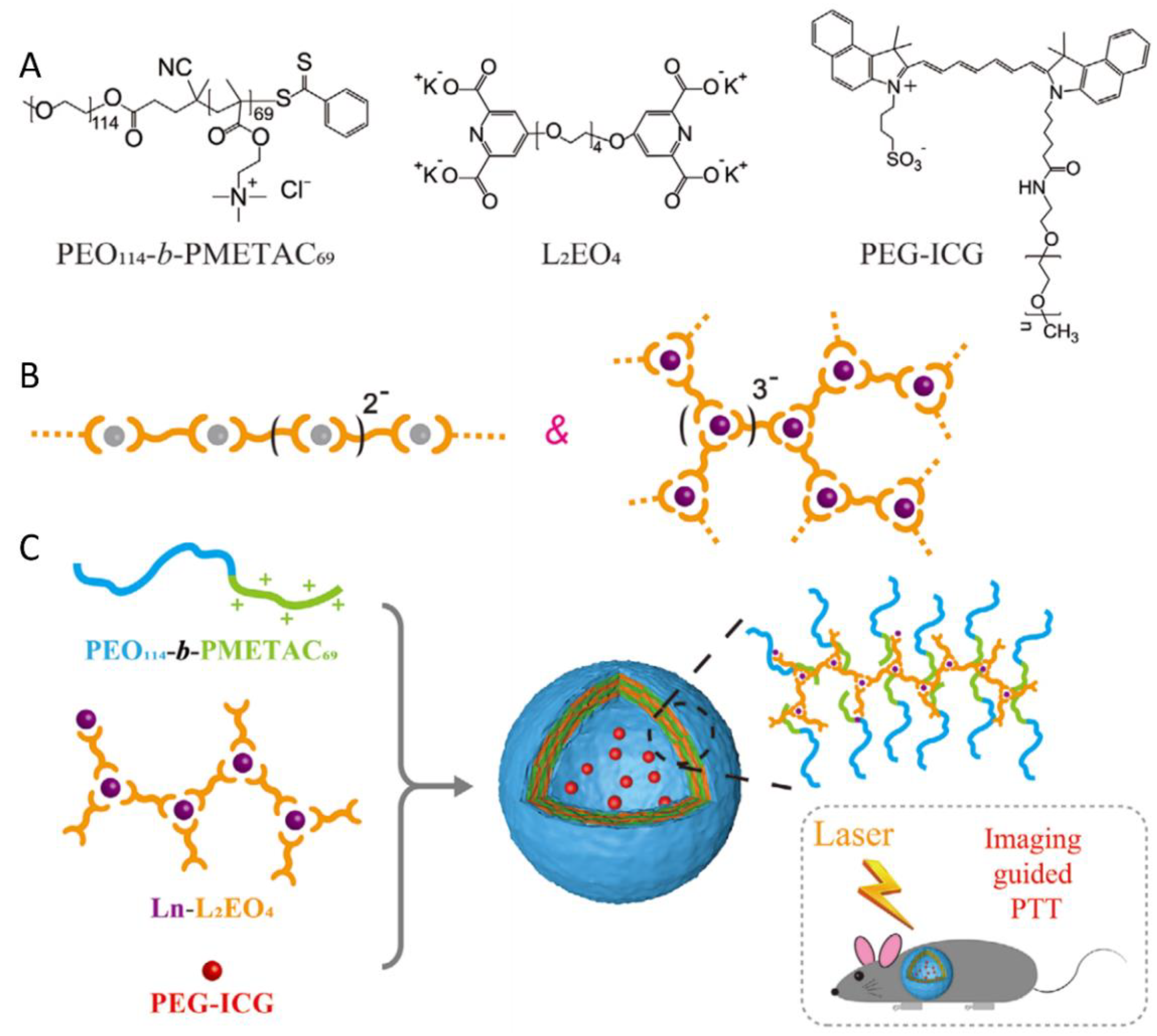
5. PIC Micelles as an MRI Contrast Agent
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Dobrynin, A.V. Polyelectrolytes: On the doorsteps of the second century. Polymer 2020, 202, 122714. [Google Scholar] [CrossRef]
- Shah, S.; Leon, L. Structural dynamics, phase behavior, and applications of polyelectrolyte complex micelles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101424. [Google Scholar] [CrossRef]
- Shah, S.; Eyler, A.; Tabandeh, S.; Leon, L. Electrostatically driven self-assembled nanoparticles and coatings. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–370. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Rieger, J.; Passirani, C.; Benoit, J.P.; Van Butsele, K.; Jérôme, R.; Jérôme, C. Synthesis of Amphiphilic Copolymers of Poly(ethylene oxide) and Poly(ϵ-caprolactone) with Different Architectures, and Their Role in the Preparation of Stealthy Nanoparticles. Adv. Funct. Mater. 2006, 16, 1506–1514. [Google Scholar] [CrossRef]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block Copolymers with Poly(ethylene glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. Part A 2019, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-G.; Jiang, Y.-W.; Chen, Z.; Yu, Z.-W. Folding Behaviors of Protein (Lysozyme) Confined in Polyelectrolyte Complex Micelle. Langmuir 2016, 32, 3655–3664. [Google Scholar] [CrossRef]
- Lee, Y.; Kataoka, K. Biosignal-sensitive polyion complex micelles for the delivery of biopharmaceuticals. Soft Matter 2009, 5, 3810–3817. [Google Scholar] [CrossRef]
- Zhao, Y.; Lord, M.S.; Stenzel, M.H. A polyion complex micelle with heparin for growth factor delivery and uptake into cells. J. Mater. Chem. B 2013, 1, 1635–1643. [Google Scholar] [CrossRef]
- Solaro, R.; Chiellini, F.; Battisti, A. Targeted Delivery of Protein Drugs by Nanocarriers. Materials 2010, 3, 1928–1980. [Google Scholar] [CrossRef]
- Lindhoud, S.; Vries, R.; Norde, W.; Stuart, M.A. Structure and stability of complex coacervate core micelles with lysozyme. Biomacromolecules 2007, 8, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ishii, T.; Kim, H.J.; Nishiyama, N.; Hayakawa, Y.; Itaka, K.; Kataoka, K. Efficient delivery of bioactive antibodies into the cytoplasm of living cells by charge-conversional polyion complex micelles. Angew. Chem. Int. Ed. Engl. 2010, 49, 2552–2555. [Google Scholar] [CrossRef]
- Kim, A.; Miura, Y.; Ishii, T.; Mutaf, O.F.; Nishiyama, N.; Cabral, H.; Kataoka, K. Intracellular Delivery of Charge-Converted Monoclonal Antibodies by Combinatorial Design of Block/Homo Polyion Complex Micelles. Biomacromolecules 2016, 17, 446–453. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Xia, W.; Quan, G.; Huang, Y.; Bai, X.; Yu, F.; Xu, Q.; Qin, W.; Liu, D.; et al. Poly(L-Glutamic Acid)-Based Brush Copolymers: Fabrication, Self-assembly, and Evaluation as Efficient Nanocarriers for Cationic Protein Drug Delivery. AAPS PharmSciTech 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Lindhoud, S. Polyelectrolyte Complex Micelles as Wrapping for Enzymes; Wur Wageningen Ur: Wageningen, The Netherlands, 2009. [Google Scholar]
- Heffernan, M.J.; Murthy, N. Disulfide-crosslinked polyion micelles for delivery of protein therapeutics. Ann. Biomed. Eng. 2009, 37, 1993–2002. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, J.; Gao, H.; Liu, G.; Ma, R.; An, Y.; Kong, D.; Shi, L. pH/sugar dual responsive core-cross-linked PIC micelles for enhanced intracellular protein delivery. Biomacromolecules 2013, 14, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Anraku, Y.; Kataoka, K. Self-Boosting Catalytic Nanoreactors Integrated with Triggerable Crosslinking Membrane Networks for Initiation of Immunogenic Cell Death by Pyroptosis. Angew. Chem. Int. Ed. Engl. 2020, 59, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Thao, P.T.; Rintaro, T.; Duc, P.T.; Shin-Ichi, Y. Stable Water-soluble Polyion Complex Micelles Composed of Oppositely Charged Diblock Copolymers and Reinforced by Hydrophobic Interactions. Chem. Lett. 2022, 8, 877–880. [Google Scholar]
- Li, K.; Chen, F.; Wang, Y.; Stenzel, M.H.; Chapman, R. Polyion Complex Micelles for Protein Delivery Benefit from Flexible Hydrophobic Spacers in the Binding Group. Macromol. Rapid Commun. 2020, 41, e2000208. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving Forces for Oppositely Charged Polyion Association in Aqueous Solutions: Enthalpic, Entropic, but Not Electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Chuanoi, S.; Anraku, Y.; Hori, M.; Kishimura, A.; Kataoka, K. Fabrication of polyion complex vesicles with enhanced salt and temperature resistance and their potential applications as enzymatic nanoreactors. Biomacromolecules 2014, 15, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust Polyion Complex Vesicles (PICsomes) under Physiological Conditions Reinforced by Multiple Hydrogen Bond Formation Derived by Guanidinium Groups. Biomacromolecules 2018, 19, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nii, T.; Mori, T.; Katayama, Y.; Toyofuku, M.; Kishimura, A. Nanostructure Control of an Antibiotic-Based Polyion Complex Using a Series of Polycations with Different Side-Chain Modification Rates. Macromol. Rapid Commun. 2022, 43, e2200316. [Google Scholar] [CrossRef]
- Fay, J.M.; Lim, C.; Finkelstein, A.; Batrakova, E.V.; Kabanov, A.V. PEG-Free Polyion Complex Nanocarriers for Brain-Derived Neurotrophic Factor. Pharmaceutics 2022, 14, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Sakaue, S.; Nagasaki, Y. Redox-active injectable gel using polyion complex to achieve sustained release of exenatide and enhance therapeutic efficacy for the treatment of type 2 diabetes. J. Biomed. Mater. Res. A 2019, 107, 1107–1113. [Google Scholar] [CrossRef]
- Ishii, S.; Kaneko, J.; Nagasaki, Y. Dual Stimuli-Responsive Redox-Active Injectable Gel by Polyion Complex Based Flower Micelles for Biomedical Applications. Macromolecules 2015, 48, 3088–3094. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Zablocka, M.; Majoral, J.P. Dendritic Macromolecular Architectures: Dendrimer-Based Polyion Complex Micelles. Biomacromolecules 2021, 22, 262–274. [Google Scholar] [CrossRef]
- Du, Y.; Yan, W.; Lian, H.; Xiang, C.; Duan, L.; Xiao, C. 2,2′-Dithiodisuccinic acid-stabilized polyion complex micelles for pH and reduction dual-responsive drug delivery. J. Colloid Interface Sci. 2018, 522, 74–81. [Google Scholar] [CrossRef]
- Weiguo, X.; Gao, L.; Jianxun, D.; Jinjin, C.; Yang, L.; Pan, Z. Targeted pH-responsive polyion complex micelle for controlled intracellular drug delivery. Chin. Chem. Lett. 2020, 31, 1178–1182. [Google Scholar] [CrossRef]
- Kalinova, R.; Dimitrov, I. Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery. Molecules 2022, 27, 2178. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, S.F.; Su, L.; Song, J. Gas-Mediated Cancer Bioimaging and Therapy. ACS Nano 2019, 13, 10887–10917. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, J.; Liu, Y.; Chen, T.; Qi, J.; Sun, S.; Hao, H.; Zeng, W.; Zhao, J.; Wu, M. Hyperthermia-triggered NO release based on Cu-doped polypyrrole for synergistic catalytic/gas cancer therapy. Acta Biomater. 2023, 167, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, H.; Liu, Y.; Wan, L.; Liu, S.; Zhang, H. L-Arginine self-delivery supramolecular nanodrug for NO gas therapy. Acta Biomater. 2023, 169, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, D.; Yang, J.; Zhu, S.; Shi, J.; Qin, X.; Yin, W.; Wang, J.; Ding, Y.; Chen, T.; et al. Glucose oxidase-amplified CO generation for synergistic anticancer therapy via manganese carbonyl-caged MOFs. Acta Biomater. 2022, 154, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Nagasaki, Y. Facile and Quantitative Synthesis of a Poly(ethylene glycol)-b-Poly(l-arginine) Block Copolymer and Its Use for the Preparation of Polyion Complex Micelles with Polyanions for Biomedical Applications. Macromol. Rapid Commun. 2015, 36, 1916–1922. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, C.; Du, J.; Jin, H. Endogenous Sulfur Dioxide: A New Member of Gasotransmitter Family in the Cardiovascular System. Oxid. Med. Cell Longev. 2016, 2016, 8961951. [Google Scholar] [CrossRef]
- Zhou, W.; Ding, P.; Zhang, C.; Wu, B.; Guo, X.; Cohen Stuart, M.A.; Zhou, X.; Wang, J. Regulated Polyion Complex Vesicles for Efficient Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2108729. [Google Scholar] [CrossRef]
- Lee, S.M.; Jang, W.D. Polyion complex micelle formed from tetraphenylethene containing block copolymer. Biomater. Res. 2017, 21, 17. [Google Scholar] [CrossRef]
- Karayianni, M.; Koufi, D.; Pispas, S. Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles. Polymers 2022, 14, 5186. [Google Scholar] [CrossRef]
- Sinani, G.; Durgun, M.E.; Cevher, E.; Özsoy, Y. Polymeric-Micelle-Based Delivery Systems for Nucleic Acids. Pharmaceutics 2023, 15, 2021. [Google Scholar] [CrossRef]
- Yang, W.; Chen, P.; Boonstra, E.; Hong, T.; Cabral, H. Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery. Pharmaceutics 2022, 14, 1205. Available online: https://www.mdpi.com/1999-4923/14/6/1205 (accessed on 6 June 2022). [CrossRef] [PubMed]
- Yoshinaga, N.; Uchida, S.; Dirisala, A.; Naito, M.; Osada, K.; Cabral, H.; Kataoka, K. mRNA loading into ATP-responsive polyplex micelles with optimal density of phenylboronate ester crosslinking to balance robustness in the biological milieu and intracellular translational efficiency. J. Control Release 2021, 330, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Capelôa, L.; Yazdi, M.; Zhang, H.; Chen, X.; Nie, Y.; Wagner, E.; Lächelt, U.; Barz, M. Cross-Linkable Polyion Complex Micelles from Polypept(o)ide-Based ABC-Triblock Copolymers for siRNA Delivery. Macromol. Rapid Commun. 2022, 43, 2100698. [Google Scholar] [CrossRef]
- Aydinlioglu, E.; Abdelghani, M.; Le Fer, G.; van Hest, J.C.M.; Sandre, O.; Lecommandoux, S. Robust Polyion Complex Vesicles (PICsomes) Based on PEO-b-poly(amino acid) Copolymers Combining Electrostatic and Hydrophobic Interactions: Formation, siRNA Loading and Intracellular Delivery. Macromol. Chem. Phys. 2023, 224, 2200306. [Google Scholar] [CrossRef]
- Richter, F.; Leer, K.; Martin, L.; Mapfumo, P.; Solomun, J.I.; Kuchenbrod, M.T.; Hoeppener, S.; Brendel, J.C.; Traeger, A. The impact of anionic polymers on gene delivery: How composition and assembly help evading the toxicity-efficiency dilemma. J. Nanobiotechnol. 2021, 19, 292. [Google Scholar] [CrossRef]
- Richter, F.; Mapfumo, P.; Martin, L.; Solomun, J.I.; Hausig, F.; Frietsch, J.J.; Ernst, T.; Hoeppener, S.; Brendel, J.C.; Traeger, A. Improved gene delivery to K-562 leukemia cells by lipoic acid modified block copolymer micelles. J. Nanobiotechnol. 2021, 19, 70. [Google Scholar] [CrossRef]
- Bhowmik, S.; Pham, T.T.; Takahashi, R.; Kim, D.; Matsuoka, H.; Ishihara, K.; Yusa, S.I. Preparation of Water-Soluble Polyion Complex (PIC) Micelles with Random Copolymers Containing Pendant Quaternary Ammonium and Sulfonate Groups. Langmuir 2023, 39, 8120–8129. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Osada, K.; Hiki, S.; Dirisala, A.; Ishii, T.; Kataoka, K. Polyplex Micelles with Double-Protective Compartments of Hydrophilic Shell and Thermoswitchable Palisade of Poly(oxazoline)-Based Block Copolymers for Promoted Gene Transfection. Biomacromolecules 2016, 17, 354–361. [Google Scholar] [CrossRef]
- Yan, Y.; Besseling, N.A.; de Keizer, A.; Marcelis, A.T.; Drechsler, M.; Cohen Stuart, M.A. Hierarchical self-assembly in solutions containing metal ions, ligand, and diblock copolymer. Angew. Chem. Int. Ed. Engl. 2007, 46, 1807–1809. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ding, P.; Zhou, W.; Li, Y.; Drechsler, M.; Guo, X.; Cohen Stuart, M.A. A Supramolecular Crosslinker To Give Salt-Resistant Polyion Complex Micelles and Improved MRI Contrast Agents. Angew. Chem. Int. Ed. Engl. 2018, 57, 12680–12684. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Tanaka, Y.; Yakou, S.; Takayama, K. In vivo drug release from hydrophilic dextran tablets capable of forming polyion complex. J. Control Release 2006, 114, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kim, H.J.; Takemoto, H.; Miyata, K.; Kataoka, K. Precisely regulated nanoarchitecture comprised of gold nanotemplate and unimer polyion complex for systemic delivery of siRNA. J. Control Release 2015, 213, e75–e76. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Kim, H.J.; Ahn, J.; Naito, M.; Hayashi, K.; Toh, K.; Kim, B.S.; Matsumura, Y.; Kwon, I.C.; Miyata, K.; et al. Tuned Density of Anti-Tissue Factor Antibody Fragment onto siRNA-Loaded Polyion Complex Micelles for Optimizing Targetability into Pancreatic Cancer Cells. Biomacromolecules 2018, 19, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhao, C.-W.; Xiao, C.-S.; Tang, Z.-H.; Chen, X.-S. Thermosensitive polyion complex micelles prepared by self-assembly of two oppositely charged diblock copolymers. Chin. J. Polym. Sci. 2013, 31, 318–324. [Google Scholar] [CrossRef]
- Xia, J.; Chen, C.; Dong, M.; Zhu, Y.; Wang, A.; Li, S.; Zhang, R.; Feng, C.; Jiang, X.; Xu, X.; et al. Ginsenoside Rg3 endows liposomes with prolonged blood circulation and reduced accelerated blood clearance. J. Control Release 2023, 364, 23–36. [Google Scholar] [CrossRef]
- Giulimondi, F.; Vulpis, E.; Digiacomo, L.; Giuli, M.V.; Mancusi, A.; Capriotti, A.L.; Laganà, A.; Cerrato, A.; Zenezini Chiozzi, R.; Nicoletti, C.; et al. Opsonin-Deficient Nucleoproteic Corona Endows UnPEGylated Liposomes with Stealth Properties In Vivo. ACS Nano 2022, 16, 2088–2100. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, E.; Sun, X.; Huang, P.; Long, H.; Wang, H.; Yu, X.; Zheng, C.; Huang, Y. Pluronic L61 as a long-circulating modifier for enhanced liposomal delivery of cancer drugs. Polym. Chem. 2013, 4, 2958–2962. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhao, T.; Ren, X.; Lin, H.; He, P. Recent Progress in Polyion Complex Nanoparticles with Enhanced Stability for Drug Delivery. Polymers 2024, 16, 1871. https://doi.org/10.3390/polym16131871
Ma X, Zhao T, Ren X, Lin H, He P. Recent Progress in Polyion Complex Nanoparticles with Enhanced Stability for Drug Delivery. Polymers. 2024; 16(13):1871. https://doi.org/10.3390/polym16131871
Chicago/Turabian StyleMa, Xinlin, Tianyi Zhao, Xiaoyue Ren, Hui Lin, and Pan He. 2024. "Recent Progress in Polyion Complex Nanoparticles with Enhanced Stability for Drug Delivery" Polymers 16, no. 13: 1871. https://doi.org/10.3390/polym16131871
APA StyleMa, X., Zhao, T., Ren, X., Lin, H., & He, P. (2024). Recent Progress in Polyion Complex Nanoparticles with Enhanced Stability for Drug Delivery. Polymers, 16(13), 1871. https://doi.org/10.3390/polym16131871





