Novel Reactive Polyhedral Oligomeric Silsesquioxane-Reinforced and Toughened Epoxy Resins for Advanced Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Octa (Mercaptopropyl) Polyhedral Oligomeric Silsesquioxane (OMPPS)
2.3. Synthesis of Octa (Carboxyl Decyl Thiopropyl) Polyhedral Oligomeric Silsesquioxane (OCOPS)
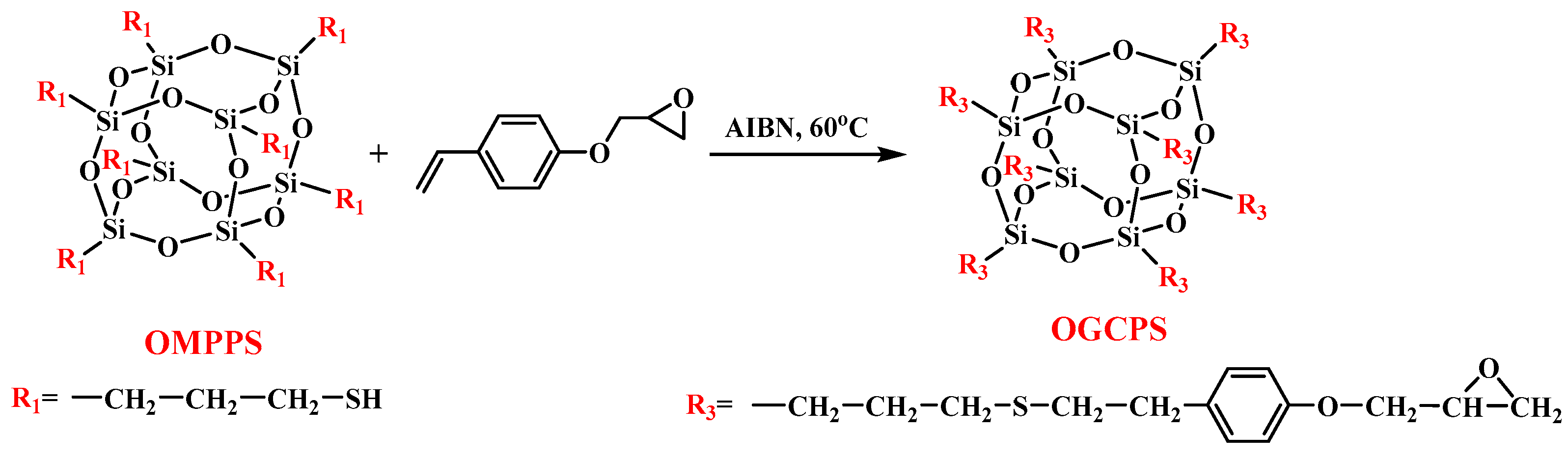
2.4. Synthesis of Octa (Glycidyl Phenyl Ethyl Thiopropyl) Polyhedral Oligomeric Silsesquioxane (OGCPS)
2.5. Preparation of Hybrid Resins
2.5.1. Preparation of OMPPS- or OGCPS-Modified Epoxy Hybrid Resins
2.5.2. Preparation of OCOPS-Modified Epoxy Hybrid Resins
2.6. Preparation of Cured Hybrid Resins
2.7. Instruments and Measurements
3. Results and Discussion
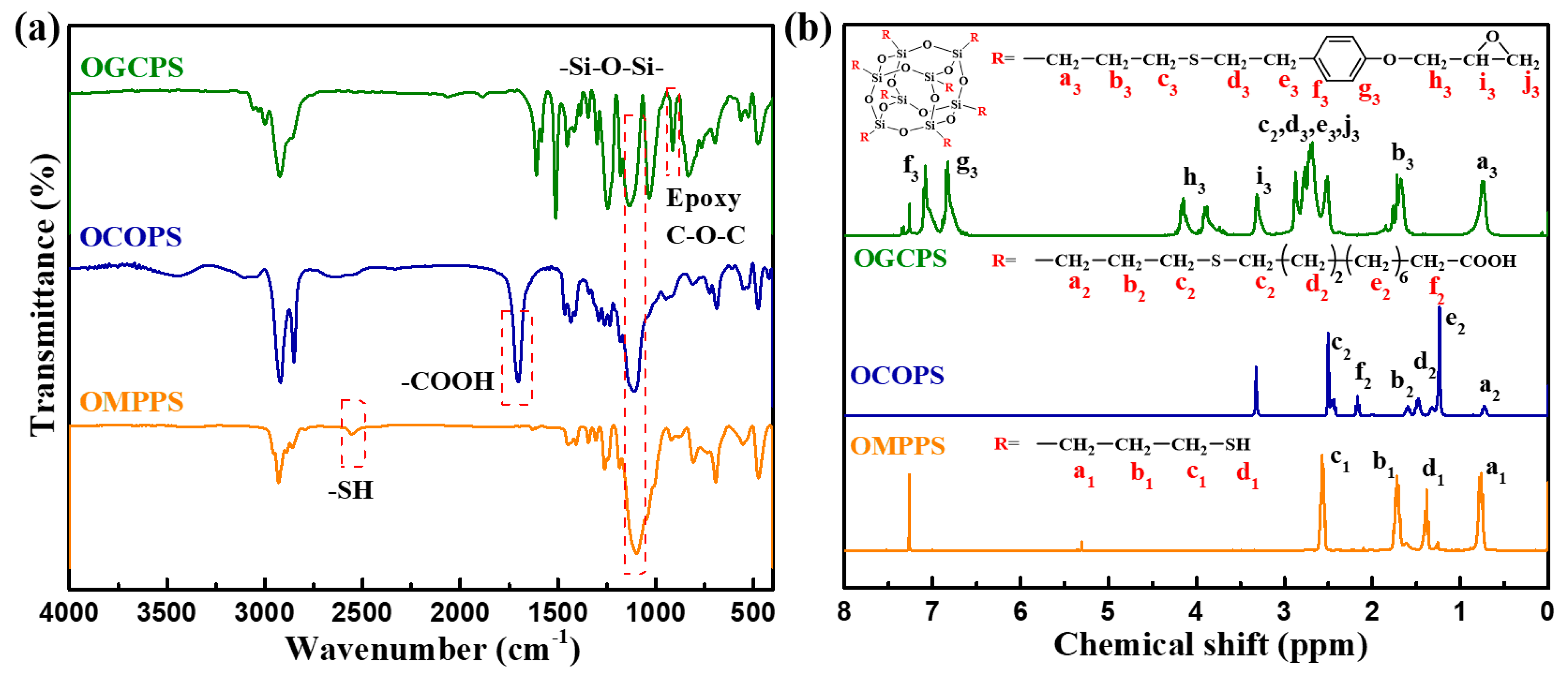
3.1. The Structural Characterization of OMPPS, OCOPS, and OGCPS
3.2. The Processability of Hybrid Resins
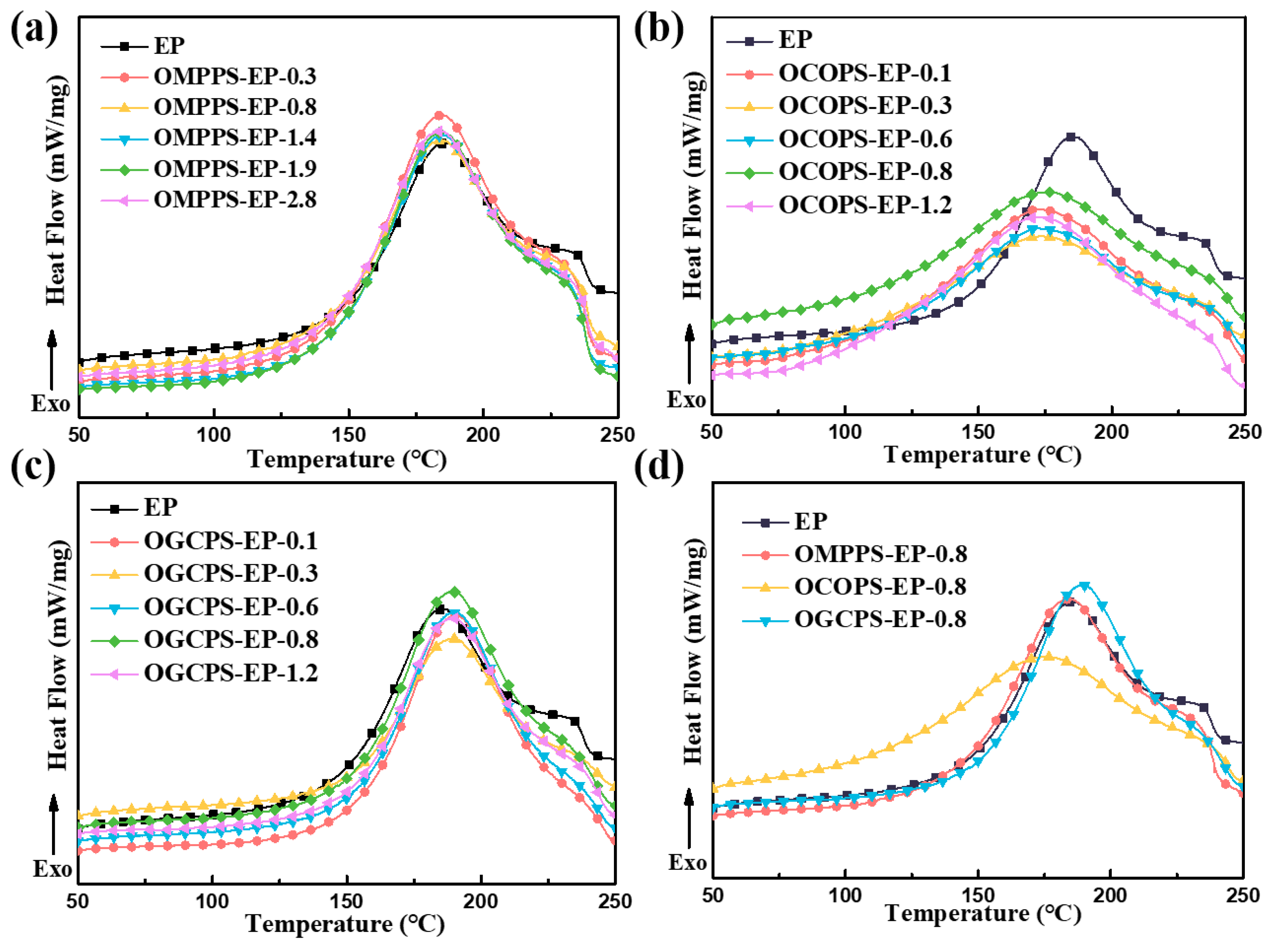
3.3. Thermal Curing Behaviors of Hybrid Resins
3.4. Mechanical Properties of Cured Hybrid Resins
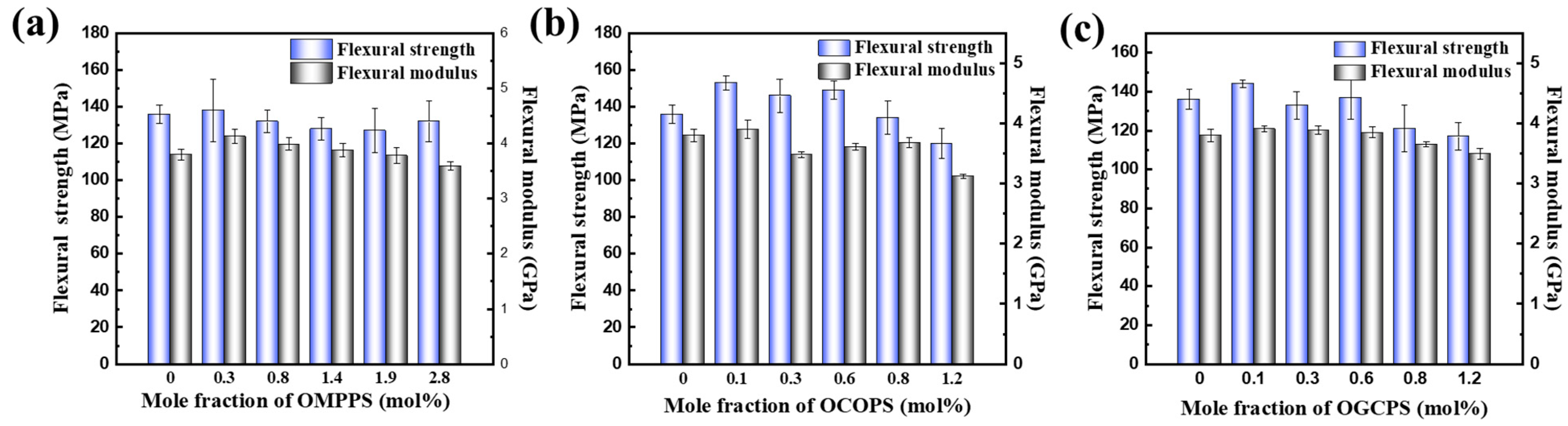
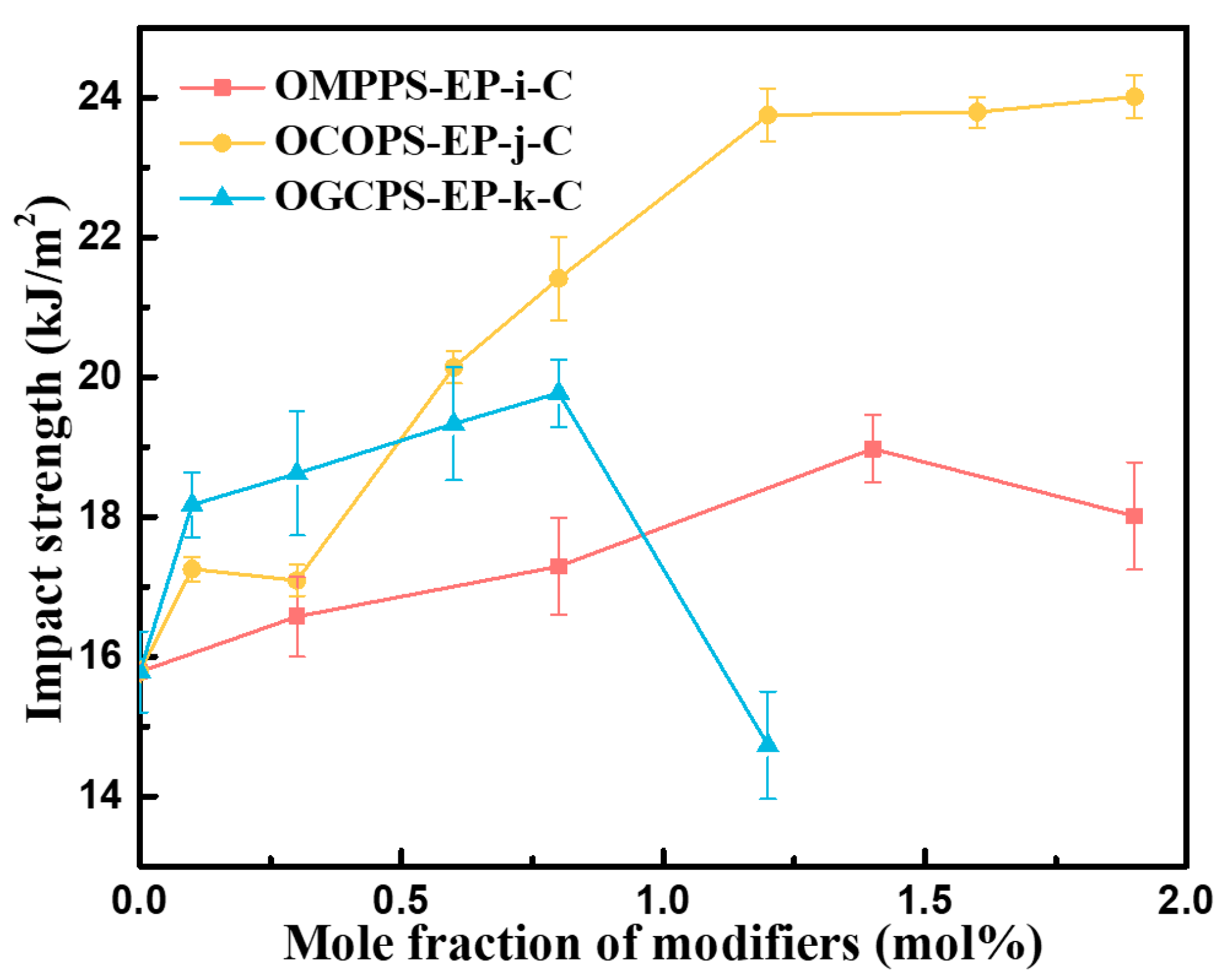
3.4.1. Flexural and Impact Properties of Cured Hybrid Resins
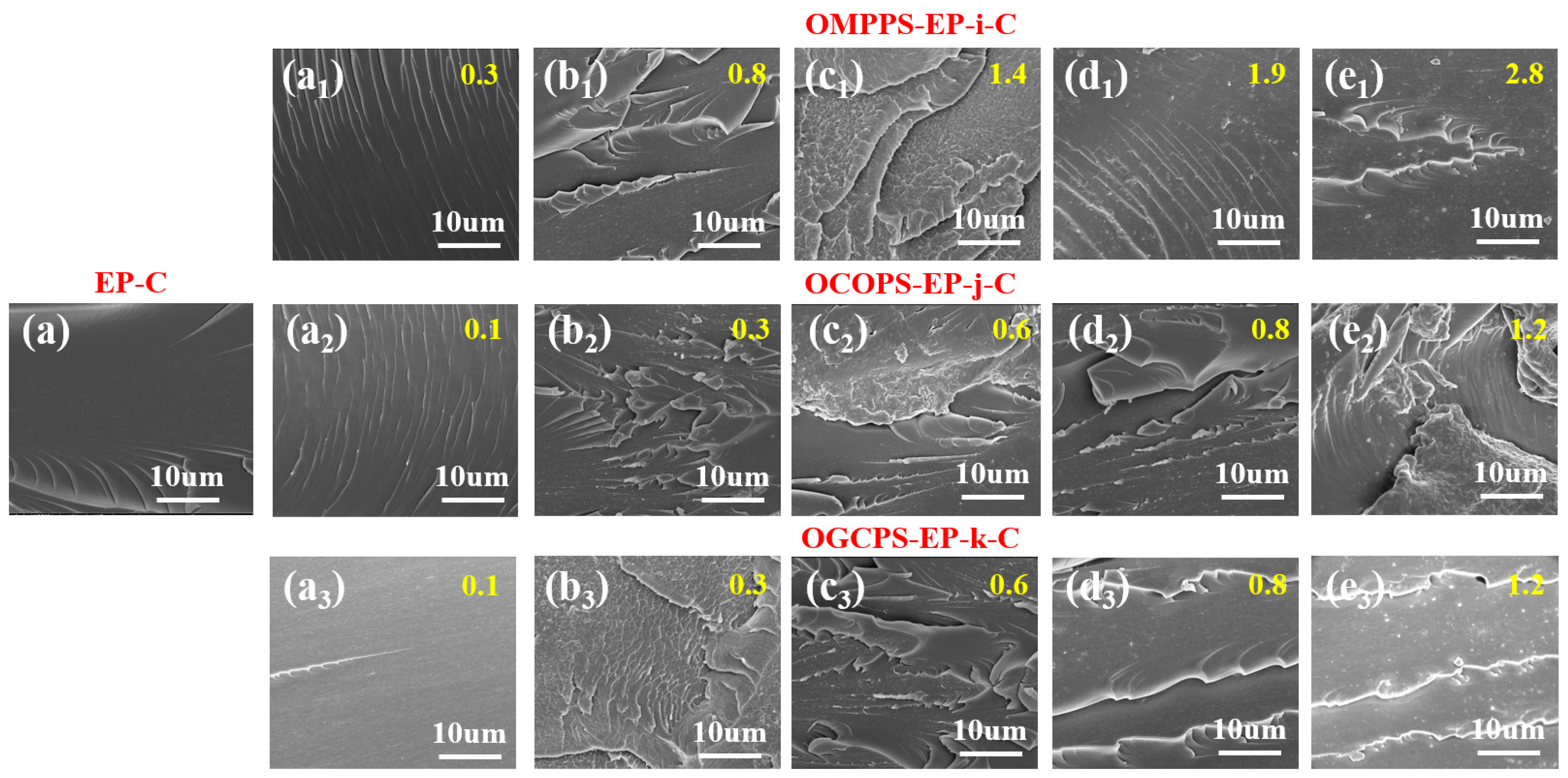
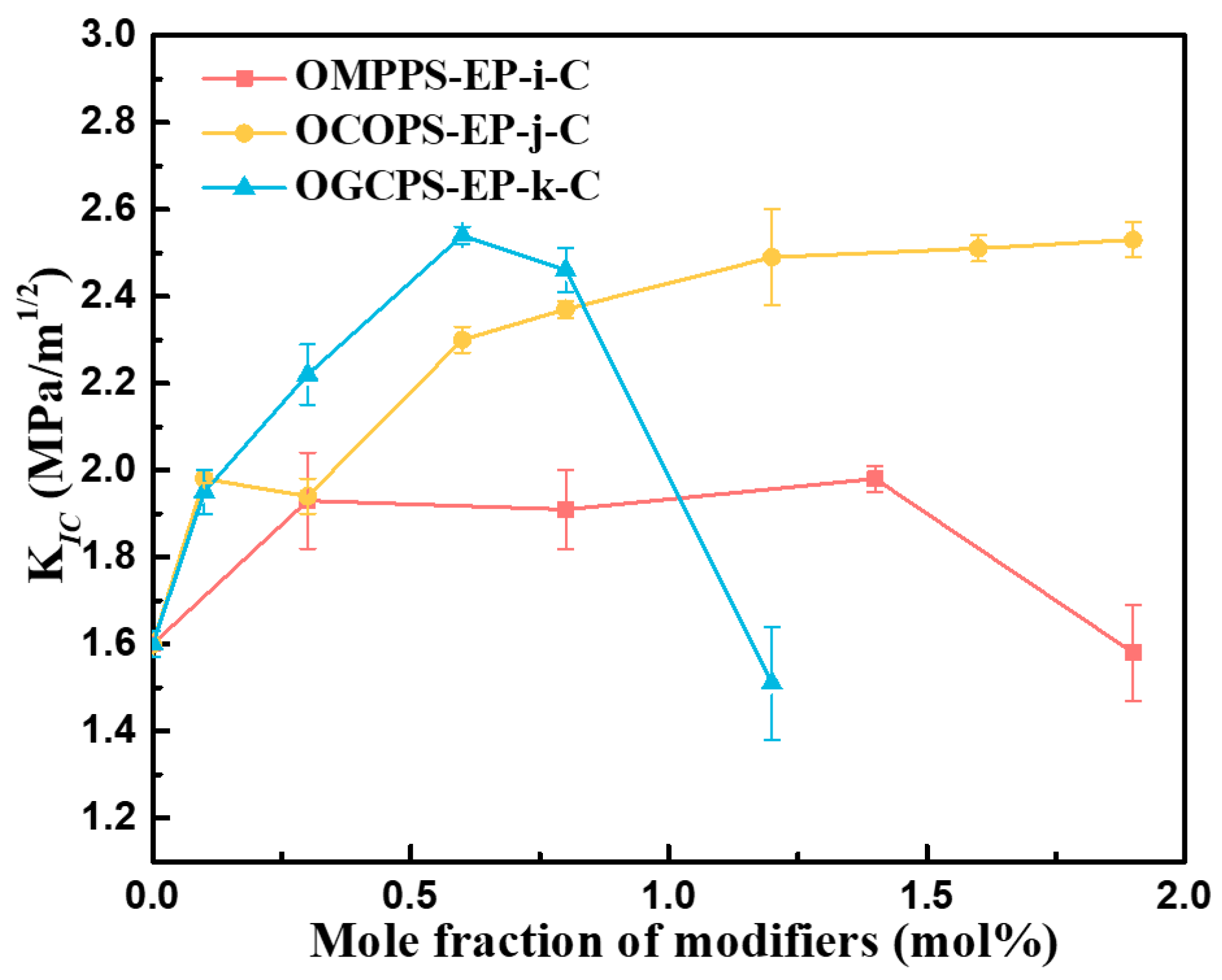
3.4.2. Fracture Toughness of Cured Hybrid Resins
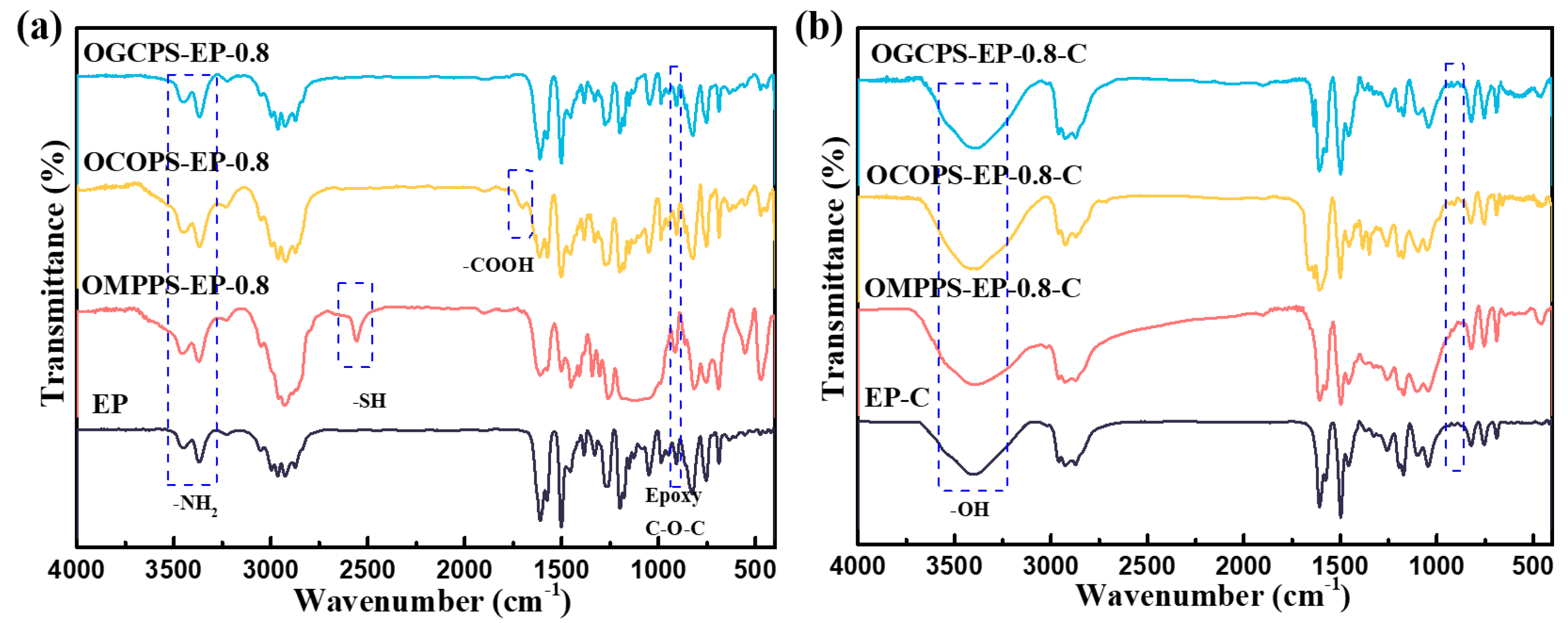
3.5. Thermal Properties of Cured Hybrid Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic self-healing epoxies in polymer matrix composites (PMCs) for aerospace applications. Polymers 2021, 13, 2073–4360. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, T.; Yang, Z.; Han, M. Fabrication and optimization of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ electrode for symmetric solid oxide fuel cell with zirconia based electrolyte. J. Mater. Sci. Technol. 2017, 33, 1329–1333. [Google Scholar]
- Zhao, Y.S.; He, Y.H.; Yang, K.R.; Wang, X.P.; Bai, J.H.; Du, B. Improving the surface insulating performance of epoxy resin/Al2O3 composite materials by extending chain of liquid epoxy resin with Me-THPA. High Volt. 2020, 5, 472–481. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Xu, R.; Xiao, Y.; Wang, H.L.; Zhang, W.; Zhang, G.Y. Mechanical performances of phenolic modified epoxy resins at room and high temperatures. Coatings 2022, 12, 643. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, M.; Chen, Y.; He, J.C.; Wang, X.L.; Xie, J.; Li, Z.W.; Chen, Z.M.; Fu, Y.H.; Xiong, C.X.; et al. Epoxy resin/hollow glass microspheres composite materials with low dielectric constant and excellent mechanical performance. J. Appl. Polym. Sci. 2022, 139, E52787. [Google Scholar] [CrossRef]
- Qin, J.Y.; Zhao, H.P.; Qin, Z.L.; Zhang, W.C.; Yang, R.J. Effect of polyhedral oligomeric silsesquioxanes with different structures on dielectric and mechanical properties of epoxy resin. Polym. Compos. 2021, 42, 3445–3457. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L., Jr.; Zattera, A.J.; Amico, S.C. Toughening epoxy resin with liquid rubber and its hybrid composites: A systematic review. J. Polym. Res. 2022, 29, 340. [Google Scholar] [CrossRef]
- Thomas, R.; Yumei, D.; Yuelong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Jones, A.R.; Watkins, C.A.; White, S.R.; Sottos, N.R. Self-healing thermoplastic-toughened epoxy. Polymer 2015, 74, 254–261. [Google Scholar] [CrossRef]
- Wu, Z.J.; Yi, X.S.; Wilkinson, A. Interlaminar fracture toughness of carbon fibre/RTM6-2 composites toughened with thermoplastic-coated fabric reinforcement. Compos. Part B 2017, 130, 192–199. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Eissa, M.M.; Samy, M.; Ramadan, A.M.; Amin, A. Amino-terminated hyperbranched polymer for toughness improvement of epoxy/clay nanocomposites. Polym. Bull. 2015, 72, 3147–3168. [Google Scholar] [CrossRef]
- Shiravand, F.; Ascione, L.; Persico, P.; Carfagna, C.; Brocks, T.; Cioffi, M.O.H.; Puglisi, C.; Samperi, F.; Ambrogi, V. A novel hybrid linear-hyperbranched poly(butylene adipate) copolymer as an epoxy resin modifier with toughening effect. Polym. Int. 2016, 65, 308–319. [Google Scholar] [CrossRef]
- Qi, Y.; Fan, Q.; Li, J.; Cao, Q.; Pan, X.; Pan, Y.; Jian, X.; Weng, Z. Toughened and reinforced the petroleum-based epoxy resin via thermotropic liquid crystal bio-based counterpart. Compos. Commun. 2023, 44, 101771. [Google Scholar] [CrossRef]
- Wang, T.T.; Huang, P.; Li, Y.Q.; He, N.; Fu, S.Y. Epoxy nanocomposites significantly toughened by both poly(sulfone) and graphene oxide. Compos. Commun. 2019, 14, 55–60. [Google Scholar]
- Xu, Z.G.; Song, P.G.; Zhang, J.; Guo, Q.P.; Mai, Y.W. Epoxy nanocomposites simultaneously strengthened and toughened by hybridization with graphene oxide and block ionomer. Compos. Sci. Technol. 2018, 168, 363–370. [Google Scholar] [CrossRef]
- Park, Y.T.; Qian, Y.Q.; Chan, C.; Suh, T.; Nejhad, M.G.; Macosko, C.W.; Stein, A. Epoxy toughening with low graphene loading. Adv. Funct. Mater. 2015, 25, 575–585. [Google Scholar] [CrossRef]
- Wang, F.Z.; Drzal, L.T.; Qin, Y.; Huang, Z.X. Enhancement of fracture toughness, mechanical and thermal properties of rubber/epoxy composites by incorporation of graphene nanoplatelets. Compos. Part A 2016, 87, 10–22. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, C.S.; Yin, H.Y.; Wang, L.L.; Xie, H.F.; Cheng, R.S. Carboxyl-terminated butadiene-acrylonitrile-toughened epoxy/carboxyl-modified carbon nanotube nanocomposites: Thermal and mechanical properties. Express. Polym. Lett. 2012, 6, 719–728. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosur, M.; Ludwick, A.G.; Zainuddin, S.; Kumar, A.; Trovillion, J.; Jeelani, S. Thermo-mechanical behavior of epoxy composites modified with reactive polyol diluent and randomly-oriented amino-functionalized multi-walled carbon nanotubes. Polym. Test. 2012, 31, 777–784. [Google Scholar] [CrossRef]
- Dharmavarapu, P.; Reddy, M. Mechanical, low velocity impact, fatigue and tribology behaviour of silane grafted aramid fibre and nano-silica toughened epoxy eomposite. Silicon 2021, 13, 1741–1750. [Google Scholar] [CrossRef]
- Pham, T.D.; Vu, C.M.; Choi, H.J. Enhanced fracture toughness and mechanical properties of epoxy resin with rice husk-based nano-silica. Polym. Sci. Ser. A 2017, 59, 437–444. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Mohanty, S.; Nayak, S.K. Study of thermal stability and thermo-mechanical behavior of functionalized soybean oil modified toughened epoxy/organo clay nanocomposite. Prog. Org. Coat. 2015, 88, 263–271. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Liu, Y.; Wang, Q. Synergistic effect of piperazine pyrophosphate and epoxy-octavinyl silsesquioxane on flame retardancy and mechanical properties of epoxy resin. Compos. Part B Eng. 2021, 223, 109115. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Zhang, P.; Xue, K.; Yao, T.; Liu, L.; Huang, Y. POSS-polyurethane prepolymer strengthened and toughened CF/epoxy resin composites for room and simulated arctic ambient temperature. Polymer 2024, 294, 126692. [Google Scholar] [CrossRef]
- Han, R.; Ma, X.; Cai, L.; Zhang, Z.; Fang, Y.; Wang, J. Low viscosity and low temperature curing reactive POSS/epoxy hybrid resin with enhanced toughness and comprehensive thermal performance. RSC Adv. 2024, 14, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Ni, G.; Zhang, L.; Mi, J.; Yao, B.; Zhu, C. Syntheses of silsesquioxane (POSS)-based inorganic/organic hybrid and the application in reinforcement for an epoxy resin. J. Colloid Interface Sci. 2011, 362, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, T.X.; Song, H.; Han, Y.Q.; Su, H.B.; Wang, Y.M.; Wang, Q. Epoxy Resin/POSS Nanocomposites with Toughness and Thermal Stability. J. Photopolym. Sci. Technol. 2017, 30, 25–31. [Google Scholar] [CrossRef]
- Cao, J.; Fan, H.; Li, B.-G.; Zhu, S. Synthesis and evaluation of Double-Decker Silsesquioxanes as modifying agent for epoxy resin. Polymer 2017, 124, 157–167. [Google Scholar] [CrossRef]
- Konnola, R.; Parameswaranpillai, J.; Joseph, K. Mechanical, thermal, and viscoelastic response of novel in situ CTBN/POSS/epoxy hybrid composite system. Polymer. Compos. 2016, 37, 2109–2120. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Song, H.; Han, Y.; Dong, Y.; Wang, Y.; Wang, Q. Improving the thermal conductivity and mechanical property of epoxy composites by introducing polyhedral oligomeric silsesquioxane-grafted graphene oxide. Polymer. Compos. 2018, 39, E1890–E1899. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Hao, M.; Zhi, J.; Qian, X. Synergistically toughened epoxy resin based on modified-POSS triggered interpenetrating network. Polymer 2023, 268, 125719. [Google Scholar] [CrossRef]
- Misasi, J.M.; Jin, Q.; Knauer, K.M.; Morgan, S.E.; Wiggins, J.S. Hybrid POSS-hyperbranched polymer additives for simultaneous reinforcement and toughness improvements in epoxy networks. Polymer 2017, 117, 54–63. [Google Scholar] [CrossRef]
- Kopesky, E.T.; Haddad, T.S.; McKinley, G.H.; Cohen, R.E. Miscibility and viscoelastic properties of acrylic polyhedral oligomeric silsesquioxane–poly(methyl methacrylate) blends. Polymer 2005, 46, 4743–4752. [Google Scholar] [CrossRef]
- Hao, N.; Böhning, M.; Schönhals, A. Dielectric properties of nanocomposites based on polystyrene and polyhedral oligomeric phenethyl-silsesquioxanes. Macromolecules 2007, 40, 9672–9679. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, C.; Guang, S.; Xu, H. POSS Core star-shape molecular hybrid materials: Effect of the chain length and POSS content on dielectric properties. J. Appl. Polym. Sci. 2013, 127, 2628–2634. [Google Scholar] [CrossRef]
- Robertson, R.E.; Mindroiu, V.E.; Cheung, M.-F. Fracture in epoxy matrix resins. Compos. Sci. Technol. 1985, 22, 197–207. [Google Scholar] [CrossRef]
- Sahagun, C.M.; Morgan, S.E. Thermal control of nanostructure and molecular network development in epoxy-amine thermosets. ACS Appl. Mater. Inter. 2012, 4, 564–572. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Guo, X.; Zhang, W.; Qin, Z.; Yang, R. Preparation and mechanism of toughening and flame retardance of epoxy resin using novel silsesquioxane molecules. React. Funct. Polym. 2023, 190, 105645. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS particles on the mechanical, thermal, and morphological properties of PLA and plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Abbasi, A.; Salimi, A.; Bouhendi, H.; Karimi, M. A study of the thermal properties of alumina/glycidoxy propyl POSS/epoxy adhesives. Int. J. Adhes. Adhes. 2023, 124, 103368. [Google Scholar] [CrossRef]
- Lian, Q.; Chen, H.; Luo, Y.; Li, Y.; Cheng, J.; Liu, Y. Toughening mechanism based on the physical entanglement of branched epoxy resin in the non-phase-separated inhomogeneous crosslinking network: An experimental and molecular dynamics simulation study. Polymer 2022, 247, 124754. [Google Scholar] [CrossRef]
- Zeng, K.; Zheng, S. Nanostructures and surface dewettability of epoxy thermosets containing hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(ethylene oxide). J. Phys. Chem. B 2007, 111, 13919–13928. [Google Scholar] [CrossRef]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and characterization of cyanate ester functional benzoxazine and its polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]








| Solvent | Solubility | ||
|---|---|---|---|
| OMPPS | OCOPS | OGCPS | |
| Methanol | − | − | − |
| Ethanol | − | − | − |
| Tetrahydrofuran | + | + | + |
| Ethyl acetate | + | − | + |
| Dimethylformamide | + | + | + |
| Dimethyl sulfoxide | + | + | + |
| Dichloromethane | + | − | + |
| Petroleum ether | − | − | − |
| Chloroform | + | − | + |
| Sample | Tg (°C) | Td5 (°C) |
|---|---|---|
| EP-C | 169 | 317 |
| OMPPS-EP-0.3-C | 170 | 341 |
| OMPPS-EP-0.8-C | 173 | 347 |
| OMPPS-EP-1.4-C | 173 | 348 |
| OMPPS-EP-1.9-C | 171 | 349 |
| OMPPS-EP-2.8-C | 170 | 345 |
| OCOPS-EP-0.1-C | 174 | 356 |
| OCOPS-EP-0.3-C | 156 | 333 |
| OCOPS-EP-0.6-C | 155 | 204 |
| OCOPS-EP-0.8-C | 157 | 136 |
| OCOPS-EP-1.2-C | 158 | 125 |
| OGCPS-EP-0.1-C | 166 | 355 |
| OGCPS-EP-0.3-C | 168 | 356 |
| OGCPS-EP-0.6-C | 167 | 357 |
| OGCPS-EP-0.8-C | 167 | 356 |
| OGCPS-EP-1.2-C | 170 | 353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, C.; Feng, Y.; Chen, Y.; Wan, L.; Huang, F.; Liu, Z.; Qian, J.; Liu, W. Novel Reactive Polyhedral Oligomeric Silsesquioxane-Reinforced and Toughened Epoxy Resins for Advanced Composites. Polymers 2024, 16, 1877. https://doi.org/10.3390/polym16131877
Liu W, Wang C, Feng Y, Chen Y, Wan L, Huang F, Liu Z, Qian J, Liu W. Novel Reactive Polyhedral Oligomeric Silsesquioxane-Reinforced and Toughened Epoxy Resins for Advanced Composites. Polymers. 2024; 16(13):1877. https://doi.org/10.3390/polym16131877
Chicago/Turabian StyleLiu, Weibo, Caiyun Wang, Yu Feng, Yongfeng Chen, Liqiang Wan, Farong Huang, Zuozhen Liu, Jianhua Qian, and Weiping Liu. 2024. "Novel Reactive Polyhedral Oligomeric Silsesquioxane-Reinforced and Toughened Epoxy Resins for Advanced Composites" Polymers 16, no. 13: 1877. https://doi.org/10.3390/polym16131877






