Sequential Cascade Doping of Conjugated-Polymer-Wrapped Carbon Nanotubes for Highly Electrically Conductive Platforms
Abstract
:1. Introduction
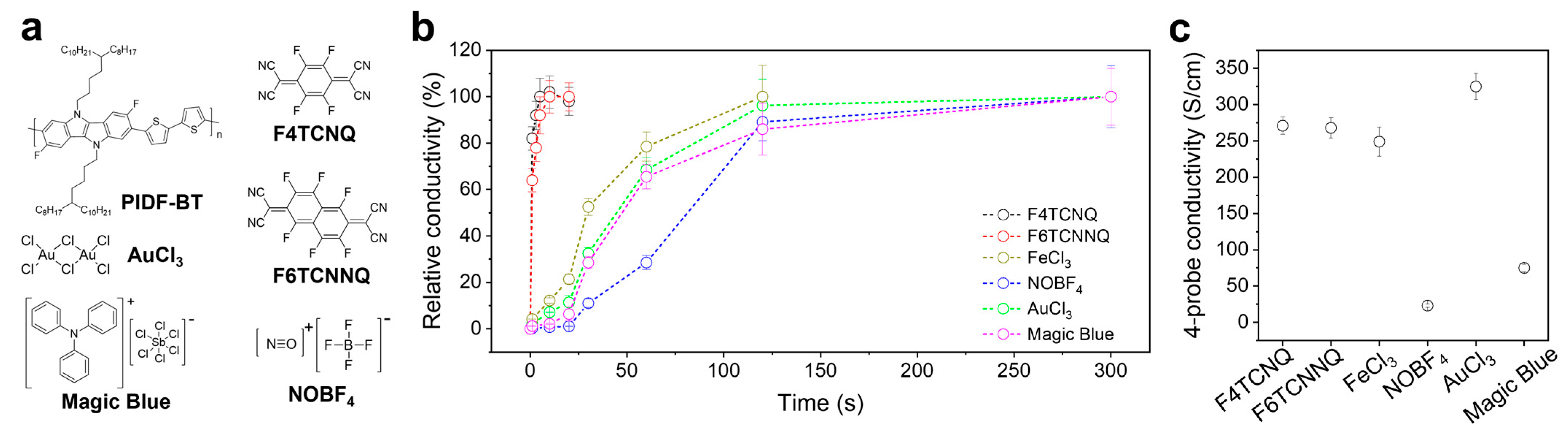
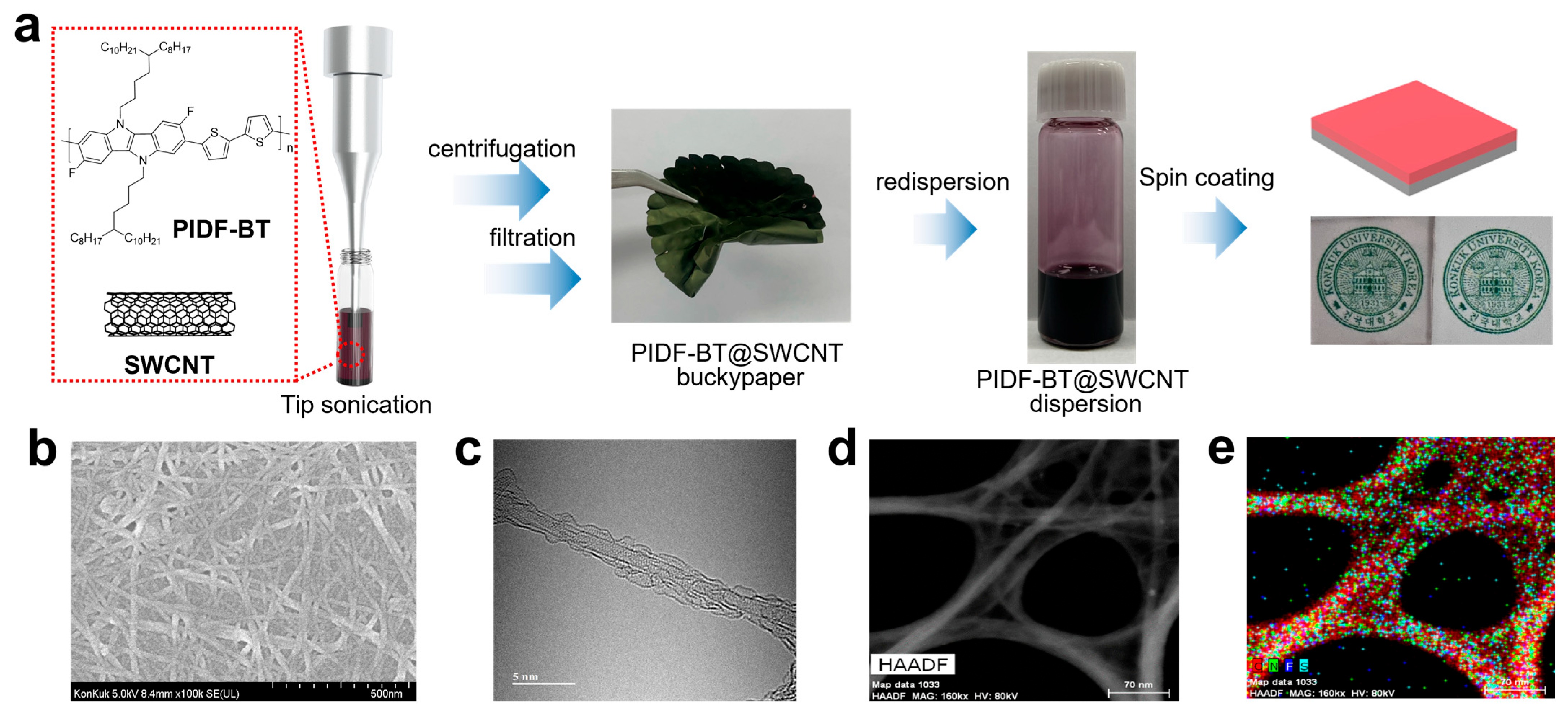
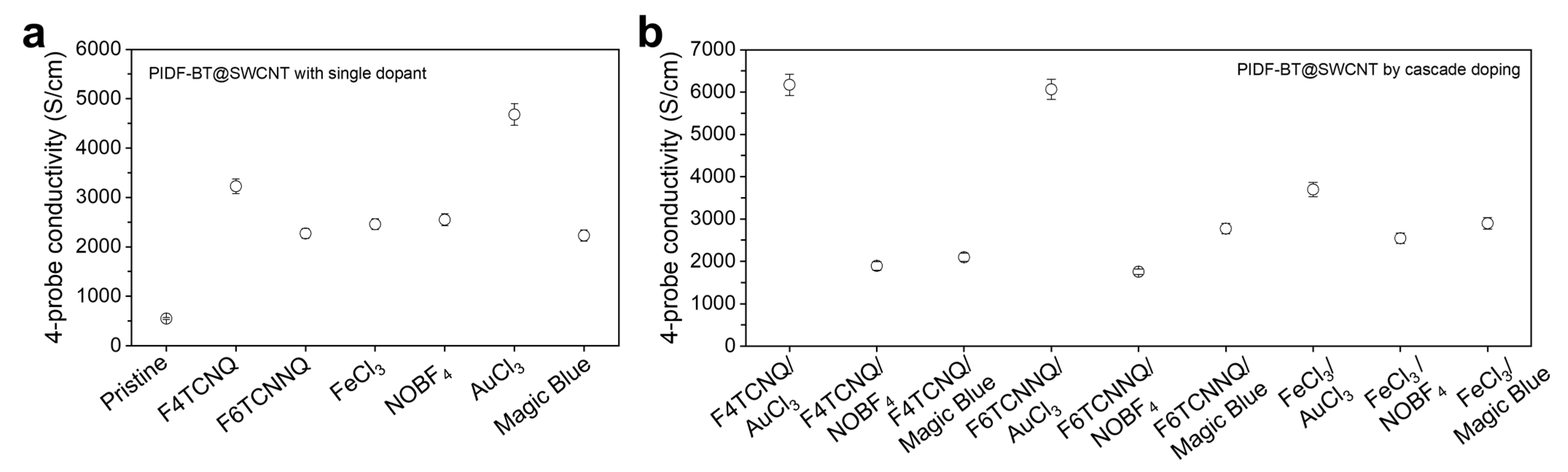
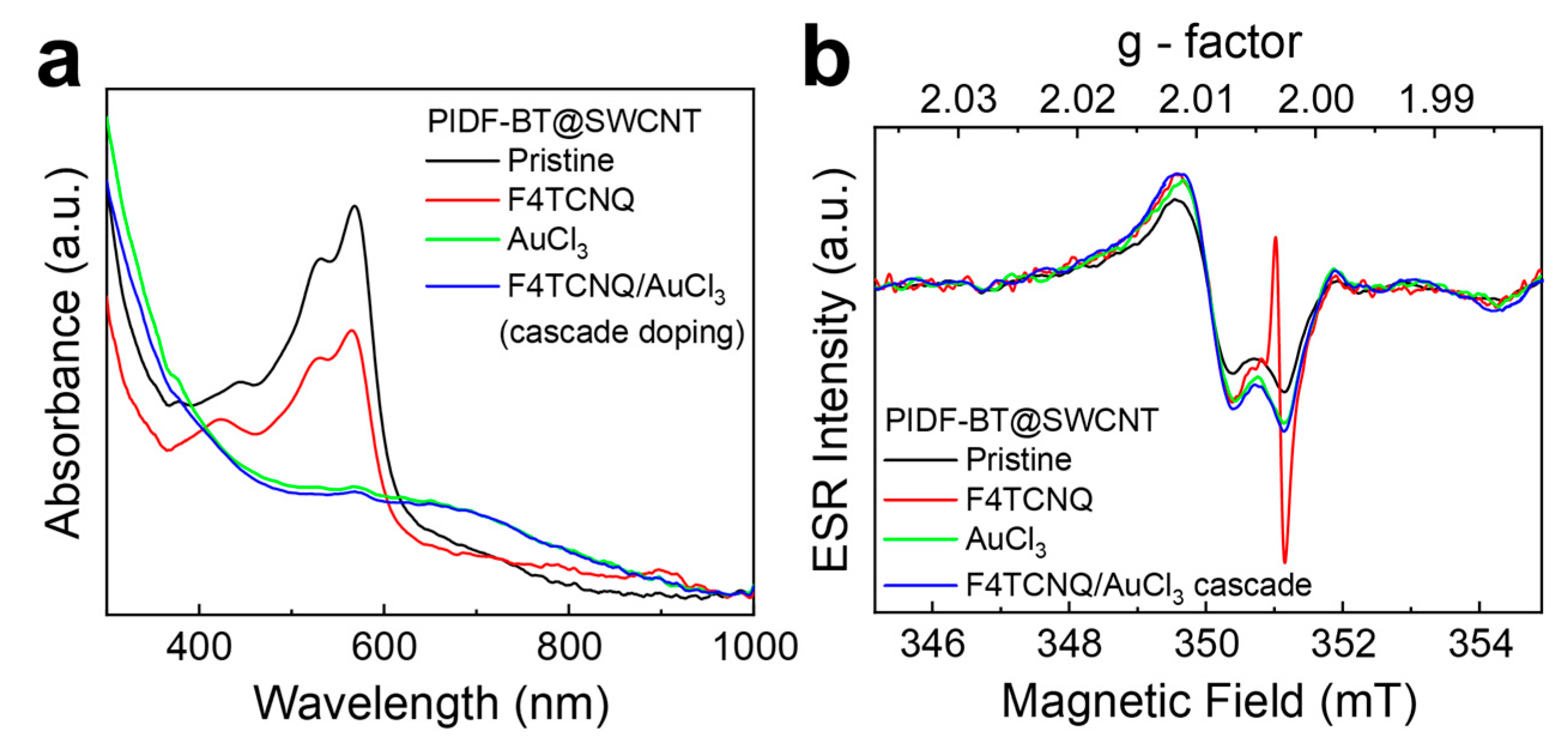
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. PIDF-BT@SWCNT Preparation
3.3. Sequential Doping and Conductivity Measurement
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Cui, Y.; Xu, Y.; Xian, K.; Bi, P.; Chen, Z.; Zhou, K.; Ma, L.; Zhang, T.; Yang, Y.; et al. A New Polymer Donor Enables Binary All-Polymer Organic Photovoltaic Cells with 18% Efficiency and Excellent Mechanical Robustness. Adv. Mater. 2022, 34, 2205009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; An, C.; Cui, Y.; Zhang, J.; Bi, P.; Yang, C.; Zhang, S.; Hou, J. A Universal Nonhalogenated Polymer Donor for High-Performance Organic Photovoltaic Cells. Adv. Mater. 2022, 34, 2105803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Sheriff, H.K.M.; Forrest, S.R. Semitransparent Organic Photovoltaics for Building Integrated Photovoltaic Applications. Nat. Rev. Mater. 2023, 8, 186. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, S.; Kim, M.; Yoo, D.; Ok, E.; Kim, S.; Song, K.C.; Song, Y.J.; Kang, B.; Cho, K. Designing a Length-Modulated Azide Photocrosslinker to Improve the Stretchability of Semiconducting Polymers. Adv. Funct. Mater. 2023, 33, 2212127. [Google Scholar] [CrossRef]
- Kang, S.; Kim, G.-H.; Park, S.-Y. Conjugated Block Copolymers for Functional Nanostructures. Acc. Chem. Res. 2022, 55, 2224. [Google Scholar] [CrossRef] [PubMed]
- Mikie, T.; Okamoto, K.; Iwasaki, Y.; Koganezawa, T.; Sumiya, M.; Okamoto, T.; Osaka, I. Naphthobispyrazine Bisimide: A Strong Acceptor Unit for Conjugated Polymers Enabling Highly Coplanar Backbone, Short π−π Stacking, and High Electron Transport. Chem. Mater. 2022, 34, 2717. [Google Scholar] [CrossRef]
- Yu, Z.-D.; Lu, Y.; Wang, Z.-Y.; Un, H.-I.; Zelewski, S.J.; Cui, Y.; You, H.-Y.; Liu, Y.; Xie, K.-F.; Yao, Z.-F.; et al. High n-Type and p-Type Conductivities and Power Factors Achieved in a Single Conjugated Polymer. Sci. Adv. 2023, 9, eadf3495. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.E.; Kang, Y.; Im, J.; Lee, J.; Lee, S.Y.; Park, J.; Gao, Y.J.; Jeon, D.; Son, J.Y.; Kim, J.; et al. Enhancing dopant diffusion for ultrahigh electrical conductivity and efficient thermoelectric conversion in conjugated polymers. Joule 2023, 7, 2291. [Google Scholar] [CrossRef]
- Yoon, S.E.; Kang, Y.; Noh, S.Y.; Park, J.; Lee, S.Y.; Park, J.; Lee, D.W.; Whang, D.R.; Kim, T.; Kim, G.-H.; et al. High Efficiency Doping of Conjugated Polymer for Investigation of Intercorrelation of Thermoelectric Effects with Electrical and Morphological Properties. ACS Appl. Mater. Interfaces 2020, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.A.; Arnold, L.; Rasheed, A.; Khan, I.A.; Wang, L. Textronics—A Review of Textile-Based Wearable Electronics. Adv. Eng. Mater. 2021, 23, 2100469. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.-S.; Ha, J.S. Flexible/Stretchable Supercapacitors with Novel Functionality for Wearable Electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Yoon, S.E.; Jung, K.H.; Bae, O.; Kim, D.; Kim, U.; Seo, H.; Kim, F.S.; Kim, K.C.; Kim, J.H.; et al. Dopant-dependent thermoelectric performance of indoloindole-selenophene based conjugated polymer. Chem. Eng. J. 2022, 431, 133779. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, J.; Zou, Y.; Di, C.-A.; Zhu, D. Chemical Doping of Organic Semiconductors for Thermoelectric Applications. Chem. Soc. Rev. 2020, 49, 7210. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Tsurumi, J.; Ohno, M.; Fujimoto, R.; Kumagai, S.; Kurosawa, T.; Okamoto, T.; Takeya, J.; Watanabe, S. Efficient Molecular Doping of Polymeric Semiconductors Driven by Anion Exchange. Nature 2019, 572, 634. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Watanabe, S.; Broch, K.; Sepe, A.; Brown, A.; Nasrallah, I.; Nikolka, M.; Fei, Z.; Heeney, M.; Matsumoto, D.; et al. 2D Coherent Charge Transport in Highly Ordered Conducting Polymers Doped by Solid-State Diffusion. Nat. Mater. 2016, 15, 896. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.E.; Lin, Y.; Huang, Y.; Ren, X.; Simatos, D.; Chen, C.; Tjhe, D.; Statz, M.; Lai, L.; Finn, P.A.; et al. High-Efficiency Ion-Exchange Doping of Conducting Polymers. Adv. Mater. 2022, 34, 2102988. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.E.; Kang, Y.; Jeon, G.G.; Jeon, D.; Lee, S.Y.; Ko, S.-J.; Kim, T.; Seo, H.; Kim, B.-G.; Kim, J.H. Exploring wholly doped conjugated polymer films based on hybrid doping: Strategic approach for optimizing electrical conductivity and related thermoelectric properties. Adv. Funct. Mater. 2020, 30, 2004598. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A Review of Fabrication and Applications of Carbon Nanotube Film-Based Flexible Electronics. Nanoscale 2013, 5, 1727. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, R.; Yang, Y.; Liao, G. A Hierarchical Helical Carbon Nanotube Fiber Artificial Ligament. Adv. Fiber Mater. 2023, 5, 1549. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, X.; Yang, J.; Liu, P.; Ding, G.; Chen, Z.; Liao, G. Size effect of ruthenium nanoparticles on water cracking properties with different crystal planes for boosting electrocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 644, 238. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, J.; Wang, Z.; Xu, Y.; Xing, Z.; Zhang, X.; Guan, Y.; Liao, G.; Li, X. High-loaded single Cu atoms decorated on N-doped graphene for boosting Fenton-like catalysis under neutral pH. J. Mater. Chem. A 2020, 8, 13685. [Google Scholar] [CrossRef]
- Choi, D.E.; Im, J.; Ahn, Y.; Hwang, K.; Kim, J.; Kwon, J.E.; Park, S.K.; Choi, H.H.; Kim, B.-G. Sequential Doping of Carbon Nanotube Wrapped by Conjugated Polymer for Highly Conductive Platform and Thermoelectric Application. Small Struct. 2024, 5, 2300321. [Google Scholar] [CrossRef]
- Kiefer, D.; Kroon, R.; Hofmann, A.I.; Sun, H.; Liu, X.; Giovannitti, A.; Stegerer, D.; Cano, A.; Hynynen, J.; Yu, L.; et al. Double doping of conjugated polymers with monomer molecular dopants. Nat. Mater. 2019, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.E.; Park, J.; Kwon, J.E.; Lee, S.Y.; Han, J.M.; Go, C.Y.; Choi, S.; Kim, K.C.; Seo, H.; Kim, J.H.; et al. Improvement of Electrical Conductivity in Conjugated Polymers through Cascade Doping with Small-Molecular Dopants. Adv. Mater. 2020, 32, 2005129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Broch, K.; Lee, W.; Ahn, H.; Lee, J.; Yoo, D.; Kim, J.; Chung, S.; Sirringhaus, H.; Kang, K.; et al. Highly Stable Contact Doping in Organic Field EffectTransistors by Dopant-Blockade Method. Adv. Funct. Mater. 2020, 30, 2000058. [Google Scholar] [CrossRef]
- Zhang, F.; Kahn, A. Investigation of the High Electron Affinity Molecular Dopant F6-TCNNQ for Hole-Transport Materials. Adv. Funct. Mater. 2018, 28, 1703780. [Google Scholar] [CrossRef]
- Li, H.; DeCoster, M.E.; Ming, C.; Wang, M.; Chen, Y.; Hopkins, P.E.; Chen, L.; Katz, H.E. Enhanced Molecular Doping for High Conductivity in Polymers with Volume Freed for Dopants. Macromolecules 2019, 52, 9804. [Google Scholar] [CrossRef]
- Tripathi, A.; Lee, Y.; Jung, C.; Kim, S.; Lee, S.; Choi, W.; Park, C.; Kwon, Y.W.; Lee, H.; Woo, H.Y. A heavily doped D–D′-type polymer with metal-like carrier transport via hybrid doping. J. Mater. Chem. C 2023, 11, 5646. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Chai, H.; Xu, Q.; Chen, Y.; Chen, L. Anion-Dependent Molecular Doping and Charge Transport in Ferric Salt-Doped P3HT for Thermoelectric Application. ACS Appl. Electron. Mater. 2021, 3, 1252. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, K.; Jang, J.; Baek, K.-Y.; Kim, J.; Seo, J.; Song, M.; Shin, J.; Kim, J.; Parkin, S.S.P.; et al. Molecular Dopant-Dependent Charge Transport in Surface-Charge-Transfer-Doped Tungsten Diselenide Field Effect Transistors. Adv. Mater. 2021, 33, 2101598. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yoon, S.E.; Lee, J.; Whang, D.R.; Lee, S.Y.; Shin, S.J.; Han, J.M.; Seo, H.; Park, H.J.; Kim, J.H.; et al. Unraveling doping capability of conjugated polymers for strategic manipulation of electric dipole layer toward efficient charge collection in perovskite solar cells. Adv. Funct. Mater. 2020, 30, 2001560. [Google Scholar] [CrossRef]
- Marqués, P.S.; Londi, G.; Yurash, B.; Nguyen, T.-Q.; Barlow, S.; Marder, S.R.; Beljonne, D. Understanding how Lewis acids dope organic semiconductors: A “complex” story. Chem. Sci. 2021, 12, 7012. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.L.; Vezin, H.; Nguyen, T.-Q.; Reddy, G.N.M. Structural insights into Lewis acid- and F4TCNQ-doped conjugated polymers by solid-state magnetic resonance spectroscopy. Mater. Horiz. 2022, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Fediai, A.; Emering, A.; Symalla, F.; Wenzel, W. Disorder-driven doping activation in organic semiconductors. Phys. Chem. Chem. Phys. 2020, 22, 10256. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Hofmann, A.; Brütting, W. Controlling Charge Accumulation Properties of Organic Light-Emitting Diodes using Dipolar Doping of Hole Transport Layers. Adv. Optical Mater. 2022, 10, 2201278. [Google Scholar] [CrossRef]
- Talipov, M.R.; Hossain, M.M.; Boddeda, A.; Thakur, K.; Rathore, R. A search for blues brothers: X-ray crystallographic/spectroscopic characterization of the tetraarylbenzidine cation radical as a product of aging of solid magic blue. Org. Biomol. Chem. 2016, 14, 2961. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Bae, J.J.; Park, H.K.; Kim, S.M.; Geng, H.-Z.; Park, K.A.; Shin, H.-J.; Yoon, S.-M.; Benayad, A.; Choi, J.-Y.; et al. Fermi Level Engineering of Single-Walled Carbon Nanotubes by AuCl3 Doping. J. Am. Chem. Soc. 2008, 130, 12757. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, K.K.; Jo, Y.W.; Park, M.H.; Chae, S.J.; Duong, D.L.; Yang, C.W.; Kong, J.; Lee, Y.H. Role of Anions in the AuCl3-Doping of Carbon Nanotubes. ACS Nano 2011, 5, 1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-H.; Jiang, R.; Fan, C.-Y.; Xie, D.; Li, B.; Zhang, J.-P.; Wu, X.-L. Engineering All-Purpose Amorphous Carbon Nanotubes with High N/O-Co-Doping Content to Bridge the Alkali-Ion Batteries and Li Metal Batteries. Small 2021, 17, 2006566. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Shahzad, A.; Rasool, K.; Mateen, F.; Oh, J.-M.; Shim, J.W. 2D MXene: A Potential Candidate for Photovoltaic Cells? A Critical Review. Adv. Sci. 2022, 9, 2104743. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Maleski, K.; Shuck, C.E.; Yang, Y.; Glazar, J.T.; Foucher, A.C.; Hantanasirisakul, K.; Sarycheva, A.; Frey, N.C.; May, S.J.; et al. Tailoring Electronic and Optical Properties of MXenes through Forming Solid Solutions. J. Am. Chem. Soc. 2020, 142, 19110. [Google Scholar] [CrossRef] [PubMed]
- Badía-Domínguez, I.; Canola, S.; Hernández Jolín, V.; López Navarrete, J.T.; Sancho-García, J.C.; Negri, F.; Ruiz Delgado, M.C. Tuning the Diradical Character of Indolocarbazoles: Impact of Structural Isomerism and Substitution Position. J. Phys. Chem. Lett. 2022, 13, 6003. [Google Scholar] [CrossRef] [PubMed]
- Paternò, G.M.; Robbiano, V.; Fraser, K.J.; Frost, C.; García Sakai, V.; Cacialli, F. Neutron Radiation Tolerance of Two Benchmark Thiophene-Based Conjugated Polymers: The Importance of Crystallinity for Organic Avionics. Sci. Rep. 2017, 7, 41013. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Xu, Z.; Li, H.; Zhong, F.; Bai, S.; Chen, L. Sequential-Twice-Doping Approach toward Synergistic Optimization of Carrier Concentration and Mobility in Thiophene-Based Polymers. ACS Appl. Electron. Mater. 2022, 4, 4947. [Google Scholar] [CrossRef]
- Jayasundara, W.J.M.J.S.R.; Schreckenbach, G. Theoretical Study of p- and n-Doping of Polythiophene- and Polypyrrole-Based Conjugated Polymers. J. Phys. Chem. C 2020, 124, 17528. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, L.; Jiao, X.; Zou, Y.; McNeill, C.R.; Xu, W.; Zhu, X.; Zhu, D. Quinoid-Resonant Conducting Polymers Achieve High Electrical Conductivity over 4000 S cm−1 for Thermoelectrics. Adv. Sci. 2018, 5, 1800947. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Young, R.J.; Halsall, M. Investigation into the Deformation of Carbon Nanotubes and Their Composites through the Use of Raman Spectroscopy. Compos. Part A Appl. 2001, 32, 401. [Google Scholar] [CrossRef]
- Park, J.; Kang, Y.; Lee, I.; Kim, B.-G. Doping Characteristics of Isoindoloindole-Based Conjugated Polymer toward Robust Transformable Organic Conductor. Org. Electron. 2019, 75, 105435. [Google Scholar] [CrossRef]
- Cho, I.; Jeon, N.J.; Kwon, O.K.; Kim, D.W.; Jung, E.H.; Noh, J.H.; Seo, J.; Seok, S.I.; Park, S.Y. Indolo[3,2-b]indole-based crystalline hole-transporting material for highly efficient perovskite solar cells. Chem. Sci. 2017, 8, 734. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.Y.; Choi, D.E.; Ahn, Y.; Kye, H.; Kim, M.S.; Kim, B.-G. Sequential Cascade Doping of Conjugated-Polymer-Wrapped Carbon Nanotubes for Highly Electrically Conductive Platforms. Polymers 2024, 16, 1884. https://doi.org/10.3390/polym16131884
Lee DY, Choi DE, Ahn Y, Kye H, Kim MS, Kim B-G. Sequential Cascade Doping of Conjugated-Polymer-Wrapped Carbon Nanotubes for Highly Electrically Conductive Platforms. Polymers. 2024; 16(13):1884. https://doi.org/10.3390/polym16131884
Chicago/Turabian StyleLee, Da Young, Da Eun Choi, Yejin Ahn, Hyojin Kye, Min Seon Kim, and Bong-Gi Kim. 2024. "Sequential Cascade Doping of Conjugated-Polymer-Wrapped Carbon Nanotubes for Highly Electrically Conductive Platforms" Polymers 16, no. 13: 1884. https://doi.org/10.3390/polym16131884




