Development of Polymeric Films Based on Sunflower Seed Proteins and Locust Bean Gum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Film Characterization
2.3.1. Digital Images, Chromatic and Microscopic Properties
2.3.2. FTIR Spectroscopy
2.3.3. X-Ray Diffraction (XRD)
2.3.4. Thermal Profile
2.3.5. Thickness and Mechanical Properties
2.3.6. Moisture Content (MC), Water Vapor and Oxygen Permeabilities (WVP, OXP), and Water Solubility (WS)
2.3.7. Film Biodegradation
2.3.8. Statistical Analysis
3. Results
3.1. Digital Images, Chromatic and Microscopic Properties
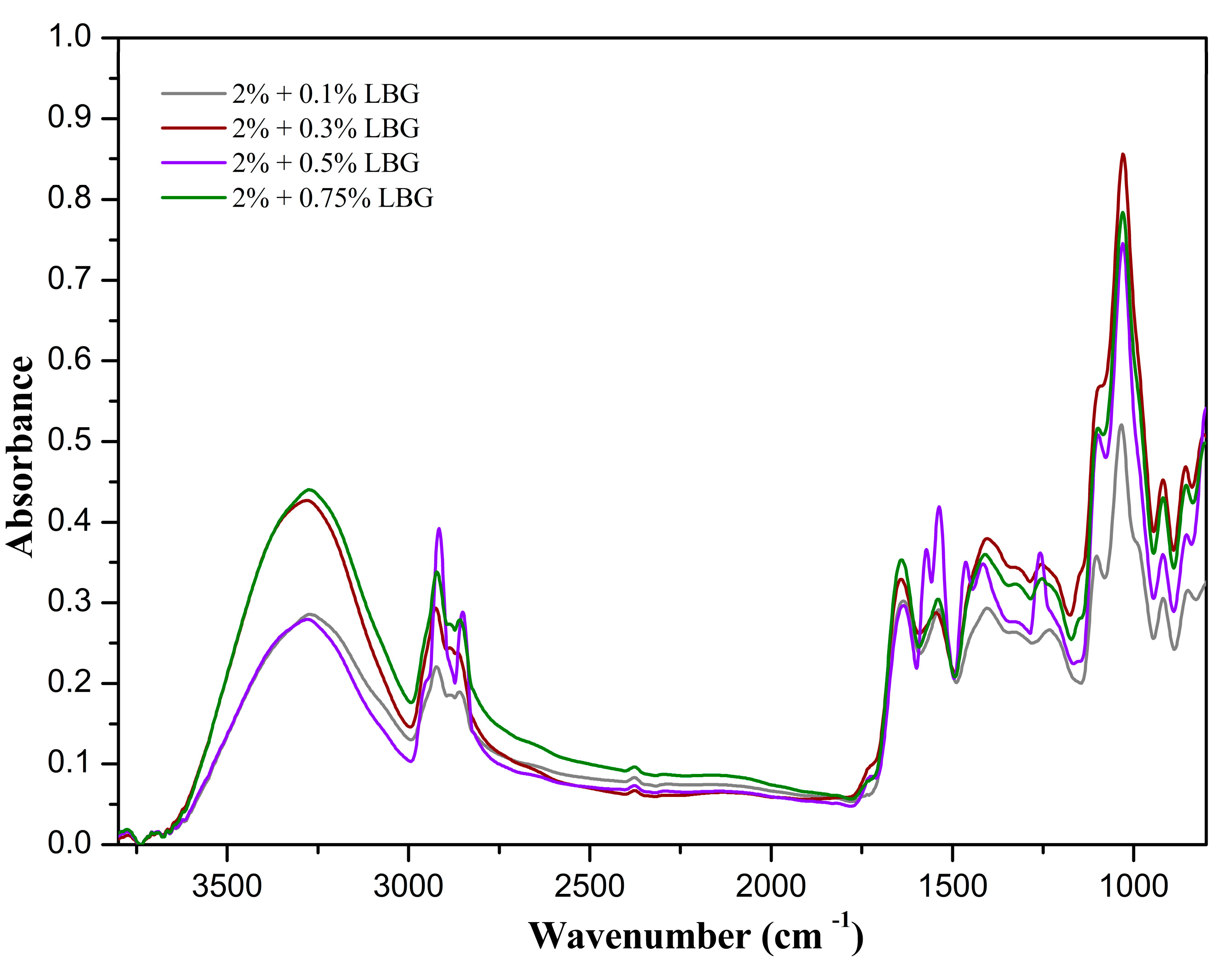
3.2. FTIR Spectroscopy
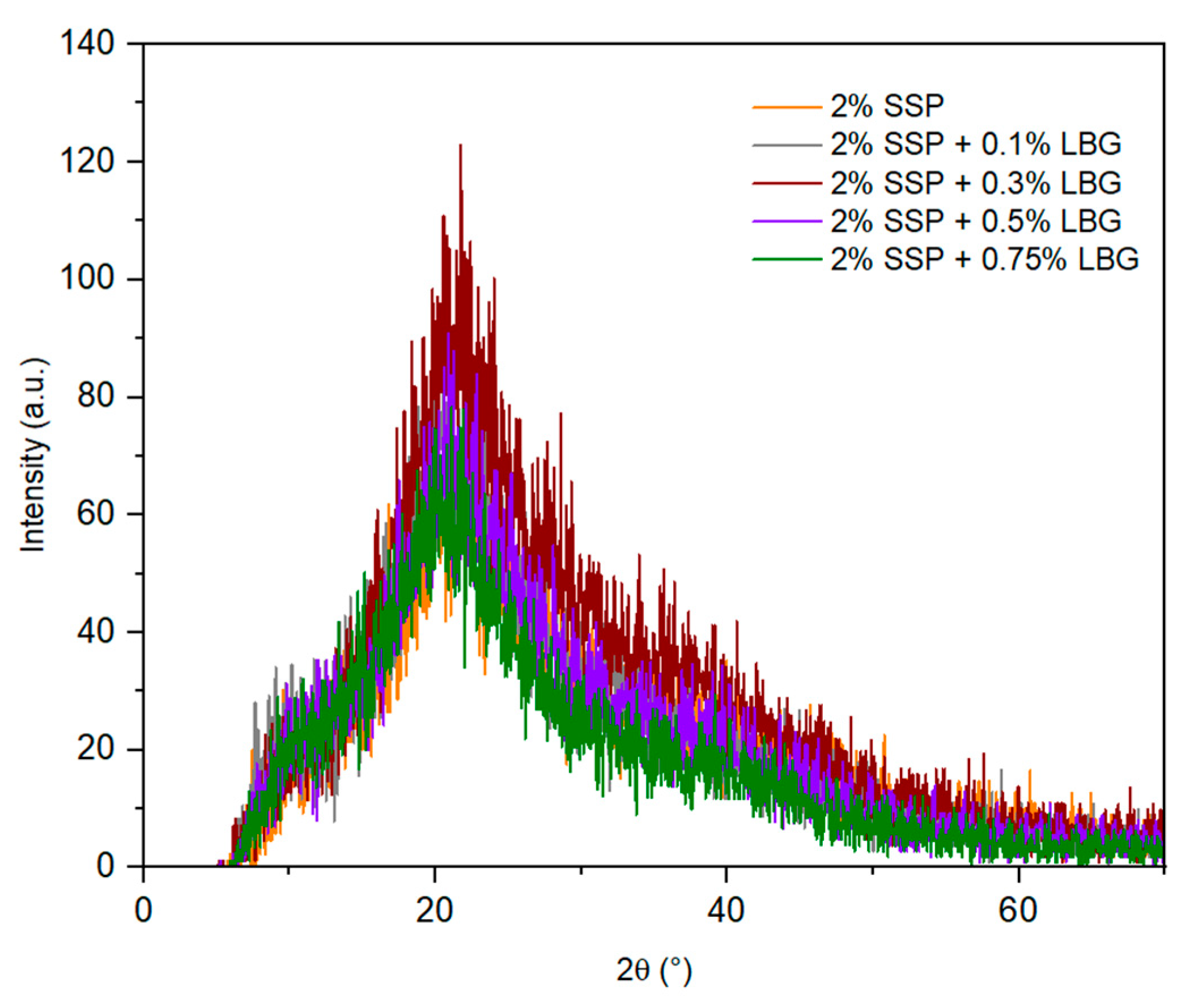
3.3. X-ray Diffraction
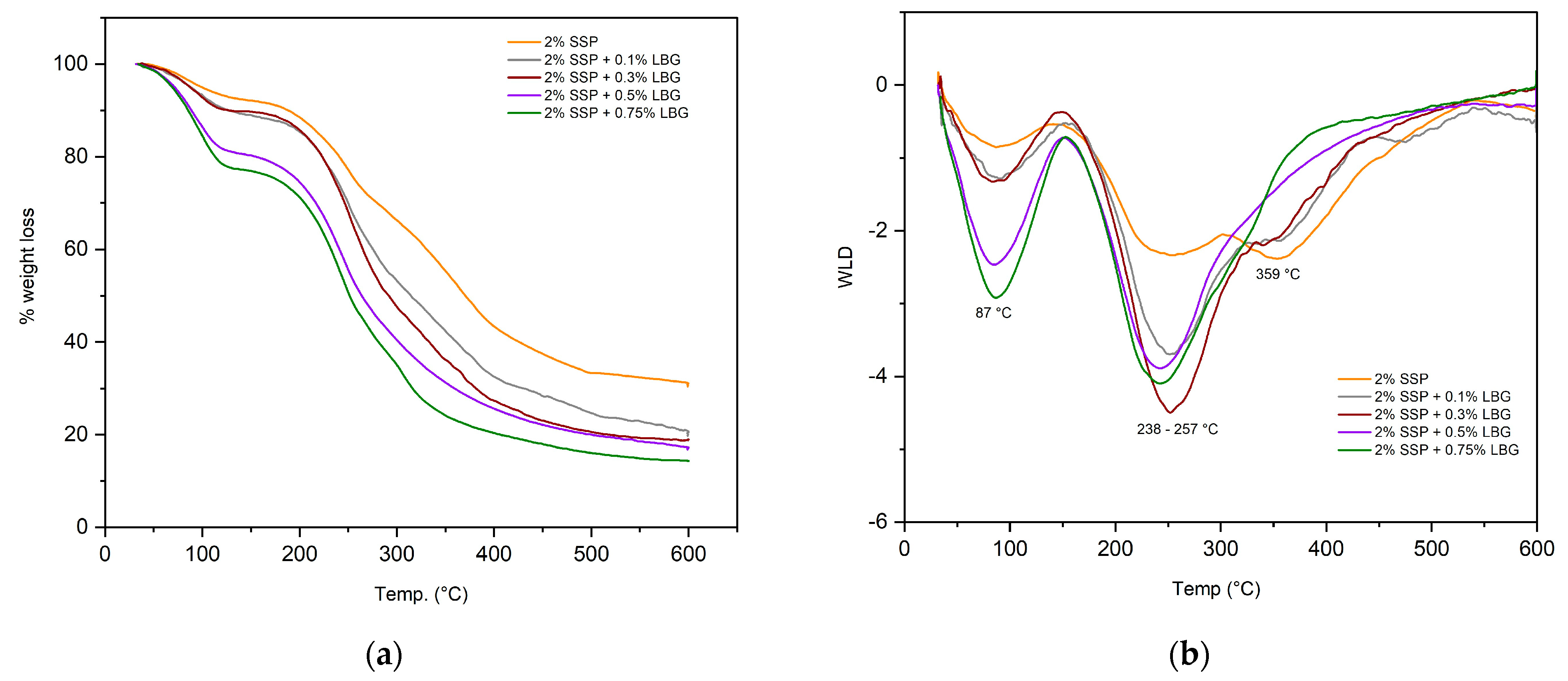
3.4. Thermal Behavior
3.5. Thickness and Mechanical Properties
3.6. Moisture Content (MC), Water Solubility (WS), and Water Vapor and Oxygen Permeabilities (WVP, OXP)
3.7. Film Biodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.O.; Batista, M.J.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. J. Food Eng. 2021, 289, 110083. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- dos Santos Alves, J.; Canabarro, N.I.; Boeira, C.P.; Melo, P.T.; de Moura Aouada, M.R.; da Rosa, C.S. Design of biodegradable films using pecan nut cake extracts for food packing. Foods 2023, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Kumar, U.S.; Khalil, H.P. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.-G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of biodegradable films using sunflower protein isolates and bacterial nanocellulose as innovative food packaging materials for fresh fruit preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid -based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Gay, J.-P.; Karbowiak, T.; Debeaufort, F. Tuning the functional properties of polysaccharide-protein bio-based edible films by chemical, enzymatic, and physical cross-linking. Compr. Rev. Food Sci. Food Saf. 2016, 15, 739–752. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.; Teixeira, J.A.; Vicente, A.A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. 2011, 22, 662–671. [Google Scholar] [CrossRef]
- Liu, W.; Gu, J.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. Fabrication of hydrophobic and high-strength packaging films based on the esterification modification of galactomannan. Int. J. Biol. Macromol. 2021, 167, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.J.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-rich fraction of spent coffee grounds as promising biomaterial for films fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, J.; Liu, W.; Mei, J.; Xie, J. Characterization of sodium alginate—Locust bean gum films reinforced with daphnetin emulsions for the development of active packaging. Polymers 2020, 14, 731. [Google Scholar] [CrossRef]
- Petitjean, M.; Isasi, J.R. Locust bean gum, a vegetable hydrocolloid with industrial and biopharmaceutical applications. Molecules 2022, 27, 8265. [Google Scholar] [CrossRef] [PubMed]
- Başyiğit, B.; Altun, G.; Yücetepe, M.; Karaaslan, A.; Karaaslan, M. Locust bean gum provides excellent mechanical and release attributes to soy protein-based natural hydrogels. Int. J. Biol. Macromol. 2023, 231, 123352. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Pettersen, M.K.; Grøvlen, M.S.; Sharmin, N.; Li, K.D.; Wetterhus, E.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Heat-sealable bioplastic films of blended locust bean and potato byproducts for active packaging of fatty foods: Cheese and oat cookies as case studies. Food Hydrocoll. 2024, 147, 109322. [Google Scholar] [CrossRef]
- Lopes, J.; Malheiro, C.; Prodana, M.; Loureiro, S.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Locust bean milling-derived dust as a raw material for the development of biodegradable bioplastics with antioxidant activity. J. Sci. Food Agric. 2022, 103, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from agri-food industrial biowastes or co-products and their applications as green materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef]
- Das, P.; Borah, P.P.; Badwaik, L.S. Transformation of chicken feather keratin and pomelo peel pectin into biodegradable composite film. J. Polym. Environ. 2017, 26, 2120–2129. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of protein and pectin from pumpkin industry by-products and their utilization for developing edible film. J. Food Sci. Technol. 2019, 57, 1807–1816. [Google Scholar] [CrossRef]
- Pedroche, J. Utilization of Sunflower Proteins. In Sunflower Chemistry, Production, Processing, and Utilization; Martínez-Force, E., Dunford, N.T., Salas, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 395–439. [Google Scholar] [CrossRef]
- Silva, K.S.; Mauro, M.A.; Gonçalves, M.P.; Rocha, C.M. Synergistic interactions of locust bean gum with whey proteins: Effect on physicochemical and microstructural properties of whey protein-based films. Food Hydrocoll. 2016, 54, 179–188. [Google Scholar] [CrossRef]
- Kaur, R.; Ghoshal, G. Sunflower protein isolates-composition, extraction and functional properties. Adv. Colloid Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef]
- Ayhllon-Meixueiro, F.; Vaca-Garcia, C.; Silvestre, F. Biodegradable films from isolate of sunflower (Helianthus annuus) proteins. J. Agric. Food Chem. 2000, 48, 3032–3036. [Google Scholar] [CrossRef]
- Molina, M.I.; Petruccelli, S.; Añón, M.C. Effect of pH and ionic strength modifications on thermal denaturation of the 11S globulin of sunflower (Helianthus annuus). J. Agric. Food Chem. 2004, 52, 6023–6029. [Google Scholar] [CrossRef]
- Geneau-Sbartaï, C.; Leyris, J.; Silvestre, F.; Rigal, L. Sunflower cake as a natural composite: Composition and plastic properties. J. Agric. Food Chem. 2008, 56, 11198–11208. [Google Scholar] [CrossRef]
- Kurt, A.; Toker, O.S.; Tornuk, F. Effect of xanthan and locust bean gum synergistic interaction on characteristics of biodegradable edible film. Int. J. Biol. Macromol. 2017, 102, 1035–1044. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018.
- Association of Official Analytical Chemists International. Official Methods of Analysis of AOAC International, 22nd ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- do Lago, R.C.; de Oliveira, A.L.; Dias, M.C.; de Carvalho, E.E.; Tonoli, G.H.; de Barros Vilas Boas, E.V. Obtaining cellulosic nanofibrils from oat straw for biocomposite reinforcement: Mechanical and barrier properties. Ind. Crops Prod. 2020, 148, 112264. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice-Hernández, C.; Martins, V.G. Active and sustainable materials from rice starch, fish protein and oregano essential oil for food packaging. Ind. Crops Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Fronza, P.; Batista, M.J.; Franca, A.S.; Oliveira, L.S. Bionanocomposite based on cassava waste starch, locust bean galactomannan, and cassava waste cellulose nanofibers. Foods 2024, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- ASTM G160-03; Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Materials by Laboratory Soil Burial. ASTM: West Conshohocken, PA, USA, 2019.
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Q.; Hu, X.; Sheng, L. Structure and properties of egg white protein films modified by high-intensity ultrasound: An effective strategy. Food Res. Int. 2022, 157, 111264. [Google Scholar] [CrossRef]
- Shanesazzadeh, E.; Kadivar, M.; Fathi, M. Production and characterization of hydrophilic and hydrophobic sunflower protein isolate nanofibers by electrospinning method. Int. J. Biol. Macromol. 2018, 119, 1–7. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys Acta—Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Liang, N.; Lu, X.; Hu, Y.; Kitts, D.D. Application of Attenuated Total Reflectance–Fourier Transformed Infrared (ATR-FTIR) spectroscopy to determine the chlorogenic acid isomer profile and antioxidant capacity of coffee beans. J. Agric. Food Chem. 2016, 64, 681–689. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic acid oxidation and its reaction with sunflower proteins to form green-colored complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Schlegel, H.B. On the physical origin of blue-shifted hydrogen bonds. J. Am. Chem. Soc. 2002, 124, 9639–9647. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol 2020, 153, 892–901. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Medeiros, K.A.; Amado, L.R.; Silva, K.D. Influence of locust bean gum addition on properties of soy protein based films. Rev. Gest. Ambient. Sustentabilidade 2022, 11, 238–255. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2016, 104, 50–58. [Google Scholar] [CrossRef]
- Alves-Silva, G.F.; Romani, V.P.; Martins, V.G. Jatobá (Hymenaea stigonocarpa) pulp films: Properties, antioxidant potential and biodegradability. Food Packag. Shelf Life 2022, 34, 100923. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Nogueira, D.; Martins, V.G. Use of different proteins to produce biodegradable films and blends. J. Environ. Polym. Degrad. 2019, 27, 2027–2039. [Google Scholar] [CrossRef]
- Batista, M.J.; Torres, S.S.; Franca, A.S.; Oliveira, L.S. Effect of zinc chloride solution assisted by ultrasound on polysaccharides of spent coffee grounds. Carbohydr. Polym. Technol. Appl. 2023, 5, 100298. [Google Scholar] [CrossRef]

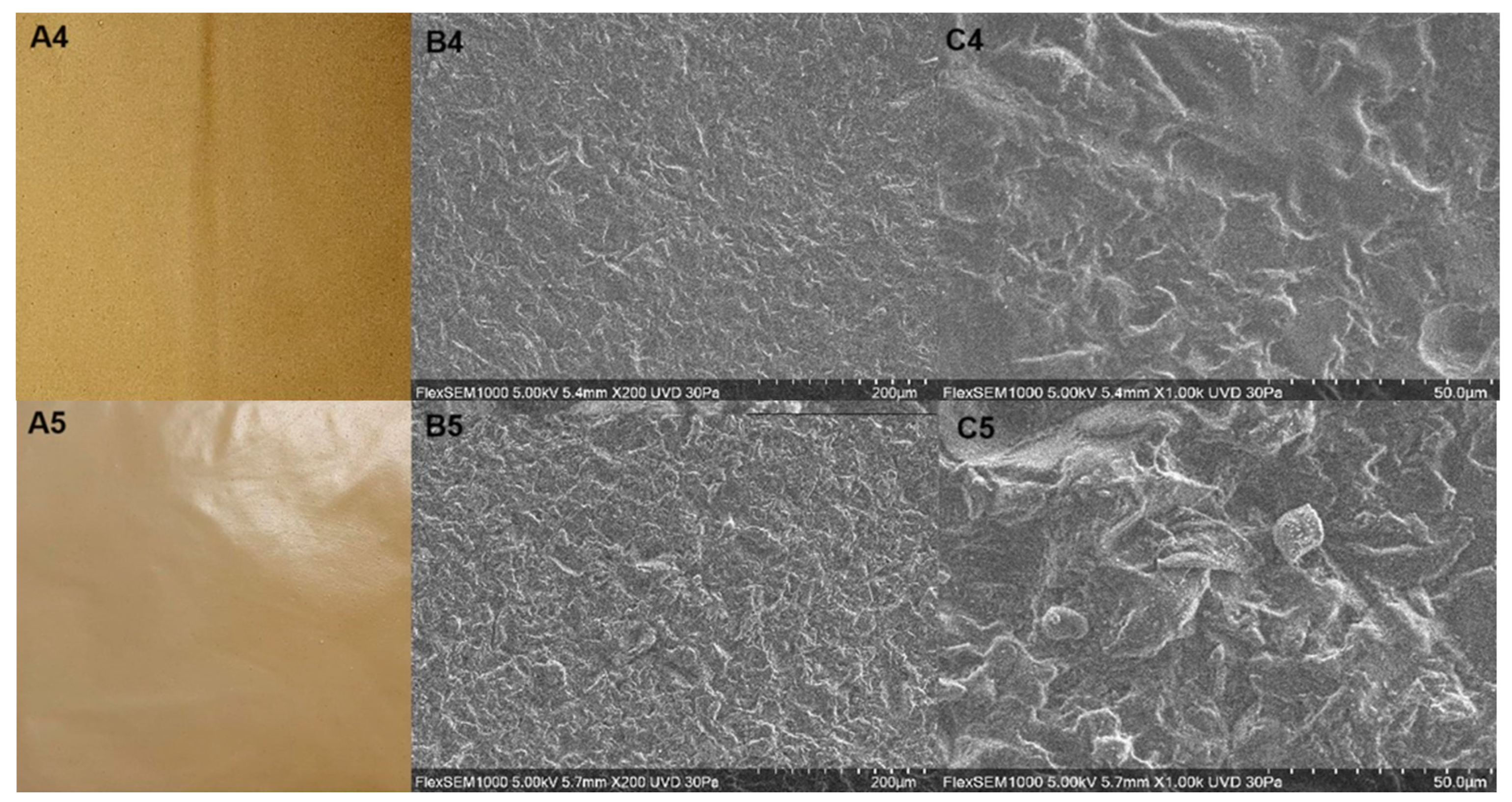


| Treatment | Color Measurements | Opacity (mm−1) | ||
|---|---|---|---|---|
| L* | h | c* | ||
| 2% SSP | 23.75 ± 0.05 d | 61.23 ± 1.25 c | 9.09 ± 0.11 a | 6.57 ± 0.05 d |
| 2% SSP + 0.1%LBG | 25.68 ± 0.05 c | 58.22 ± 2.17 c | 6.61 ± 0.19 b | 8.86 ± 0.10 c |
| 2% SSP + 0.3%LBG | 25.78 ± 0.05 c | 72.08 ± 1.29 b | 5.81 ± 0.08 c | 8.86 ± 0.10 c |
| 2% SSP + 0.5%LBG | 27.66 ± 0.11 b | 73.69 ± 2.48 b | 5.49 ± 0.26 c | 9.50 ± 0.17 b |
| 2% SSP + 0.75%LBG | 28.63 ± 0.04 a | 78.03 ± 2.36 b | 4.73 ± 0.29 d | 12.11 ± 0.18 a |
| Treatment | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) | Elasticity Modulus (MPa) |
|---|---|---|---|---|
| 2% SSP | 0.13 ± 0.01 a | 3.45 ± 0.40 b | 125.81 ± 3.11 a | 18.58 ± 0.90 a |
| 2% SSP + 0.1% LBG | 0.11 ± 0.01 b | 1.89 ± 0.23 c | 116.13 ± 6.22 b | 4.00 ± 0.28 b |
| 2% SSP + 0.3% LBG | 0.09 ± 0.01 c | 3.28 ± 0.21 b | 113.10 ± 5.44 b | 4.37 ± 0.33 b |
| 2% SSP + 0.5% LBG | 0.10 ± 0.01 bc | 3.47 ± 0.74 b | 112.53 ± 2.87 b | 4.03 ± 0.59 b |
| 2% SSP + 0.75% LBG | 0.09 ± 0.01 c | 6.31 ± 0.72 a | 131.87 ± 1.39 a | 3.60 ± 0.62 b |
| Treatment | MC (%) | WS (%) | WVP (g mm kPa−1 d−1m−2) | OXP (×10−7 g mm−2s−1) |
|---|---|---|---|---|
| 2% SSP | 16.20 ± 0.53 f | 33.21 ± 0.96 a | 0.62 ± 0.01 d | 0.99 ± 0.03 bc |
| 2% SSP + 0.1% LBG | 42.97 ± 1.17 d | 31.43 ± 0.86 a | 1.16 ± 0.02 a | 1.15 ± 0.04 a |
| 2% SSP + 0.3% LBG | 48.10 ± 0.20 c | 24.39 ± 0.97 b | 1.04 ± 0.02 bc | 1.10 ± 0.07 ab |
| 2% SSP + 0.5% LBG | 51.40 ± 1.11 b | 18.30 ± 0.89 c | 1.06 ± 0.02 b | 1.02 ± 0.01 bc |
| 2% SSP + 0.75% LBG | 56.23 ± 0.15 a | 7.18 ± 0.65 d | 1.01 ± 0.02 c | 0.96 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, L.T.d.O.; Fronza, P.; Gonçalves, I.; da Silva, W.A.; Oliveira, L.S.; Franca, A.S. Development of Polymeric Films Based on Sunflower Seed Proteins and Locust Bean Gum. Polymers 2024, 16, 1905. https://doi.org/10.3390/polym16131905
Alves LTdO, Fronza P, Gonçalves I, da Silva WA, Oliveira LS, Franca AS. Development of Polymeric Films Based on Sunflower Seed Proteins and Locust Bean Gum. Polymers. 2024; 16(13):1905. https://doi.org/10.3390/polym16131905
Chicago/Turabian StyleAlves, Layla Talita de Oliveira, Pãmella Fronza, Idalina Gonçalves, Washington Azevêdo da Silva, Leandro S. Oliveira, and Adriana S. Franca. 2024. "Development of Polymeric Films Based on Sunflower Seed Proteins and Locust Bean Gum" Polymers 16, no. 13: 1905. https://doi.org/10.3390/polym16131905
APA StyleAlves, L. T. d. O., Fronza, P., Gonçalves, I., da Silva, W. A., Oliveira, L. S., & Franca, A. S. (2024). Development of Polymeric Films Based on Sunflower Seed Proteins and Locust Bean Gum. Polymers, 16(13), 1905. https://doi.org/10.3390/polym16131905






