3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gelatin Methacryloyl (GelMA)
2.3. Design and Manufacturing of 3D Composite Scaffolds
2.4. Characterization Methods for GelMA and Composite Scaffolds
3. Results
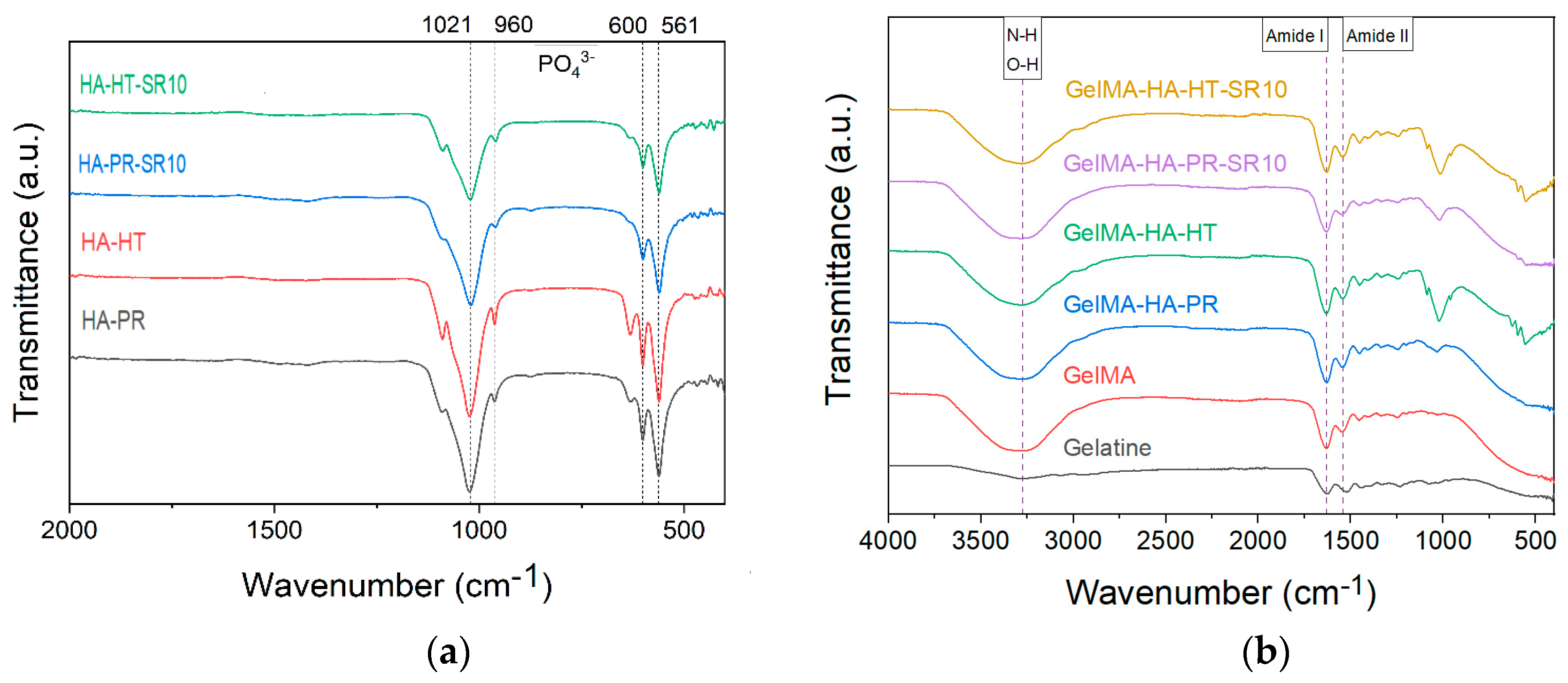
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Thermogravimetric Analyses (TGA)
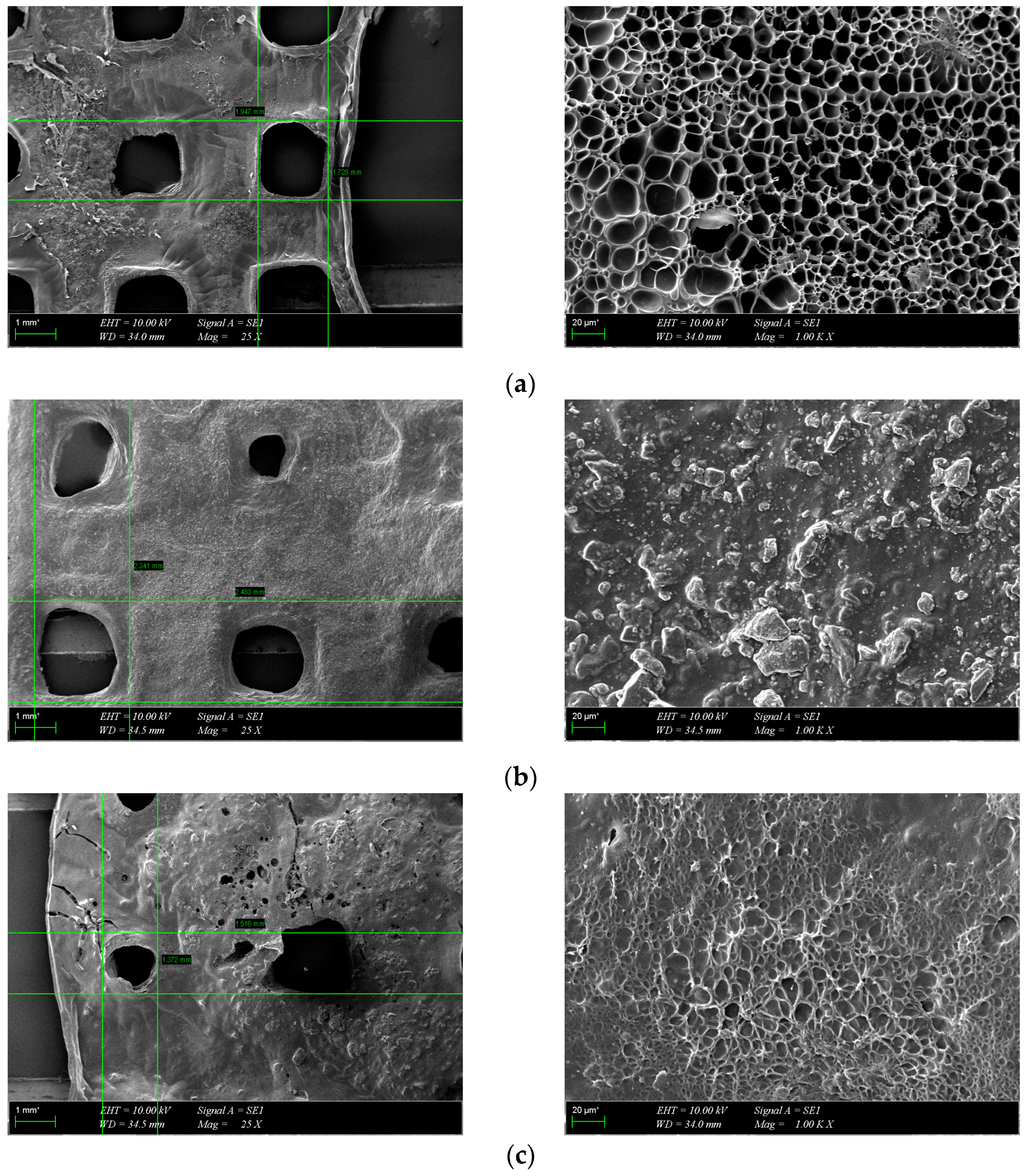
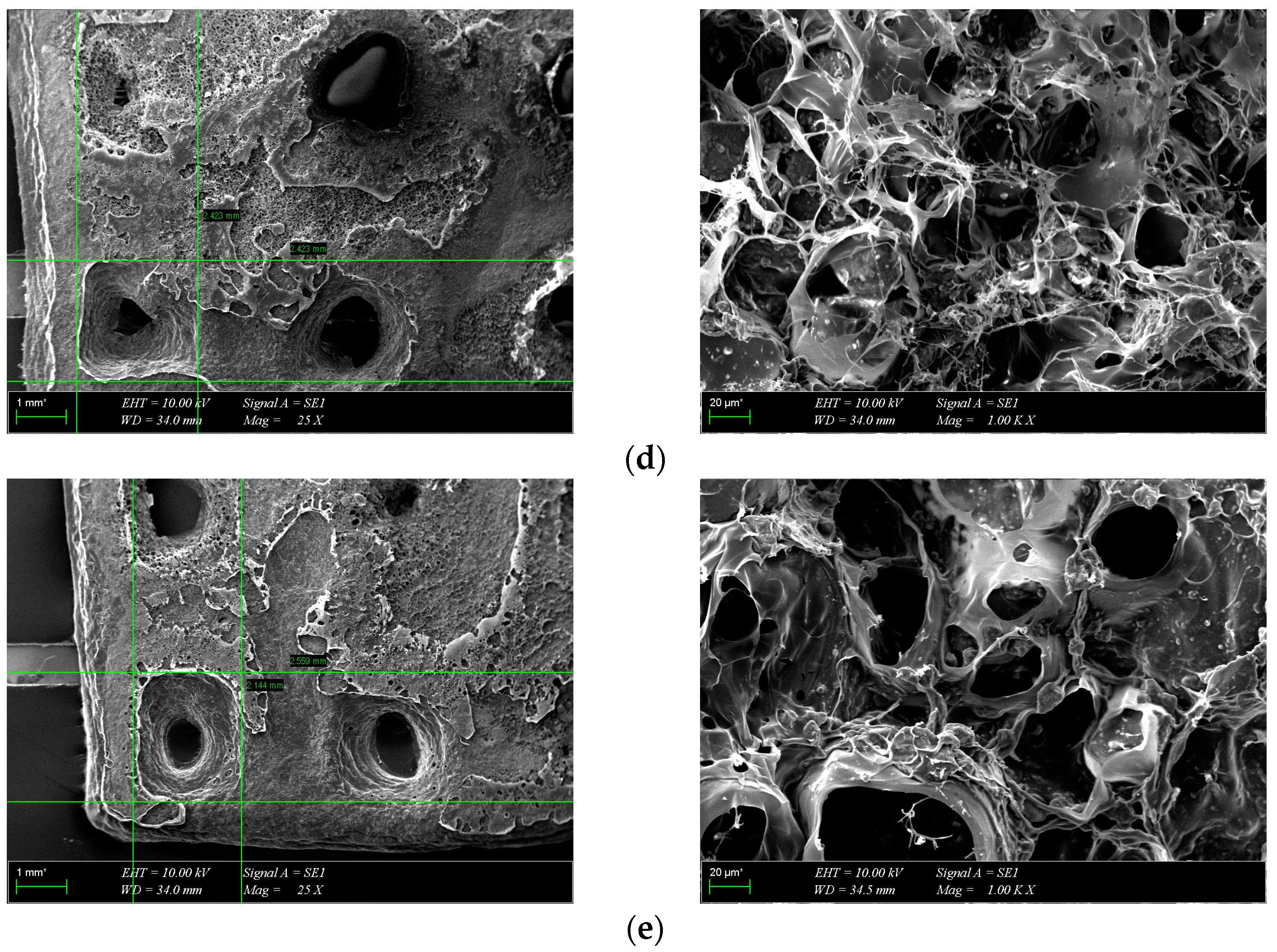
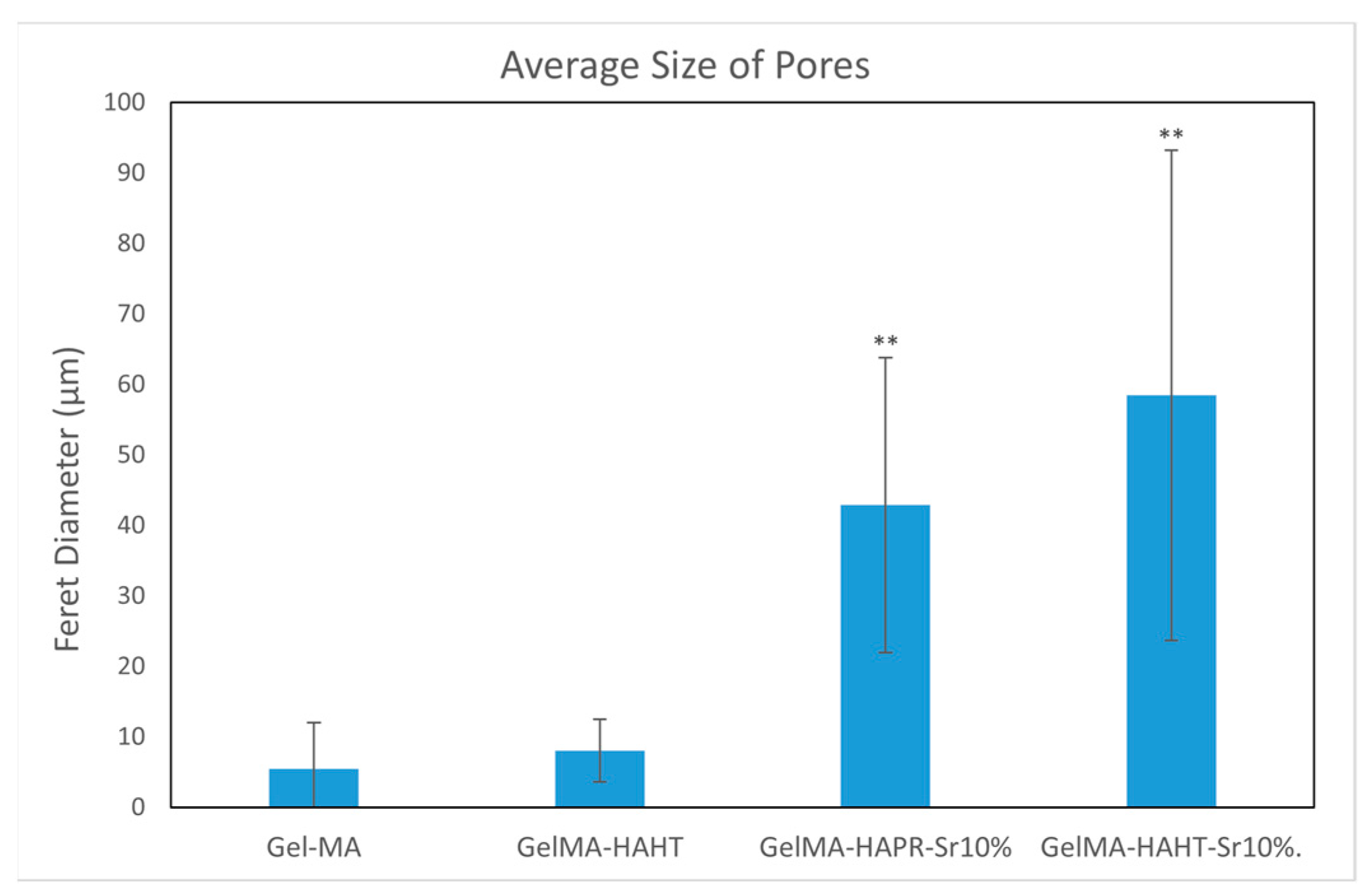
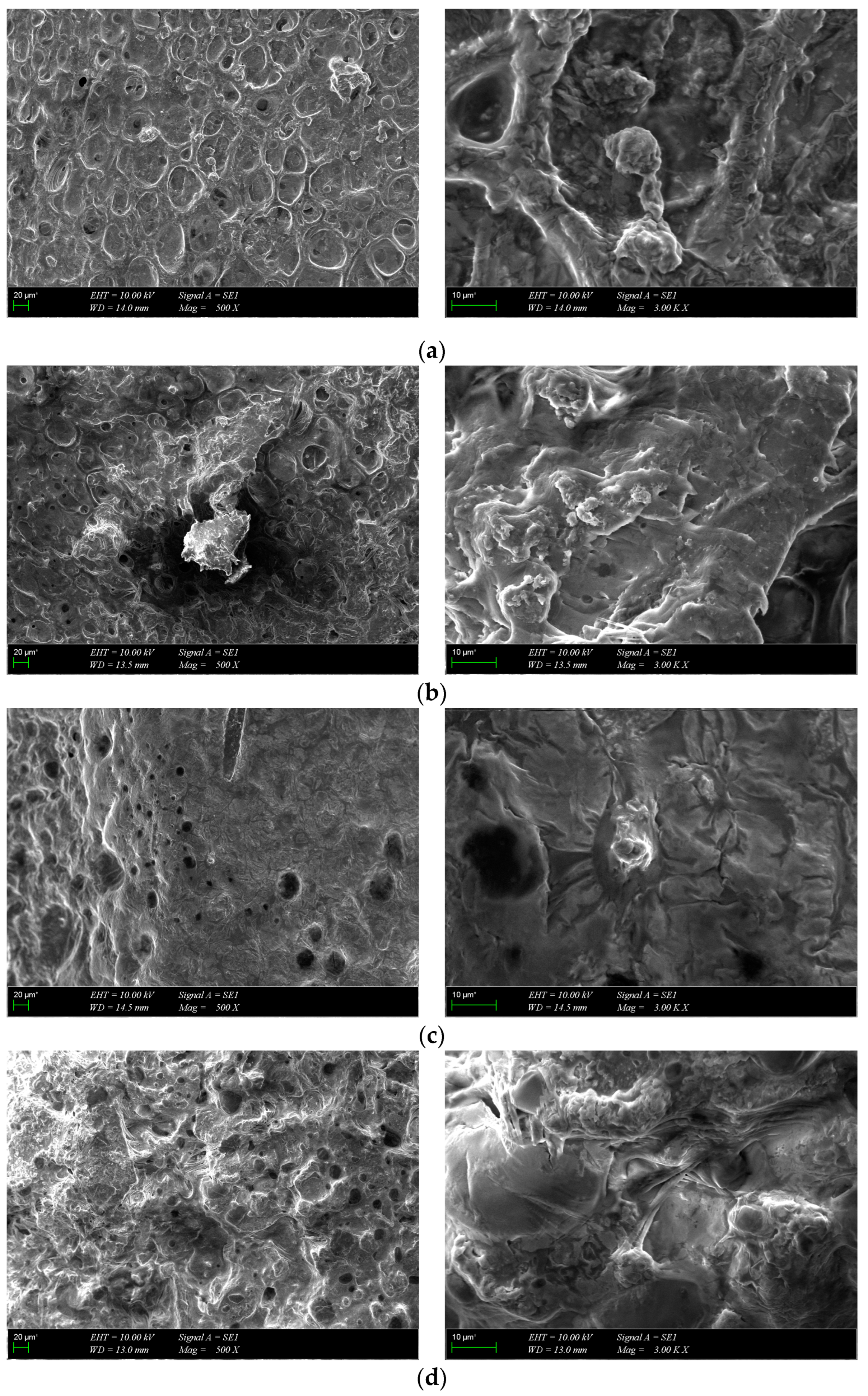
3.3. Morphological Characterization
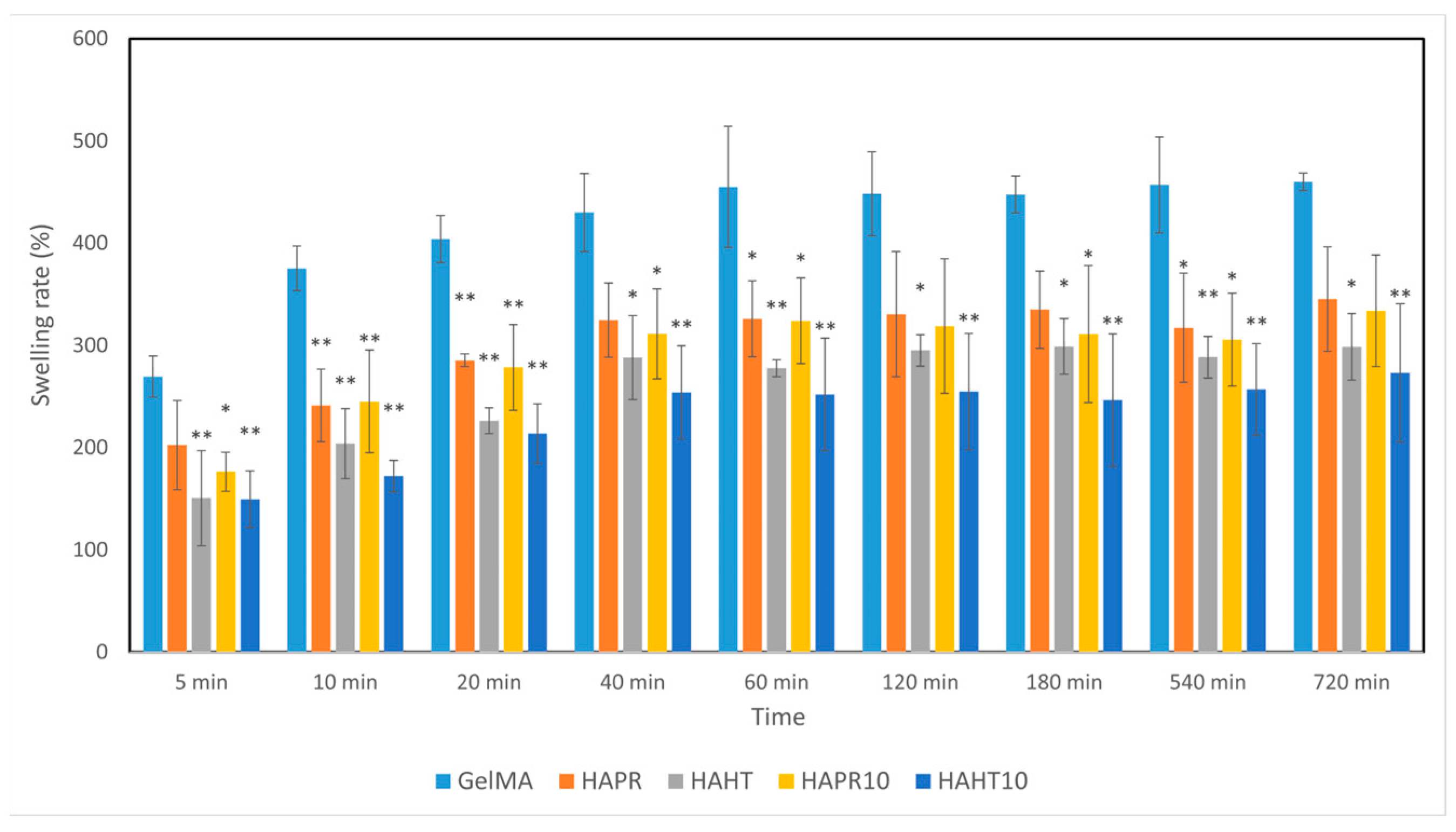
3.4. Swelling Behavior
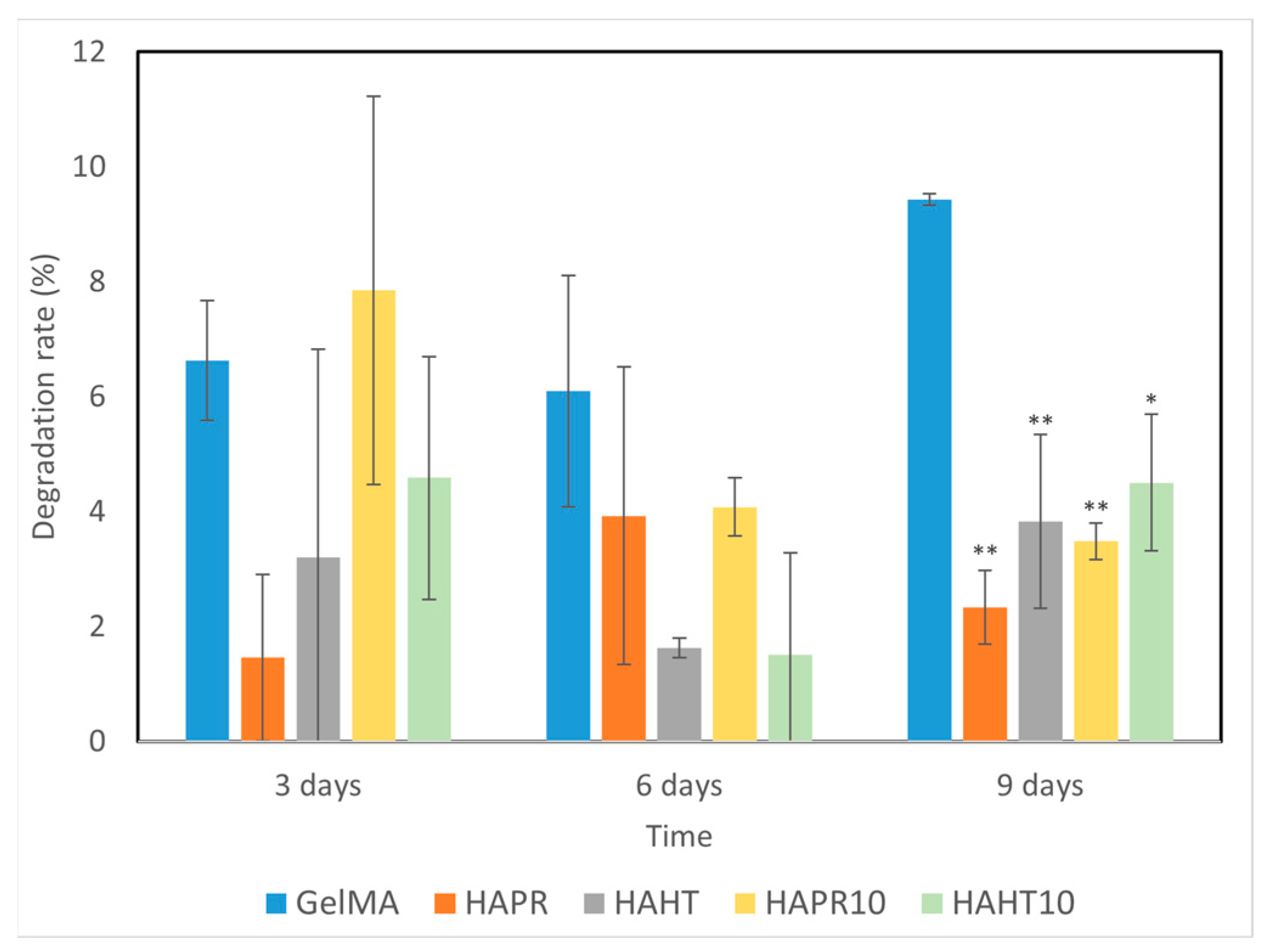
3.5. Degradation Performance
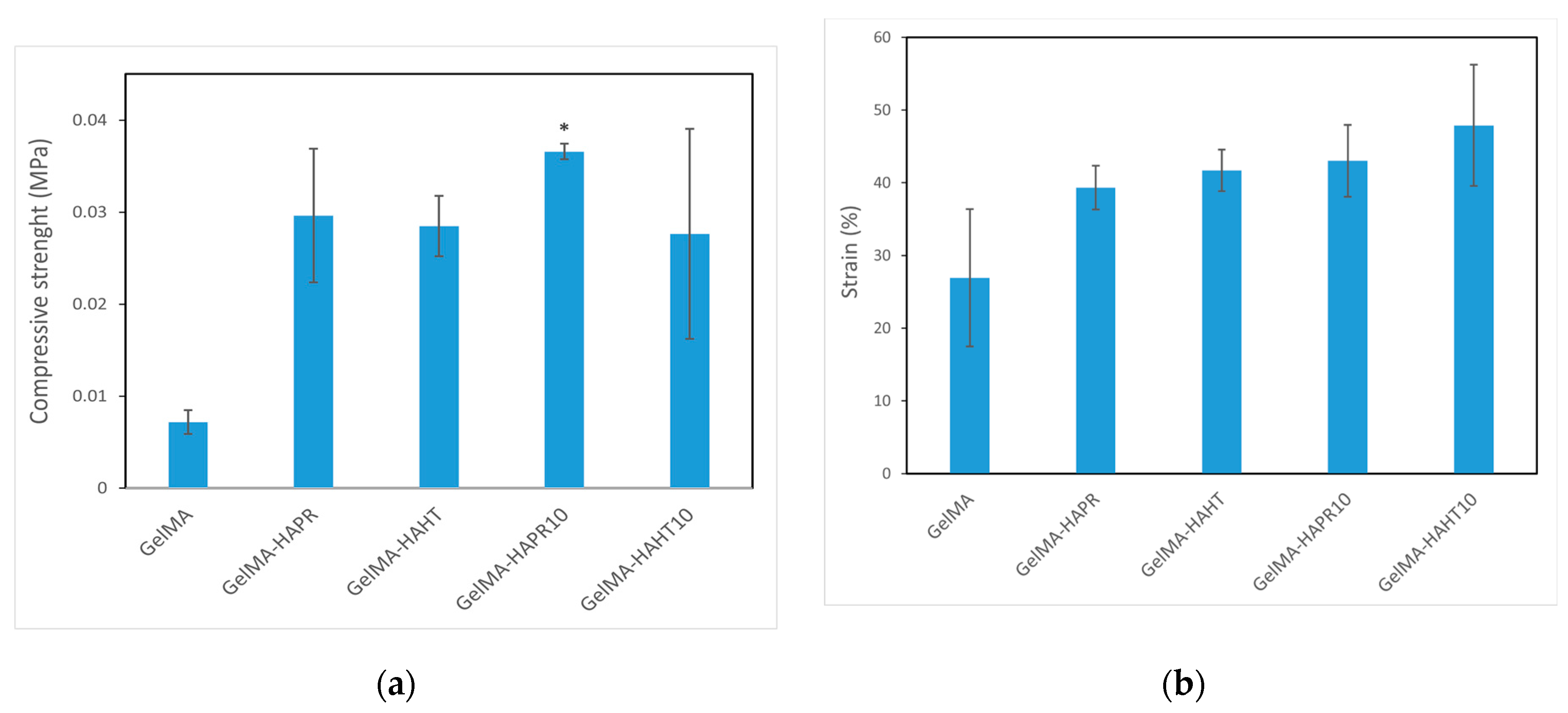
3.6. Mechanical Analysis
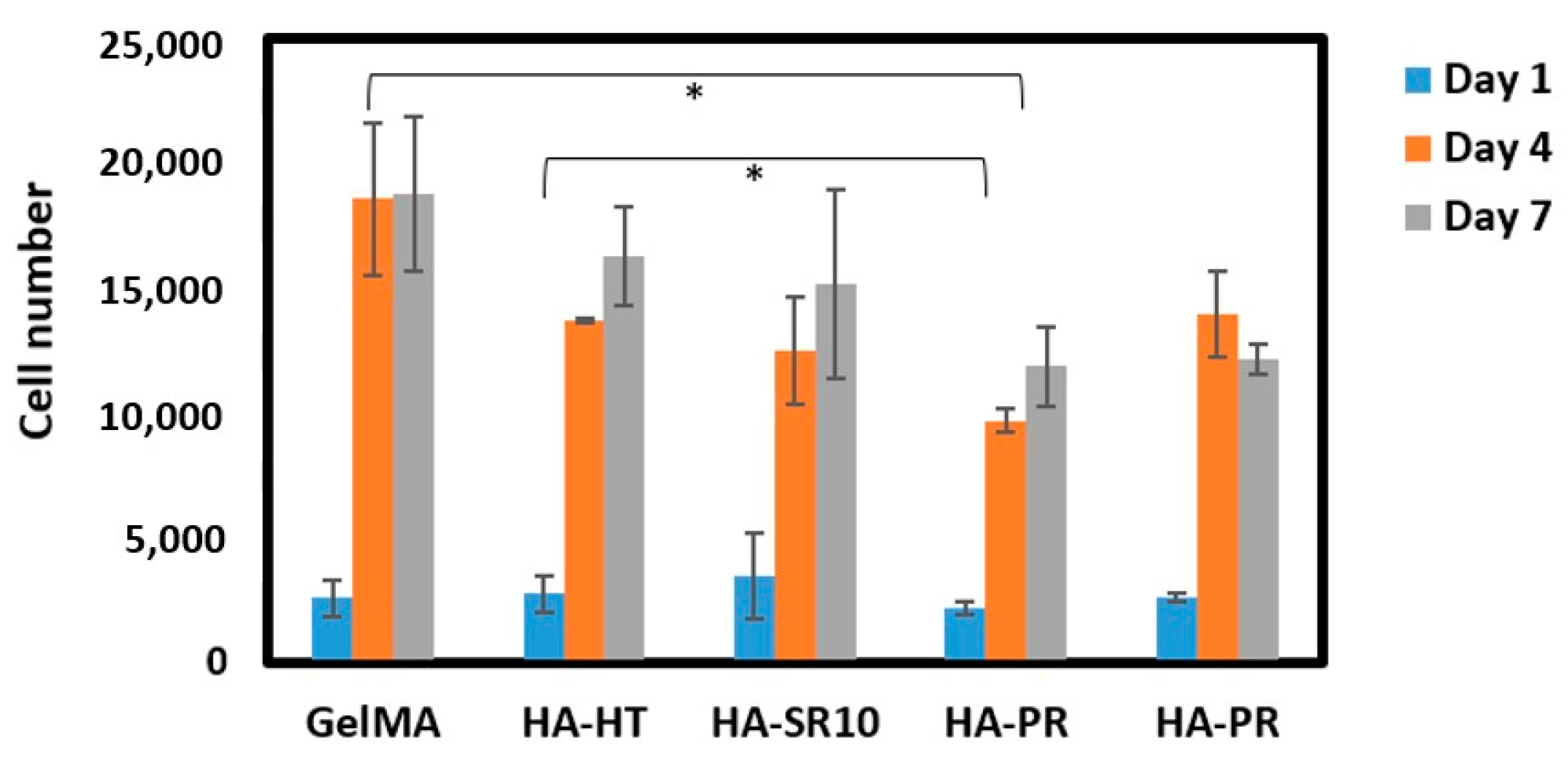
3.7. In Vitro Biological Evaluation of GelMA Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, N.B.; Abar, B.; Johnson, L.; Burbano, J.; Danilkowicz, R.M.; Adams, S.B. 3D-Bioprinted GelMA-Gelatin-Hydroxyapatite Osteoblast-Laden Composite Hydrogels for Bone Tissue Engineering. Bioprinting 2022, 26, e00196. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, A.; Upadhyay, N.K.; Singh, G.; Lal, U.R.; Goyal, R. In-Vitro Osteoblast Proliferation and in-Vivo Anti-Osteoporotic Activity of Bombax Ceiba with Quantification of Lupeol, Gallic Acid and β-Sitosterol by HPTLC and HPLC. BMC Complement. Altern. Med. 2018, 18, 233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, X.; Ma, M.; Lu, W.; Zhang, B.; Guo, Y. A GelMA-PEGDA-nHA Composite Hydrogel for Bone Tissue Engineering. Materials 2020, 13, 3735. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Shi, C.; Wu, T.; Cui, Y.; Wang, S.; Liu, P.; Feng, X.; He, Y.; Fu, D. Remote-Controllable Bone-Targeted Delivery of Estradiol for the Treatment of Ovariectomy-Induced Osteoporosis in Rats. J. Nanobiotechnol. 2021, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Jerdioui, S.; Elansari, L.L.; Jaradat, N.; Jodeh, S.; Azzaoui, K.; Hammouti, B.; Lakrat, M.; Tahani, A.; Jama, C.; Bentiss, F. Effects of Gallic Acid on the Nanocrystalline Hydroxyapatite Formation Using the Neutralization Process. J. Trace Elem. Miner. 2022, 2, 100009. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, A.; Azher, K.; Rehman, A.U.; Juan, W.; Aktürk, N.; Tüfekci, C.S.; Salamci, M.U. Vat Photopolymerization-Based 3D Printing of Polymer Nanocomposites: Current Trends and Applications. RSC Adv. 2023, 13, 1456–1496. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/Hydroxyapatite Composite Bone Tissue Engineering Scaffolds with Dual and Decoupled Therapeutic Ion Delivery: Copper and Strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [PubMed]
- Loca, D.; Locs, J.; Dubnika, A.; Zalite, V.; Berzina-Cimdina, L. 9—Porous Hydroxyapatite for Drug Delivery. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 189–209. [Google Scholar] [CrossRef]
- Stolarov, P.; de Vries, J.; Stapleton, S.; Morris, L.; Martyniak, K.; Kean, T.J. Suitability of Gelatin Methacrylate and Hydroxyapatite Hydrogels for 3D-Bioprinted Bone Tissue. Materials 2024, 17, 1218. [Google Scholar] [CrossRef]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin Methacryloyl (GelMA)-Based Biomaterials for Bone Regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Ferreira, J.A.; Dubey, N.; Ribeiro, J.S.; de Souza Costa, C.A.; Soares, D.G.; Bottino, M.C. Injectable Multifunctional Drug Delivery System for Hard Tissue Regeneration under Inflammatory Microenvironments. ACS Appl. Bio Mater. 2021, 4, 6993–7006. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl. Mater. Interfaces 2016, 8, 30017–30026. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D Printing of Biomimetic Multi-Layered GelMA/nHA Scaffold for Osteochondral Defect Repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghițulică, C.-D.; Voicu, G.; Stamat (Balahura), L.-R.; Dinescu, S.; Vlasceanu, G.M.; Iovu, H.; Serafim, A.; Ianchis, R.; et al. 3D Printed Composite Scaffolds of GelMA and Hydroxyapatite Nanopowders Doped with Mg/Zn Ions to Evaluate the Expression of Genes and Proteins of Osteogenic Markers. Nanomaterials 2022, 12, 3420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Wang, X.; Zhou, X.; Yang, G.; Lai, Q.; Zhao, Y. SLA-3d Printed Building and Characteristics of GelMA/HAP Biomaterials with Gradient Porous Structure. J. Mech. Behav. Biomed. Mater. 2024, 155, 106553. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Liu, T.; Jin, M.; Zhang, Y.; Weng, W.; Wang, T.; Yang, H.; Zhou, L. K+/Sr2+/Na+ Triple-Doped Hydroxyapatites/GelMA Composite Hydrogel Scaffold for the Repair of Bone Defects. Ceram. Int. 2021, 47, 30929–30937. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, X.; Song, X. Engineering Materials with Light: Recent Progress in Digital Light Processing Based 3D Printing. J. Mater. Chem. C 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Baykara, D.; Bedir, T.; Ilhan, E.; Mutlu, M.E.; Gunduz, O.; Narayan, R.; Ustundag, C.B. Fabrication and Optimization of 3D Printed Gelatin Methacryloyl Microneedle Arrays Based on Vat Photopolymerization. Front. Bioeng. Biotechnol. 2023, 11, 1157541. [Google Scholar] [CrossRef]
- Codrea, C.I.; Lincu, D.; Atkinson, I.; Culita, D.C.; Croitoru, A.-M.; Dolete, G.; Trusca, R.; Vasile, B.S.; Stan, M.S.; Ficai, D.; et al. Comparison between Two Different Synthesis Methods of Strontium-Doped Hydroxyapatite Designed for Osteoporotic Bone Restoration. Materials 2024, 17, 1472. [Google Scholar] [CrossRef] [PubMed]
- Sahadat Hossain, M.; Ahmed, S. FTIR Spectrum Analysis to Predict the Crystalline and Amorphous Phases of Hydroxyapatite: A Comparison of Vibrational Motion to Reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Mujahid, M.; Amin, S.; Rawat, R.S.; Nusair, A.; Deen, G.R. Effect of Surfactant and Heat Treatment on Morphology, Surface Area and Crystallinity in Hydroxyapatite Nanocrystals. Ceram. Int. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.-M.; Ianchis, R. 3D-Printed Gelatin Methacryloyl-Based Scaffolds with Potential Application in Tissue Engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between Gelatin and Sodium Alginate: UV and FTIR Studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Hong, C.; Chung, H.; Lee, G.; Kim, C.; Kim, D.; Oh, S.J.; Kim, S.-H.; Lee, K. Hydrogel/Nanofiber Composite Wound Dressing Optimized for Skin Layer Regeneration through the Mechanotransduction-Based Microcellular Environment. ACS Appl. Bio Mater. 2023, 6, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, J.; Ning, C. Biomimetic Mineralization of Anionic Gelatin Hydrogels: Effect of Degree of Methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Jakus, A.E.; Geisendorfer, N.R.; Lewis, P.L.; Shah, R.N. 3D-Printing Porosity: A New Approach to Creating Elevated Porosity Materials and Structures. Acta Biomater. 2018, 72, 94–109. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liang, Z.; Lan, W.; Wei, Y.; Hu, Y.; Wang, L.; Lei, Q.; Huang, D. Injectable Antibacterial Ag-HA/GelMA Hydrogel for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1219460. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold Degradation in Bone Tissue Engineering: An Overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, C. Study on Properties of 3D-Printed GelMA Hydrogel Scaffolds with Different nHA Contents. J. Bioact. Compat. Polym. 2022, 37, 392–405. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Li, T.; Liu, Z.; Sun, X.; Wang, W.; Chen, L.; He, C. Enhanced Tissue Infiltration and Bone Regeneration through Spatiotemporal Delivery of Bioactive Factors from Polyelectrolytes Modified Biomimetic Scaffold. Mater. Today Bio 2023, 20, 100681. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, Y.; Luo, W.; Jiang, J.; Zhao, J.; Liu, C. Controllable Synthesis of Biomimetic Hydroxyapatite Nanorods with High Osteogenic Bioactivity. ACS Biomater. Sci. Eng. 2020, 6, 320–328. [Google Scholar] [CrossRef]



| Sample | GelMA (% wt.) | HA/SrHA (% wt.) |
|---|---|---|
| GelMA-HA HT | 62.0 | 38.0 |
| GelMA-HA HT-SR-10 | 63.1 | 36.9 |
| GelMA-HA PR | 62.6 | 37.4 |
| GelMA-HA PR SR-10 | 67.4 | 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codrea, C.I.; Baykara, D.; Mitran, R.-A.; Koyuncu, A.C.Ç.; Gunduz, O.; Ficai, A. 3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration. Polymers 2024, 16, 1932. https://doi.org/10.3390/polym16131932
Codrea CI, Baykara D, Mitran R-A, Koyuncu ACÇ, Gunduz O, Ficai A. 3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration. Polymers. 2024; 16(13):1932. https://doi.org/10.3390/polym16131932
Chicago/Turabian StyleCodrea, Cosmin Iulian, Dilruba Baykara, Raul-Augustin Mitran, Ayşe Ceren Çalıkoğlu Koyuncu, Oguzhan Gunduz, and Anton Ficai. 2024. "3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration" Polymers 16, no. 13: 1932. https://doi.org/10.3390/polym16131932
APA StyleCodrea, C. I., Baykara, D., Mitran, R.-A., Koyuncu, A. C. Ç., Gunduz, O., & Ficai, A. (2024). 3D-Bioprinted Gelatin Methacryloyl-Strontium-Doped Hydroxyapatite Composite Hydrogels Scaffolds for Bone Tissue Regeneration. Polymers, 16(13), 1932. https://doi.org/10.3390/polym16131932










