Various Morphologies of Graphitic Carbon Nitride (g-C3N4) and Their Effect on the Thermomechanical Properties of Thermoset Epoxy Resin Composites
Abstract
:1. Introduction and Literature Review
2. Methodology
2.1. Materials
2.2. Preparation Process of the Particles
2.2.1. Bulk g-C3N4
2.2.2. g-C3N4 Nanotubes
2.2.3. g-C3N4 Nanosheets
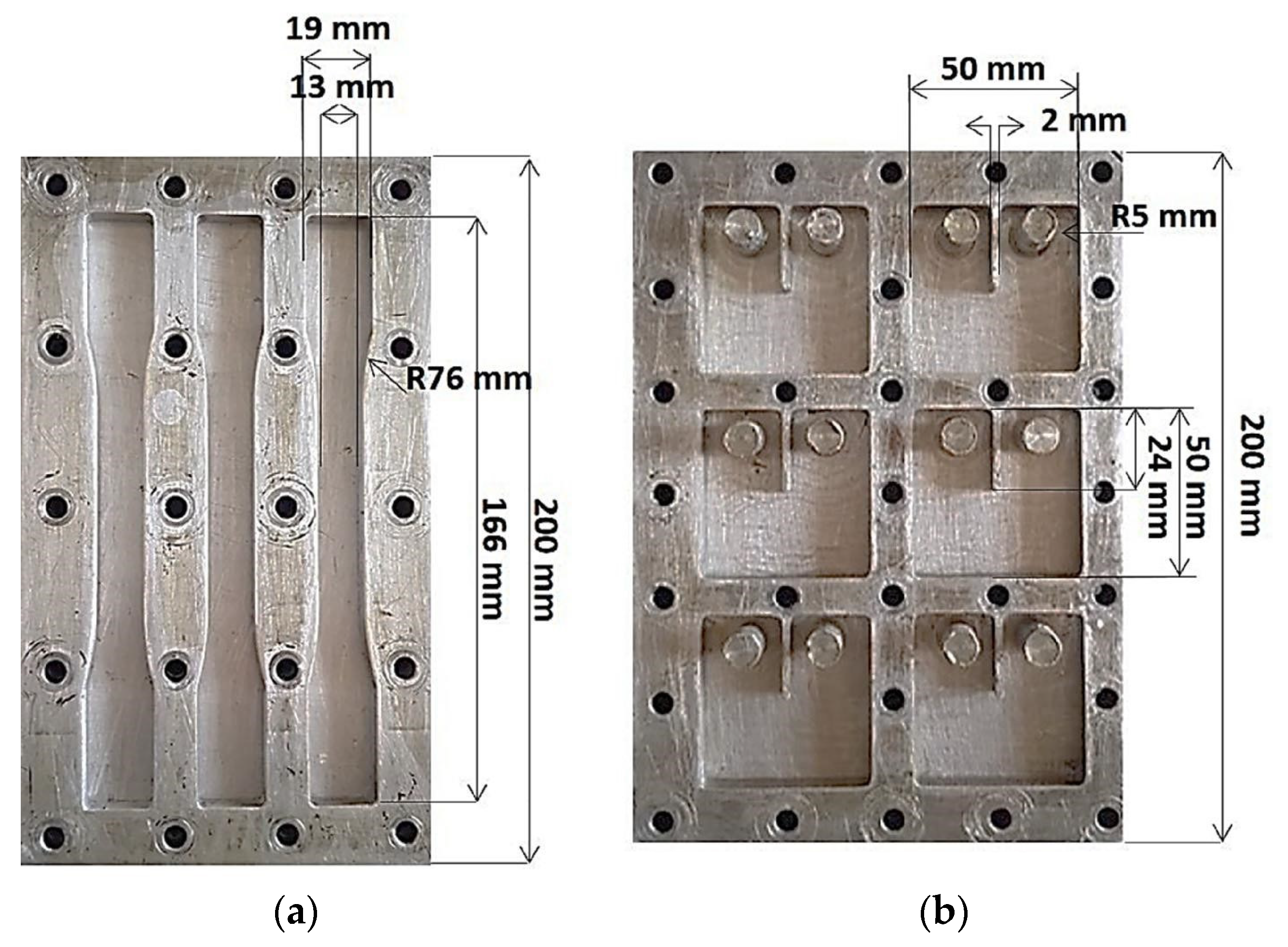
2.3. Preparation of g-C3N4/Epoxy Composites
2.4. Characterization
3. Results and Discussion
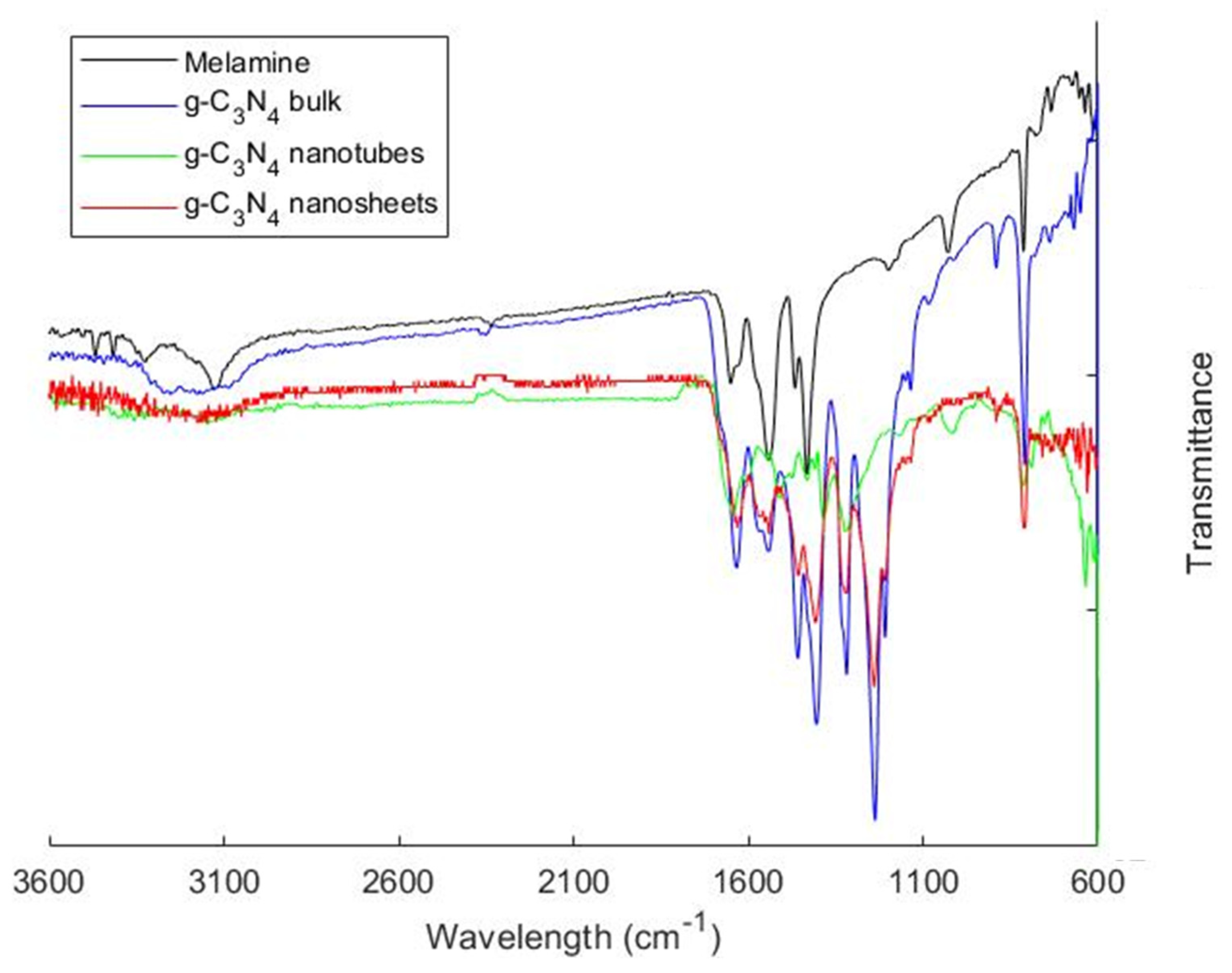
3.1. Characterization of the Synthesized Particles
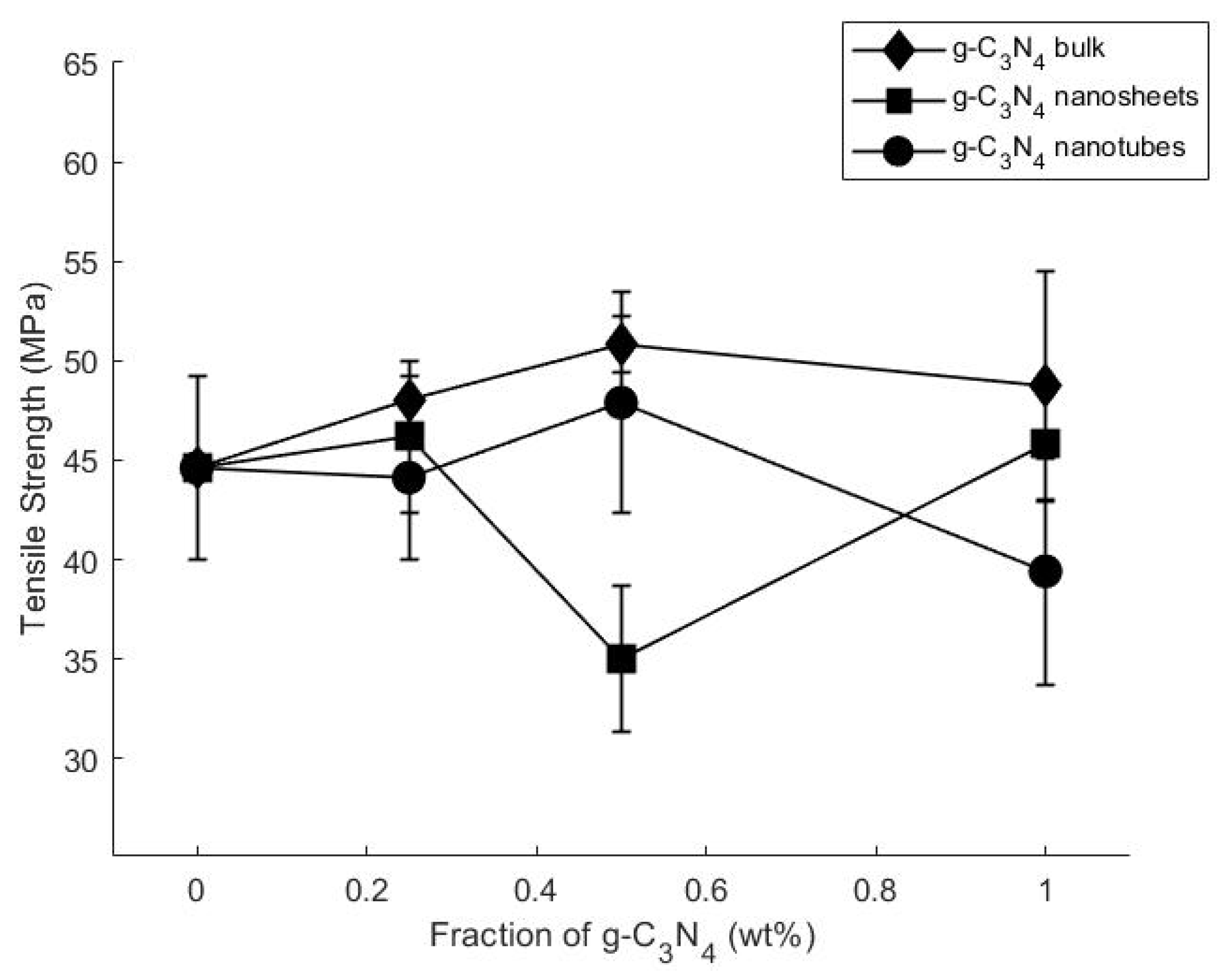
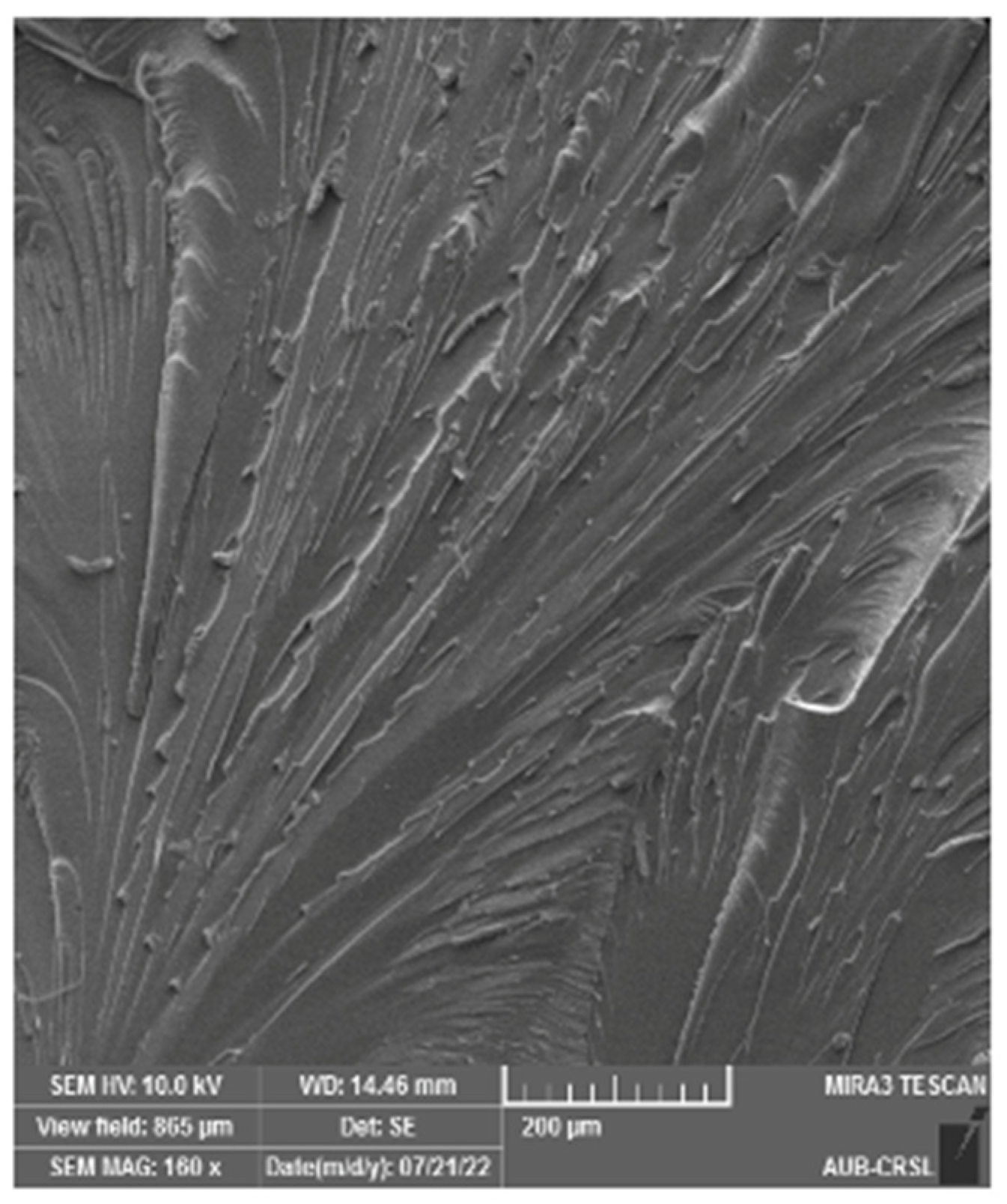
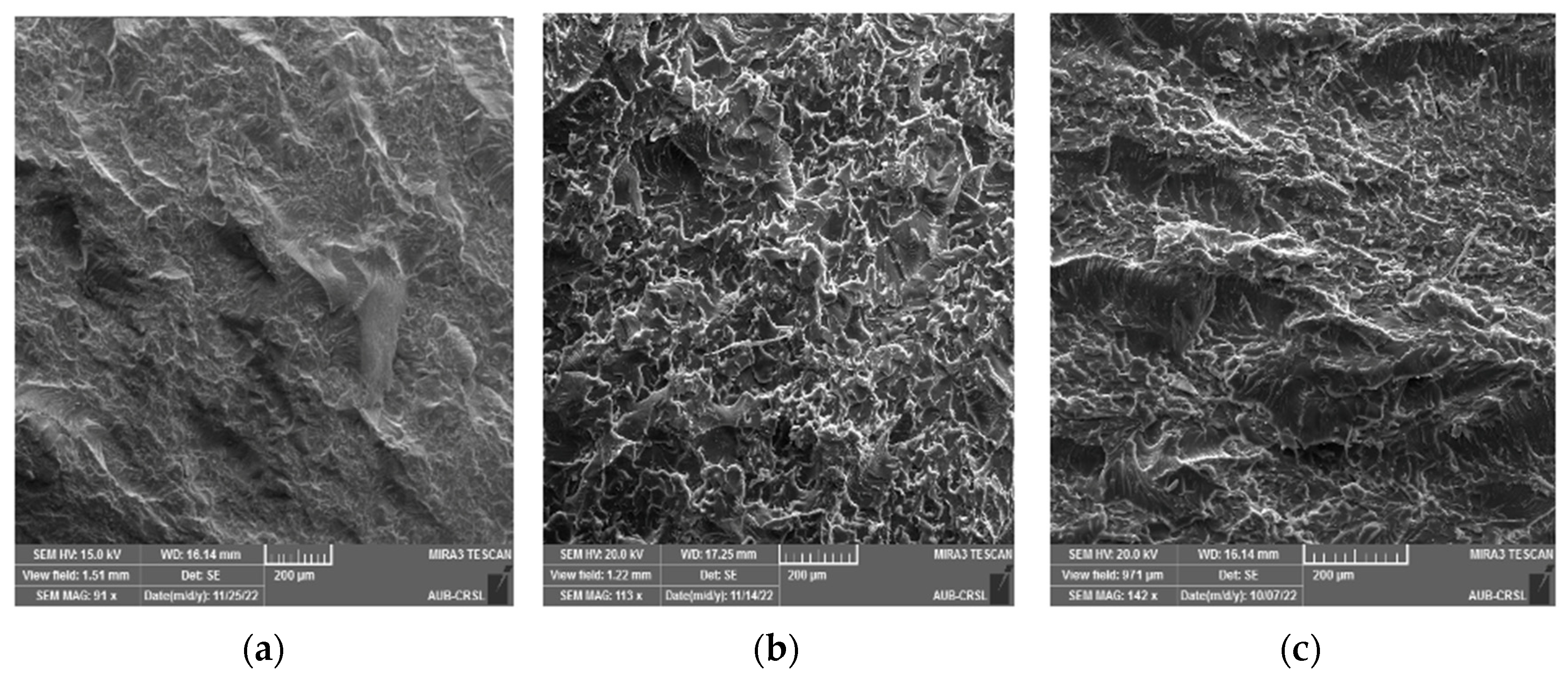
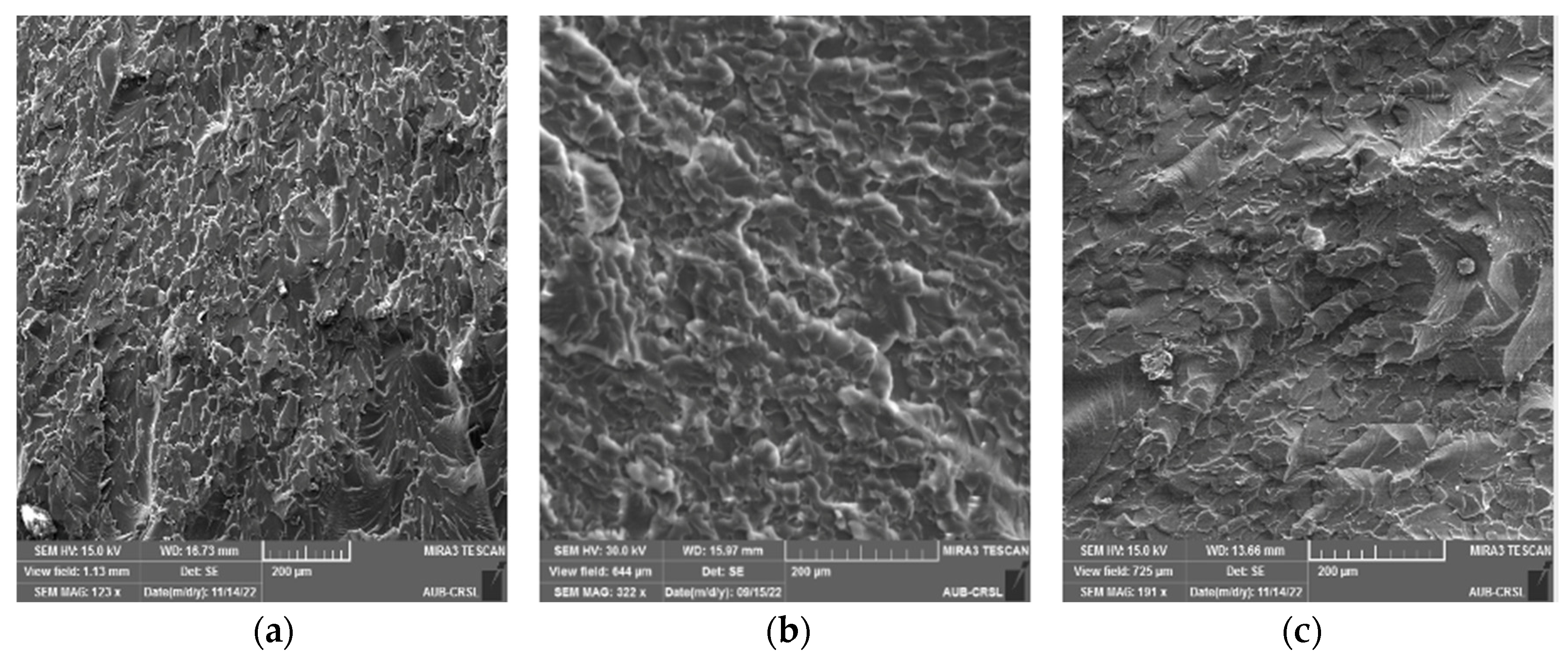
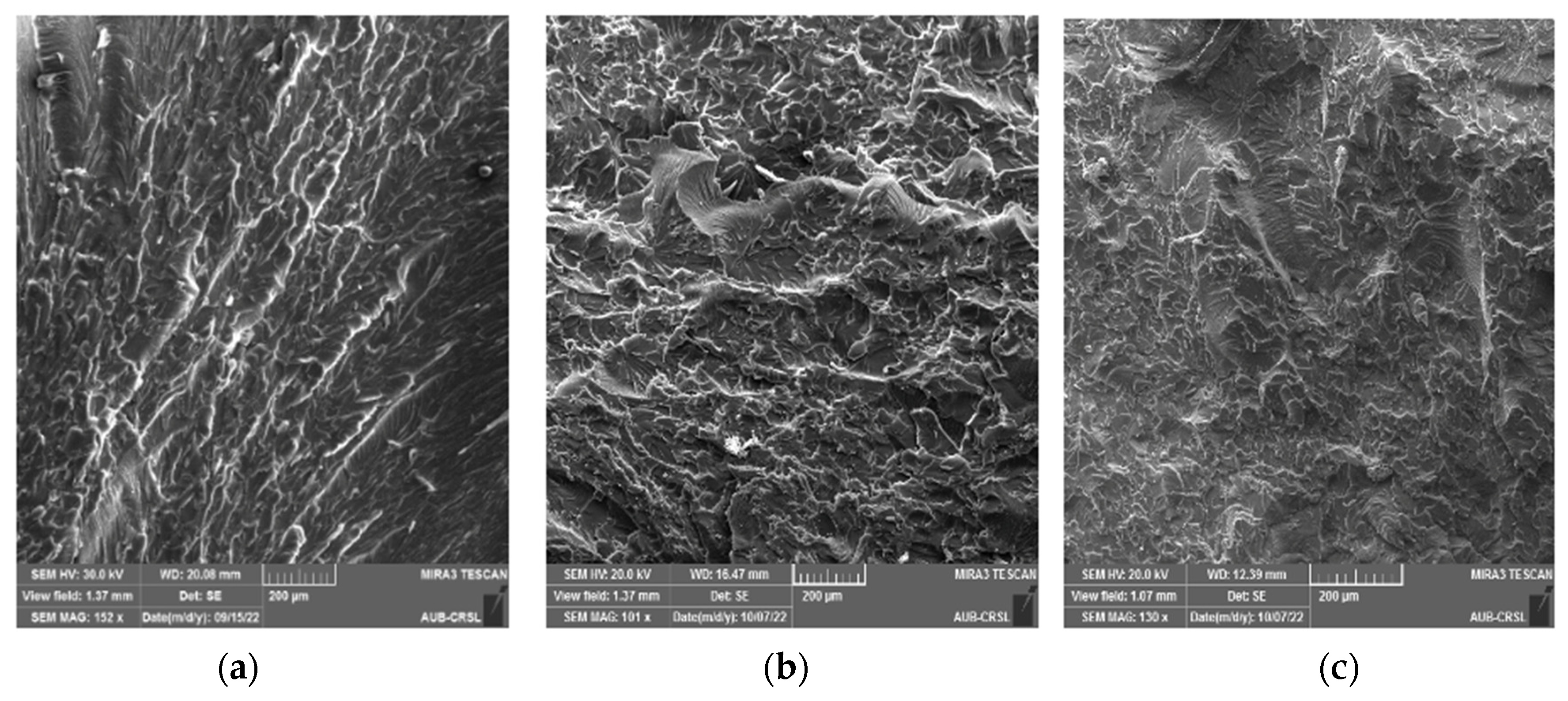
3.2. Static Mechanical Properties and Fracture Morphology
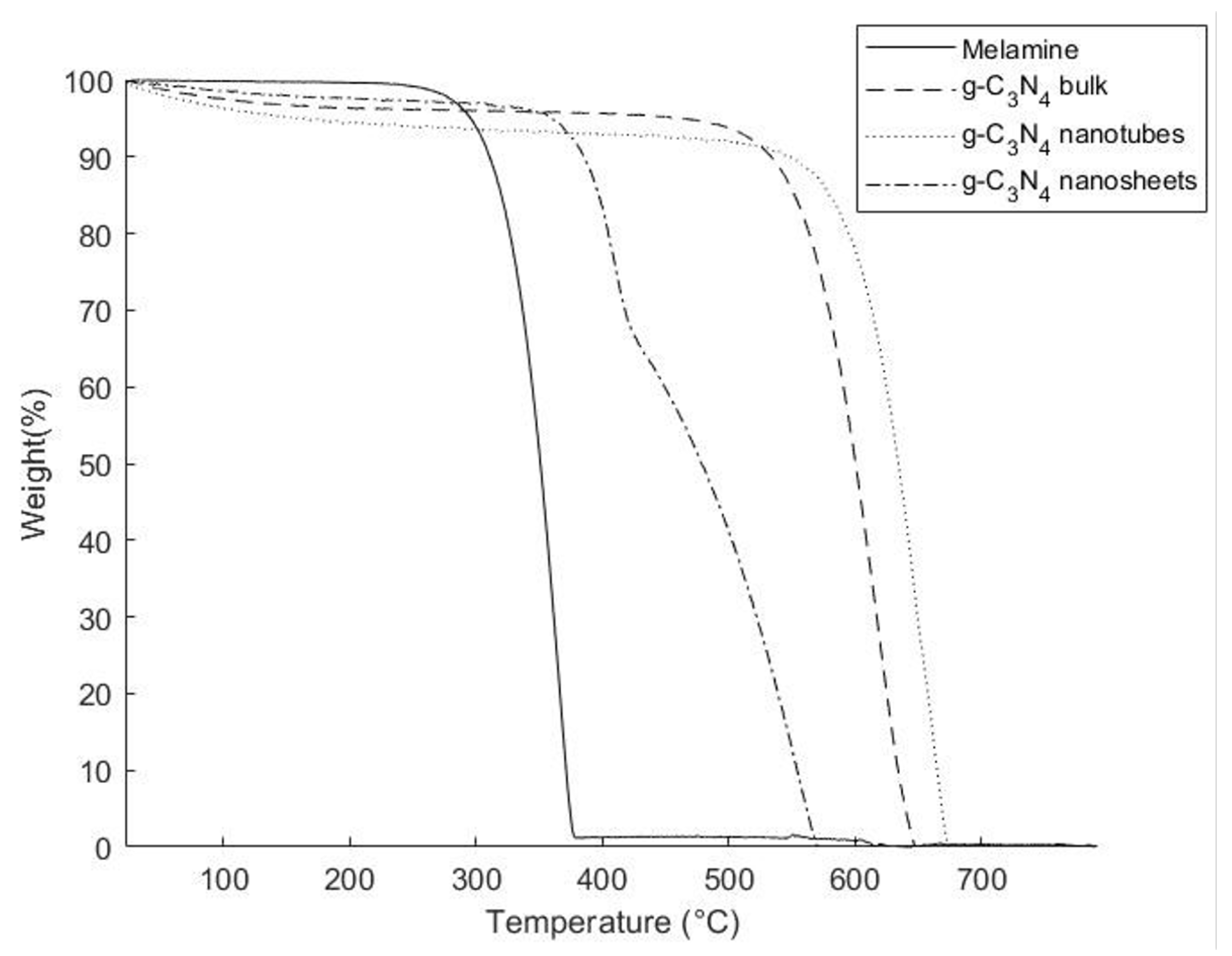
3.3. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gowda, T.Y.; Sanjay, M.; Bhat, K.S.; Madhu, P.; Senthamaraikannan, P.; Yogesha, B. Polymer matrix-natural fiber composites: An overview. Cogent Eng. 2018, 5, 1446667. [Google Scholar] [CrossRef]
- Ticoalu, A.; Aravinthan, T.; Cardona, F. A review of current development in natural fiber composites for structural and infrastructure applications. In Proceedings of the Southern Region Engineering Conference (SREC 2010); 2010; pp. 113–117. [Google Scholar]
- Khalil, H.P.S.A.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Fazita, M.R.N.; Haafiz, M.K.M.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2018, 136, 47251. [Google Scholar] [CrossRef]
- Semaan, P. Micro-and Nano-Crystalline Cellulose for Enhancing the Mechanical Properties of Epoxy. Ph.D. Thesis, American University of Beirut, Beirut, Lebanon, 2021. [Google Scholar]
- Kiran, M.; Govindaraju, H.; Jayaraju, T.; Kumar, N. Review-effect of fillers on mechanical properties of polymer matrix composites. Mater. Today Proc. 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- Wu, N.; Xu, D.; Wang, Z.; Wang, F.; Liu, J.; Liu, W.; Shao, Q.; Liu, H.; Gao, Q.; Guo, Z. Achieving superior electromagnetic wave absorbers through the novel metal-organic frameworks derived magnetic porous carbon nanorods. Carbon 2019, 145, 433–444. [Google Scholar] [CrossRef]
- Vaithylingam, R.; Ansari, M.N.M.; Shanks, R.A. Recent advances in polyurethane-based nanocomposites: A review. Polym. Technol. Eng. 2017, 56, 1528–1541. [Google Scholar] [CrossRef]
- Rajsekhar, V.; Gattu, M. Size-effect testing: Nano-alumina enhances fracture toughness of epoxy resins. Theor. Appl. Fract. Mech. 2023, 125, 103859. [Google Scholar] [CrossRef]
- Wang, E.; Dong, Y.; Islam, M.Z.; Yu, L.; Liu, F.; Chen, S.; Qi, X.; Zhu, Y.; Fu, Y.; Xu, Z.; et al. Effect of graphene oxide-carbon nanotube hybrid filler on the mechanical property and thermal response speed of shape memory epoxy composites. Compos. Sci. Technol. 2019, 169, 209–216. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Zeng, S.; Shen, M.; Xue, Y.; Zheng, Y.; Zhang, K.; Han, Y.; Yang, L. Controllable mechanical properties of epoxy composites by incorporating self-assembled carbon nanotube–montmorillonite. Compos. Part B Eng. 2018, 164, 368–376. [Google Scholar] [CrossRef]
- Zeng, S.; Shen, M.; Yang, L.; Xue, Y.; Lu, F.; Chen, S. Self-assembled montmorillonite–carbon nanotube for epoxy composites with superior mechanical and thermal properties. Compos. Sci. Technol. 2018, 162, 131–139. [Google Scholar] [CrossRef]
- Kumar, A.; Chouhan, D.K.; Alegaonkar, P.S.; Patro, T.U. Graphene-like nanocarbon: An effective nanofiller for improving the mechanical and thermal properties of polymer at low weight fractions. Compos. Sci. Technol. 2016, 127, 79–87. [Google Scholar] [CrossRef]
- Khan, A.; Puttegowda, M.; Jagadeesh, P.; Marwani, H.M.; Asiri, A.M.; Manikandan, A.; Khan, A.A.P.; Ashraf, G.M.; Rangappa, S.M.; Siengchin, S. Review on nitride compounds and its polymer composites: A multifunctional material. J. Mater. Res. Technol. 2022, 18, 2175–2193. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Nawaz, F.; Wang, Y.; Du, P.; Cao, H. Preparation of short, robust and highly ordered titanium dioxide nanotube arrays and their applications as electrode. Appl. Catal. B Environ. 2016, 183, 417–425. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mater. Sci. 2020, 46, 189–217. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Sun, H.; Liu, H.; Mai, X.; Wang, X.; Wang, L.; Wang, N.; Huang, Y.; Guo, Z. Graphitic carbon nitride (g-C3N4) interfacially strengthened carbon fiber epoxy composites. Compos. Sci. Technol. 2018, 167, 515–521. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y.; et al. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2018, 158, 259–268. [Google Scholar] [CrossRef]
- Baghdadi, Y.N.; Sinno, J.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. The mechanical and thermal properties of graphitic carbon nitride (g-C3N4)-based epoxy composites. J. Appl. Polym. Sci. 2021, 138, 51324. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhu, J.; Mahmood, A.; Butt, F.K.; Rizwan, S.; Aslam, I.; Tanveer, M.; Idrees, F.; Shakir, I.; et al. One Dimensional Graphitic Carbon Nitrides as Effective Metal-Free Oxygen Reduction Catalysts. Sci. Rep. 2015, 5, srep12389. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Fang, Z.; Luo, B.; Shi, W. Rational synthesis of ultrathin graphitic carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Carbon 2017, 121, 463–471. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Zeng, H.; Zou, G.; Zhao, Y.; Huang, L.; Bi, J.; Gao, D.; Lin, Z. Surfactant-thermal synthesis of amino acid-templated zinc phosphates with 3-connected nets related to zeolite ABW. Inorg. Chem. 2019, 58, 4089–4092. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Corporation: North Chelmsford, MA, USA, 2000. [Google Scholar]
- Zeng, C.; Lu, S.; Song, L.; Xiao, X.; Gao, J.; Pan, L.; He, Z.; Yu, J. Enhanced thermal properties in a hybrid graphene–alumina filler for epoxy composites. RSC Adv. 2015, 5, 35773–35782. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, B.; Huang, L.; Ma, D.; Jiao, Z.; Xie, Y.; Tan, S.; Cai, X. The utilization of carbon nitride to reinforce the mechanical and thermal properties of UV-curable waterborne polyurethane acrylate coatings. Prog. Org. Coatings 2015, 89, 35–41. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, X.; Fan, K.; Peng, T.; Wei, B. Hydrothermal synthesis of single-walled carbon nanotube–TiO2 hybrid and its photocatalytic activity. Appl. Surf. Sci. 2013, 270, 238–244. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Williams, J.G. Crack blunting mechanisms in polymers. J. Mater. Sci. 1980, 15, 987–996. [Google Scholar] [CrossRef]
- Garg, A.C.; Mai, Y.-W. Failure mechanisms in toughened epoxy resins—A review. Compos. Sci. Technol. 1988, 31, 179–223. [Google Scholar] [CrossRef]
- Chatterjee, S.; A Nüesch, F.; Chu, B.T.T. Comparing carbon nanotubes and graphene nanoplatelets as reinforcements in polyamide 12 composites. Nanotechnology 2011, 22, 275714. [Google Scholar] [CrossRef]
- Tee, Z.Y.; Yeap, S.P.; Hassan, C.S.; Kiew, P.L. Nano and non-nano fillers in enhancing mechanical properties of epoxy resins: A brief review. Polym. Technol. Mater. 2021, 61, 709–725. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging chemical functionalization of g-C3N4: Covalent/noncovalent modifications and applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]

| Time (min) | t1 = 0 | t2 = 15 | t3 = 60 | ||||
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 0.25 | 0.125 | 0.25 | 0.125 | 0.25 | 0.125 | |
| Size (µm) | g-C3N4Bulk | 9.6 ± 0.1 | 9.3 ± 0.1 | 9.2 ± 0.2 | 9.2 ± 0.09 | 3.1 ± 0.09 | 2.8 ± 0.06 |
| g-C3N4 Nanotubes | 3.7 ± 0.07 | 3.3 ± 0.07 | 4.9 ± 0.1 | 3.4 ± 0.06 | 3.1 ± 0.1 | 3.0 ± 0.1 | |
| g-C3N4 Nanosheets | 5.7 ± 0.08 | 4.2 ± 0.09 | 5.1± 0.06 | 3.9 ± 0.08 | 1.7 ± 0.06 | 1.9 ± 0.03 | |
| Composite | Filler Concentration wt% | Tensile Strength (MPa) | % Change | Elastic Modulus (MPa) | % Change | Strain (mm/mm) | % Change |
|---|---|---|---|---|---|---|---|
| Pure Epoxy | 0 | 44.61 ± 4.6 | - | 853.6 ± 83.6 | - | 0.07 ± 0.002 | - |
| g-C3N4 bulk | 0.2 | 48.04 ± 1.23 | 7 | 883.4 ± 11.5 | 3 | 0.07 ± 0.002 | 0 |
| 0.5 | 50.82 ± 1.45 | 14 | 830.7 ± 76.3 | −2 | 0.06 ± 0.009 | −14 | |
| 1.0 | 48.73 ± 5.8 | 9 | 768.8 ± 95.4 | −9 | 0.07 ± 0.002 | 0 | |
| g-C3N4nanosheets | 0.25 | 46.19 ± 3.8 | 3 | 854.08 ± 22.5 | 0 | 0.09 ± 0.006 | 18 |
| 0.5 | 35 ± 3.7 | −21 | 983.5 ± 66.8 | 15 | 0.05 ± 0.006 | −29 | |
| 1.0 | 45.82 ± 2.81 | 3 | 803.5 ± 38.2 | −5 | 0.07 ± 0.002 | 0 | |
| g-C3N4nanotubes | 0.25 | 44.12 ± 4.08 | −1 | 921.7 ± 55 | 8 | 0.07 ±0.002 | 0 |
| 0.5 | 47.896 ± 5.55 | 8 | 956.8 ± 151 | 12 | 0.07 ± 0.002 | 0 | |
| 1.0 | 39.41 ± 5.7 | −11 | 755.64 ± 13.2 | −11 | 0.06 ± 0.004 | −14 |
| Composite | Filler Concentration wt% | Average Residue (%) | Average Td (°C) |
|---|---|---|---|
| Pure Epoxy | 0 | 1.12 | 396.1 ± 0.4 |
| g-C3N4 bulk | 0.25 | 1.14 | 401.7 ± 0.1 |
| 0.5 | 1.38 | 401.3 ± 0.5 | |
| 1.0 | 1.17 | 402.9 ± 0.8 | |
| g-C3N4 nanosheets | 0.25 | 1.24 | 400.1 ± 0.4 |
| 0.5 | 1.77 | 400.5 ± 0.9 | |
| 1.0 | 1.72 | 401.6 ± 0.3 | |
| g-C3N4 nanotubes | 0.25 | 1.63 | 398.7 ± 0.3 |
| 0.5 | 1.21 | 399.6 ± 0.6 | |
| 1.0 | 1.36 | 399.4 ± 0.4 |
| Composite | Filler Concentration wt% | Average Onset Tg (°C) |
|---|---|---|
| Pure Epoxy | 0 | 132 ± 0.44 |
| g-C3N4 bulk | 0.25 | 152 ± 0.21 |
| 0.5 | 153 ± 0.33 | |
| 1.0 | 155 ± 0.43 | |
| g-C3N4 nanosheets | 0.25 | 153 ± 0.57 |
| 0.5 | 154 ± 0.36 | |
| 1.0 | 151 ± 0.41 | |
| g-C3N4 nanotubes | 0.25 | 145 ± 0.39 |
| 0.5 | 149 ± 0.52 | |
| 1.0 | 152 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mais, D.; Mustapha, S.; Baghdadi, Y.N.; Bouhadir, K.; Tehrani-Bagha, A.R. Various Morphologies of Graphitic Carbon Nitride (g-C3N4) and Their Effect on the Thermomechanical Properties of Thermoset Epoxy Resin Composites. Polymers 2024, 16, 1935. https://doi.org/10.3390/polym16131935
Al Mais D, Mustapha S, Baghdadi YN, Bouhadir K, Tehrani-Bagha AR. Various Morphologies of Graphitic Carbon Nitride (g-C3N4) and Their Effect on the Thermomechanical Properties of Thermoset Epoxy Resin Composites. Polymers. 2024; 16(13):1935. https://doi.org/10.3390/polym16131935
Chicago/Turabian StyleAl Mais, Dina, Samir Mustapha, Yasmine N. Baghdadi, Kamal Bouhadir, and Ali R. Tehrani-Bagha. 2024. "Various Morphologies of Graphitic Carbon Nitride (g-C3N4) and Their Effect on the Thermomechanical Properties of Thermoset Epoxy Resin Composites" Polymers 16, no. 13: 1935. https://doi.org/10.3390/polym16131935







