Flexible Resistive Gas Sensor Based on Molybdenum Disulfide-Modified Polypyrrole for Trace NO2 Detection
Abstract
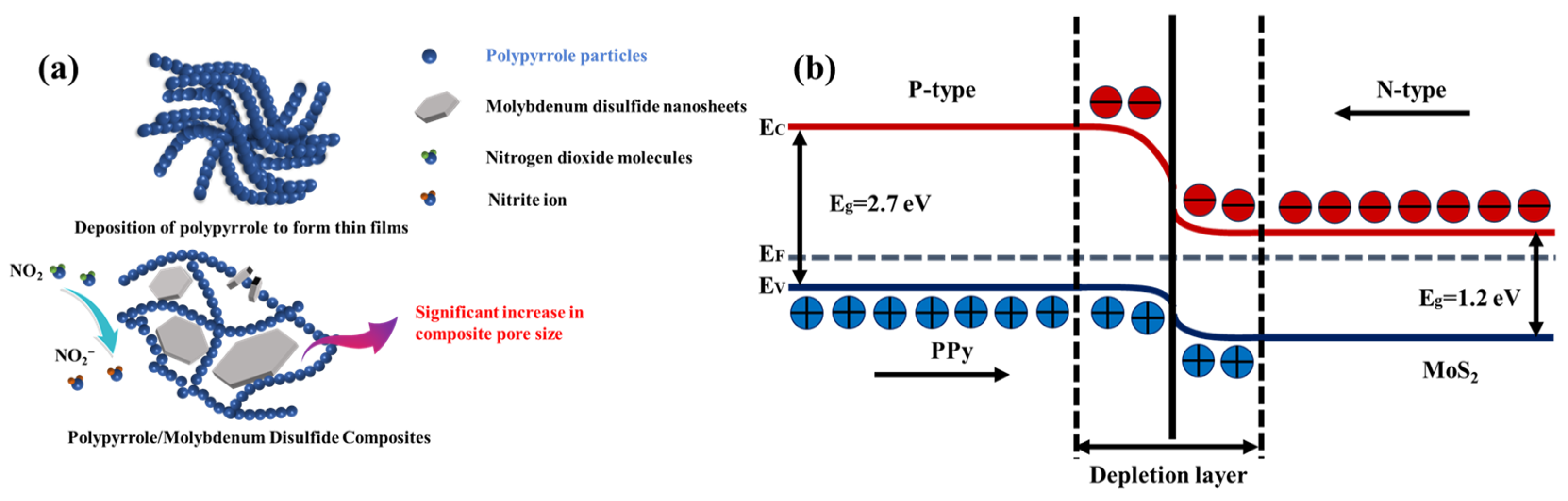
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
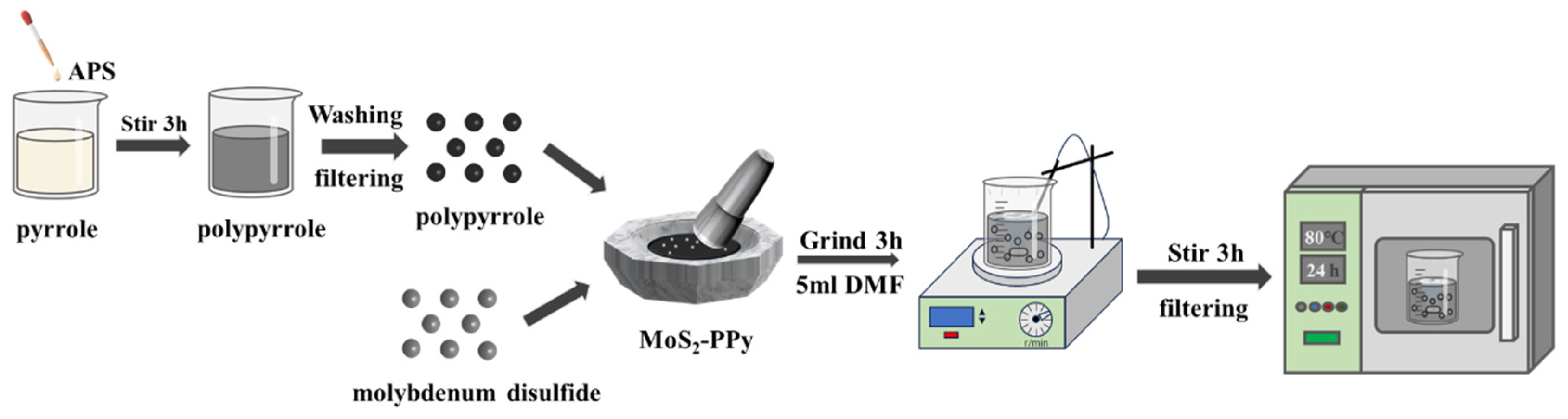
2.3. Preparation of Sensing Material Composites
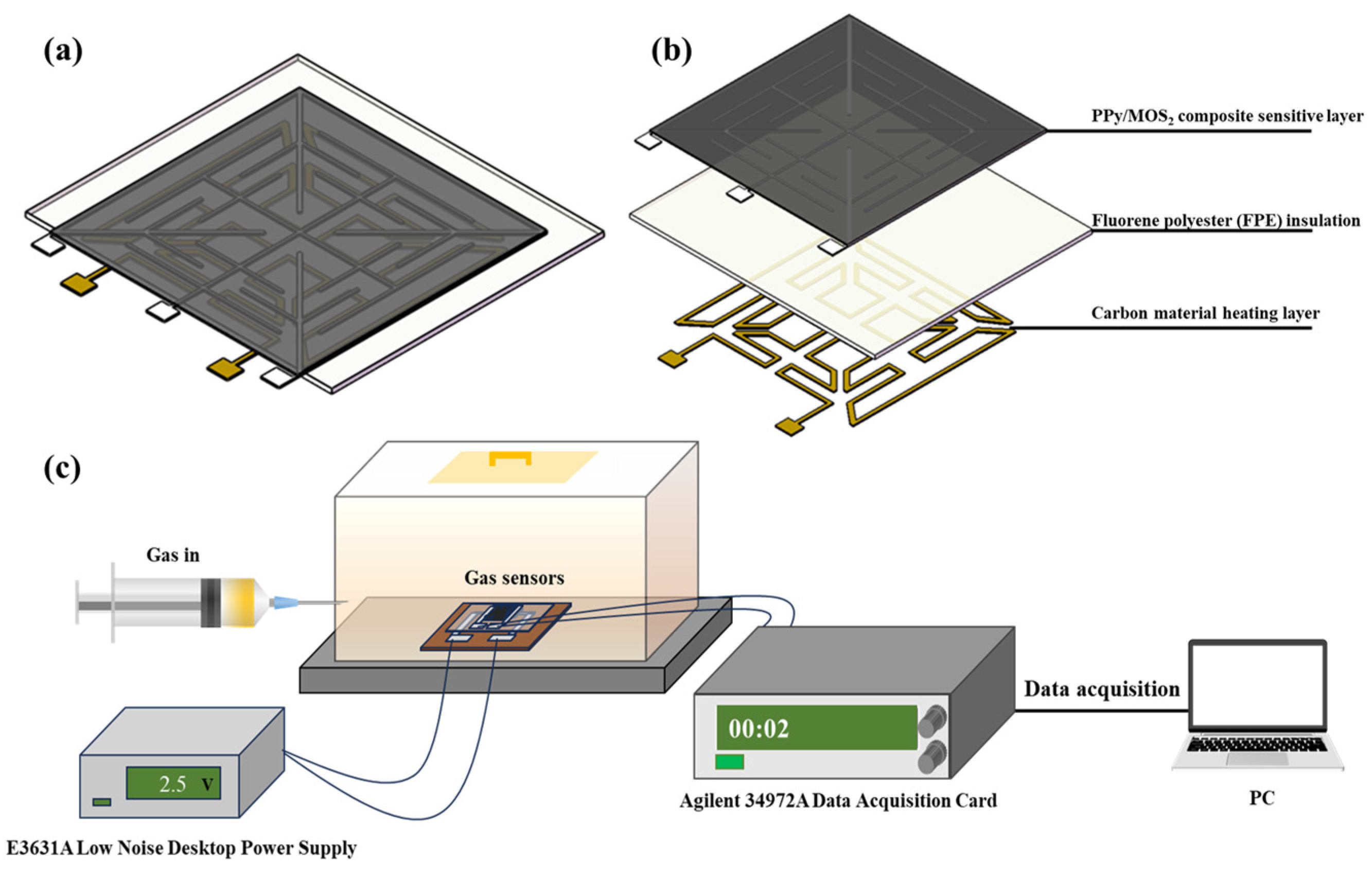
2.4. Sensor Structure Design and Gas Sensing Test Platform
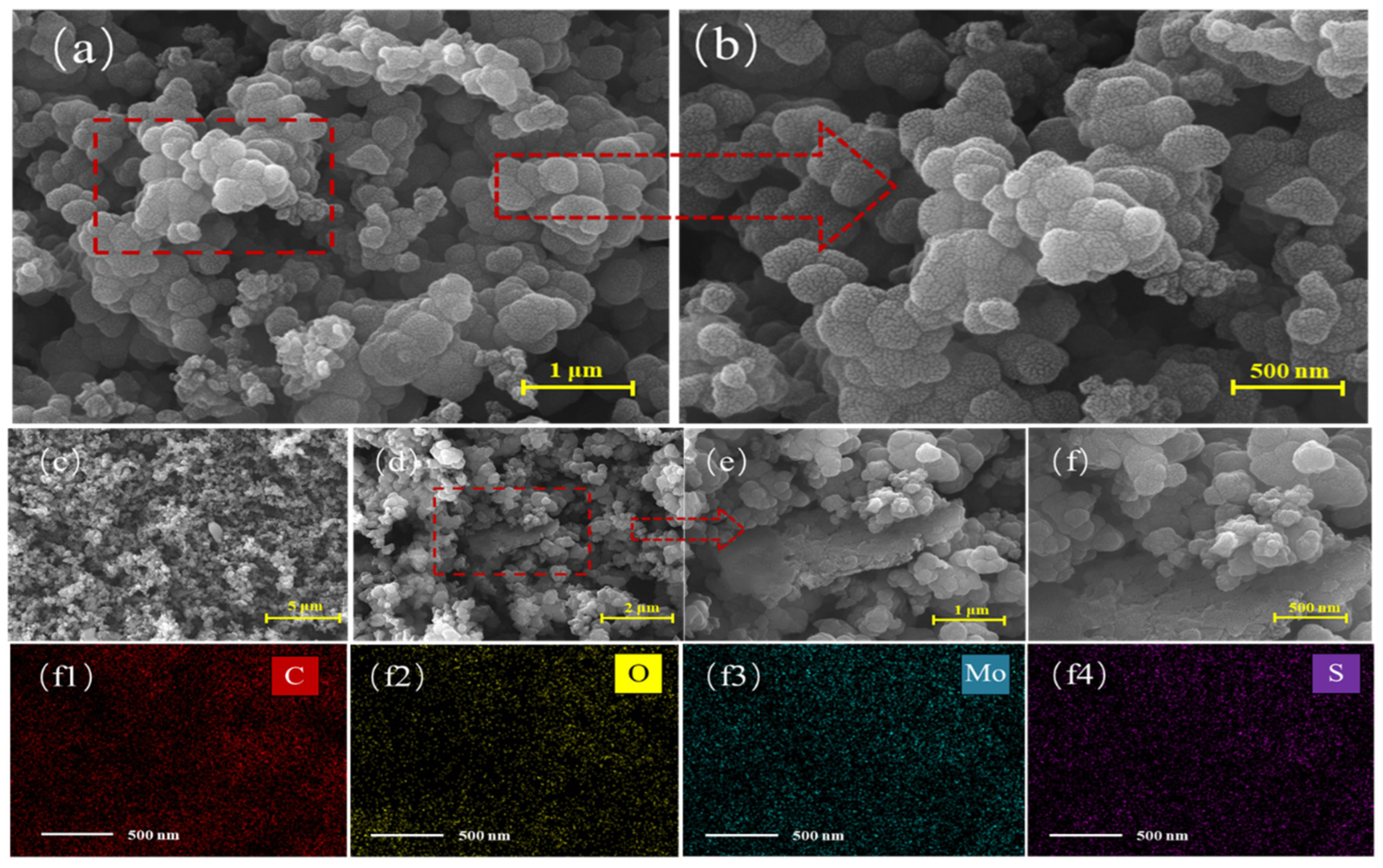
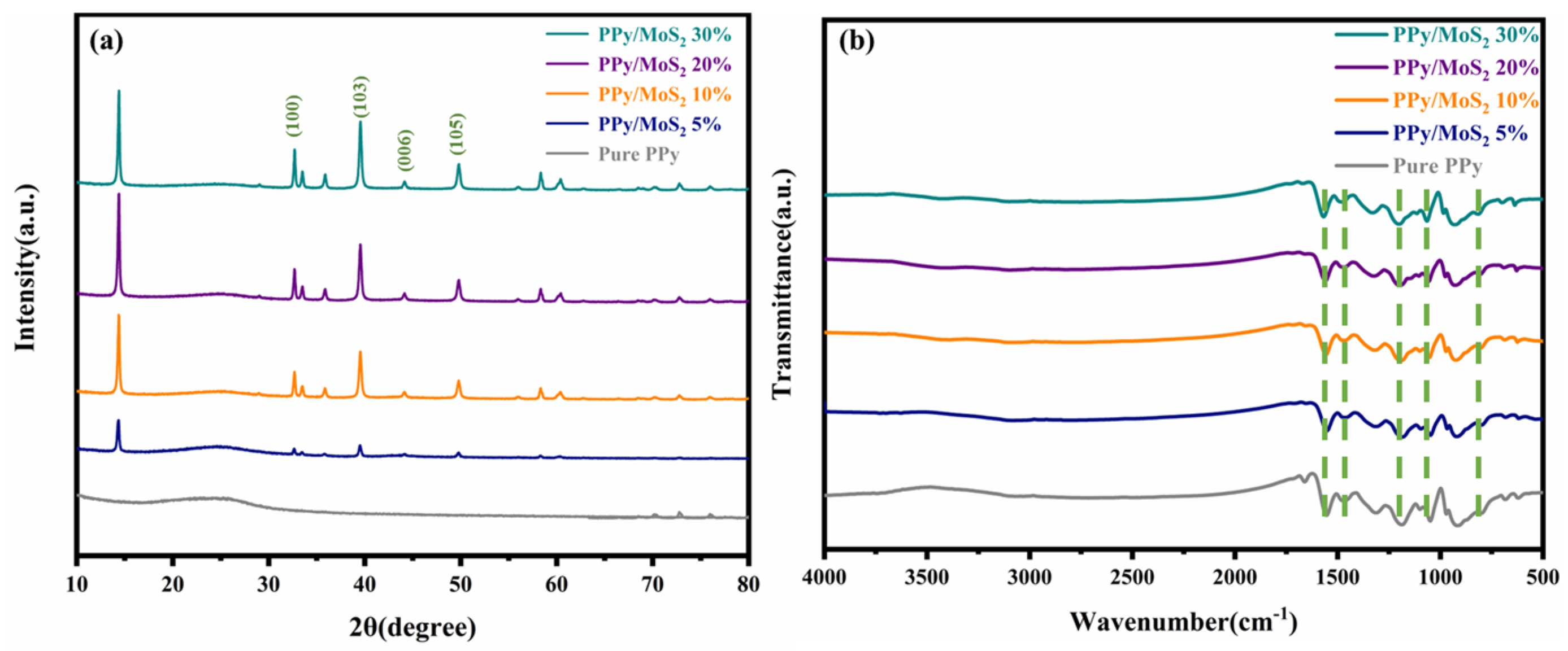
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González Riquelme, F. Air pollution with nitrogen dioxide and its impact on health. Rev. Med. Chil. 2021, 149, 1391–1398. [Google Scholar]
- Zhu, Z.; Hsieh, C.Y.; Chiang, Z.X.; Lin, Y.S.; Wu, R.J. Nanoarchitectonics of three-dimensional ZnO–BiVO4 for trace nitrogen dioxide gas detection. Ceram. Int. 2022, 48, 7706–7714. [Google Scholar] [CrossRef]
- Rigi Jangjoo, M.; Berahman, M. Room-temperature nitrogen dioxide gas sensor based on graphene oxide nanoribbons decorated with MoS2 nanospheres. Appl. Phys. A 2022, 128, 523. [Google Scholar] [CrossRef]
- Jasim, S.A.; Banimuslem, H.A.; Alsultany, F.H.; Al-Bermany, E.; Mohammed, R.M. Ammonia and nitrogen dioxide detection using ZnO/CNT nanocomposite synthesized by sol–gel technique. J. Sol-Gel Sci. Technol. 2023, 108, 734–741. [Google Scholar] [CrossRef]
- Marjani, A.; Ghambarian, M.; Ghashghaee, M. Alkali metal doping of black phosphorus monolayer for ultrasensitive capture and detection of nitrogen dioxide. Sci. Rep. 2021, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, H.; Harvey, M.A.; Stemn, E.; Cliff, D. Focusing on coal workers’ lung diseases: A comparative analysis of China, Australia, and the United States. Int. J. Environ. Res. Public Health 2018, 15, 2565. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical conductivity based ammonia sensing properties of polypyrrole/MoS2 nanocomposite. Polymers 2020, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.R.; Yang, M.; Khandaker, S.; Bashar, M.M.; Alsukaibi, A.K.D.; Hassan, H.M.; Znad, H.; Awual, M.R. Polypyrrole-based sensors for volatile organic compounds (VOCs) sensing and capturing: A comprehensive review. Sens. Actuators A Phys. 2022, 347, 113933. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Han, D.; Han, X.; Liu, L.; Li, D.; Liu, Y.; Liu, Z.; Liu, D.; Chen, Y.; Zhuo, K.; Sang, S. Sub-ppb-level detection of nitrogen dioxide based on high-quality black phosphorus. ACS Appl. Mater. Interfaces 2022, 14, 13942–13951. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Luo, H.; Li, B.; Shi, J.; Fan, C.; Yang, J.; Zeng, M.; Zhou, Z.; Hu, N.; et al. Highly sensitive and recoverable room-temperature NO2 gas detection realized by 2D/0D MoS2/ZnS heterostructures with synergistic effects. Sens. Actuators B Chem. 2021, 347, 130608. [Google Scholar] [CrossRef]
- Quan, W.; Shi, J.; Luo, H.; Fan, C.; Lv, W.; Chen, X.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; et al. Fully flexible MXene-based gas sensor on paper for highly sensitive room-temperature nitrogen dioxide detection. ACS Sens. 2023, 8, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Kang, S.J.; Cho, H.; Jeong, H.M.; Kim, S.; Park, J.S.; Lee, H.J. Highly sensitive and selective gas sensors based on metal iodates: Material characterization and sensor performance evaluation. ACS Appl. Mater. Interfaces 2023, 15, 33721–33731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J.L.; Cao, Q.; Cheng, X.F.; Chen, Z.K.; He, J.H. Chemresistive detection of NO2 of ppb level in humid air at 350 K using azo-spaced polycroconamide. ACS Sens. 2023, 9, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Navale, S.T.; Chougule, M.A.; Patil, V.B.; Mane, A.T. Highly sensitive, reproducible, selective and stable CSA-polypyrrole NO2 sensor. Synth. Met. 2014, 189, 111–118. [Google Scholar] [CrossRef]
- Navale, S.T.; Khuspe, G.D.; Chougule, M.A.; Patil, V.B. Room temperature NO2 gas sensor based on PPy/α-Fe2O3 hybrid nanocomposites. Ceram. Int. 2014, 40, 8013–8020. [Google Scholar] [CrossRef]
- Hong, W.K.; Sohn, J.I.; Hwang, D.K.; Kwon, S.S.; Jo, G.; Song, S.; Kim, S.M.; Ko, H.J.; Park, S.J.; Well, M.E.; et al. Tunable electronic transport 775 characteristics of surface-architecture-controlled ZnO nanowire 776 field effect transistors. Nano Lett. 2008, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room temperature conductive type metal oxide semiconductor gas sensors for NO2 detection. Sens. Actuators A 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xu, M.; Wang, Y.; Xia, H.; Zhang, S.; Guo, X.; Wu, S. Polypyrrole-coated SnO2 hollow spheres and their application for ammonia sensor. J. Phys. Chem. C 2009, 113, 1662–1665. [Google Scholar] [CrossRef]
- Mane, A.T.; Navale, S.T.; Patil, V.B. Room temperature NO2 gas sensing properties of DBSA doped PPy–WO3 hybrid nanocomposite sensor. Org. Electron. 2015, 19, 15–25. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-dimensional transition metal dichalcogenides and metal oxide hybrids for gas sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-performance chemical sensing using Schottky contacted chemical vapor deposition grown mono layer MoSz transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew. Chem. Int. Ed. 2016, 55, 8816–8838. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Design of hetero-nanostructures on MoS2 nanosheets to boost NO2 room-temperature sensing. ACS Appl. Mat. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, Y.; Liu, Y.; Xu, S.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Construction of MoS2/SnO2 heterostructures for sensitive NO2 detection at room temperature. Appl. Surf. Sci. 2019, 493, 613–619. [Google Scholar] [CrossRef]
- Liu, J.B.; Hu, J.Y.; Liu, C.; Tan, Y.M.; Peng, X.; Zhang, Y. Mechanically exfoliated MoS2 nanosheets decorated with SnS2 nanoparticles for high-stability gas sensors at room temperature. Rare Met. 2021, 40, 1536–1544. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Kuang, D.; Hu, X.; Zhou, Y.; He, Y.; Zang, Z. Hollow Cu2O nanospheres loaded with MoS2/reduced graphene oxide nanosheets for ppb-level NO2 detection at room temperature. J. Hazard. Mater. 2021, 416, 126218. [Google Scholar] [CrossRef] [PubMed]
- Vasiljević, D.Z.; Mansouri, A.; Anzi, L.; Sordan, R.; Stojanović, G.M. Performance analysis of flexible ink-jet printed humidity sensors based on graphene oxide. IEEE Sens. J. 2018, 18, 4378–4383. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, K.; Guan, F.; Yin, J.; Feng, Y.; Li, J.; Li, Y. Microstructure and electrical properties of fluorene polyester based nanocomposite dielectrics. Polymers 2021, 13, 3053. [Google Scholar] [CrossRef]
- Das, M.; Roy, S. Polypyrrole and associated hybrid nanocomposites as chemiresistive gas sensors: A comprehensive review. Mater. Sci. Semicond. Process. 2021, 121, 105332. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Santhi, V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 18804–18814. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Pawar, S.A.; Chougule, M.A.; Patil, V.B. Ammonia gas sensing properties of CSA doped PANi-SnO2 nanohybrid thin films. Synth. Met. 2013, 185–186, 1–8. [Google Scholar] [CrossRef]
- Kite, S.V.; Kadam, A.N.; Sathe, D.J.; Patil, S.; Mali, S.S.; Hong, C.K.; Garadkar, K.M. Nanostructured TiO2 sensitized with MoS2 nanoflowers for enhanced photodegradation efficiency toward methyl orange. ACS Omega 2021, 6, 17071–17085. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Li, Y.; Liu, X.; Zhang, W.; Wang, C.; Al-Deyab, S.S.; El-Newehy, M. The application of novel spindle-like polypyrrole hollow nanocapsules containing Pt nanoparticles in electrocatalysis oxidation of nicotinamide adenine dinucleotide (NADH). J. Colloid. Interface Sci. 2011, 356, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Khuspe, G.D.; Navale, S.T.; Pawar, S.A.; Chougule, M.A.; Patil, V.B. Polyaniline SnO2 hybrid nanocomposite: Synthesis, structural, morphological, optical and electrical properties. Synth. Met. 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.Z.; Chew, S.Y.; Chen, J.; Guo, Z.P.; Zhao, L.; Konstantinov, K.; Liu, H.K. Synthesis and characterization of SnO2–polypyrrole composite for lithium-ion battery. J. Power Sources 2007, 174, 1183. [Google Scholar] [CrossRef]
- Su, P.-G.; Pan, T.-T. Fabrication of a room-temperature NO2 gas sensor based on WO3 films and WO3/MWCNT nanocomposite films by combining polyol process with metal organic decomposition method. Mat. Chem. Phys. 2011, 125, 351–357. [Google Scholar] [CrossRef]
- Halfaya, Y.; Bishop, C.; Soltani, A.; Sundaram, S.; Aubry, V.; Voss, P.L.; Salvestrini, J.-P.; Ougazzaden, A. Investigation of the performance of HEMT-based NO, NO2 and NH3 exhaust gas sensors for automotive antipollution systems. Sensors 2016, 16, 273. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.S.M.I.; Chung, G.-S. A high-performance flexible NO2 sensor based on WO3 NPs decorated on MWCNTs and RGO hybrids on PI/PET substrates. Sens. Actuators B Chem. 2016, 224, 738–746. [Google Scholar] [CrossRef]
- Navale, S.T.; Mane, A.T.; Khuspe, G.D.; Chougule, M.A.; Patil, V.B. Room temperature NO2 sensing properties of polythiophene films. Synth. Met. 2014, 195, 228–233. [Google Scholar] [CrossRef]
- Karmakar, N.; Fernandes, R.; Jain, S.; Patil, U.V.; Shimpi, N.G.; Bhat, N.V.; Kothari, D.C. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silverpolypyrrole nanocomposite. Sens. Actuators B 2017, 242, 118–126. [Google Scholar] [CrossRef]
- Malook, K.; Khan, H.; Shah, M.; Haque, I.U. Highly selective and sensitive response of Polypyrrole-MnO2 based composites towards ammonia gas. Polym. Compos. 2018, 40, 1676–1683. [Google Scholar] [CrossRef]
- Jamalabadi, H.; Varnosfaderani, A.M.; Alizadeh, N. Detection of alkyl amine vapors using PPy-ZnO hybrid nanocomposite sensor array and artificial neural network. Sens. Actuators A 2018, 280, 228–237. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, M.F.; Yang, M.J. In-situ grown nanostructured ZnO via a green approach and gas sensing properties of polypyrrole/ZnO nanohybrids. Sens. Actuators B 2017, 238, 596–604. [Google Scholar] [CrossRef]
- Sultan, A.; Mohammad, F. Chemical sensing, thermal stability, electrochemistry and electrical conductivity of silver nanoparticles decorated and polypyrrole enwrapped boron nitride nanocomposite. Polymer 2017, 113, 221–232. [Google Scholar] [CrossRef]
- Sharique, A.; Mujahid, A.K.M.; Faiz, M. Graphene/nickel oxide-based nanocomposite of polyaniline with special reference to ammonia sensing. ACS Omega 2018, 3, 9378–9387. [Google Scholar]
- Tian, S.; Yang, F.; Zeng, D.; Xie, C. Solution-processed gas sensors based on ZnO nanorods array with an exposed (0001) facet for enhanced gas-sensing properties. J. Phys. Chem. C 2012, 116, 10586–10591. [Google Scholar] [CrossRef]
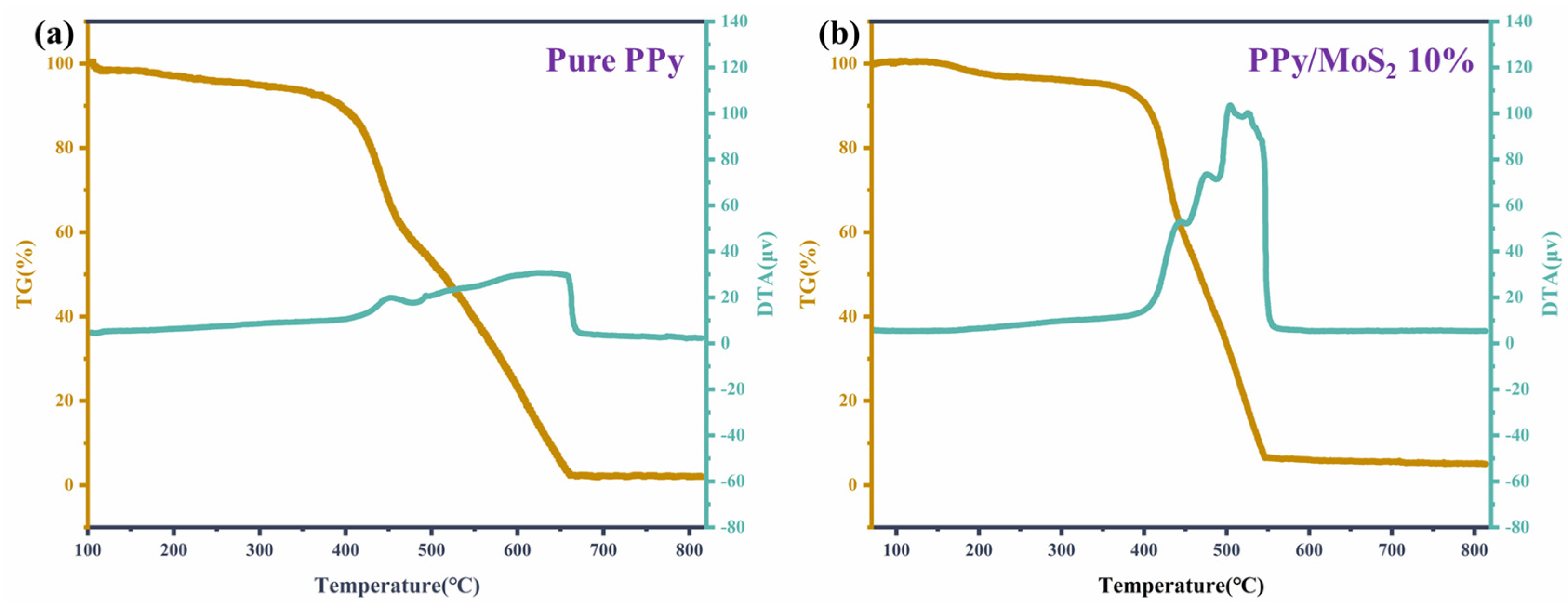
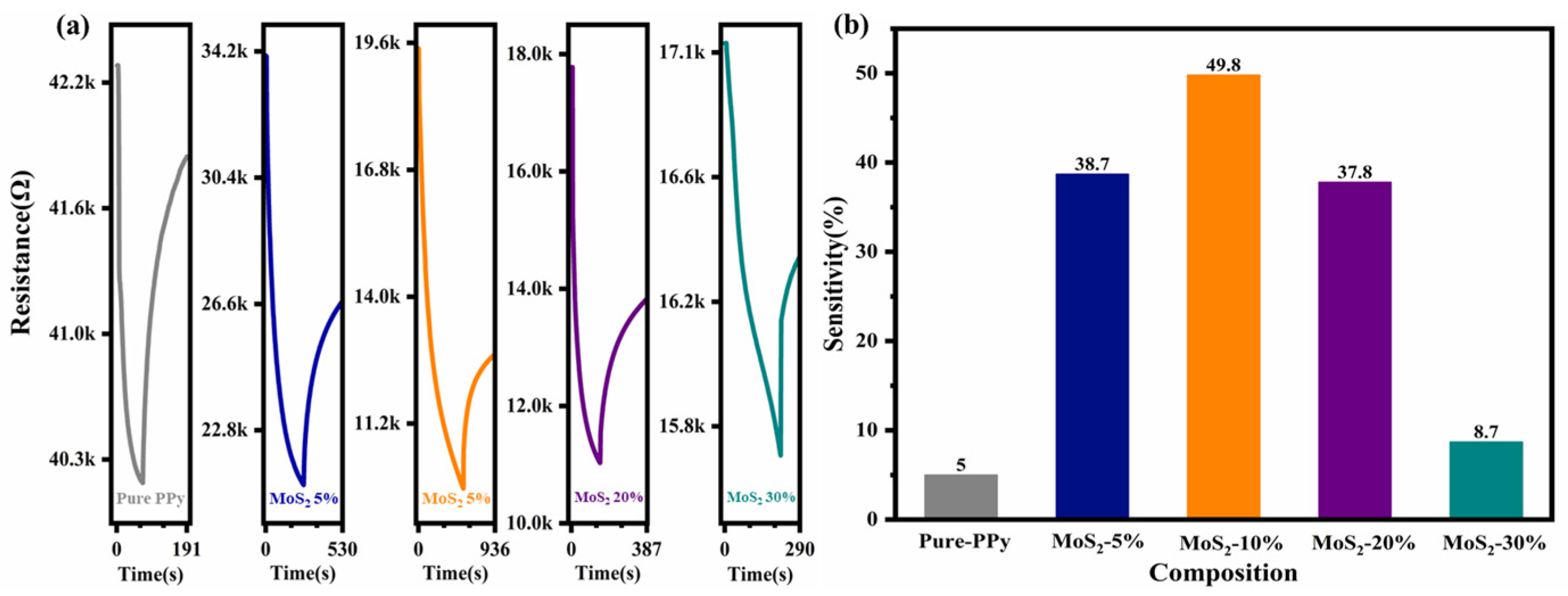
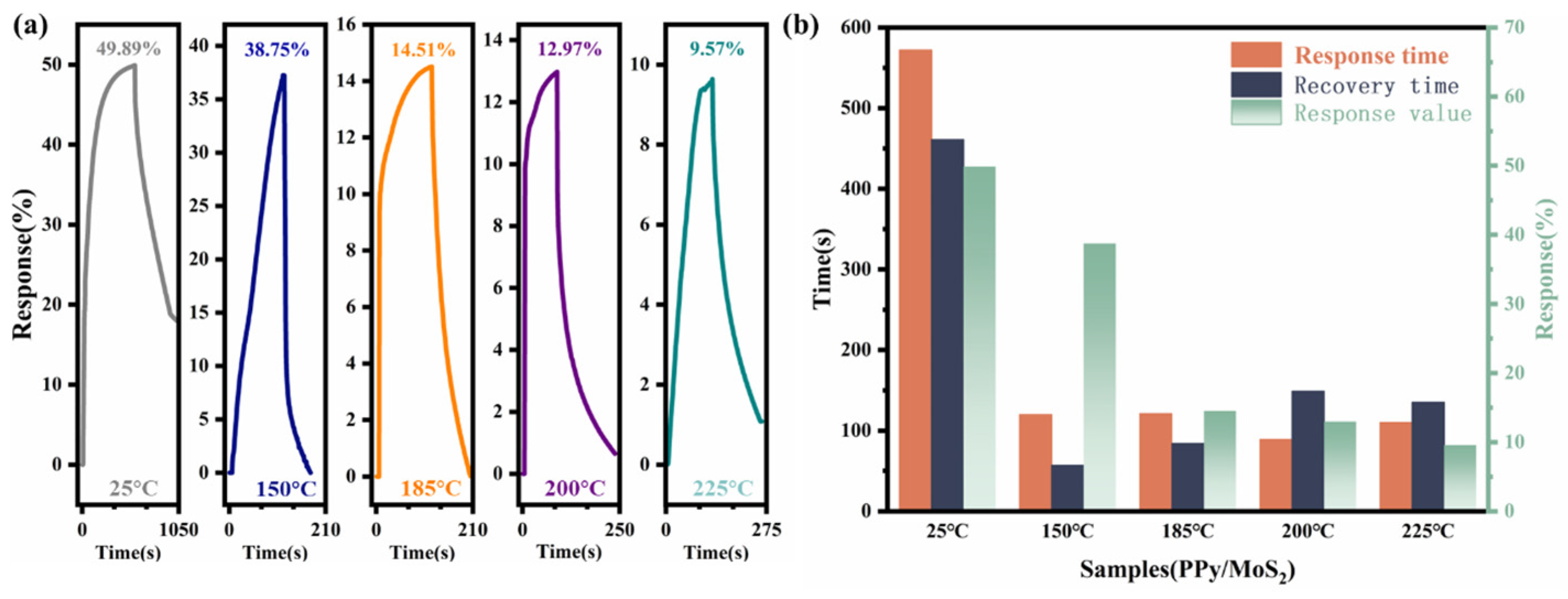


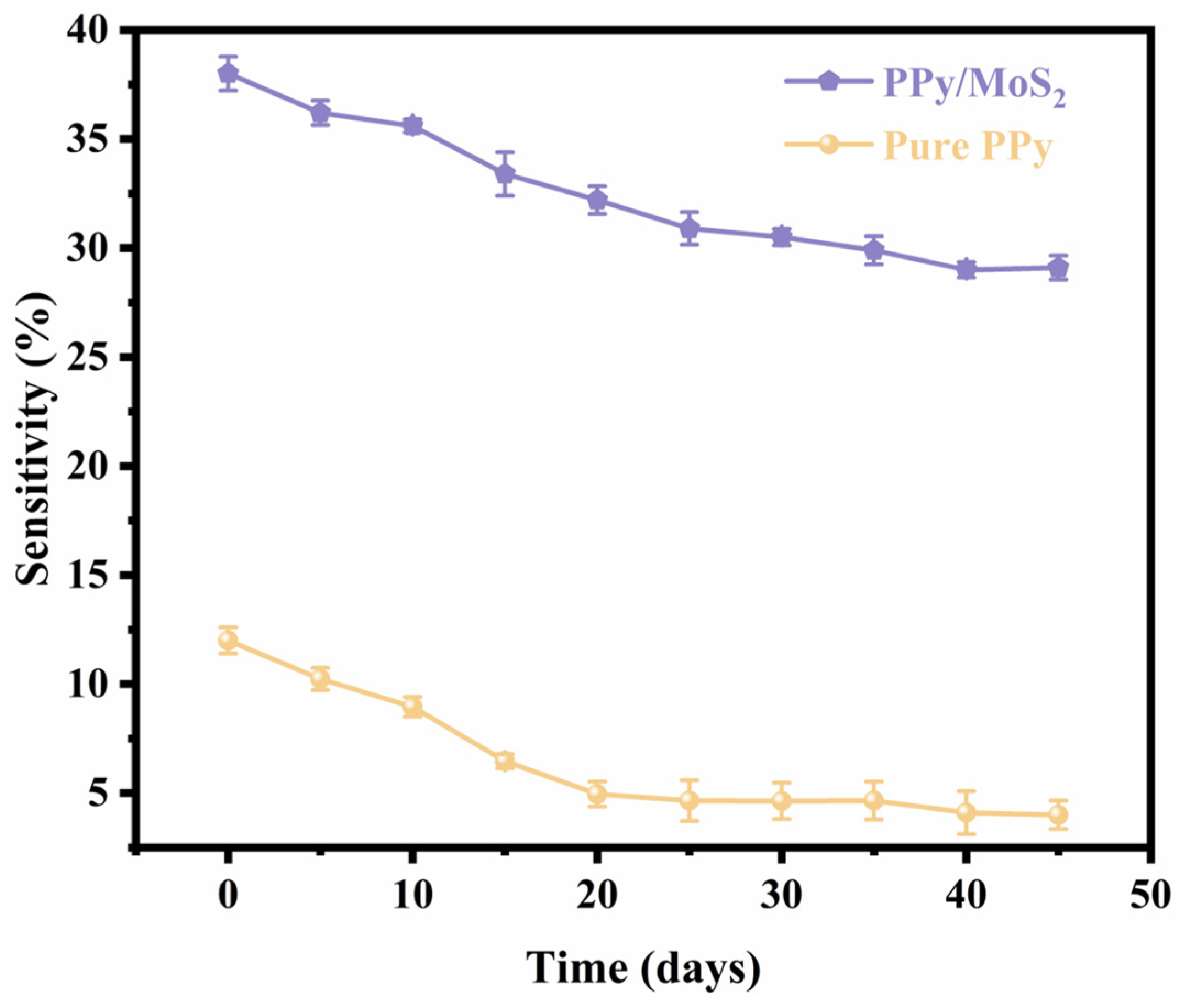
| Material | Structure | Temperature | Concentration | Response | Response/Recovery Time | Reference |
|---|---|---|---|---|---|---|
| MWCNT-WO3 | Nanocomposite | RT | 100 ppm | 96% | 10 s/20 min | [38] |
| Pt/AlGaN/GaN | HEMT | 300 °C | 900 ppm | 33 | 27 min/- | [39] |
| MWCNT and rGO-WO3 | Nanoparticles | RT | 5 ppm | 17% | 7 min/15 min | [40] |
| PTH | Film | RT | 100 ppm | 33% | 400 s/1600 s | [41] |
| PPy/MoS2 | Film | 150 °C | 100 ppm | 48.9% | 120 s/57 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Shi, Y.; Cui, M.; Tang, B.; Zheng, C.; Chen, Q.; Hu, Y. Flexible Resistive Gas Sensor Based on Molybdenum Disulfide-Modified Polypyrrole for Trace NO2 Detection. Polymers 2024, 16, 1940. https://doi.org/10.3390/polym16131940
Zhao K, Shi Y, Cui M, Tang B, Zheng C, Chen Q, Hu Y. Flexible Resistive Gas Sensor Based on Molybdenum Disulfide-Modified Polypyrrole for Trace NO2 Detection. Polymers. 2024; 16(13):1940. https://doi.org/10.3390/polym16131940
Chicago/Turabian StyleZhao, Kuo, Yunbo Shi, Mingrui Cui, Bolun Tang, Canda Zheng, Qinglong Chen, and Yuhan Hu. 2024. "Flexible Resistive Gas Sensor Based on Molybdenum Disulfide-Modified Polypyrrole for Trace NO2 Detection" Polymers 16, no. 13: 1940. https://doi.org/10.3390/polym16131940
APA StyleZhao, K., Shi, Y., Cui, M., Tang, B., Zheng, C., Chen, Q., & Hu, Y. (2024). Flexible Resistive Gas Sensor Based on Molybdenum Disulfide-Modified Polypyrrole for Trace NO2 Detection. Polymers, 16(13), 1940. https://doi.org/10.3390/polym16131940






