Dual First and Second Surface Solar Mirrors of Polished WS2 and Silver by Dynamical Chemical Plating Technique on Polycarbonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Metallization of PC Samples
2.2. Surface Treatment of PC Samples
2.3. Coating of WS2 by Polishing
2.4. Characterization
3. Results and Discussion
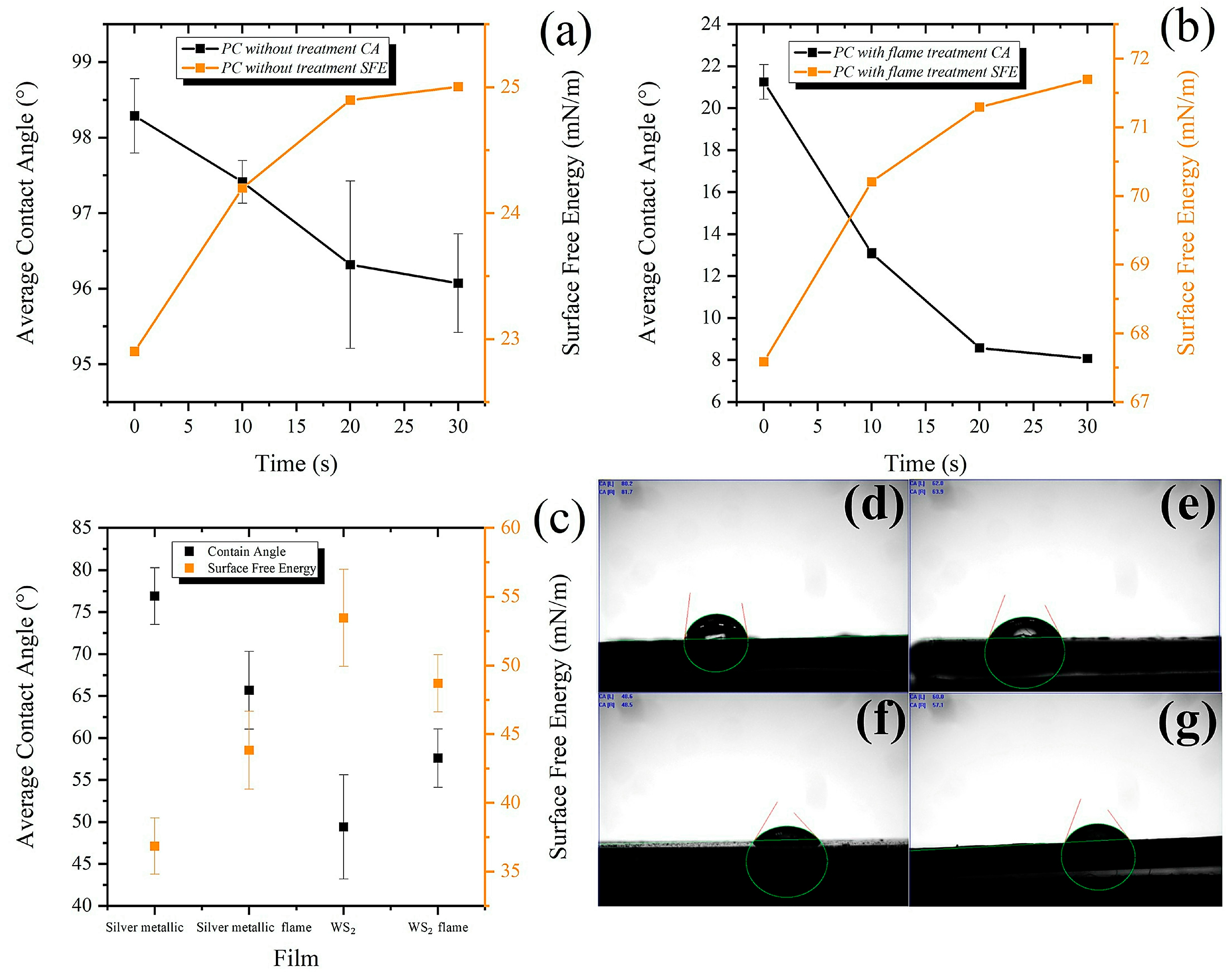
3.1. Effect of Flame Activation on PC Surface Behavior
3.2. Adhesion Testing
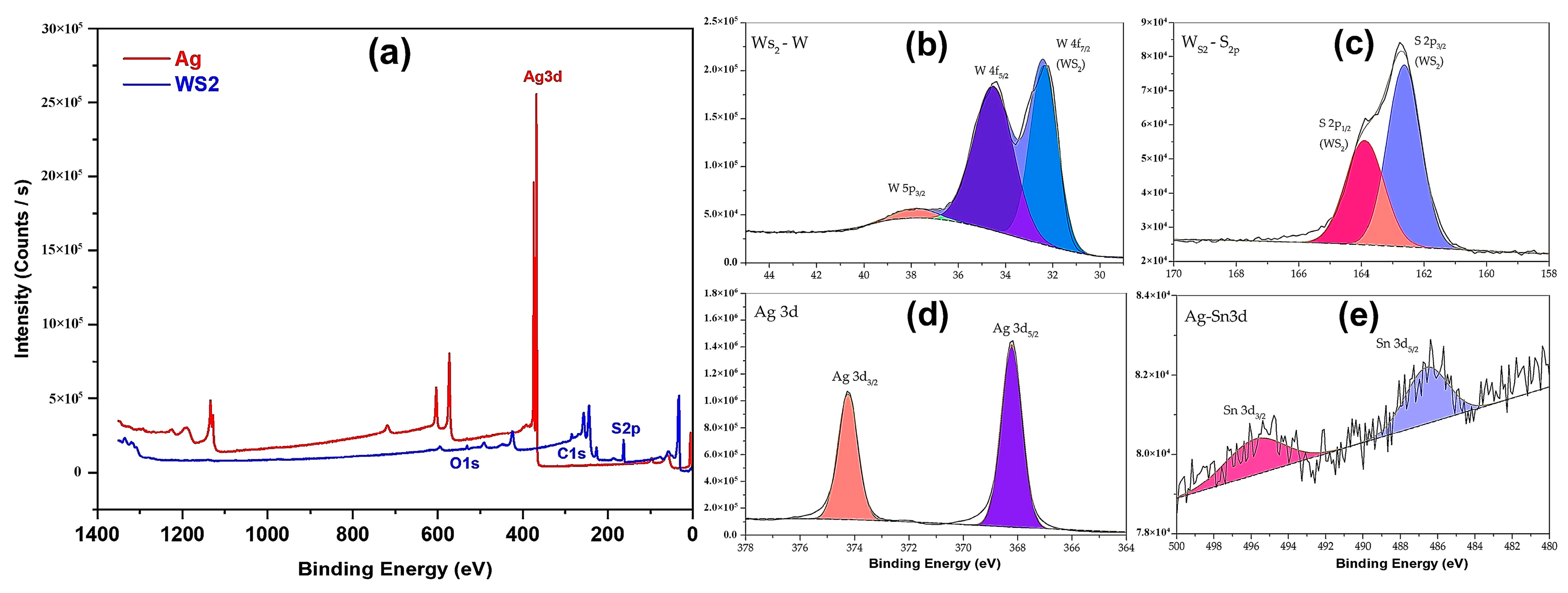
3.3. XPS Analysis of Silver Metallic Film
4. Conclusions
- DCP is a suitable process for depositing metal layers of Ag on non-conductive polycarbonate surfaces, using double-nozzle spray guns at room temperature. The layers obtained were deposited homogeneously on the substrate and had a uniform thickness and excellent adhesion properties as the deposits obtained on the polycarbonate were uniform. Therefore, DCP is a feasible method that does not require plating tanks, racks, drums, or external electric currents.
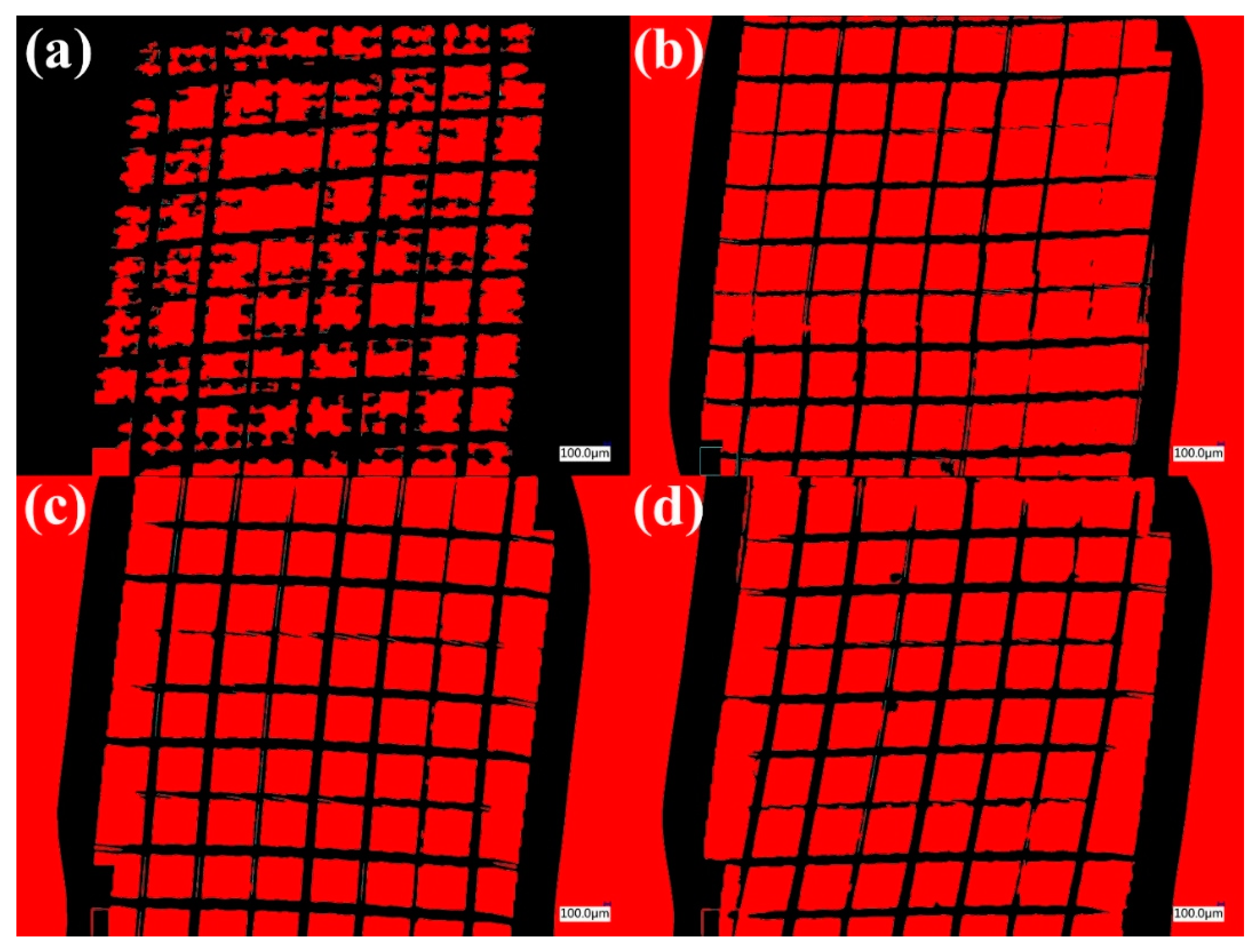
- The surface treatment by flame changed the wettability of the surface as the static contact angle with the water decreased. Generally, adequate adhesion was found between the coating and the substrate, quantified accordingly with ASTM D 3359-23 40, obtained from the shear test.
- Analysis of the chemical composition of the DCP deposit on the PC surface showed metallic silver.
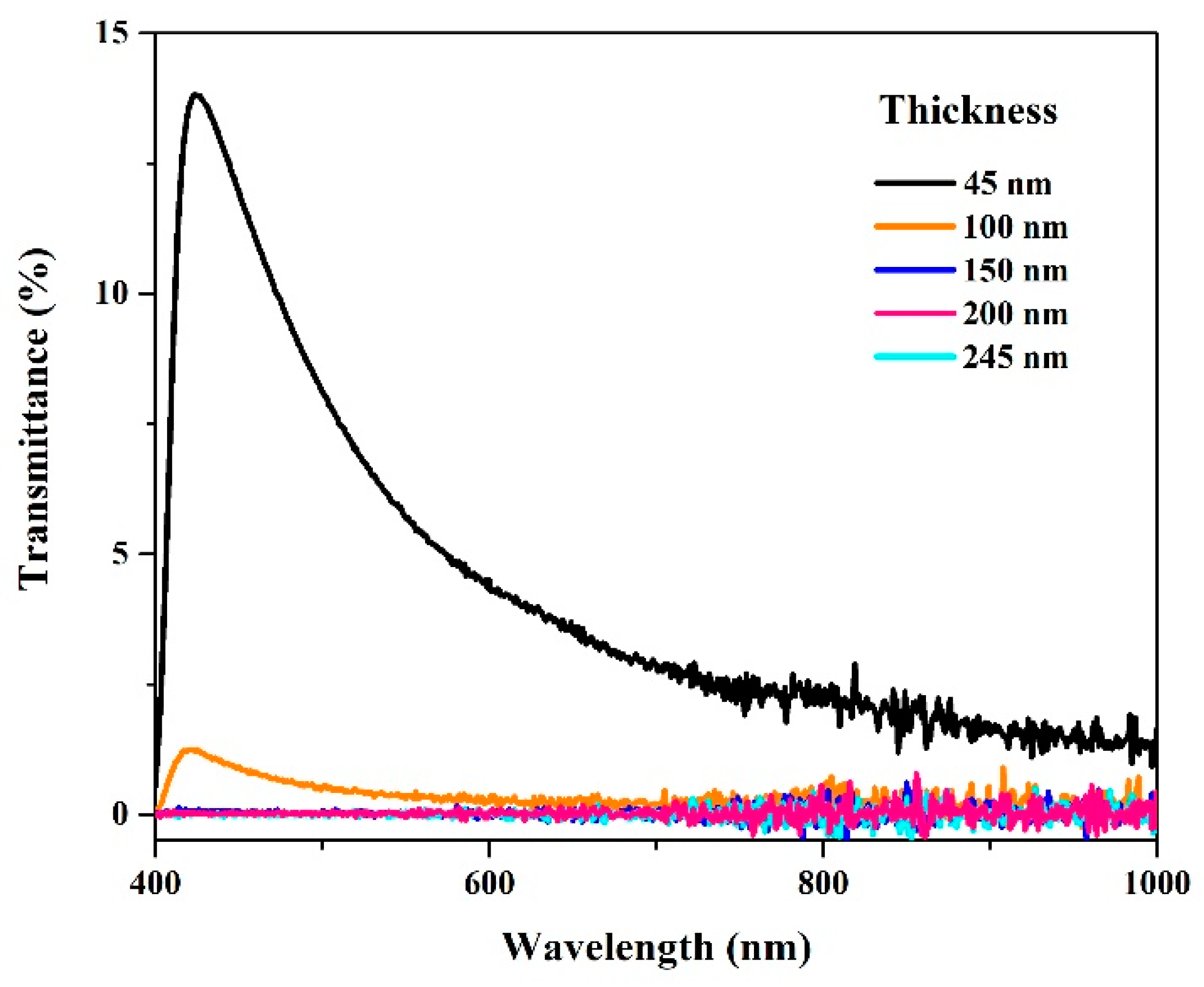
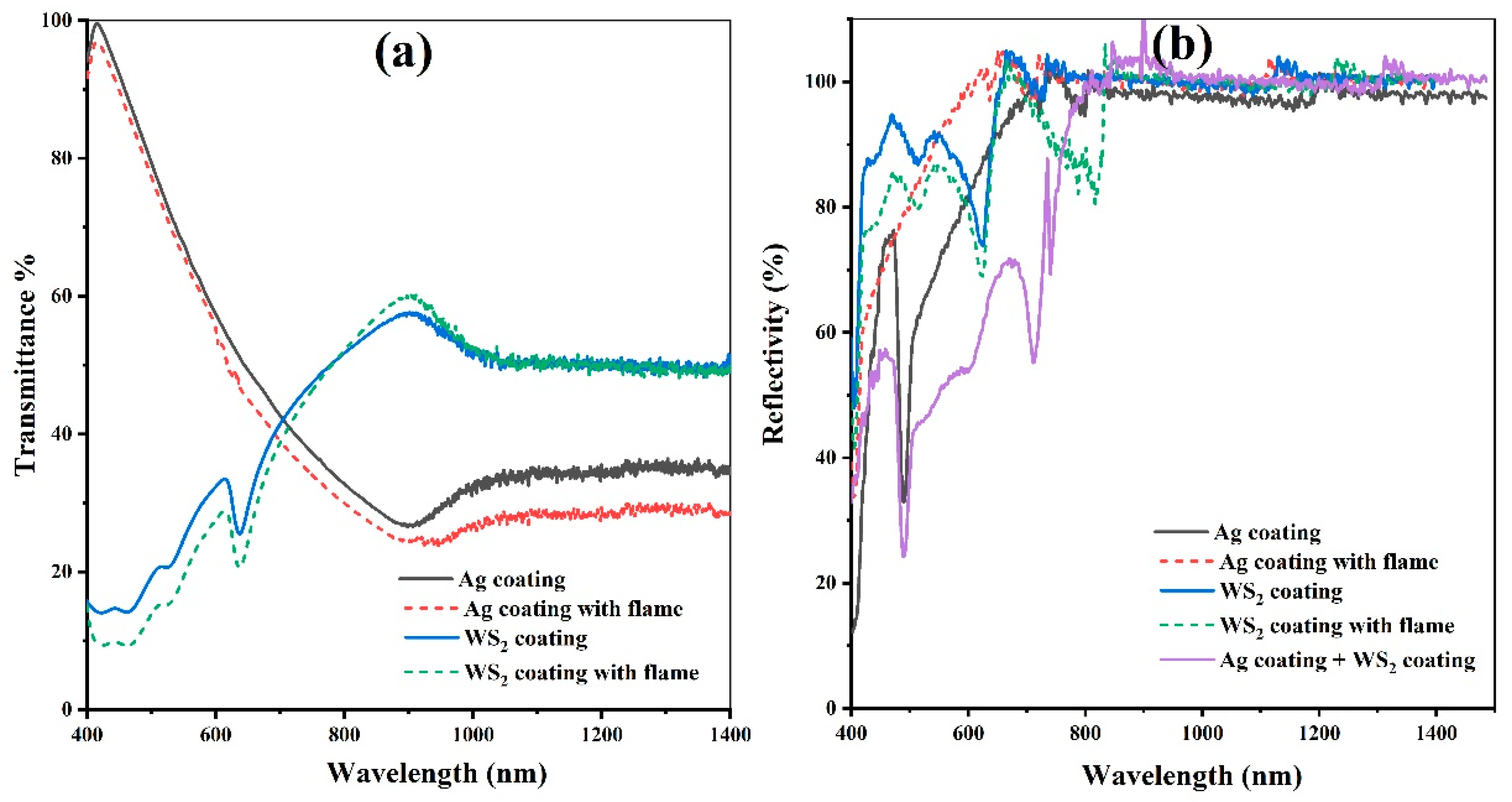
- Most solar mirrors used in commercial concentrating solar plants are made with a thin reflective layer of silver (<200 nm) between glass and paints to ensure high reflectance across the solar spectrum, with high durability for decades. In this work, the polycarbonate/Ag system showed an excellent reflectivity value of >90%, comparable to commercial rigid reflectors used in concentrated solar power. The flame treatment improved this reflectivity, which was attributed to the elimination of residues of silver compounds that could be photoactive, darken the surface, or even cause different types of dark spots.
- A specular reflective coating type based on WS2 lamellar powder was achieved using easy, dry, and one-step mechanical polishing. Results showed reflectance higher than 90% for the visible region and >99% for the NIR region. Also, it showed adequate adhesion with and without flame treatment, which caused a slight reflectance reduction attributed to lamellar orientation on the surface.
- The combination of a silver coating on the back side of the PC and WS2 on the front reduced reflectance, as was expected. However, the change on the external surface could be more extensively studied, as desirable properties can be foreseen. Alternatively, the silver and WS2 specular surfaces could also be used separately.
- This work, for the first time, proposes protecting–reflecting on both sides of mirrors and a solution to PC surface vulnerability to weathering and scratching. Even more, using mechanical polishing, WS2 is used for reflection and surface protection and can be applied to polymers (PC) and metals (aluminum). The PC pieces were the industrially available substrates that resisted pretreatment with a flame for the silver electroless process.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alder, F.A.; Charrault, E.; Zuber, K.; Fabretto, M.; Patil, A.; Murphy, P.; Llusca, M. Fabrication of Robust Solar Mirrors on Polymeric Substrates by Physical Vapor Deposition Technique. Sol. Energy Mater. Sol. Cells 2020, 209, 110476. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, Y.; Wu, J.; Du, E.; Zhang, S.; Xiao, J. Optimal Configuration of Concentrating Solar Power Generation in Power System with High Share of Renewable Energy Resources. Renew. Energy 2024, 220, 119535. [Google Scholar] [CrossRef]
- Xu, R.; He, Z.; Yang, L.; Xu, S.; Wang, R.; Wang, H. Concentration Performance of Solar Collector Integrated Compound Parabolic Concentrator and Flat Microchannel Tube with Tracking System. Renew. Energy 2022, 200, 809–820. [Google Scholar] [CrossRef]
- Jamali, H. Investigation and Review of Mirrors Reflectance in Parabolic Trough Solar Collectors (PTSCs). Energy Rep. 2019, 5, 145–158. [Google Scholar] [CrossRef]
- Achkari, O.; El Fadar, A. Latest Developments on TES and CSP Technologies—Energy and Environmental Issues, Applications and Research Trends. Appl. Therm. Eng. 2020, 167, 114806. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, N.; Abdullah, A.B.; Saidur, R. A Comprehensive Review of State-of-the-Art Concentrating Solar Power (CSP) Technologies: Current Status and Research Trends. Renew. Sustain. Energy Rev. 2018, 91, 987–1018. [Google Scholar] [CrossRef]
- García-Segura, A.; Fernández-García, A.; Ariza, M.J.; Sutter, F.; Valenzuela, L. Durability Studies of Solar Reflectors: A Review. Renew. Sustain. Energy Rev. 2016, 62, 453–467. [Google Scholar] [CrossRef]
- García-Segura, A.; Fernández-García, A.; Ariza, M.J.; Sutter, F.; Diamantino, T.C.; Martínez-Arcos, L.; Reche-Navarro, T.J.; Valenzuela, L. Influence of Gaseous Pollutants and Their Synergistic Effects on the Aging of Reflector Materials for Concentrating Solar Thermal Technologies. Sol. Energy Mater. Sol. Cells 2019, 200, 109955. [Google Scholar] [CrossRef]
- Fernández, A.G.; Gomez-Vidal, J.; Oró, E.; Kruizenga, A.; Solé, A.; Cabeza, L.F. Mainstreaming Commercial CSP Systems: A Technology Review. Renew. Energy 2019, 140, 152–176. [Google Scholar] [CrossRef]
- García-Segura, A.; Fernández-García, A.; Ariza, M.J.; Sutter, F.; Valenzuela, L. Effects of Reduced Sulphur Atmospheres on Reflector Materials for Concentrating Solar Thermal Applications. Corros. Sci. 2018, 133, 78–93. [Google Scholar] [CrossRef]
- Múgica-Vidal, R.; Alba-Elías, F.; Sainz-García, E.; Pantoja-Ruiz, M. Atmospheric Pressure Air Plasma Treatment of Glass Substrates for Improved Silver/Glass Adhesion in Solar Mirrors. Sol. Energy Mater. Sol. Cells 2017, 169, 287–296. [Google Scholar] [CrossRef]
- James, R.A.; Stapleton, A.J.; Hughes, A.; Charrault, E.; Zuber, K.; Switalska, E.; Evans, D.; Murphy, P.; Llusca, M. Metallic Adhesive Layers for Ag-Based First Surface Mirrors. Adv. Eng. Mater. 2018, 20, 1800106. [Google Scholar] [CrossRef]
- McElwain, M.W.; Feinberg, L.D.; Perrin, M.D.; Clampin, M.; Mountain, C.M.; Lallo, M.D.; Lajoie, C.-P.; Kimble, R.A.; Bowers, C.W.; Stark, C.C.; et al. The James Webb Space Telescope Mission: Optical Telescope Element Design, Development, and Performance. Publ. Astron. Soc. Pac. 2023, 135, 058001. [Google Scholar] [CrossRef]
- Sutter, F.; Wette, J.; Wiesinger, F.; Fernández-García, A.; Ziegler, S.; Dasbach, R. Lifetime Prediction of Aluminum Solar Mirrors by Correlating Accelerated Aging and Outdoor Exposure Experiments. Solar Energy 2018, 174, 149–163. [Google Scholar] [CrossRef]
- Arias, N.; Jaramillo, F. Highly Reflective Aluminum Films on Polycarbonate Substrates by Physical Vapor Deposition. Appl. Surf. Sci. 2020, 505, 144596. [Google Scholar] [CrossRef]
- Palacios, A.; Barreneche, C.; Navarro, M.E.; Ding, Y. Thermal Energy Storage Technologies for Concentrated Solar Power—A Review from a Materials Perspective. Renew. Energy 2020, 156, 1244–1265. [Google Scholar] [CrossRef]
- Peinado Gonzalo, A.; Pliego Marugán, A.; García Márquez, F.P. A Review of the Application Performances of Concentrated Solar Power Systems. Appl. Energy 2019, 255, 113893. [Google Scholar] [CrossRef]
- Grosjean, A.; Soum-Glaude, A.; Thomas, L. Replacing Silver by Aluminum in Solar Mirrors by Improving Solar Reflectance with Dielectric Top Layers. Sustain. Mater. Technol. 2021, 29, e00307. [Google Scholar] [CrossRef]
- İkizler, B.; Erden, S. Simple and Additive-Free Synthesis of Highly Reflective Thin Silver Films and Their Application on Large-Scale Surfaces. Mater. Today Chem. 2024, 35, 101859. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Mirror-Finished Superhydrophobic Aluminum Surfaces Modified by Anodic Alumina Nanofibers and Self-Assembled Monolayers. Appl. Surf. Sci. 2018, 440, 506–513. [Google Scholar] [CrossRef]
- Ulitschka, M.; Bauer, J.; Frost, F.; Arnold, T. Improvement of the Optical Properties after Surface Error Correction of Aluminium Mirror Surfaces. J. Eur. Opt. Soc.-Rapid Publ. 2021, 17, 1. [Google Scholar] [CrossRef]
- Abouelata, A.M.A.; Gaballah, S.A. Nano/Micro Smoothing and Reflectivity Enhancement for Preparation of Aluminum Surface Mirror. ARPN J. Eng. Appl. Sci. 2022, 17, 1026–1033. [Google Scholar]
- Mao, L.; Geng, Y.; Cao, Y.; Yan, Y. Uniform High-Reflectivity Silver Film Deposited by Planar Magnetron Sputtering. Vacuum 2021, 185, 109999. [Google Scholar] [CrossRef]
- Ju, H.; Kong, F.; Xu, J.; Geng, Y.; Zhang, C.; Luan, J. Influence of Ag on the Microstructure, Mechanical and Tribological Properties of SiC–Ag Composite Film Deposited by the Industrial DC Magnetron Sputtering System. Vacuum 2023, 218, 112672. [Google Scholar] [CrossRef]
- Pan, Y.; Fan, Y.; Niu, J. Optical Properties of Ultra-Thin Silver Films Deposited by Thermal Evaporation and Its Application in Optical Filters. Infrared Phys. Technol. 2020, 104, 103123. [Google Scholar] [CrossRef]
- Alves, M.S.; Melo, J.C.S.; Costa, C.V.; Ula, M.; de Freitas, J.D.; Tonholo, J.; Hillman, A.R.; de Assis, A.M.L.; Ribeiro, A.S. Latent Fingerprint Enhancement by Ag Nanoparticle Electrodeposition on Metal Surfaces. Electrochim. Acta 2024, 484, 143925. [Google Scholar] [CrossRef]
- Bragaglia, M.; Pascale, V.; Rinaldi, M.; Nanni, F. Silver Electroless Plating on 3D Printed Resins via Stereolithography: A Sustainable Solution. Thin Solid Films 2022, 757, 139417. [Google Scholar] [CrossRef]
- Riaz, K.; Imran Ali, F.; Arslan Wasim, A.; Rafique, F.; Naveed Javed, M.; Abid Ali, S.; Ali Hashmi, I. Ionic Liquid Based Electroless Silver Plating Bath for Printable Circuit Boards (PCBs) Finishing. J. Mol. Liq. 2024, 394, 123704. [Google Scholar] [CrossRef]
- Chitvoranund, N.; Jiemsirilers, S.; Kashima, D.P. Effects of Surface Treatment on Adhesion of Silver Film on Glass Substrate Fabricated by Electroless Plating. Adv. Mater. Res. 2013, 664, 566–573. [Google Scholar] [CrossRef]
- Lien, W.-F.; Huang, P.-C.; Tseng, S.-C.; Cheng, C.-H.; Lai, S.-M.; Liaw, W.-C. Electroless Silver Plating on Tetraethoxy Silane-Bridged Fiber Glass. Appl. Surf. Sci. 2012, 258, 2246–2254. [Google Scholar] [CrossRef]
- Magallón Cacho, L.; Pérez Bueno, J.J.; Meas Vong, Y.; Stremsdoerfer, G.; Espinoza Beltrán, F.J.; Martínez Vega, J. Novel Green Process to Modify ABS Surface before Its Metallization: Optophysic Treatment. J. Coat. Technol. Res. 2015, 12, 313–323. [Google Scholar] [CrossRef]
- Magallón-Cacho, L.; Pérez-Bueno, J.J.; Meas-Vong, Y.; Stremsdoerfer, G.; Espinoza-Beltrán, F.J. Surface Modification of Acrylonitrile-Butadiene-Styrene (ABS) with Heterogeneous Photocatalysis (TiO2) for the Substitution of the Etching Stage in the Electroless Process. Surf. Coat. Technol. 2011, 206, 1410–1415. [Google Scholar] [CrossRef]
- Cacho, L.M.; de Jesús Pérez Bueno, J.; Vong, Y.M.; Stremsdoerfer, G. Effect of Swelling of Chemical Reagents and the Sulfuric-Chromic Acid Bath on Surface Texturizing of Poly(Acrylonitrile-Butadiene-Styrene). Acta Chim. Slov. 2019, 66, 638–647. [Google Scholar] [CrossRef]
- López, J.R.; Méndez, P.F.; Pérez-Bueno, J.J.; Trejo, G.; Stremsdoerfer, G.; Meas, Y.; Stremsdoerfer, G.; López, J.R.; Pérez-Bueno, J.J. Hardness and Corrosion Resistance of Ni/NiB Bi-Layer and Ni/NiB/NiB-PTFE Tri-Layer Coatings Prepared by Electrodeposition and Dynamic Chemical Plating (DCP) Techniques. J. Electrochem. Soc. 2018, 165, D753–D760. [Google Scholar] [CrossRef]
- Magdaleno López, C.; Pérez Bueno, J.d.J.; Cabello Mendez, J.A.; Hernández Leos, R.; Mendoza López, M.L.; Sosa Domínguez, A.; Meas Vong, Y. Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power. Sustainability 2022, 14, 16360. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Xiao, S.; Yang, C.; Zhou, J.; Xiao, B. Single Ni Atom Doped WS2 Monolayer as Sensing Substrate for Dissolved Gases in Transformer Oil: A First-Principles Study. Appl. Surf. Sci. 2022, 579, 152141. [Google Scholar] [CrossRef]
- Hur, Y.G.; Kim, M.-S.; Lee, D.-W.; Kim, S.; Eom, H.-J.; Jeong, G.; No, M.-H.; Nho, N.S.; Lee, K.-Y. Hydrocracking of Vacuum Residue into Lighter Fuel Oils Using Nanosheet-Structured WS 2 Catalyst. Fuel 2014, 137, 237–244. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, D.; Fu, Y.; Qiao, D.; Gao, X.; Feng, D.; Sun, J.; Weng, L.; Wang, H. Vacuum Tribological Performance of WS2–MoS2 Composite Film against Oil-Impregnated Porous Polyimide: Influence of Oil Viscosity. Tribol. Lett. 2019, 67, 2. [Google Scholar] [CrossRef]
- Krasian, T.; Punyodom, W.; Worajittiphon, P. A Hybrid of 2D Materials (MoS2 and WS2) as an Effective Performance Enhancer for Poly(Lactic Acid) Fibrous Mats in Oil Adsorption and Oil/Water Separation. Chem. Eng. J. 2019, 369, 563–575. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Yin, J.; Zhang, X.; Guo, T.; Yan, P. High-Damage-Resistant Tungsten Disulfide Saturable Absorber Mirror for Passively Q-Switched Fiber Laser. Opt. Express 2016, 24, 16287. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, D.; Xu, B.; Xu, H.; Cai, Z.; Peng, J.; Weng, J.; Xu, S.; Zhu, C.; Wang, F.; et al. Two-Dimensional Material-Based Saturable Absorbers: Towards Compact Visible-Wavelength All-Fiber Pulsed Lasers. Nanoscale 2016, 8, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, K.; Persaud, P.D.; Xing, X.; Liu, W.; Long, H.; Li, F.; Wang, B.; Singh, M.R.; Lu, P. Harmonic Resonance Enhanced Second-Harmonic Generation in the Monolayer WS 2—Ag Nanocavity. ACS Photonics 2020, 7, 562–568. [Google Scholar] [CrossRef]
- Huang, H.; Deng, F.; Xiang, J.; Li, S.; Lan, S. Plasmon-Exciton Coupling in Dielectric-Metal Hybrid Nanocavities with an Embedded Two-Dimensional Material. Appl. Surf. Sci. 2021, 542, 148660. [Google Scholar] [CrossRef]
- AMS2530A; Tungsten Disulfide Coating, Thin Lubricating Film, Binder-Less Impingement Applied. SAE International: Warrendale, PA, USA, 2014.
- ASTM D3359-23; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2023.
- Farris, S.; Pozzoli, S.; Biagioni, P.; Duó, L.; Mancinelli, S.; Piergiovanni, L. The Fundamentals of Flame Treatment for the Surface Activation of Polyolefin Polymers—A Review. Polymer 2010, 51, 3591–3605. [Google Scholar] [CrossRef]
- Homola, T.; Matoušek, J.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Plasma Treatment of Glass Surfaces Using Diffuse Coplanar Surface Barrier Discharge in Ambient Air. Plasma Chem. Plasma Process. 2013, 33, 881–894. [Google Scholar] [CrossRef]
- Magdaleno-López, C.; de Jesús Pérez-Bueno, J. Quantitative Evaluation for the ASTM D4541-17/D7234 and ASTM D3359 Adhesion Norms with Digital Optical Microscopy for Surface Modifications with Flame and APPJ. Int. J. Adhes. Adhes. 2020, 98, 102551. [Google Scholar] [CrossRef]
- Sabbatini, L. 7.2 CA and Surface Energy. In Polymer Surface Characterization; De Gruyter Textbook: Berlin, Germany, 2014; p. 306. ISBN 311037692X/9783110376920. [Google Scholar]
- Xavier, J.R.; Vinodhini, S.P.; Raja Beryl, J. Anti-Corrosion and Flame-Retardant Properties of Environmentally Benign Smart Functionalized WS2/RGO in Epoxy Coatings for Enhanced Steel Structural Protection in Natural Seawater. Mater. Today Commun. 2024, 38, 107842. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, S.; Liu, Y.; Lei, R.; Guo, T.; Yao, Y.; Gao, S.; Ding, J.; Zhang, Z. The Effect of Surface-Free Energy and Microstructure on the Condensation Mechanism of Water Vapor. Prog. Nat. Sci. Mater. Int. 2023, 33, 37–46. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact Angle Measurements. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–255. [Google Scholar]
- Stremsdoerfer, S. Non-Electrolytic Method for Metallizing a Substrate by the Reduction of Metallic Salt(s) and the Spraying of Aerosol(S). US8507043B2, 24 November 2006. [Google Scholar]
- Mielewczyk, L.; Liebscher, V.; Grothe, J.; Kaskel, S. Sugar-Based Electroless Copper Deposition on Pectin-Coated Alumina Microparticles. Adv. Mater. Interfaces 2023, 10, 2300496. [Google Scholar] [CrossRef]
- Powell, C.J. Elemental Binding Energies for X-ray Photoelectron Spectroscopy. Appl. Surf. Sci. 1995, 89, 141–149. [Google Scholar] [CrossRef]
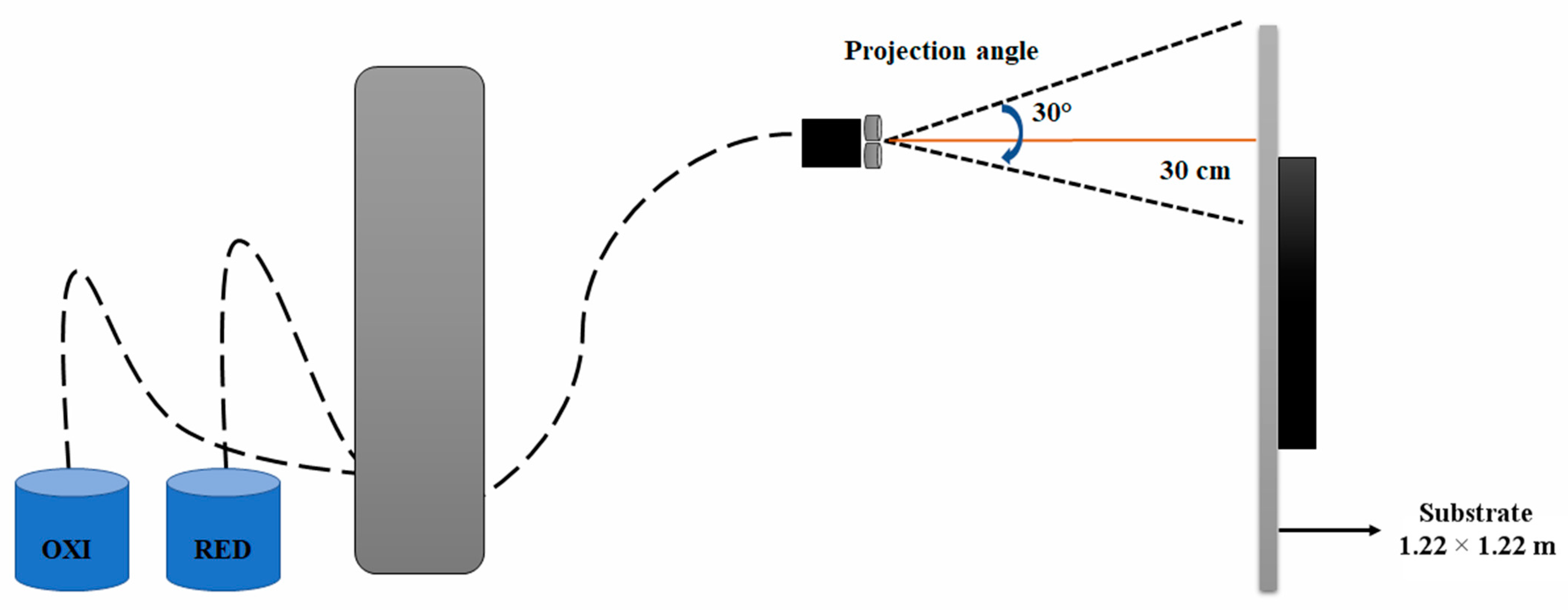
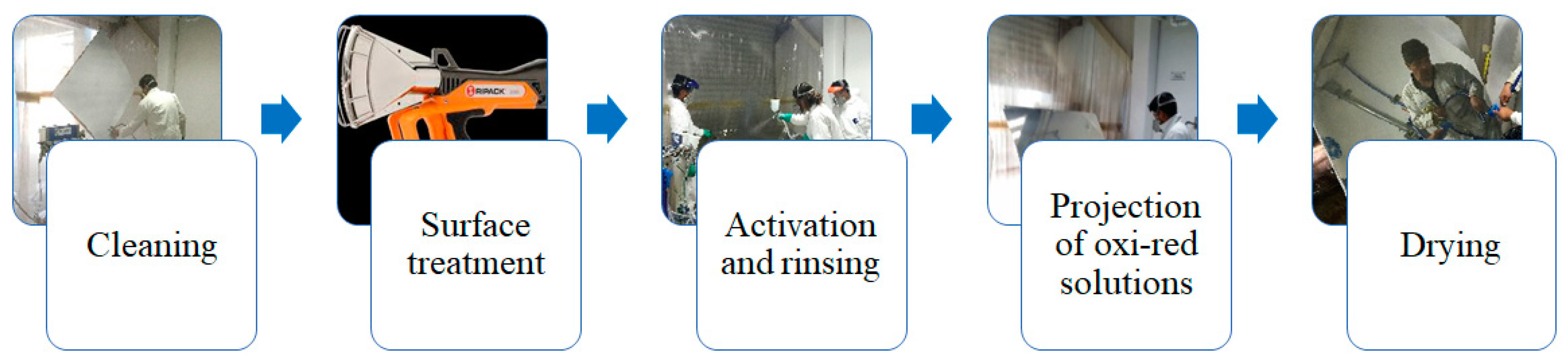
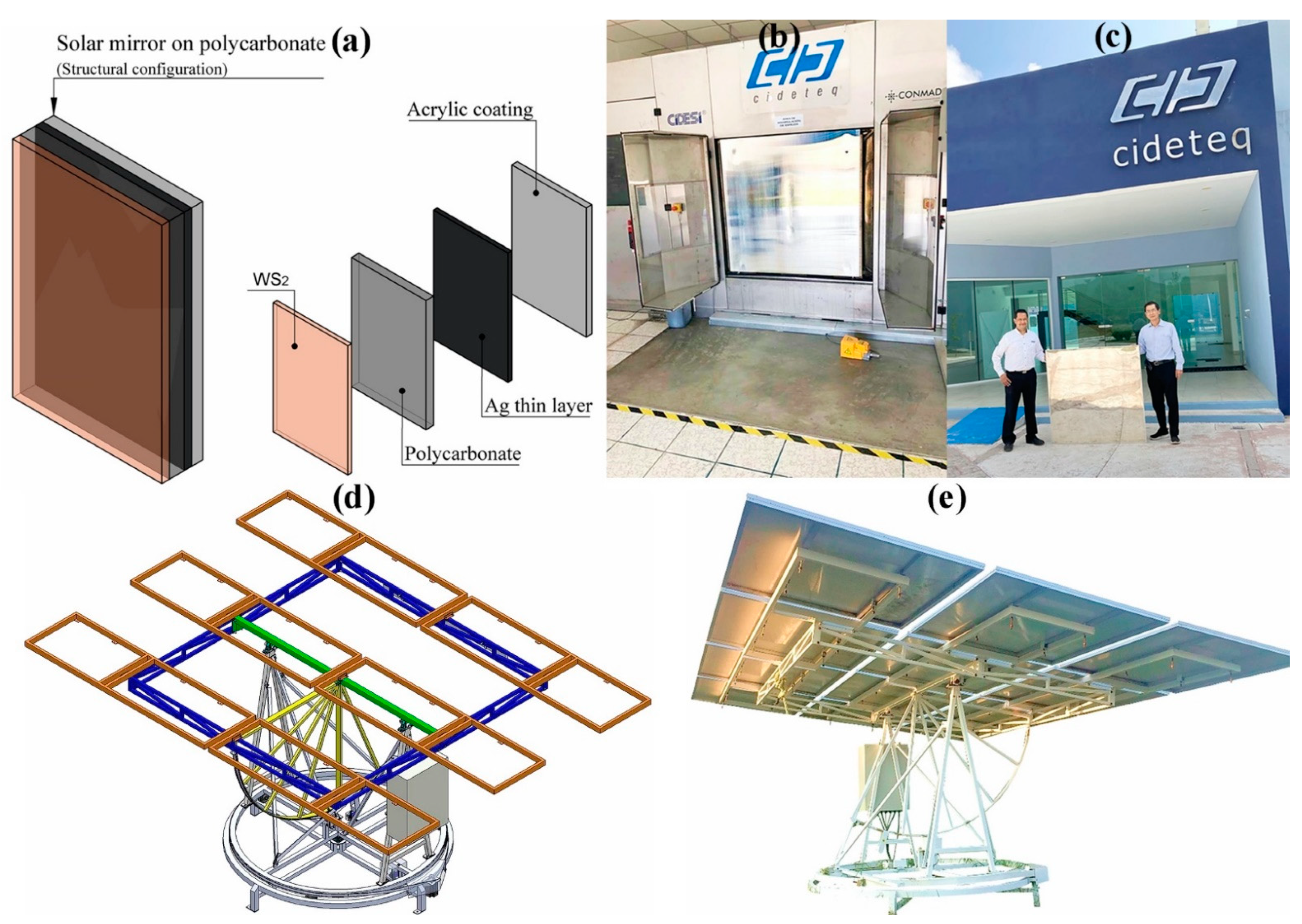

| Pressure (bar) | Power (kW) | Gas Flow Rate (kg/h) | Power Source |
|---|---|---|---|
| 1.5–3.5 | 45–76 | 2.9–5.2 | Natural gas |
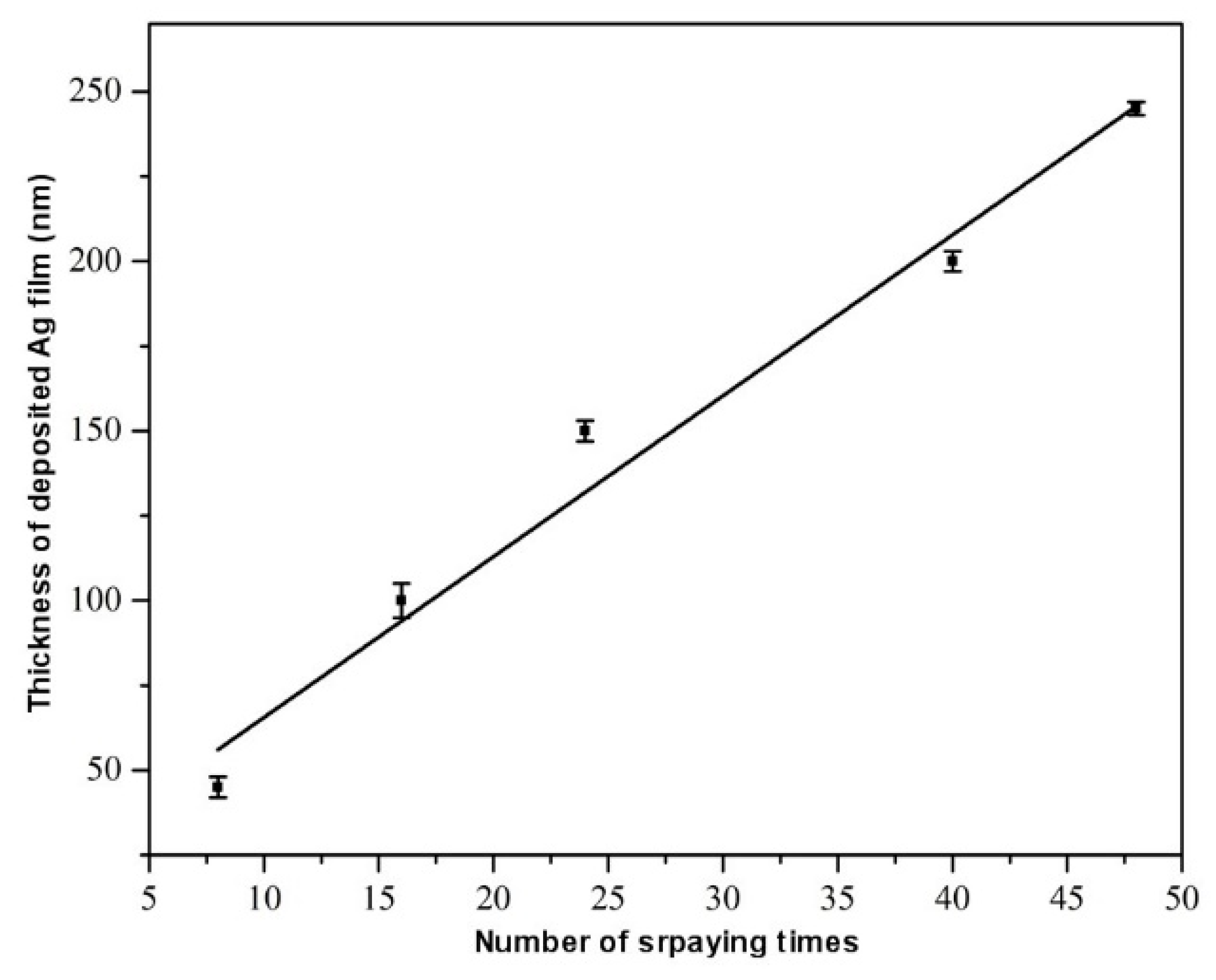
| Layers Plating | Thickness (nm) |
|---|---|
| 8 | 45 |
| 16 | 100 |
| 24 | 150 |
| 40 | 200 |
| 48 | 245 |
| Number of Layers Added to the Substrate | % Area Removed | Classification |
|---|---|---|
| 8 layers | <5% | 4B |
| 16 layers | 15–30% | 2B |
| 24 layers | 35–65% | 1B |
| 40 layers | 35–65% | 1B |
| 48 layers | >65% | 0B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdaleno López, C.; Pérez Bueno, J.d.J.; Maldonado Pérez, A.X.; Meas Vong, Y.; Morales Hernández, J.; Ambrosio Juárez, J.E.; Toledo Manuel, I.; Cabello Mendez, J.A.; Meneses Rodríguez, D. Dual First and Second Surface Solar Mirrors of Polished WS2 and Silver by Dynamical Chemical Plating Technique on Polycarbonate. Polymers 2024, 16, 1951. https://doi.org/10.3390/polym16131951
Magdaleno López C, Pérez Bueno JdJ, Maldonado Pérez AX, Meas Vong Y, Morales Hernández J, Ambrosio Juárez JE, Toledo Manuel I, Cabello Mendez JA, Meneses Rodríguez D. Dual First and Second Surface Solar Mirrors of Polished WS2 and Silver by Dynamical Chemical Plating Technique on Polycarbonate. Polymers. 2024; 16(13):1951. https://doi.org/10.3390/polym16131951
Chicago/Turabian StyleMagdaleno López, Coraquetzali, José de Jesús Pérez Bueno, Alejandra Xochitl Maldonado Pérez, Yunny Meas Vong, Jorge Morales Hernández, José Emanuel Ambrosio Juárez, Iván Toledo Manuel, José Antonio Cabello Mendez, and David Meneses Rodríguez. 2024. "Dual First and Second Surface Solar Mirrors of Polished WS2 and Silver by Dynamical Chemical Plating Technique on Polycarbonate" Polymers 16, no. 13: 1951. https://doi.org/10.3390/polym16131951
APA StyleMagdaleno López, C., Pérez Bueno, J. d. J., Maldonado Pérez, A. X., Meas Vong, Y., Morales Hernández, J., Ambrosio Juárez, J. E., Toledo Manuel, I., Cabello Mendez, J. A., & Meneses Rodríguez, D. (2024). Dual First and Second Surface Solar Mirrors of Polished WS2 and Silver by Dynamical Chemical Plating Technique on Polycarbonate. Polymers, 16(13), 1951. https://doi.org/10.3390/polym16131951










