Development of 3D Printable Gelatin Methacryloyl/Chondroitin Sulfate/Hyaluronic Acid Hydrogels as Implantable Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Fabrication
2.3. Mechanical Properties
2.4. Swelling Behaviour
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Rheological Properties
2.7. Printability
2.8. Freeze Drying
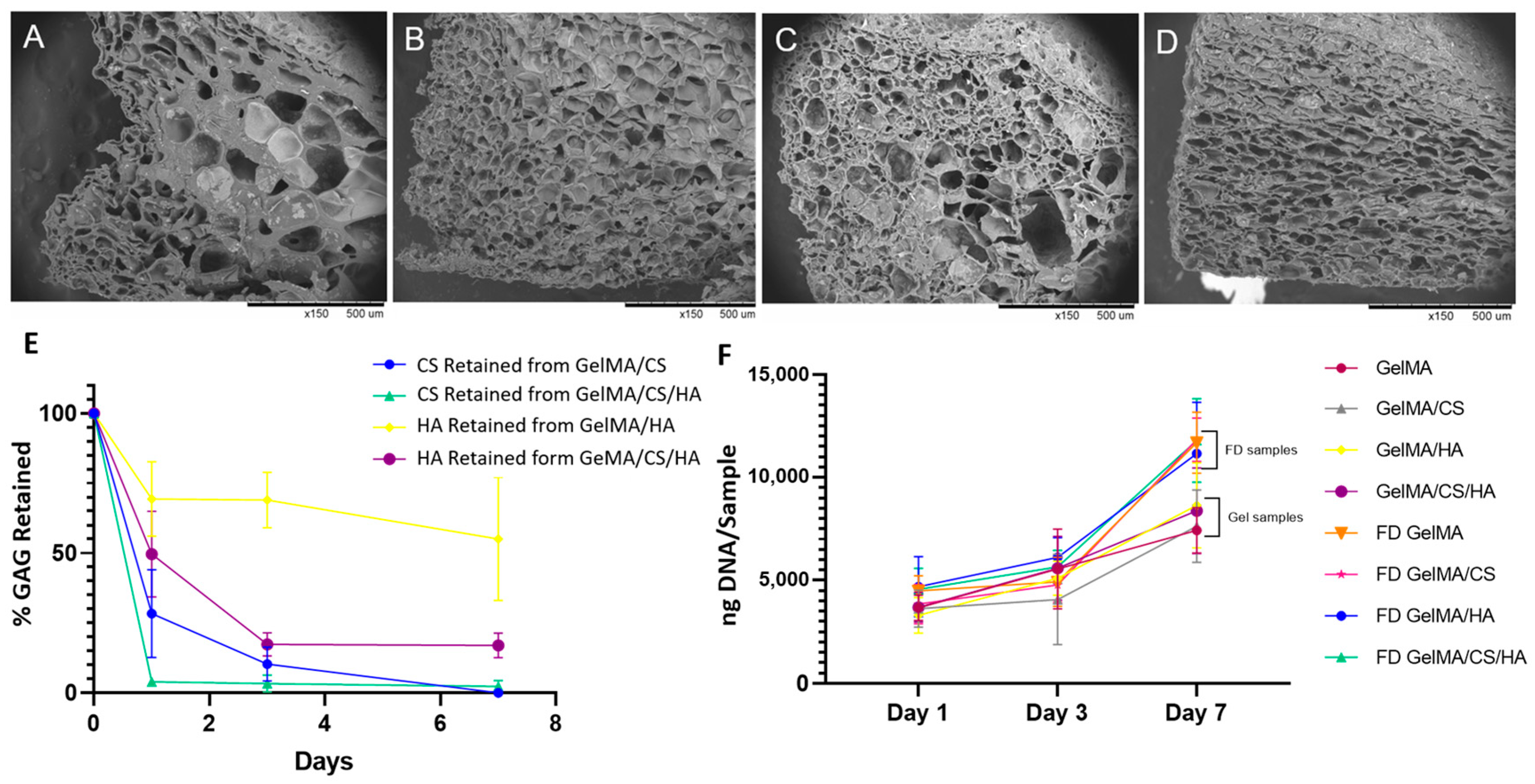
2.9. Morphological Analysis
2.10. GAG Retention Analysis
2.11. In Vitro Cell Investigation
2.11.1. Cell Expansion, Seeding and Culture
2.11.2. Cell Viability
2.11.3. Metabolic Activity
2.11.4. Cell Proliferation: DNA Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. GAG Concentration and Crosslinking Effectiveness
3.2. Mechanical Properties
3.3. Swelling Behaviour
3.4. FTIR
3.5. Rheological and Viscoelastic Properties
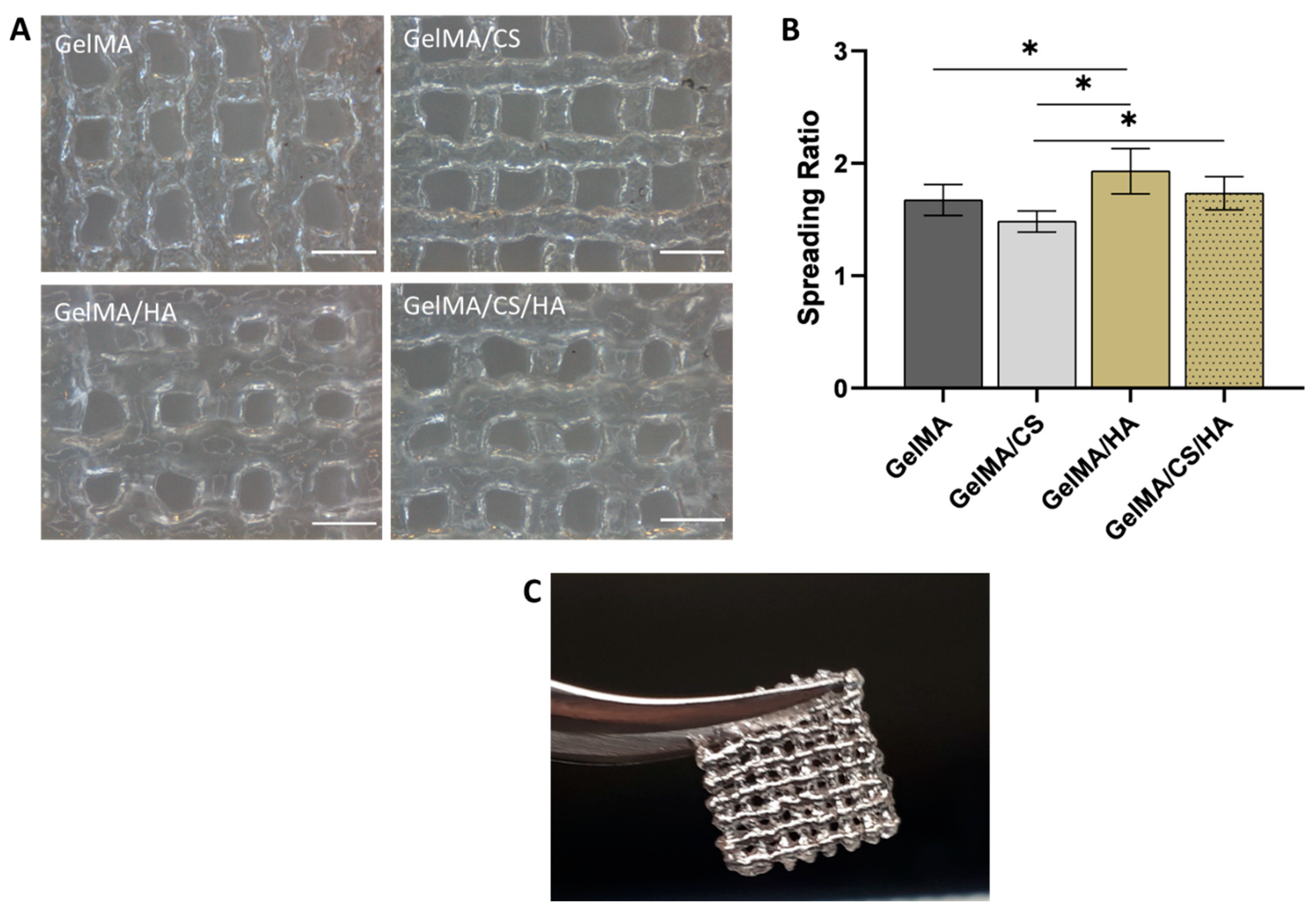
3.6. Printability
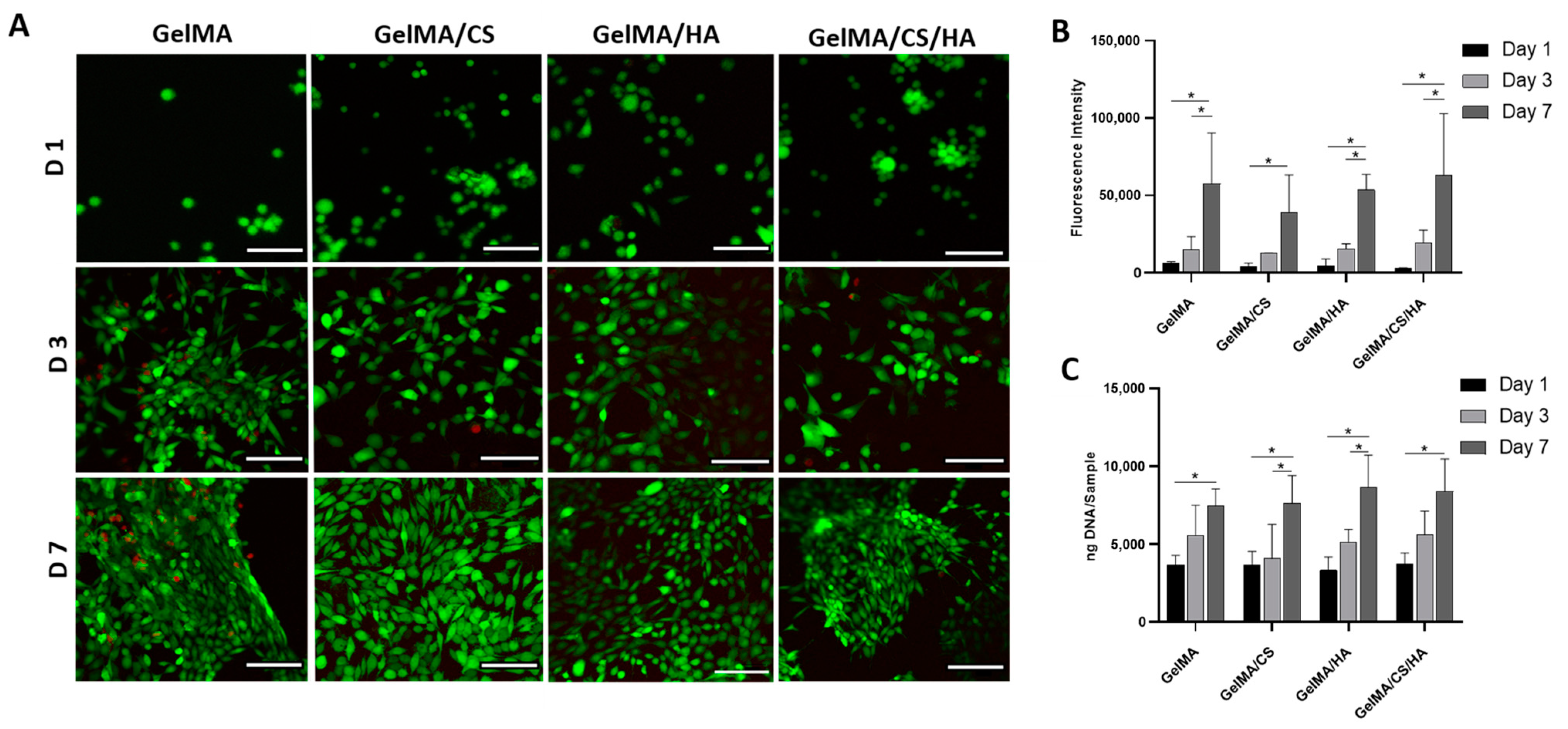
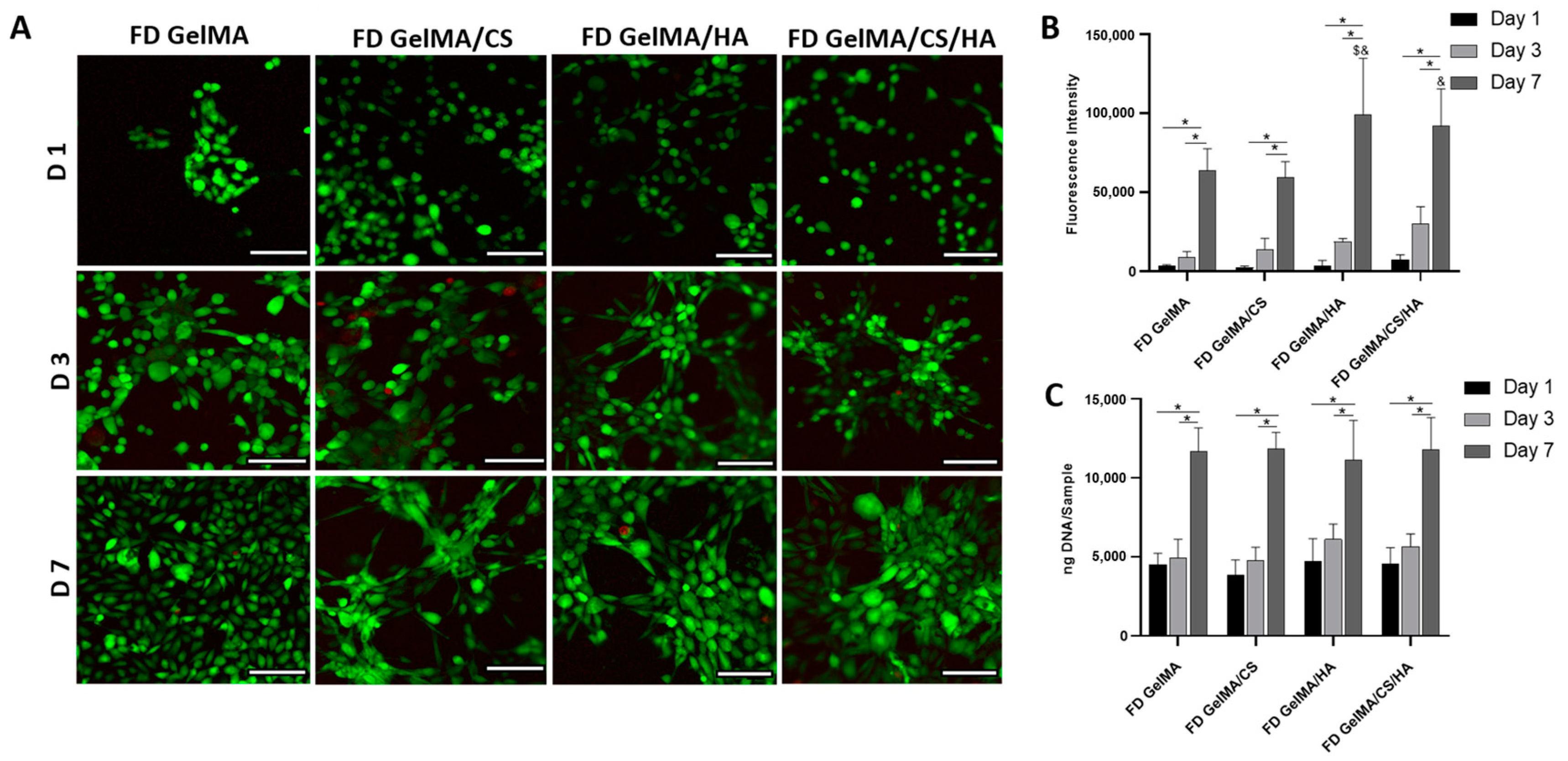
3.7. In Vitro Viability
3.8. Morphological Influence on In Vitro Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, C.A.; Garg, A.K.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. The Meniscus in Normal and Osteoarthritic Tissues: Facing the Structure Property Challenges and Current Treatment Trends. Annu. Rev. Biomed. Eng. 2019, 21, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Rey-rico, A.; Klich, A.; Cucchiarini, M.; Madry, H. Biomedical-grade, high mannuronic acid content (BioMVM) alginate enhances the proteoglycan production of primary human meniscal fibrochondrocytes in a 3-D microenvironment. Sci. Rep. 2016, 6, 28170. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Serafin, A.; Carvalho, M.R.; Culebras, M.; Cantarero, A.; Beaucamp, A.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. Electroconductive poly(3,4-ethylenedioxythiophene) (PEDOT) nanoparticle-loaded silk fibroin biocomposite conduits for peripheral nerve regeneration. Adv. Compos. Hybrid Mater. 2023, 6, 118. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Stocco, T.D.; Rodrigues, B.V.M.; Marciano, F.R.; Lobo, A.O. Design of a novel electrospinning setup for the fabrication of biomimetic scaffolds for meniscus tissue engineering applications. Mater. Lett. 2017, 196, 221–224. [Google Scholar] [CrossRef]
- Fisher, M.B.; Henning, E.A.; Söegaard, N.; Bostrom, M.; Esterhai, J.L.; Mauck, R.L. Engineering meniscus structure and function via multi-layered mesenchymal stem cell-seeded nanofibrous scaffolds. J. Biomech. 2015, 48, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Bussel, E.M.V.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Chen, M.; Feng, Z.; Guo, W.; Yang, D.; Gao, S.; Li, Y.; Shen, S.; Yuan, Z.; Huang, B.; Zhang, Y.; et al. PCL-MECM-based hydrogel hybrid scaffolds and meniscal fibrochondrocytes promote whole meniscus regeneration in a rabbit meniscectomy model. ACS Appl. Mater. Interfaces 2019, 11, 41626–41639. [Google Scholar] [CrossRef]
- Tienen, T.G.; Heijkants, R.G.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; Veth, R.P.; Buma, P. Replacement of the Knee Meniscus by a Porous Polymer Implant: A Study in Dogs. Am. J. Sports Med. 2006, 34, 64–71. [Google Scholar] [CrossRef]
- Cook, J.L.; Fox, D.B. A novel bioabsorbable conduit augments healing of avascular meniscal tears in a dog model. Am. J. Sports Med. 2007, 35, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Li, X.; Sun, M.; He, Y.; Zhao, F.; Wu, T.; Wang, J.; Ren, S.; Shi, W.; Xu, L.; et al. Meniscal transplantation and regeneration using functionalized polyurethane bionic scaffold and digital light processing 3D printing. Chem. Eng. J. 2022, 431, 133861. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M. Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater. Sci. Eng. C 2013, 33, 4777–4785. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Burdick, J.A.; Cho, D.w.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Lee, S.S.; Choi, Y.J.; Gao, G.; Wang, J.H.; Cho, D.W. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2021, 267, 120466. [Google Scholar] [CrossRef]
- Yan, R.; Chen, Y.; Gu, Y.; Tang, C.; Huang, J.; Hu, Y.; Zheng, Z.; Ran, J.; Heng, B.; Chen, X.; et al. A collagen-coated sponge silk scaffold for functional meniscus regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 56–173. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Oliveira, J.M.; Koffler, J.; Collins, M.N. 3D printable electroconductive gelatin-hyaluronic acid materials containing polypyrrole nanoparticles for electroactive tissue engineering. Adv. Compos. Hybrid Mater. 2023, 6, 109. [Google Scholar] [CrossRef]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of Gelatin Macromers to Synthesize Porous Hydrogels That Promote Valvular Interstitial Cell Function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Inci, F.; Mullick, O.; Gurkan, U.A.; Sung, Y.; Kavaz, D.; Li, B.; Denkbas, E.B.; Demirci, U. Release of Magnetic Nanoparticles from Cell-Encapsulating Biodegradable Nanobiomaterials. ACS Nano 2012, 6, 6640–6649. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds. Int. J. Biol. Macromol. 2019, 122, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Liu, H.C.; Lin, C.C.; Chou, C.H.; Lin, F.H. Gelatin-chondroitin-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Marques, C.F.; Martins, E.; Alves, A.L.; Reis, R.L.; Silva, T.H. Marine Gelatin-Methacryloyl-Based Hydrogels as Cell Templates for Cartilage Tissue Engineering. Polymers 2023, 15, 1674. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Ghalandari, B.; Khajehmohammadi, M.; Bakhtiary, N.; Tolabi, H.; Sahranavard, M.; Fathi-Karkan, S.; Nazar, V.; Hasan Niari Niar, S.; Armoon, A.; et al. Photo-cross-linkable hyaluronic acid bioinks for bone and cartilage tissue engineering applications. Int. Mater. Rev. 2023, 68, 901–942. [Google Scholar] [CrossRef]
- Ai, C.; Liu, L.; Wong, K.; Tan, X.H.; Goh, J.C.H. The effect of chondroitin sulfate concentration and matrix stiffness on chondrogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2023, 11, 4557–4573. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; An, Y.; Zhang, X.; Ding, P.; Bi, H.; Zhao, Z. Chondrocyte Spheroids Laden in GelMA/HAMA Hybrid Hydrogel for Tissue-Engineered Cartilage with Enhanced Proliferation, Better Phenotype Maintenance, and Natural Morphological Structure. Gels 2021, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Zamboni, F.; Serafin, A.; Ren, G.; Thanusha, A.V.; Culebras, M. The Role of Hyaluronic Acid in Tissue Engineering. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J.M., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1063–1116. [Google Scholar] [CrossRef]
- Herwig, J.; Egner, E.; Buddecke, E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann. Rheum. Dis. 1984, 43, 635–640. [Google Scholar] [CrossRef]
- Habuchi, H.; Yamagata, T.; Iwata, H.; Suzuki, S. The occurrence of a wide variety of dermatan sulfate chondroitin sulfate copolymers in fibrous cartilage. J. Biol. Chem. 1973, 248, 6019–6028. [Google Scholar] [CrossRef]
- Valachova, K.; Svik, K.; Biro, C.; Collins, M.N.; Jurcik, R.; Ondruska, L.; Soltes, L. Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing. Polymers 2021, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Topol’ská, D.; Mendichi, R.; Collins, M.N.; Sasinková, V.; Šoltés, L. Hydrogen peroxide generation by the Weissberger biogenic oxidative system during hyaluronan degradation. Carbohydr. Polym. 2016, 148, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Rubio, M.C.; Carsi, M.; Ortiz-Serna, P.; Sanchis, M.J.; Garg, A.K.; Oliveira, J.M.; Koffler, J.; Collins, M.N. Electroconductive PEDOT nanoparticle integrated scaffolds for spinal cord tissue repair. Biomater. Res. 2022, 26, 63. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, J.; Hall, C.L.; Turley, E.A. HA receptors: Regulators of signalling to the cytoskeleton. J. Cell. Biochem. 1990, 577, 569–577. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Cartilage regeneration using principles of tissue engineering. Clin. Orthop. Relat. Res. 2001, 391, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen–Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Waller, M.A.; Yannas, V.I.; Gibson, L.J.; Prendergast, P.J. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2007, 15, 3–17. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Pan, Z.; Duan, P.; Liu, X.; Wang, H.; Cao, L.; He, Y.; Dong, J.; Ding, J. Effect of porosities of bilayered porous scaffolds on spontaneous osteochondral repair in cartilage tissue engineering. Regen. Biomater. 2015, 2, 9–20. [Google Scholar] [CrossRef]
- Searles, J.A. Freezing and annealing phenomena in lyophilization. In Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products; CRC Press: New York, NY, USA, 2004; pp. 109–146. [Google Scholar]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid solutions—A processing method for efficient chemical modification. J. Appl. Polym. Sci. 2013, 130, 145–152. [Google Scholar] [CrossRef]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Möbius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Heal. Mater 2019, 8, e1801501. [Google Scholar] [CrossRef]
- Murphy, C.A.; Cunniffe, G.M.; Garg, A.K.; Collins, M.N. Regional dependency of bovine meniscus biomechanics on the internal structure and glycosaminoglycan content. J. Mech. Behav. Biomed. Mater. 2019, 94, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Erickson, I.E.; Huang, A.H.; Sengupta, S.; Kestle, S.; Burdick, J.A.; Mauck, R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthr. Cartil. 2009, 17, 1639–1648. [Google Scholar] [CrossRef]
- Amann, E.; Wolff, P.; Breel, E.; van Griensven, M.; Balmayor, E.R. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater 2017, 52, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Muddasar, M.; Menéndez, N.; Quero, Á.; Nasiri, M.A.; Cantarero, A.; García-Cañadas, J.; Gómez, C.M.; Collins, M.N.; Culebras, M. Highly-efficient sustainable ionic thermoelectric materials using lignin-derived hydrogels. Adv. Compos. Hybrid Mater. 2024, 7, 47. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. High performance all lignin derived supercapacitors for energy storage applications. Mater. Today Sustain. 2024, 26, 100767. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Seitz, M.A.; Galbusera, F.; Krais, C.; Ignatius, A.; Dürselen, L. Stress-relaxation response of human menisci under confined compression conditions. J. Mech. Behav. Biomed. Mater. 2013, 26, 68–80. [Google Scholar] [CrossRef]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Tissakht, M.; Ahmed, A.M. Tensile stress-strain characteristics human meniscal material. J. Biomech. 1995, 28, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Goertzen, D.J.; Budney, D.R.; Cinats, J.G. Methodology and apparatus to determine material properties of the knee joint meniscus. J. Med. Eng. Phys. 1997, 19, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lakes, E.H.; Kline, C.L.; McFetridge, P.S.; Allen, K.D. Comparing the mechanical properties of the porcine knee meniscus when hydrated in saline versus synovial fluid. J. Biomech. 2015, 48, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Gălăţeanu, B.; Albu, M.; Lungu, A.; Radu, E.; Hermenean, A.; Costache, M. Biocompatibility assessment of novel collagen-sericin scaffolds improved with hyaluronic acid and chondroitin sulfate for cartilage regeneration. BioMed Res. Int. 2013, 2013, 598056. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, Y.; Ding, C.; Li, G. Interaction study in homogeneous collagen/chondroitin sulfate blends by two-dimensional infrared spectroscopy. Carbohydr. Polym. 2012, 89, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef]
- Servaty, R.; Schiller, J.; Binder, H.; Arnold, K. Hydration of polymeric components of cartilage--an infrared spectroscopic study on hyaluronic acid and chondroitin sulfate. Int. J. Biol. Macromol. 2001, 28, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.K.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Dursun Usal, T.; Yucel, D.; Hasirci, V. A novel GelMA-pHEMA hydrogel nerve guide for the treatment of peripheral nerve damages. Int. J. Biol. Macromol. 2019, 121, 699–706. [Google Scholar] [CrossRef]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki-Modaress, M.; Mirzadeh, H.; Zandi, M.; Rajabi-Zeleti, S.; Sodeifi, N.; Aghdami, N.; Mofrad, M.R.K. Gelatin/chondroitin sulfate nanofibrous scaffolds for stimulation of wound healing: In-vitro and in-vivo study. J. Biomed. Mater. Res. A 2017, 105, 2020–2034. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Yeong, W.Y.; Win Naing, M. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2016, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Ren, G.; Culebras, M.; O’Driscoll, J.; O’Dwyer, J.; Ryan, E.J.; Collins, M.N. Curcumin encapsulated polylactic acid nanoparticles embedded in alginate/gelatin bioinks for in situ immunoregulation: Characterization and biological assessment. Int. J. Biol. Macromol. 2022, 221, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M. How can genipin assist gelatin/carbohydrate chitosan scaffolds to act as replacements of load-bearing soft tissues? Carbohydr. Polym. 2013, 93, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Wong, C.K.; Collins, M.N. Hyaluronic acid association with bacterial, fungal and viral infections: Can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact. Mater. 2023, 19, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Allemann, F.; Eid, K.; Yates, K.E.; Zaleske, D.; Glowacki, J.; Mizuno, S. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J. Biomed. Mater. Res. 2001, 55, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS ONE 2014, 9, e113216. [Google Scholar] [CrossRef] [PubMed]



| Name | GelMA | CS | HA |
|---|---|---|---|
| GelMA | 10% | ||
| GelMA/CS | 10% | 1% | |
| GelMA/HA | 10% | 0.5% | |
| GelMA/CS/HA | 10% | 1% | 0.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, C.A.; Serafin, A.; Collins, M.N. Development of 3D Printable Gelatin Methacryloyl/Chondroitin Sulfate/Hyaluronic Acid Hydrogels as Implantable Scaffolds. Polymers 2024, 16, 1958. https://doi.org/10.3390/polym16141958
Murphy CA, Serafin A, Collins MN. Development of 3D Printable Gelatin Methacryloyl/Chondroitin Sulfate/Hyaluronic Acid Hydrogels as Implantable Scaffolds. Polymers. 2024; 16(14):1958. https://doi.org/10.3390/polym16141958
Chicago/Turabian StyleMurphy, Caroline A., Aleksandra Serafin, and Maurice N. Collins. 2024. "Development of 3D Printable Gelatin Methacryloyl/Chondroitin Sulfate/Hyaluronic Acid Hydrogels as Implantable Scaffolds" Polymers 16, no. 14: 1958. https://doi.org/10.3390/polym16141958
APA StyleMurphy, C. A., Serafin, A., & Collins, M. N. (2024). Development of 3D Printable Gelatin Methacryloyl/Chondroitin Sulfate/Hyaluronic Acid Hydrogels as Implantable Scaffolds. Polymers, 16(14), 1958. https://doi.org/10.3390/polym16141958









