Transitioning to Microplastic-Free Seed Coatings: Challenges and Solutions
Abstract
:1. Introduction
2. State of the Art on Current Initiatives and Regulations against Microplastics
2.1. Actions to Restrict the Discharge of Plastic Waste
2.1.1. Restrict the Use and Production of Plastic Objects
2.1.2. Restrict the Discharge into the Environment
- Using recyclable plastic materials (such as PET, PE, or PP), simplifying the polymer blends used, and reducing the use of materials that are difficult to recycle, from the design stage of plastic products onwards.
- Raising public awareness of the importance of recycling, the associated benefits, and good sorting practices.
- Setting up selective waste collection infrastructures, and improving the efficiency of existing infrastructures.
- Expanding the range of recycled plastics as much as possible.
- Investing in the research and development of new recycling technologies.
2.2. Actions to Limit the Release of Manufactured Microplastics
2.2.1. Plastic Microbeads
2.2.2. Manufactured Microplastics
3. Definitions of Microplastics (MPs) and Their Differences
3.1. Comparison of Definitions: REACH versus Literature for Each Criterion
- Either contained in particles and constituting at least 1% by weight of said particles, or building a continuous surface coating on said particles.
- At least 1% by weight of the particles described in 1. must have (i) all dimensions between 5 mm and 0.1 µm; (ii) in the case of fibers, a length less than or equal to 15 mm, any dimension greater than or equal to 0.3 µm, and a length/diameter ratio > 3.
- Polymers which are the result of a polymerization mechanism that already occurs in nature, and which are not chemically modified substances (within the meaning of the REACH regulation).
- Polymers that are biodegradable as proven by certain criteria detailed further.
- Polymers with a water solubility greater than 2 g/L as proven by certain methods detailed further.
- Polymers containing no carbon atoms in their chemical structure.
3.1.1. Size
3.1.2. Physical State
3.1.3. Chemical Structure
- Be composed of more than 50% polymer molecules (i.e., molecules containing at least three monomer units covalently bonded to at least one other monomer unit or reagent).
- The mass percentage of molecules with the same molecular weight must not exceed 50%.
3.1.4. Origin
3.1.5. Persistence
- Solubility in Water
- 2.
- Biodegradability
- Ultimate: complete degradation of the compound into fully oxidized or reduced single molecules (such as CO2, CH4, NH3, and H2O)
- Primary (or biotransformation): modification of the chemical structure of a substance, resulting in the loss of a specific property.
- Ready: the product has passed certain selection tests specified for final biodegradability. These tests are so stringent that it is assumed that the compound will biodegrade rapidly and completely in an aerobic aquatic environment (in the presence of O2)
- Intrinsic: there is unequivocal evidence of biodegradation (primary or total) of the substance in any biodegradability test.
- According to its half-life (t0.5): the time required for 50% of the test substance to be transformed, when this transformation can be described according to a first-order kinetic law. This time is independent of the initial concentration.
- According to its disappearance time (DT50), i.e., the time required for its initial concentration to be divided by 2.
3.1.6. Conclusions
- Persistent in the environment;
- Small in size;
- Able to transport toxic elements;
- Rich in additives.
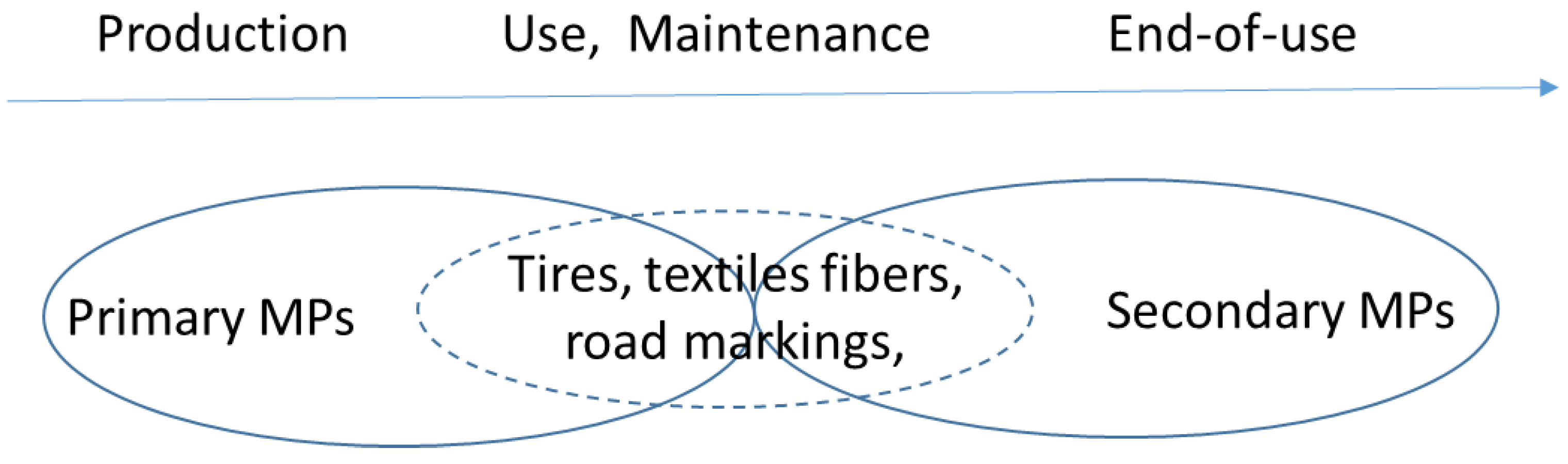
3.2. Types of Microplastics
3.2.1. Primary Microplastics
- (i)
- They can be generated during the production phase of a manufactured product (e.g., intentionally added microplastics, such as polymer capsules used to encapsulate certain active ingredients, or polymer dispersions used as film-forming agents).
- (ii)
- They can also be generated during the product’s use phase. The latter category includes, for example, tire particles resulting from road abrasion, or laundry water containing textile fibers.
3.2.2. Secondary Microplastics
- (i)
- During use and maintenance (tires, textiles) and/or end of life (degradation and fragmentation of plastic waste in the environment).
- (ii)
- Only at the end-of-life stage.
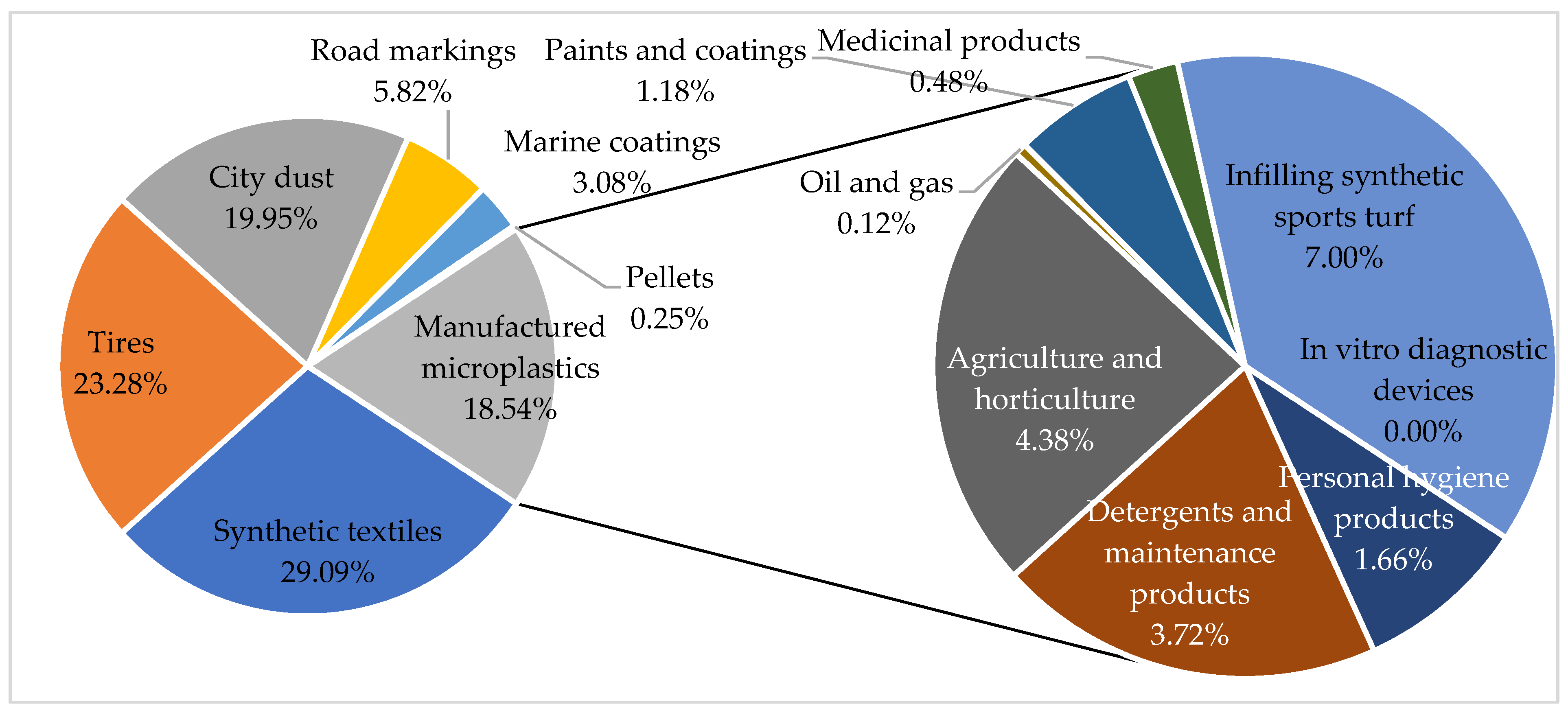
3.3. Most Common Microplastics
4. Seeds
4.1. Seed Production Processes
4.1.1. General Production Diagram
- (i)
- Physiological preconditioning: This improves germination speed and capacity, resulting in faster, more uniform field emergence and a more uniform level of final establishment, particularly under unfavorable germination and growth conditions. A variety of techniques exist, as listed by Khan [157], which can be divided into two categories: those based on seed hydration (pre-soaking, wetting and drying, humidification, osmotic treatment, conditioning on a matrix, pre-germination), and those based on chemical or physical stimulation in addition to hydration (cold stratification, thermal shock, irradiation, oxygenation, hormonal treatment, salt treatment [158]).
- (ii)
- Seed treatment: Crop protection products are applied to seeds for several purposes, including the control of seed-borne diseases and the protection of seeds and young plants from early attacks (diseases, parasites, pests) [159]. Biostimulants, fertilizers, and/or microorganisms can also be applied in addition to the treatment.
- (iii)
- The addition of seed-coating agents to improve the distribution of the treatment on the seed, its adhesion, and any morphological changes.
4.1.2. Seed Treatment
- (i)
- Seed disinfection: This is a treatment against a pathogen that has infected the seed and is established in the seed coat or deeper parts. The seed is already infected by the pathogen.
- (ii)
- Seed disinfestation: When the surface of the seed is contaminated with spores or other forms of pathogens, without being penetrated or infected, the seed is said to be infested with the pathogen. Chemical and fungicide dips, applied in powder or spray form, are effective disinfectants. Copper sulfate is a particularly effective disinfestant.
- (iii)
- Seed protection: The seed is protected by coating it with crop protection products (fungicides, insecticides, bactericides) to prevent infection and damage by soil organisms, which it is particularly prone to during the early stages of its growth.
4.1.3. Seed Coating
- (i)
- (ii)
- (iii)
- Finally, it gives the seed a distinctive color, which enables it to be (a) differentiated from untreated seed, (b) easily identified, and (c) attractively presented. This also facilitates their visibility in the soil to check sowing quality [172].
4.2. Seed Treatment Formulation
5. Seed Film-Coating Agents and Associated Functionalities
5.1. Functionalities of Seed Film-Coating Agents
5.1.1. Adhesion to Seed
5.1.2. Reducing Dust-Off
5.1.3. Improving Flowability
5.1.4. Fast Drying
5.1.5. Improving Coverage
5.1.6. Ensuring Neutral or Good Effect on Seed Physiological Quality
Germination
- (i)
- Water availability and uptake rate
- (ii)
- Presence of certain germination-inhibiting or germination-promoting molecules
- (iii)
- Presence of growth regulators
- (iv)
- Influence on gas exchange
Vigor
- (i)
- Rate and uniformity of seed germination and seedling growth
- (ii)
- Seed emergence under adverse environmental conditions
- (iii)
- Performance after storage, particularly maintenance of germination capacity.” [175]
5.1.7. Effective Active Molecule Release and Resistance to Leaching
5.2. Seed Film-Coating Formulation
6. Seed Film-Coating Agent Substitution
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Regional Activity Centre for Sustainable Consumption and Production (SPC/RAC); Stockholm Convention Regional Centre in Spain (CSRC-Spain). Plastic’s Toxic Additives and the Circular Economy; International Pollutants Elimination Network (IPEN): Stockholm, Sweden, 2020; p. 55. [Google Scholar]
- Lackner, M.; Mukherjee, A.; Koller, M. What Are “Bioplastics”? Defining Renewability, Biosynthesis, Biodegradability, and Biocompatibility. Polymers 2023, 15, 4695. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe AIBSL. EPRO Plastics—The Facts 2022; Plastics Europe: Brussels, Belgique, 2022; p. 81. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of Plastic Polymer Types in the Marine Environment; A Meta-Analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature International Union for Conservation of Nature (IUCN)-Issues Brief-Plastic Pollution. Available online: https://www.iucn.org/resources/issues-brief/plastic-pollution (accessed on 24 July 2023).
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The Polymers and Their Additives in Particulate Plastics: What Makes Them Hazardous to the Fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef] [PubMed]
- Bhusare, B.P.; Zambare, V.P.; Jaweed, T.H.; Shahnawaz, M. Ecotoxicological Impact of Plastic Waste on Marine Flora. In Impact of Plastic Waste on the Marine Biota; Shahnawaz, M., Sangale, M.K., Daochen, Z., Ade, A.B., Eds.; Springer Nature: Singapore, 2022; pp. 257–286. ISBN 9789811654022. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Ryan, P.G. Plastic and Other Artefacts on South African Beaches: Temporal Trends in Abundance and Composition. South Afr. J. Sci. 1990, 86, 450–452. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Bermúdez, J.R.; Swarzenski, P.W. A Microplastic Size Classification Scheme Aligned with Universal Plankton Survey Methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Thomposon, R.C. Microplastics in the Ocean. Arch. Env. Contam. Toxicol. 2015, 69, 265–268. [Google Scholar] [CrossRef]
- Mennekes, D.; Nowack, B. Predicting Microplastic Masses in River Networks with High Spatial Resolution at Country Level. Nat. Water 2023, 1, 523–533. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous Microplastic Characteristics and Its Role as a Vector of Heavy Metal in Groundwater and Surface Water of Coastal South India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and Microplastics from Beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the Soil Environment: A Critical Review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and Wonderful? Microplastics Prevail in Snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed]
- Villanova-Solano, C.; Hernández-Sánchez, C.; Díaz-Peña, F.J.; González-Sálamo, J.; González-Pleiter, M.; Hernández-Borges, J. Microplastics in Snow of a High Mountain National Park: El Teide, Tenerife (Canary Islands, Spain). Sci. Total Environ. 2023, 873, 162276. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful Effects of the Microplastic Pollution on Animal Health: A Literature Review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef] [PubMed]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Tang, C. A Review of Possible Pathways of Marine Microplastics Transport in the Ocean. Anthr. Coasts 2020, 3, 6–13. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of Sample Preparation Methods for Microplastic Analysis in Wastewater Matrices—Reproducibility and Standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A Standardized Method for Sampling and Extraction Methods for Quantifying Microplastics in Beach Sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Naji, A.; Al-Jandal, N. Standardized Protocols for Microplastics Determinations in Environmental Samples from the Gulf and Marginal Seas. Mar. Pollut. Bull. 2020, 158, 111374. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A Growing Plastic Smog, Now Estimated to Be over 170 Trillion Plastic Particles Afloat in the World’s Oceans—Urgent Solutions Required. PLoS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Azuma, T.; Cordova, M.R.; Cózar, A.; Galgani, F.; Hagita, R.; Kanhai, L.D.; Imai, K.; Iwasaki, S.; Kako, S.; et al. A Multilevel Dataset of Microplastic Abundance in the World’s Upper Ocean and the Laurentian Great Lakes. Micropl. Nanopl. 2021, 1, 16. [Google Scholar] [CrossRef]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of Plastics: Entry into the Environment and Potential Risks to Human and Ecological Health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic Occurrence in a Soil Amended with Plastic Debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef]
- Moon, S.; Martin, L.M.A.; Kim, S.; Zhang, Q.; Zhang, R.; Xu, W.; Luo, T. Direct Observation and Identification of Nanoplastics in Ocean Water. Sci. Adv. 2024, 10, eadh1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, H.; Liu, Y.; Jin, L.; Peng, R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics 2023, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Niu, S.; Yu, J. Microplastics as Vectors of Organic Pollutants in Aquatic Environment: A Review on Mechanisms, Numerical Models, and Influencing Factors. Sci. Total Environ. 2023, 887, 164008. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Zero Waste Hierarchy of Highest and Best Use 8.0—Zero Waste International Alliance. Available online: https://zwia.org/zwh/ (accessed on 4 February 2024).
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017. [Google Scholar]
- Shawon, I.H.; Haider, M.Z.; Oni, F.A. Effectiveness of Banning Plastic Bag in Bangladesh for Environmental Protection. Khulna Univ. Stud. 2023, 112–118. [Google Scholar] [CrossRef]
- New South Wales-Plastic Reduction and Circular Economy Bill 2021. Available online: https://legislation.nsw.gov.au/view/pdf/bill/efc59978-8583-47c7-aa00-f46cb67e1adc (accessed on 7 June 2024).
- Buchholz, K. The Countries Banning Plastic Bags. Available online: https://www.statista.com/chart/14120/the-countries-banning-plastic-bags/ (accessed on 31 January 2024).
- Directive (UE) 2015/720 du Parlement Européen et du Conseil du 29 avril 2015—Modifiant la directive 94/62/CE en ce qui concerne la réduction de la consommation de sacs en plastique légers. J. Off. L’union Eur. 2015, 115, 11–15.
- Legal Limits on Single-Use Plastics and Microplastics: A Global Review of National Laws and Regulations; United Nations Environment Progeamme: Nairobi, Kenya, 2018; p. 118.
- Green Procurement Guidelines of the Free and Hanseatic City of Hamburg (Green Procurement Guidelines). Available online: https://www.hamburg.de/resource/blob/170608/684ede9b4cf2953fd68d3c94c8a945d3/d-umweltleitfaden-kurz-englisch-data.pdf (accessed on 1 February 2024).
- Chapter 20:27 Environmental Management (Plastic Packaging & Plastic Bottles) Regulations. Available online: https://swm-programme.info/c/document_library/get_file?uuid=37f37bdb-4437-dce0-74da-15fd0bdb47aa&groupId=20142 (accessed on 1 February 2024).
- Journal Officiel de La République de Vanuatu-No 10-2 Fév 2018. Available online: https://environment.gov.vu/images/Environmental.Protection/Official-Gazette-No.-10-of-2018-dated-2-February-2018.pdf (accessed on 1 February 2024).
- Lutte Pour le Réemploi et Contre le Gaspillage (Articles L541-15-3 à L541-15-17)–Légifrance. Available online: https://www.legifrance.gouv.fr/codes/section_lc/LEGITEXT000006074220/LEGISCTA000032043245/ (accessed on 1 February 2024).
- JORF n° 0142 du 21 juin 2023. Available online: https://www.legifrance.gouv.fr/jorf/jo/2023/06/21/0142 (accessed on 1 February 2024).
- Parlement Européen, Conseil Européen, Directive (UE) 2019/du Parlement européen et du Conseil du 5 juin 2019 relative à la réduction de l’incidence de certains produits en plastique sur l’environnement. J. Off. L’union Eur. 2019, 155, 1–19.
- UNEA Resolution 5/14 Entitled “End Plastic Pollution: Towards an International Legally Binding Instrument”; United Nations Environment Programme: Dakar, Senegal, 2022; p. 5.
- Plastic Pollution is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 1 February 2024).
- Veiga, J.; Wintersteller, A.; Murray, C. ETC/ICM Report 5/2022: Marine Litter in Europe—An Integrated Assessment from Source to Sea; EEA: Copenhagen, Denmark, 2023. [Google Scholar]
- European Chemical Agency (ECHA). Background Document to RAC and SEAC Opinions on the Annex XV Report Proposing Restrictions on Intentionally Added Microplastics; ECHA: Helsinki, Finland, 2020. [Google Scholar]
- Our Impact on the Cosmetics Industry. Available online: https://www.beatthemicrobead.org/impact/global-impact/ (accessed on 2 February 2024).
- 5 Gyres and the Ban on Microbeads. Available online: https://scaquarium.org/microbeads/ (accessed on 2 February 2024).
- H.R.1321—Microbead-Free Waters Act of 2015–Congress.gouv. Available online: https://www.congress.gov/bill/114th-congress/house-bill/1321#:~:text=is%20repeated%20here.)-,Microbead-Free%20Waters%20Act%20of%202015,beginning%20on%20July%201%2C%202017 (accessed on 2 February 2024).
- McDevitt, J.P.; Criddle, C.S.; Morse, M.; Hale, R.C.; Bott, C.B.; Rochman, C.M. Addressing the Issue of Microplastics in the Wake of the Microbead-Free Waters Act—A New Standard Can Facilitate Improved Policy. Environ. Sci. Technol. 2017, 51, 6611–6617. [Google Scholar] [CrossRef] [PubMed]
- Gouvernement du Canada, T. Publics et S. Gouvernementaux C. Gazette du Canada—Règlement sur les Microbilles dans les Produits de Toilette. Available online: https://www.gazette.gc.ca/rp-pr/p2/2017/2017-06-14/html/sor-dors111-fra.html (accessed on 2 February 2024).
- Agency, E.P. Microbeads. Available online: https://www.epa.ie/our-services/compliance--enforcement/microbeads/#d.en.118969 (accessed on 2 February 2024).
- Accord Sectoriel Pour Moins de Microplastiques. Available online: https://www.health.belgium.be/fr/news/accord-sectoriel-pour-moins-de-microplastiques (accessed on 2 February 2024).
- Plastic Soup Foundation—Annual Report 2019; Plastic Soup Foundation: Amsterdam, The Netherlands, 2019; p. 43.
- Watkins, E. Policy Approaches to Incentivise Sustainable Plastic Design; OECD Environment Working Papers; OECD: Paris, France, 2019; Volume 149. [Google Scholar]
- Parlement Européen. Conseil Européen, Règlement (UE) 2023/2055 de la Commission du 25 septembre 2023 modifiant l’annexe XVII du règlement (CE) no 1907/2006 du Parlement européen et du Conseil concernant l’enregistrement, l’évaluation et l’autorisation des substances chimiques, ainsi que les restrictions applicables à ces substances (REACH), en ce qui concerne les microparticules de polymère synthétique. J. Off. L’union Eur. 2023, 238, 67–87. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An Emerging Contaminant of Potential Concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Verschoor, A.J. Towards a Definition of Microplastics; Dutch National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2015; p. 42. [Google Scholar]
- Yuan, Z.; Nag, R.; Cummins, E. Human Health Concerns Regarding Microplastics in the Aquatic Environment—From Marine to Food Systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris; University of Washington Tacoma: Tacoma, WA, USA, 2008; p. 49. [Google Scholar]
- Chae, B.; Oh, S.; Lee, D.G. Is 5 Mm Still a Good Upper Size Boundary for Microplastics in Aquatic Environments? Perspectives on Size Distribution and Toxicological Effects. Mar. Pollut. Bull. 2023, 196, 115591. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 21960:2020(E); Plastics—Environmental Aspects—State of Knowledge and Methodologies. International Organization for Standardization: Geneva, Switzerland, 2020.
- Xu, J.; Wang, Z. Efficient and Accurate Microplastics Identification and Segmentation in Urban Waters Using Convolutional Neural Networks. Sci. Total Environ. 2024, 911, 168696. [Google Scholar] [CrossRef]
- Izar, G.M.; Tan, T.Y.; Laurino, I.R.A.; Nobre, C.R.; Vivas, M.P.M.; Gusso-Choueri, P.K.; Felix, C.S.A.; Moreno, B.B.; Abessa, D.M.S.; De Andrade, J.B.; et al. Plastic Pellets Make Excirolana Armata More Aggressive: Intraspecific Interactions and Isopod Mortality Differences between Populations. Sci. Total Environ. 2024, 911, 168611. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Liu, S.; Wei, H.; Karthikeyan, K.G. The Glowing Potential of Nile Red for Microplastics Identification: Science and Mechanism of Fluorescence Staining. Microchem. J. 2024, 197, 109708. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The Potential Toxicity of Microplastics on Human Health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Gkoulemani, N.; Hui, J.; Irvine, J.T.S.; Lawton, L.A. Aging Microplastics Enhances the Adsorption of Pharmaceuticals in Freshwater. Sci. Total Environ. 2024, 912, 169467. [Google Scholar] [CrossRef]
- Wegner, A.; Besseling, E.; Foekema, E.M.; Kamermans, P.; Koelmans, A.A. Effects of Nanopolystyrene on the Feeding Behavior of the Blue Mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Commission Européenne. Recommandation de la Commission du 18 octobre 2011 relative à la définition des nanomatériaux (Texte présentant de l’intérêt pour l’EEE. J. Off. L’union Eur. 2011, 275, 38–40. [Google Scholar]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhou, W.; Hu, J.; Wu, C.; Niu, J.; Naidu, R. Paint Has the Potential to Release Microplastics, Nanoplastics, Inorganic Nanoparticles, and Hybrid Materials. Environ. Sci. Eur. 2024, 36, 17. [Google Scholar] [CrossRef]
- Hanrahan, J.; Steeves, K.L.; Locke, D.P.; O’Brien, T.M.; Maekawa, A.S.; Amiri, R.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Simpson, A.J.; et al. Maternal Exposure to Polyethylene Micro- and Nanoplastics Impairs Umbilical Blood Flow but Not Fetal Growth in Pregnant Mice. Sci. Rep. 2024, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, D.; Edo, C.; Fernández-Piñas, F.; Rosal, R.; Aguado, S. Efficient Removal of Nanoplastics from Water Using Mesoporous Metal Organic Frameworks. Sep. Purif. Technol. 2024, 333, 125816. [Google Scholar] [CrossRef]
- Pitt, J.A.; Hahn, M.E.; Aluru, N. Implications of Exposure Route for the Bioaccumulation Potential of Nanopolystyrene Particles. Chemosphere 2024, 351, 141133. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, F.; Huo, C.; Wan, J.; Song, H.; Du, F.; Liu, R. Molecular Mechanisms of Polystyrene Nanoplastics and Alpha-Amylase Interactions and Their Binding Model: A Multidimensional Analysis. Sci. Total Environ. 2024, 915, 170036. [Google Scholar] [CrossRef] [PubMed]
- Prasittisopin, L.; Ferdous, W.; Kamchoom, V. Microplastics in Construction and Built Environment. Dev. Built Environ. 2023, 15, 100188. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic Fibers—Underestimated Threat to Aquatic Organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; An, L.; Wang, M.; Yang, Q.; Zhu, B.; Ding, J.; Ye, C.; Xu, Y. Microfiber Pollution in the Earth System. Rev. Environ. Contam. Toxicol. 2022, 260, 13. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in Oceanic Surface Waters: A Global Characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef]
- Rozman, U.; Kalčíková, G. The First Comprehensive Study Evaluating the Ecotoxicity and Biodegradability of Water-Soluble Polymers Used in Personal Care Products and Cosmetics. Ecotoxicol. Environ. Saf. 2021, 228, 113016. [Google Scholar] [CrossRef]
- ISO 9408:1999; Water Quality—Evaluation of Ultimate Aerobic Biodegradability of Organic Compounds in Aqueous Medium by Determination of Oxygen Demand in a Closed Respirometer. ISO: Geneva, Switzerland, 1999.
- Suka, M.; Kido, T.; Yoshioka, W.; Hachisuka, E.; Okoshi, H.; Yamauchi, T.; Hano, H.; Okano, T.; Yokoyama, M.; Yanagisawa, H. Single Intratracheal Administration of Cross-Linked Water-Soluble Acrylic Acid Polymer Causes Acute Alveolo-Interstitial Inflammation and the Subsequent Fibrotic Formation Possibly via the TGF-Β1 Pathway in the Lung of Rats. Toxicology 2021, 448, 152647. [Google Scholar] [CrossRef]
- Buczek, S.B.; Cope, W.G.; McLaughlin, R.A.; Kwak, T.J. Acute Toxicity of Polyacrylamide Flocculants to Early Life Stages of Freshwater Mussels. Environ. Toxicol. Chem. 2017, 36, 2715–2721. [Google Scholar] [CrossRef]
- Duggan, K.L.; Morris, M.; Bhatia, S.K.; Khachan, M.M.; Lewis, K.E. Effects of Cationic Polyacrylamide and Cationic Starch on Aquatic Life. J. Hazard. Toxic Radioact. Waste 2019, 23, 04019022. [Google Scholar] [CrossRef] [PubMed]
- Foundation, P.S. Battle on Liquid Microplastics in Cosmetics. Available online: https://www.plasticsoupfoundation.org/en/2020/03/battle-on-the-so-called-liquid-microplastics-in-cosmetics/ (accessed on 6 February 2024).
- Vidović, N.; Antić, V.; Schwarzbauer, J. Simultaneous Identification and Quantification of Three Water-Soluble Polymers (PVP, PNVCL and PEI) in Wastewater Samples by Continuous-Flow off-Line Pyrolysis GC/MS. Sci. Total Environ. 2024, 916, 170320. [Google Scholar] [CrossRef]
- Tarring, E.C.; Durance, I.; Harbottle, M.J.; Lucas, R.; Read, D.S.; Ward, B.D. Water-Soluble Polymers: Emerging Contaminants Detected, Separated and Quantified by a Novel GPC/MALDI-TOF Method. Environ. Pollut. 2024, 340, 122888. [Google Scholar] [CrossRef] [PubMed]
- ASTM D4359-90; Standard Test Method for Determining Whether a Material Is a Liquid or a Solid. ASTM International: West Conshohocken, PA, USA, 2019.
- United Nations Economic Commission for Europe, 2.3.4. Test for determining fluidity. In European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR): Applicable as from 1 January 2019, 1st ed.; United Nations: New York, NY, USA, 2018; p. 266.
- Breaking the Plastic Wave: A Comprehensive Assessment of Pathways Towards Stopping Ocean Plastic Pollution; The Pew Charitable Trusts: Philadelphia, PA, USA; SYSTEMIQ: Amsterdam, The Netherlands, 2020.
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Järlskog, I.; Jaramillo-Vogel, D.; Rausch, J.; Gustafsson, M.; Strömvall, A.-M.; Andersson-Sköld, Y. Concentrations of Tire Wear Microplastics and Other Traffic-Derived Non-Exhaust Particles in the Road Environment. Environ. Int. 2022, 170, 107618. [Google Scholar] [CrossRef] [PubMed]
- Foscari, A.; Schmidt, N.; Seiwert, B.; Herzke, D.; Sempéré, R.; Reemtsma, T. Leaching of Chemicals and DOC from Tire Particles under Simulated Marine Conditions. Front. Environ. Sci. 2023, 11, 1206449. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, T.; He, M.; Shah, K.J.; You, Z.; Zhang, T.; Zubair, M. Exploring the Toxicity of the Aged Styrene-Butadiene Rubber Microplastics to Petroleum Hydrocarbon-Degrading Bacteria under Compound Pollution System. Ecotoxicol. Environ. Saf. 2021, 227, 112903. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, X.; Tang, H.; Lu, K.; Li, H.; Liu, S.; Xing, B.; Ji, R. Steam Disinfection Releases Micro(Nano)Plastics from Silicone-Rubber Baby Teats as Examined by Optical Photothermal Infrared Microspectroscopy. Nat. Nanotechnol. 2022, 17, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Quilis, S.A.; Hernández-Martínez, A.M.; Arribas, A.J.M.; Pérez, J.G.; García-March, J.R.; Medialdea, J.T. High Prevalence of Microplastics in the Digestive Tract of Scyliorhinus Canicula (Linneaus, 1758) Shows the Species Biomonitoring Potential. Mar. Pollut. Bull. 2024, 200, 116051. [Google Scholar] [CrossRef]
- Liu, S.; You, H.; Mu, H.; Cheng, J.; Kuang, S.; Wang, F.; Chen, H.; Zheng, M.; Xu, Y.; Liu, T. Abundance, Characteristics and Risk Assessment of Microplastics in Aquatic Sediments: A Comparative Study in the Yellow River and Yellow Sea. Waste Manag. 2023, 172, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Luo, Y.; Naidu, R. Raman Imaging for the Analysis of Silicone Microplastics and Nanoplastics Released from a Kitchen Sealant. Front Chem 2023, 11, 1165523. [Google Scholar] [CrossRef] [PubMed]
- Ekvall, M.T.; Gimskog, I.; Kelpsiene, E.; Mellring, A.; Månsson, A.; Lundqvist, M.; Cedervall, T. Nanoplastics Released from Daily Used Silicone and Latex Products during Mechanical Breakdown. PLoS ONE 2023, 18, e0289377. [Google Scholar] [CrossRef]
- van Kleunen, M.; Brumer, A.; Gutbrod, L.; Zhang, Z. A Microplastic Used as Infill Material in Artificial Sport Turfs Reduces Plant Growth. Plants People Planet 2020, 2, 157–166. [Google Scholar] [CrossRef]
- European Chemicals Agency. Guidance for Monomers and Polymers: February 2023: Version 3.0.; Publications Office: Luxembourg, 2023. [Google Scholar]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An Emerging Role of Microplastics in the Etiology of Lung Ground Glass Nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are We Underestimating Anthropogenic Microfiber Pollution? A Critical Review of Occurrence, Methods, and Reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Robison-Smith, C.; Masud, N.; Tarring, E.C.; Ward, B.D.; Cable, J. A Class of Their Own? Water-Soluble Polymer Pollution Impacting a Freshwater Host-Pathogen System. Sci. Total Environ. 2024, 907, 168086. [Google Scholar] [CrossRef]
- Mondellini, S.; Schott, M.; Löder, M.G.J.; Agarwal, S.; Greiner, A.; Laforsch, C. Beyond Microplastics: Water Soluble Synthetic Polymers Exert Sublethal Adverse Effects in the Freshwater Cladoceran Daphnia Magna. Sci. Total Environ. 2022, 847, 157608. [Google Scholar] [CrossRef]
- Huppertsberg, S.; Zahn, D.; Pauelsen, F.; Reemtsma, T.; Knepper, T.P. Making Waves: Water-Soluble Polymers in the Aquatic Environment: An Overlooked Class of Synthetic Polymers? Water Res. 2020, 181, 115931. [Google Scholar] [CrossRef]
- ISO 14852:2021; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- ISO 14851:2019; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2019.
- Lagnet, C.; Monlau, F.; Jacquet-Lassus, C.; Lallement, A.; Cazaudehore, G.; Cesar, G.; Gastaldi, E.; Touchaleaume, F.; Copin, D.; Deroine, M.; et al. Revue des Normes sur la Biodégradabilité des Plastiques; ADEME: Angers, France, 2020; p. 111. [Google Scholar]
- NF U52-001; Biodegradable Materials for Use in Agriculture and Horticulture-Mulching Products-Requirements and Test Methods. AFNOR: La Plaine Saint-Denis, France, 2005.
- NF EN 13432; Packaging-Requirements for Packaging Recoverable through Compositing and Biodegradation-Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. AFNOR: La Plaine Saint-Denis, France, 2000.
- OCDE. Test No. 301: Ready Biodegradability, OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 1992. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, J.; Fan, L.; Dong, W.; Jiang, M. Simulated Gastrointestinal Digestion of Two Different Sources of Biodegradable Microplastics and the Influence on Gut Microbiota. Food Chem. Toxicol. 2024, 185, 114474. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Jiang, X.; Xu, J.; Liu, J. Source, Environmental Behavior and Ecological Impact of Biodegradable Microplastics in Soil Ecosystems: A Review. Rev. Environ. Contam. 2024, 262, 6. [Google Scholar] [CrossRef]
- Tao, S.; Li, T.; Li, M.; Yang, S.; Shen, M.; Liu, H. Research Advances on the Toxicity of Biodegradable Plastics Derived Micro/Nanoplastics in the Environment: A Review. Sci. Total Environ. 2024, 916, 170299. [Google Scholar] [CrossRef]
- Nik Mut, N.N.; Na, J.; Jung, J. A Review on Fate and Ecotoxicity of Biodegradable Microplastics in Aquatic System: Are Biodegradable Plastics Truly Safe for the Environment? Environ. Pollut. 2024, 344, 123399. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, L.; Salam, M.; Yang, B.; He, Q.; Yang, Y.; Li, H. Revealing the Environmental Hazard Posed by Biodegradable Microplastics in Aquatic Ecosystems: An Investigation of Polylactic Acid’s Effects on Microcystis Aeruginosa. Environ. Pollut. 2024, 344, 123347. [Google Scholar] [CrossRef]
- Mao, S.; He, C.; Niu, G.; Ma, Y. Effect of Aging on the Release of Di-(2-Ethylhexyl) Phthalate from Biodegradable and Petroleum-Based Microplastics into Soil. Ecotoxicol. Environ. Saf. 2024, 272, 116006. [Google Scholar] [CrossRef]
- Luangrath, A.; Na, J.; Kalimuthu, P.; Song, J.; Kim, C.; Jung, J. Ecotoxicity of Polylactic Acid Microplastic Fragments to Daphnia Magna and the Effect of Ultraviolet Weathering. Ecotoxicol. Environ. Saf. 2024, 271, 115974. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Cheng, X.; Cai, Z.; Wang, Z.; Zhou, J. Biodegradable Microplastics Reduce the Effectiveness of Biofertilizers by Altering Rhizospheric Microecological Functions. J. Environ. Manag. 2024, 352, 120071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wu, X.; Yuan, S.; Lai, C.; Bian, S.; Yu, W.; Liang, S.; Hu, J.; Huang, L.; Duan, H.; et al. A Potential Threat from Biodegradable Microplastics: Mechanism of Cadmium Adsorption and Desorption in the Simulated Gastrointestinal Environment. Front. Environ. Sci. Eng. 2023, 18, 19. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Shen, J.; Xu, F.; Hou, H.; Xie, C.; Wang, B.; An, S. Mechanism of Polyethylene and Biodegradable Microplastic Aging Effects on Soil Organic Carbon Fractions in Different Land-Use Types. Sci. Total Environ. 2024, 912, 168961. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Lu, Y.; Hua, D.; Wang, X.; Zou, X. Biodegradable Microplastics (BMPs): A New Cause for Concern? Environ. Sci. Pollut. Res. 2021, 28, 66511–66518. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- OCDE. Test No. 310: Ready Biodegradability-CO2 in Sealed Vessels (Headspace Test), OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 2014. [Google Scholar] [CrossRef]
- OCDE. Test No. 306: Biodegradability in Seawater, OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 1992. [Google Scholar] [CrossRef]
- Test No. 302C: Inherent Biodegradability: Modified MITI Test (II), OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 2009; Volume 3. [CrossRef]
- ISO 19679:2020; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sediment Interface—Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 18830:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sandy Sediment Interface—Method by Measuring the Oxygen Demand in Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 22404:2019; Plastics—Determination of the Aerobic Biodegradation of Non-Floating Materials Exposed to Marine Sediment—Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. International Organization for Standardization: Geneva, Switzerland, 2019.
- OCDE. Test No. 307: Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 2002. [Google Scholar] [CrossRef]
- OCDE. Test No. 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems; Section 3; OCDE: Paris, France, 2002. [Google Scholar] [CrossRef]
- OCDE. Test No. 309: Aerobic Mineralisation in Surface Water–Simulation Biodegradation Test, OECD Guidelines for the Testing of Chemicals; Section 3; OCDE: Paris, France, 2002. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Sangale, M.K.; Ade, A.B. Microplastics. In Bioremediation Technology for Plastic Waste; Springer: Singapore, 2019; pp. 11–19. ISBN 9789811374913. [Google Scholar]
- Jiang, J.-Q. Occurrence of Microplastics and Its Pollution in the Environment: A Review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wohlleben, W. Microplastic Regulation Should Be More Precise to Incentivize Both Innovation and Environmental Safety. Nat. Commun. 2020, 11, 5324. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 16, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B.; Copeland, L.O. Seed Production; Springer: Boston, MA, USA, 1997; ISBN 978-1-4613-6825-0. [Google Scholar]
- Turner, M. Seeds; CTA; Macmillan: Wageningen, The Netherlands; Oxford, UK, 2010; ISBN 978-0-230-02239-3. [Google Scholar]
- Black, M.; Bewley, J.D. Seed Technology and Its Biological Basis; CRC Press LLC: Boca Raton, FL, USA, 2000; ISBN 0-8493-9749-9. [Google Scholar]
- Khan, A.A. Preplant Physiological Seed Conditioning. In Horticultural Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 1992; pp. 131–181. ISBN 978-0-471-57499-6. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Pre-Sowing Seed Treatment—A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield under Saline and Non-Saline Conditions. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 88, pp. 223–271. ISBN 978-0-12-000786-8. [Google Scholar]
- Schiffers, B.; Fraselle, J. Le point sur les techniques de traitement des semences. Ann. De Gembloux J. De L’association Des Ingénieurs Sortis L’institut Agric. L’etat 1988, 94, 305–315. [Google Scholar]
- Hasanuzzaman, M.; Fotopoulos, V. (Eds.) Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; ISBN 9789811386244. [Google Scholar]
- Andrews, C.H. A History of Seed Treatment. In Proceedings of the Short Course for Seedsmen, Starkville, MS, USA, 5 January 1961. [Google Scholar]
- Matyjaszczyk, E. Comparison between Seed and Foliar Treatment as a Tool in Integrated Pest Management. J. Agric. Food Chem. 2017, 65, 6081–6086. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent Advances in Seed Coating Technologies: Transitioning toward Sustainable Agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Pill, W.G. Advances in Fluid Drilling. HortTechnology 1991, 1, 59–65. [Google Scholar] [CrossRef]
- IHS Markit–Featured Insight–Sector Briefings. Available online: https://cdn.ihsmarkit.com/www/pdf/1221/IHS-Markit-Seed-Market-Data-Featured-Insight.pdf (accessed on 4 December 2023).
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Accinelli, C.; Abbas, H.K.; Little, N.S.; Kotowicz, J.K.; Mencarelli, M.; Shier, W.T. A Liquid Bioplastic Formulation for Film Coating of Agronomic Seeds. Crop. Prot. 2016, 89, 123–128. [Google Scholar] [CrossRef]
- Pawlicki, A.A.; Borodinov, N.; Giri, N.; Moore, S.; Brown, C.; Belianinov, A.; Ievlev, A.V.; Ovchinnikova, O.S. Multimodal Chemical Imaging for Linking Adhesion with Local Chemistry in Agrochemical Multicomponent Polymeric Coatings. Anal. Chem. 2019, 91, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Halecky, A.; Ren, N.; Lu, J.; Wang, J.Q.; Lockwood, F.E. Correlation of the Mechanical Properties of Seed Coating Films and Dust-Off, Flowability, and Plantability Tests. In Pesticide Formulation and Delivery Systems: 36th Volume, Emerging Trends Building on a Solid Foundation; Poffenberger, C., Heuser, J., Eds.; ASTM International: West Conshohocken, PA, USA, 2016; pp. 183–201. ISBN 978-0-8031-7635-5. [Google Scholar]
- Rudelt, J.; Klink, H.; Verreet, J.-A. Impact of Adhesive Adjuvants Addition into Seed Treatments on the Flowability of Cereal Crop Seeds. J. Cultiv. Plants 2018, 70, 158–162. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Shier, W.T. A Bioplastic-Based Seed Coating Improves Seedling Growth and Reduces Production of Coated Seed Dust. J. Crop Improv. 2018, 32, 318–330. [Google Scholar] [CrossRef]
- Reis, L.V.; Carvalho, E.R.; Reis, V.U.V.; Nardelli, A.C.P.; de Andrade, D.B.; Oliveira Junior, A. Treatment Technologies for Soybean Seeds: Dose Effectiveness, Mechanical Damage and Seed Coating. Ciênc. Agrotec. 2023, 47, e013622. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed Enhancement: Getting Seeds Restoration-ready. Restor. Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Black, M.; Bewley, J.D.; Ha, P. The Encyclopedia of Seeds: Science, Technology and Uses; CABI: Wallingford, UK, 2008; ISBN 10: 0-85199-723-6. [Google Scholar]
- Ma, Y. Seed Coating with Beneficial Microorganisms for Precision Agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Saqlain Zaheer, M.; Faheem, M.; Haider Ali, H.; Iqbal, J. Seed Coating Technology: An Innovative and Sustainable Approach for Improving Seed Quality and Crop Performance. J. Saudi Soc. Agric. Sci. 2022, 21, 536–545. [Google Scholar] [CrossRef]
- Leuna Werke GmbH. VEB Leuna-Werke “Walter Ulbricht” Procédé d’enrobage de Semences et Semences Enrobées par le Présent Similé ou Procédé Similaire. FR1395106A, 6 April 1964.
- Cano Camacho, V.; Potter, S.W.; McDaniel, M.D.; Licht, M.A. Impact of Biological Seed Treatments on Maize (Zea mays L.) and Soil: Crop Growth and Yield. Agron. J. 2023, 115, 2877–2887. [Google Scholar] [CrossRef]
- Ferracini, C.; Blandino, M.; Rigamonti, I.E.; Jucker, C.; Busato, E.; Saladini, M.A.; Reyneri, A.; Alma, A. Chemical-Based Strategies to Control the Western Corn Rootworm, Diabrotica Virgifera Virgifera LeConte. Crop. Prot. 2021, 139, 105306. [Google Scholar] [CrossRef]
- Agatz, A.; Brown, C.D. Introducing the 2-DROPS Model for Two-Dimensional Simulation of Crop Roots and Pesticide within the Soil-Root Zone. Sci. Total Environ. 2017, 586, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, A.; Vigneshwari, R.; Sathiah, N.; Senthil Kumar, M.; Sivamurugan, A.P.; Thangachamy, P.; Ilango, S.S.; Madhu Sudhanan, E.; Karthik, P.; Shanthi, M. Persistence of Foliar Applied and Pre-Storage Seed-Treated Insecticides in Rice and Its Processed Products. Sci. Rep. 2024, 14, 2406. [Google Scholar] [CrossRef]
- Klatt, B.K.; Wurz, A.; Herbertsson, L.; Rundlöf, M.; Svensson, G.P.; Kuhn, J.; Vessling, S.; de La Vega, B.; Tscharntke, T.; Clough, Y.; et al. Seed Treatment with Clothianidin Induces Changes in Plant Metabolism and Alters Pollinator Foraging Preferences. Ecotoxicology 2023, 32, 1247–1256. [Google Scholar] [CrossRef]
- Clifton, E.H.; Tylka, G.L.; Gassmann, A.J.; Hodgson, E.W. Interactions of Effects of Host Plant Resistance and Seed Treatments on Soybean Aphid (Aphis glycines Matsumura) and Soybean Cyst Nematode (Heterodera glycines Ichinohe). Pest Manag. Sci. 2018, 74, 992–1000. [Google Scholar] [CrossRef]
- Chaudhari, R.; Parmar, G.; Juneja, R.; Parmar, S.; Mungra, K. Eco Friendly Management of Pearl Millet Downy Mildew (Sclerospora graminicola) by Using Organic Compounds. Pharma Innov. J. 2023, 12, 762–765. [Google Scholar]
- Sharma, R.; Gate, V.L.; Madhavan, S. Evaluation of Fungicides for the Management of Pearl Millet [Pennisetum glaucum (L.)] Blast Caused by Magnaporthe Grisea. Crop. Prot. 2018, 112, 209–213. [Google Scholar] [CrossRef]
- Álvarez, F.; Arena, M.; Auteri, D.; Leite, S.B.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Metalaxyl-M (Amendment of Approval Conditions). EFSA J. 2023, 21, e08373. [Google Scholar] [CrossRef] [PubMed]
- Panozzo, A.; Barion, G.; Moore, S.S.; Cobalchin, F.; Di Stefano, A.; Sella, L.; Vamerali, T. Early Morpho-Physiological Response of Oilseed Rape under Seed Applied Sedaxane Fungicide and Rhizoctonia Solani Pressure. Front. Plant Sci. 2023, 14, 1130825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, H.; Li, S.; Shang, W.; Jiang, J.; Xu, X.; Liu, D.; Hu, X. Diversity of Fungal Endophytes in American Ginseng Seeds. Plant Dis. 2023, 107, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, D.P.; Ray, R.V. Yield Loss and Integrated Disease Control of Rhizoctonia Solani AG2-1 Using Seed Treatment and Sowing Rate of Oilseed Rape. Plant Dis. 2023, 107, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Sjarpe, D.A.; Kandel, Y.R.; Chilvers, M.I.; Giesler, L.J.; Malvick, D.K.; McCarville, M.T.; Tenuta, A.U.; Wise, K.A.; Mueller, D.S. Multi-Location Evaluation of Fluopyram Seed Treatment and Cultivar on Root Infection by Fusarium Virguliforme, Foliar Symptom Development, and Yield of Soybean. Can. J. Plant Pathol. 2020, 42, 192–202. [Google Scholar] [CrossRef]
- Hakki, S.I.; Ds, U.; Js, H.; Rao, M. Efficacy of Different Seed Dressing Fungicides and Foliar Spray on Seed Quality in Durum Wheat (Triticum durum L.). Pharma Innov. J. 2023, 12, 1447–1450. [Google Scholar]
- Ye, Z.; Brûlé-Babel, A.L.; Graf, R.J.; Mohr, R.; Beres, B.L. The Role of Genetics, Growth Habit, and Cultural Practices in the Mitigation of Fusarium Head Blight. Can. J. Plant Sci. 2017, 97, 316–328. [Google Scholar] [CrossRef]
- Mongiano, G.; Zampieri, E.; Morcia, C.; Titone, P.; Volante, A.; Terzi, V.; Tamborini, L.; Valé, G.; Monaco, S. Application of Plant-Derived Bioactive Compounds as Seed Treatments to Manage the Rice Pathogen Fusarium Fujikuroi. Crop. Prot. 2021, 148, 105739. [Google Scholar] [CrossRef]
- Hysing, S.-C.; Wiik, L. The Role of Seed Infection Level and Fungicide Seed Treatments in Control of Net Blotch in Barley. Eur. J. Plant Pathol. 2013, 137, 169–180. [Google Scholar] [CrossRef]
- Nampeera, E.L.; O’Neal, M.E.; Nonnecke, G.R.; Murungi, L.K.; Abukutsa-Onyango, M.O.; Wesonga, J.M. Effects of Seed Treatments and Storage Duration on Myzus persicae (Hemiptera: Aphididae) and Amaranth Fresh Leaf Yield. Environ. Entomol. 2023, 52, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Gireesha, D.; Patil, P.V.; Kulkarni, S.; Hosmath, J.A. Management of Tropical Sugarbeet Root Rot (Sclerotium rolfsii Sacc.) by Fungicide and Rhizosphere Biocontrol Agents. Sugar Tech. 2023. [Google Scholar] [CrossRef]
- Choudhary, C.; Singh, R.; Kumar, A.; Arun, A.; Chand, P. Evaluation of Newer Molecule Fungitoxicants against Alternaria Blight, 1000 Seed Weight and Seed Yield of Oilseed Brassica in Bihar Condition. Pharma Innov. J. 2023, 12, 5353–5355. [Google Scholar]
- Kurilova, D.A.; Bushneva, N.A. Evaluation of a Combined Treatment of Soybean Seeds with Fungicides and an Inoculant, Proceedings of International Scientific and Practical Conference “Current Issues of Biology, Breeding, Technology and Processing of Agricultural Crops 2022”, Krasnodar, Russia, 1–2 June 2022; AIP Publishing: Melville, NY, USA, 2023. [Google Scholar]
- Kumar, M.; Bhadauria, V.; Singh, K.; Singh, C.; Kumar Yadav, A. Evaluation of Fungicide Efficacy for the Management of Alternaria Leaf Spot Disease on Chilli. Plant Pathol. J. 2013, 12, 32–35. [Google Scholar] [CrossRef]
- Bagri, R.; Goyal, S.; Singh, J.; Kumar, V.; Sharma, R. Management of Mosaic Disease of Bottle Gourd (Lagenaria siceraria (Mol.) Stand) through Integrated Methods. Pharma Innov. J. 2022, 11, 2984–2986. [Google Scholar]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Boawn, L.C. Zinc Seed Treatment as a Source of Zinc for Beans (Phaseolus vulgaris). Agron. J. 1969, 61, 674–676. [Google Scholar] [CrossRef]
- Lana, R.M.Q.; de Faria, M.V.; Bonotto, I.; Lana, Â.M.Q. Cobalt and Molybdenum Concentrated Suspension for Soybean Seed Treatment. Rev. Bras. Ciênc. Solo 2009, 33, 1715–1720. [Google Scholar] [CrossRef]
- Foy, C.L. Progress and Developments in Adjuvant Use since 1989 in the Usa. Pestic. Sci. 1993, 38, 65–76. [Google Scholar] [CrossRef]
- Hathcock, A.L.; Dernoeden, P.H. Seed Germination of Tall Fescue and Kentucky Bluegrass as Affected by Adhesives. HortScience 1984, 19, 442–443. [Google Scholar] [CrossRef]
- Abdelzaher, H.M.A.; Elnaghy, M.A.; Fadl-Allah, E.M. Isolation of Pythium Oligandrum from Egyptian Soil and Its Mycoparasitic Effect on Pythium Ultimum Var. Ultimum the Damping-off Organism of Wheat. Mycopathologia 1997, 139, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liang, S.; Zheng, W.; Chen, X.; Liu, J.; Wang, A. Study on the Preparation and Effect of Tomato Seedling Disease Biocontrol Compound Seed-Coating Agent. Life 2022, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Capo, L.; Sopegno, A.; Reyneri, A.; Ujvári, G.; Agnolucci, M.; Blandino, M. Agronomic Strategies to Enhance the Early Vigor and Yield of Maize Part II: The Role of Seed Applied Biostimulant, Hybrid, and Starter Fertilization on Crop Performance. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of Microalgal Extracts as Biostimulants through Seed Treatment and Foliar Spray for Tomato Cultivation. Ind. Crop. Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Mutlu-Durak, H.; Yildiz Kutman, B. Seed Treatment with Biostimulants Extracted from Weeping Willow (Salix babylonica) Enhances Early Maize Growth. Plants 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A Biostimulant Seed Treatment Improved Heat Stress Tolerance during Cucumber Seed Germination by Acting on the Antioxidant System and Glyoxylate Cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Silva, B.G.D.; Braccini, A.L.; Oliveira, S.M.D.; Coppo, C.; Bastiane, G.G.D.; Pelloso, M.F.; Borges, Y.M. Industrial Treatment of Soybean Seeds Subjected to High Volumes of Fungicides, Insecticides, Biostimulants and Micronutrients during Storage. Aust. J. Crop. Sci. 2022, 16, 539–544. [Google Scholar] [CrossRef]
- Flowable Concentrate for Seed Treatment (FS)|Croda Crop Care. Available online: https://www.crodacropcare.com/en-gb/applications/flowable-concentrate-for-seed-treatment (accessed on 6 March 2024).
- Gu, Y.; Pang, Y.; Yang, D.; Qian, Y.; Lou, H.; Zhou, M. Preparation of Lignin/Polyethylene Glycol Film-Forming Agent and Its Application in Chlorantraniliprole 5% Flowable Concentrate for Seed Coating (FS). Ind. Crops Prod. 2022, 182, 114877. [Google Scholar] [CrossRef]
- Comyn, J. Adhesion Science; RSC Paperbacks: London, UK, 1997. [Google Scholar]
- Dawood, M. Durability of Steel Components Strengthened with Fiber-Reinforced Polymer (FRP) Composites. In Rehabilitation of Metallic Civil Infrastructure Using Fiber Reinforced Polymer (FRP) Composites; Elsevier: Amsterdam, The Netherlands, 2014; pp. 96–114. ISBN 978-0-85709-653-1. [Google Scholar]
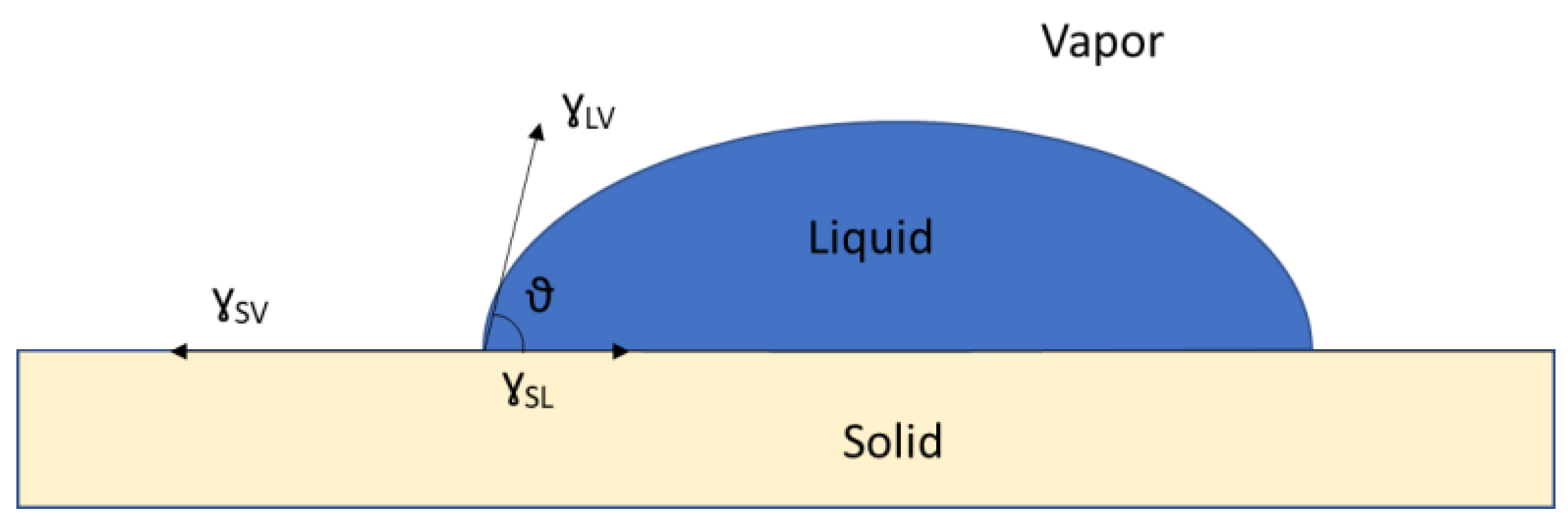
- Young, T. III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Dhungana, S.; Chhetri, G.K.; Sedhai, B.; Basnet, N.; Shakya, A.; Pandey, B.P.; Pradhan, S.P.; Joshi, U.M.; et al. Effect of Plasma Treatment on the Seed Germination and Seedling Growth of Radish (Raphanus sativus). Plasma Sci. Technol. 2022, 24, 015502. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability Increase in Plasma-Treated Agricultural Seeds and Its Relation to Germination Improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radiofrequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Reus, H.A.M.; Glas, J.; Garnier, J.S.; Samuels, P.W.J. Treatment for Plant Seeds. AU 2015276335 B2, 25 October 2018. [Google Scholar]
- Kang, B.; Liu, Y.; Yang, R.; Liang, S. Composition and Physicochemical Properties of Corn Wax. J. Am. Oil Chem. Soc. 2022, 100, 783–790. [Google Scholar] [CrossRef]
- Baümler, E.R.; Crapiste, G.H.; Carelli, A.A. Sunflower-Oil Wax Reduction by Seed Solvent Washing. J. Am. Oil Chem. Soc. 2007, 84, 603–608. [Google Scholar] [CrossRef]
- Baldanov, B.B.; Ranzhurov, T.V. Effect of Plasma Treatment on Surface Wettability of Wheat Seeds. High Energy Chem. 2022, 56, 294–298. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Morena, C.; Bruno, V.; Khambhati, V.H.; Paulk, R.T.; Little, N.S.; Bellaloui, N.; Forbes, W.; Shier, W.T. Use of a Biochar-Based Formulation for Coating Corn Seeds. Cogent Food Agric. 2023, 9, 2283956. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Bruno, V.; Vicari, A.; Little, N.S.; Ebelhar, M.W.; Shier, W.T. Minimizing Abrasion Losses from Film-Coated Corn Seeds. J. Crop. Improv. 2021, 35, 666–678. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, Y.; Tate, M.; Zhong, Z. A New Film-Forming Agent Used to Improve Seed Coating Application Performance. In Pesticide Formulation and Delivery Systems: 41st Volume, Formulation and Application Challenges of Diverse Agricultural Agrochemicals; McMullan, P., Ed.; ASTM International: West Conshohocken, PA, USA, 2022; pp. 110–118. ISBN 978-0-8031-7729-1. [Google Scholar]
- Mills, J.T. Adhesion of Seed Treatment Fungicides to Seeds of Different Crops. Can. J. Plant Sci. 1972, 52, 449–458. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.A.; Jeffs, K.A.; Tuppen, R.J. Retention and Distribution of Dry Powder and Liquid Formulations of Insecticides and Fungicides on Commercially Dressed Cereal Seed. Pestic. Sci. 1971, 2, 49–55. [Google Scholar] [CrossRef]
- Vos, P.D.; Dineur, A.; Pigeon, O. Plant Protection Products: Adhesion to and Distribution on Treated Seeds. Commun. Agric. Appl. Biol. Sci. 2011, 76, 949–953. [Google Scholar] [PubMed]
- Herbert, R. Application of Latex Emulsion Polymers in Seed Coating Technology. In Pesticide Formulations and Application Systems: 23rd Volume; Volgas, G., Downer, R., Lopez, H., Eds.; ASTM International: West Conshohocken, PA, USA, 2003; pp. 55-55–13; ISBN 978-0-8031-3468-3. [Google Scholar]
- Marzaro, M.; Vivan, L.; Targa, A.; Mazzon, L.; Mori, N.; Greatti, M.; Toffolo, E.P.; Bernardo, A.D.; Giorio, C.; Marton, D.; et al. Lethal Aerial Powdering of Honey Bees with Neonicotinoids from Fragments of Maize Seed Coat. Bull. Insectology 2011, 64, 119–126. [Google Scholar]
- ESTA Dust Reference Values Heubach Test Method. Available online: https://euroseeds.eu/esta-the-european-seed-treatment-assurance-industry-scheme/dust-reference-values-heubach-test-method/ (accessed on 20 February 2024).
- Foqué, D.; Beck, B.; Devarrewaere, W.; Verboven, P.; Nuyttens, D. Comparing Different Techniques to Assess the Risk of Dust Drift from Pesticide-Coated Seeds. Pest Manag. Sci. 2017, 73, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, J.; Zhao, X.; Pan, H.; Ali Lakhiar, I.; Wang, W. Development and Experimental Analysis of an Intelligent Sensor for Monitoring Seed Flow Rate Based on a Seed Flow Reconstruction Technique. Comput. Electron. Agric. 2019, 164, 104899. [Google Scholar] [CrossRef]
- Sidhu, H.; Wiesenborn, D.; Johnson, B.; Monono, E.; Eriksmoen, E. Coating of Hulled Seeds Improved Field Plantability and Grain Yield of Extra-Large Confectionary Sunflower Achenes. Crop. Sci. 2019, 59, 1182–1190. [Google Scholar] [CrossRef]
- Coulon, J. Lining and Shielding of Hoppers and Drums; NETCO: Jarrow, UK, 2022. [Google Scholar]
- Non-Stick Coatings for Hoppers & Chutes. Available online: https://www.fluorotec.com/news/blog/non-stick-coatings-for-hoppers-chutes/ (accessed on 21 February 2024).
- Trpělková, Ž.; Hurychová, H.; Kuentz, M.; Vraníková, B.; Šklubalová, Z. Introduction of the Energy to Break an Avalanche as a Promising Parameter for Powder Flowability Prediction. Powder Technol. 2020, 375, 33–41. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.; Loos, M.; Taylor, A. Development of Superabsorbent Polymer (SAP) Seed Coating Technology to Enhance Germination and Stand Establishment in Red Clover Cover Crop. Agronomy 2023, 13, 438. [Google Scholar] [CrossRef]
- Bueno, A.N.; Euclaire Meyer, T. Anthony Seed Coating Composition. US20180325104A1, 15 November 2018. [Google Scholar]
- D01 Committee Test Methods for Drying, Curing, or Film Formation of Organic Coatings; ASTM International: West Conshohocken, PA, USA, 2022.
- Dexter, S.T.; Miyamoto, T. Acceleration of Water Uptake and Germination of Sugarbeet Seedballs by Surface Coatings of Hydrophilic Colloids 1. Agron. J. 1959, 51, 388–389. [Google Scholar] [CrossRef]
- Baxter, L.; Waters, L. Effect of a Hydrophilic Polymer Seed Coating on the Field Performance of Sweet Corn and Cowpea. J. Am. Soc. Hort. Sci. 1986, 111, 31–34. [Google Scholar] [CrossRef]
- Manoharapaladagu, P.; Prashant Kumar, R.; Kumar, R. Effects of Polymer Seed Coating, Fungicide Seed Treatment and Packaging Materials on Seed Quality of Chilli (Capsicum annuum L.) during Storage. J. Pharmacogn. Phytochem. 2017, 6, 324–327. [Google Scholar]
- Accinelli, C.; Abbas, H.K.; Little, N.S.; Kotowicz, J.K.; Shier, W.T. Biological Control of Aflatoxin Production in Corn Using Non-Aflatoxigenic Aspergillus Flavus Administered as a Bioplastic-Based Seed Coating. Crop Prot. 2018, 107, 87–92. [Google Scholar] [CrossRef]
- Martins, A.B.N.; Suñé, A.D.S.; Migliorini, P.; Silva, R.N.O.D.; Tunes, L.V.M.D.; Soares, V.N.; Almeida, A.D.S.; Rodrigues, D.B.; Eberhardt, P.E.R.; Cavalcante, J.A. Efficiency of Zinc Sulfate (ZnSO4) Absorption, Transport and Use in Zn-Coated Seed of Wheat (Triticum aestivum). Aust. J. Crop. Sci. 2018, 12, 217–220. [Google Scholar] [CrossRef]
- Bernard, G. Implication de la concentration volumique pigmentaire dans l’étude de la pulvérulence des couches picturales mates: Approche expérimentale et études de cas. Master Thesis, Université Paris 1 Panthéon Sorbonne, Paris, France, 2018. [Google Scholar]
- Domin, M.; Kluza, F.; Góral, D.; Nazarewicz, S.; Kozłowicz, K.; Szmigielski, M.; Ślaska-Grzywna, B. Germination Energy and Capacity of Maize Seeds Following Low-Temperature Short Storage. Sustainability 2019, 12, 46. [Google Scholar] [CrossRef]
- Ngombo, W.K.; Kalonji-Mbuyi, A.; Mvumilia, R.K.; Nkongolo, K. Loss of Certified Maize (Zea mays L.) and Cowpea (Vigna unguiculata (L.) Walp.) Seed Viability during Storage in a Sub-Saharan Region: Analysis of Environmental Factors. AJPS 2021, 12, 1410–1424. [Google Scholar] [CrossRef]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of Data Analysis Methods for Seed Germination 1. Crop. Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Duan, X.; Burris, J.S. Film Coating Impairs Leaching of Germination Inhibitors in Sugar Beet Seed. Crop. Sci. 1997, 37, 515–520. [Google Scholar] [CrossRef]
- Sachs, M.; Cantliffe, D.J.; Nell, T.A. Germination Studies of Clay-Coated Sweet Pepper Seeds. J. Am. Soc. Hort. Sci. 1981, 106, 385–389. [Google Scholar] [CrossRef]
- Scott, D. Effects of Seed Coating on Establishment. N. Zeal. J. Agric. Res. 1975, 18, 59–67. [Google Scholar] [CrossRef]
- Oyekale, K.O.; Nwangburuka, C.C.; Denton, O.A.; Daramola, D.S.; Adeyeye, J.A.; Akinkuotu, A.O. Comparative Effects of Organic and Inorganic Seed Treatments on the Viability and Vigour of Sesame Seeds in Storage. J. Agric. Sci. 2012, 4, 187–195. [Google Scholar] [CrossRef]
- Kumar, B.; Zaidi, S.; Singh, V.; Venkatesh, K.T.; Ram, G.; Gupta, A.K.; Kumar, N.; Samad, A. Seed Germination Behaviour of Cannabis sativa L. under Different Temperature Regimes. J. Plant Dev. Sci. 2020, 12, 277–281. [Google Scholar]
- Shahid, M.; Singh, A.; Srivastava, M.; Sachan, C.P.; Biswas, S.K. Effect of Seed Treatment on Germination and Vigour in Chickpea. Trends Biosci. 2011, 4, 205–207. [Google Scholar]
- Jacob, S.R.; Kumar, M.B.A.; Varghese, E.; Sinha, S.N. Hydrophilic Polymer Film Coat as a Micro-Container of Individual Seed Facilitates Safe Storage of Tomato Seeds. Sci. Hortic. 2016, 204, 116–122. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Wheat, R.; McGahan, D.G.; Mitchell, F. Evaluation of Leaching Potential of Three Systemic Neonicotinoid Insecticides in Vineyard Soil. J. Contam. Hydrol. 2014, 170, 86–94. [Google Scholar] [CrossRef]
- Hladik, M.L.; Kolpin, D.W.; Kuivila, K.M. Widespread Occurrence of Neonicotinoid Insecticides in Streams in a High Corn and Soybean Producing Region, USA. Environ. Pollut. 2014, 193, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and Analysis of Neonicotinoids in River Waters—Development of a Passive Sampler for Three Commonly Used Insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Starner, K.; Goh, K.S. Detections of the Neonicotinoid Insecticide Imidacloprid in Surface Waters of Three Agricultural Regions of California, USA, 2010–2011. Bull. Environ. Contam. Toxicol. 2012, 88, 316–321. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Thompson, B.; Cooper, E.; Moore, J.; Fulton, M.H. A Long-Term Monitoring Study of Chlorophyll, Microbial Contaminants, and Pesticides in a Coastal Residential Stormwater Pond and Its Adjacent Tidal Creek. Environ. Monit. Assess. 2012, 184, 343–359. [Google Scholar] [CrossRef]
- Alford, A.; Krupke, C.H. Translocation of the Neonicotinoid Seed Treatment Clothianidin in Maize. PLoS ONE 2017, 12, e0173836. [Google Scholar] [CrossRef]
- Film Forming Agent with Outstanding Water Resistance POWERBLOXTM Filmer-17 Film Forming Agent. Available online: https://news.agropages.com/News/Detail-31746.htm (accessed on 6 March 2024).
- Kyriakoudes, G.; Turner, A. Suspended and Deposited Microplastics in the Coastal Atmosphere of Southwest England. Chemosphere 2023, 343, 140258. [Google Scholar] [CrossRef] [PubMed]
- Accinelli, C.; Abbas, H.K.; Shier, W.T.; Vicari, A.; Little, N.S.; Aloise, M.R.; Giacomini, S. Degradation of Microplastic Seed Film-Coating Fragments in Soil. Chemosphere 2019, 226, 645–650. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Ghiglione, J.-F.; Gastaldi, E.; ter Halle, A.; Huvet, A.; Bruzaud, S.; Lagarde, F.; Galgani, F.; Duflos, G.; George, M.; et al. Discussion about Suitable Applications for Biodegradable Plastics Regarding Their Sources, Uses and End of Life. Waste Manag. 2023, 157, 242–248. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Samal, A.; Mohanty, S.; Das, S.; Das, B.; Beura, J. Polymer Coating of Cowpea Seeds (Vigna unguiculata) for Improving Its Storability and Performance. Pharma Innov. J. 2021, 10, 592–597. [Google Scholar]
- Safety Data Sheet-TEXTURECEL™ 30000 P Sodium. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwixq-vixJmHAxWwT6QEHSnBBc8QFnoECA4QAQ&url=https%3A%2F%2Fwww.industrialcellulosics.com%2Fproducts%2Fdownload%3Fgrade%3D73330%26doctype%3Dmsds%26documentid%3D0103000257072307143290&usg=AOvVaw2AXa1vmyTJeI4lqp2TCpE4&opi=89978449(accessed on 11 March 2024).
- Safety Data Sheet (SDS)—KlucelTM MXF PHARM. Available online: https://sds.ashland.com/sds-web/materialDocumentResults (accessed on 11 March 2024).
- Safety Data Sheet Sodium Alginate Manutex, F. Available online: https://www.kraftkolour.net.au/assets/files/SDS%20Sodium%20Alginate%20Manutex%20F%202022.pdf (accessed on 11 March 2024).
- de Castro, V.A.; Duarte, V.G.O.; Nobre, D.A.C.; Silva, G.H.; Constantino, V.R.L.; Pinto, F.G.; Macedo, W.R.; Tronto, J. Plant Growth Regulation by Seed Coating with Films of Alginate and Auxin-Intercalated Layered Double Hydroxides. Beilstein J. Nanotechnol. 2020, 11, 1082–1091. [Google Scholar] [CrossRef]
- Piatkowski, M.; Janus, L.; Radwan-Praglowska, J.; Raclavsky, K. Microwave-Enhanced Synthesis of Biodegradable Multifunctional Chitosan Hydrogels for Wastewater Treatment. Express Polym. Lett. 2017, 11, 809–819. [Google Scholar] [CrossRef]
- Thobunluepop, P. The Inhibitory Effect of the Various Seed Coating Substances against Rice Seed Borne Fungi and Their Shelf-Life during Storage. Pak. J. Biol. Sci. 2009, 12, 1102–1110. [Google Scholar] [CrossRef]
- Satefy Data Sheet LYCATAB® PGS. Available online: https://www.roquette.com/-/media/roquette-sharepoint-libraries/safety-data-sheet/2023/07/03/14/11/roquette_sds_fr_lycatab-pgs_000000200252_fr.pdf (accessed on 11 March 2024).
- Safety Data Sheet—Guar Gum (Kraftkolour). Available online: https://www.kraftkolour.net.au/assets/files/SDS%20Guar%20Gum%202022.pdf (accessed on 11 March 2024).
- Chen, Z.; Castaing, J.-C.; Ji, P.F.; Cristobal, G. Seed Coatings, Coating Compositions and Methods for Use. US20120220454A1, 30 August 2012. [Google Scholar]
- Zvinavashe, A.T.; Laurent, J.; Mhada, M.; Sun, H.; Fouda, H.M.E.; Kim, D.; Mouhib, S.; Kouisni, L.; Marelli, B. Programmable Design of Seed Coating Function Induces Water-Stress Tolerance in Semi-Arid Regions. Nat. Food 2021, 2, 485–493. [Google Scholar] [CrossRef]
- Safety Data Sheet Luvitec K90 Powder. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjN_KeUupKHAxVlfKQEHdRhAdsQFnoECA8QAQ&url=https%3A%2F%2Fdownload.basf.com%2Fp1%2F000000000030161432_SDS_GEN_TH%2Fen%2FLuvitec%2BK%2B90%2BP_000000000030161432_SDS_GEN_TH_en_3-0.pdf&usg=AOvVaw2BD9YY7SLDKpxI6QpJ5D9Q&opi=89978449 (accessed on 11 March 2024).
- Kimmelshue, C.; Goggi, A.S.; Cademartiri, R. The Use of Biological Seed Coatings Based on Bacteriophages and Polymers against Clavibacter Michiganensis subsp. Nebraskensis Maize Seeds. Sci. Rep. 2019, 9, 17950. [Google Scholar] [CrossRef] [PubMed]
- Material Safety Data Sheet-VWR-Polyvinyl Alcohol, partially hydrolyzed, (Mw approx. 70000) for Synthesis. Available online: https://us.vwr.com/assetsvc/asset/en_US/id/12021075/contents (accessed on 2 July 2024).
- Su, L.-Q.; Li, J.-G.; Xue, H.; Wang, X.-F. Super Absorbent Polymer Seed Coatings Promote Seed Germination and Seedling Growth of Caragana Korshinskii in Drought. J. Zhejiang Univ. Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Safety Data Sheet—Crystalit. Available online: https://www.pharmacal.com/SDS/French/Crystalit_fra.pdf (accessed on 11 March 2024).




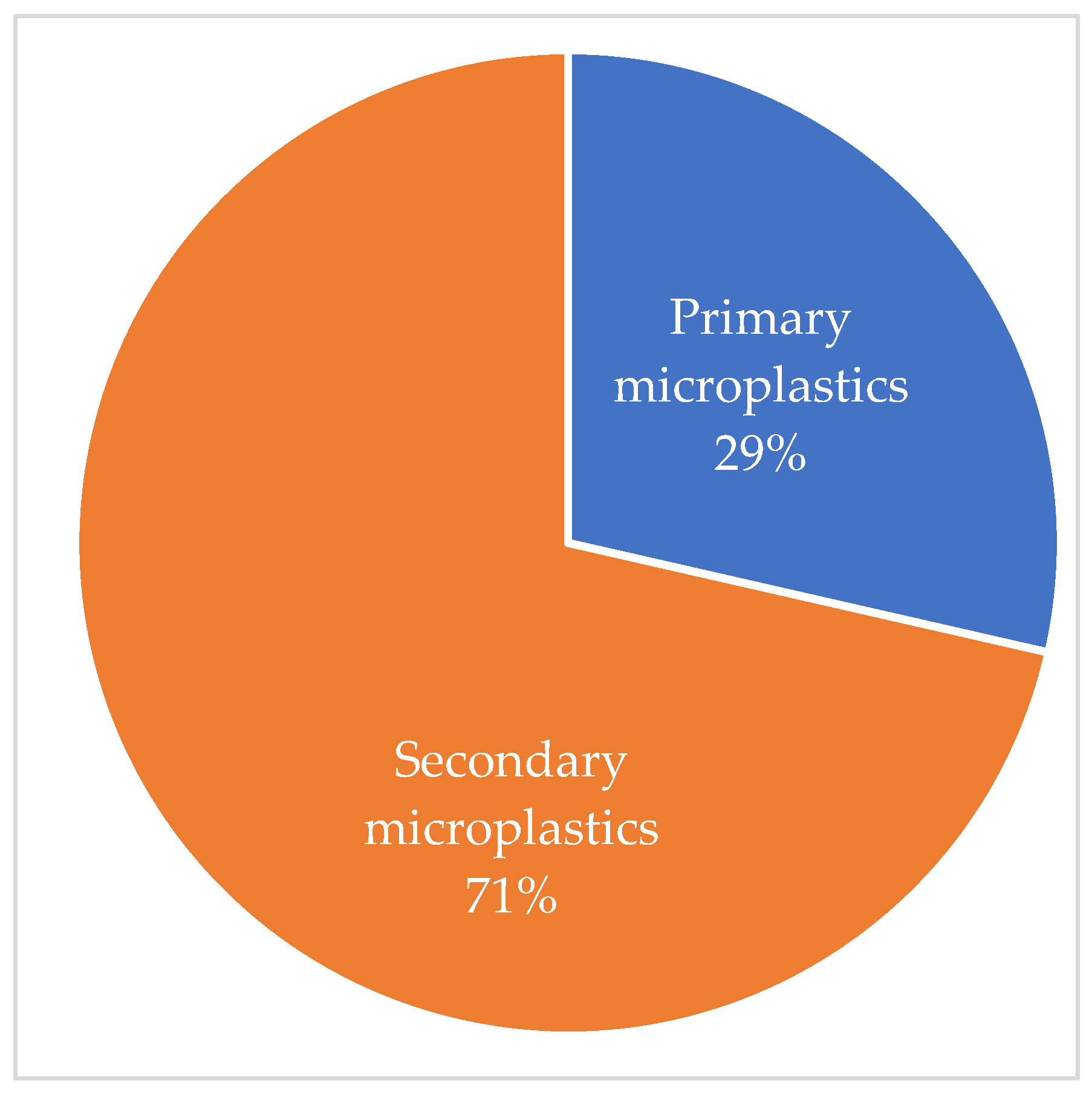
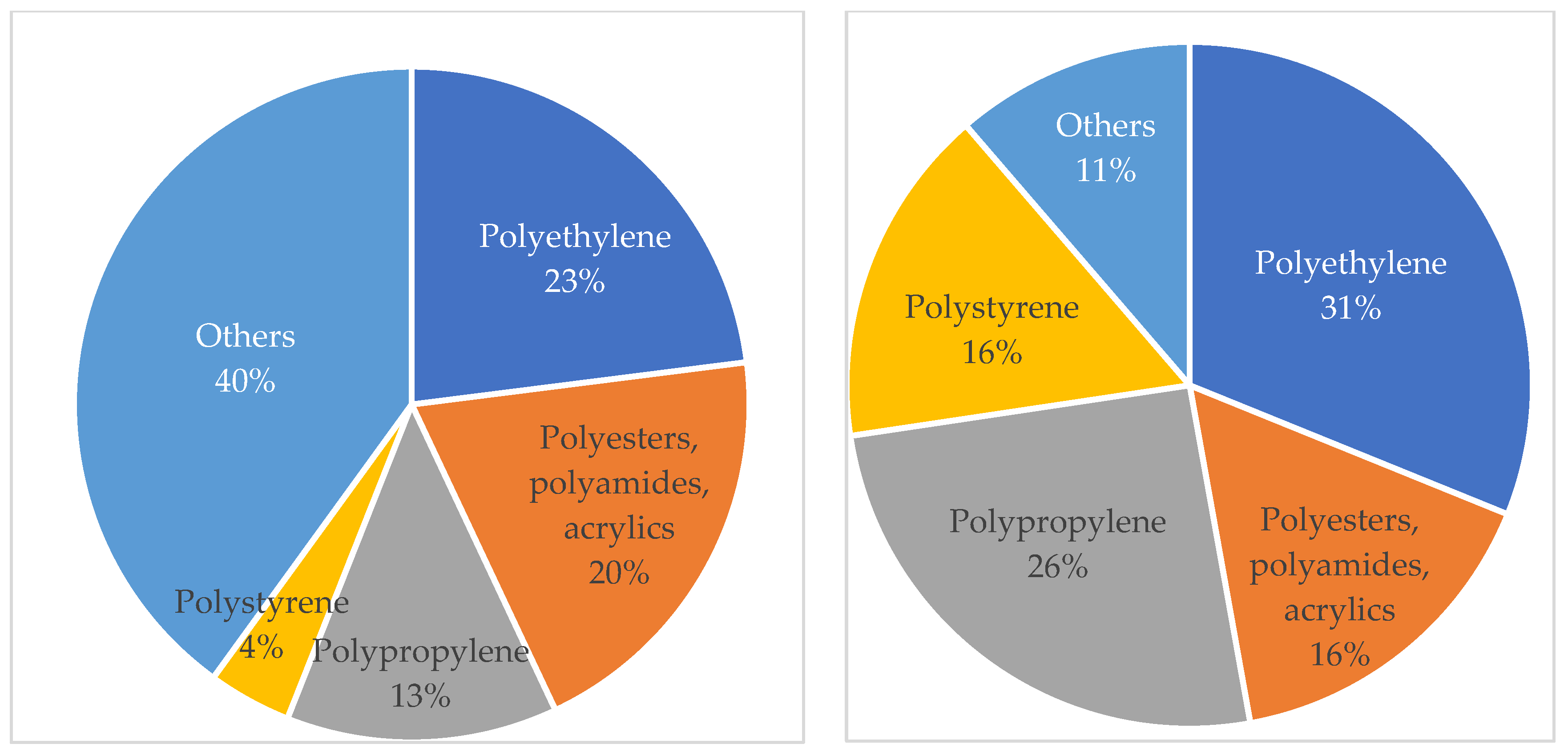


| Status Property | Liquid | Gaseous | Solid |
|---|---|---|---|
| Saturation vapor pressure at 50 °C | <300 kPa | >300 kPa | <300 kPa |
| Physical state at 20 °C and 101.3 kPa | Not completely gaseous | Completely gaseous | / |
| Melting temperature at 101.3 kPa | <20 °C | / | >20 °C |
| OECD LD 105 | OECD LD 120 | ||
|---|---|---|---|
| Low solubility (<10−2 g/L) | Sample loading | 10 g/L | / |
| Conditions | 20 °C ± 0.5 °C, pH = 7, 24 h solubilization, N = 5 replicates | ||
| Solubilization method | Elution in water on a micro-column | ||
| Non-soluble part separation | Column filtration | ||
| Quantification method | GPC, HPLC, or dry matter content determination with gravimetric method | ||
| High solubility (>10−2 g/L) | Sample loading | 10 g/L | 10 g/L |
| Conditions | 30 °C, then 20 ± 0.5 °C, pH = 7 24 h solubilization, then 24 h cooling N = 3 replicates | 20 °C ± 0.5 °C, pH = 7 24 h solubilization N = 3 replicates | |
| Solubilization method | Flask method (heated stirring in closed flask then cooling) | Flask method (stirring in closed flask) | |
| Non-soluble part separation | Filtration or centrifugation | Filtration or centrifugation | |
| Quantification method | GPC, HPLC, or dry matter content determination with gravimetric method | GPC, HPLC, or dry matter content determination with gravimetric method |
| Name | Criteria (Biodegradability Rate and Duration) | Simulated Degradation Environment | Test Substance | Measurement Method |
|---|---|---|---|---|
| Group 1—Screening test methods and success criteria for demonstrating ready biodegradation | ||||
| OECD LD 301 B, C, D, F [127] | 60% mineralization, 28 days | Aerobic aqueous environment | Organic substance soluble ≥ 100 mg/L in water, non-volatile, non-adsorbable | B: released CO2 C, D, F: biochemical oxygen demand (BOD) |
| OECD LD 310 [140] | Produced CO2 | |||
| Group 2—Modified and improved screening test methods and success criteria for demonstrating ready biodegradation | ||||
| OECD LD 301 B, C, D, F [127] | 60% mineralization, 28 days | Aerobic aqueous environment | Organic substance soluble ≥ 100 mg/L in water, non-volatile, non-adsorbable | B: CO2 released C, D, F: biochemical oxygen demand (BOD) |
| OECD LD 310 [140] | Produced CO2 | |||
| OECD LD 306 [141] | Sea | Organic substance soluble ≥ 25–40 mg/L in water, non-volatile, non-adsorbable on glass | Dissolved organic carbon (DOC) | |
| Group 3—Screening test method and success criteria for demonstrating intrinsic degradation | ||||
| OECD LD 302 C [142] | 70% mineralization, 14 days | Aerobic aqueous environment | Organic substance dispersible/soluble in the medium | BOD |
| Group 4—Screening test methods and success criteria for demonstrating degradation against a reference material | ||||
| EN ISO 14852:2021 [122] | 90% degradation compared with reference material, 6 months | Natural aquatic environment | Natural and/or synthetic polymers, copolymers, or mixtures thereof. Plastic materials containing additives Water-soluble polymers | Released CO 2 |
| EN ISO 14851:2019 [123] | ||||
| EN ISO 19679:2020 [143] | 90% degradation compared with reference material, 24 months | Sediment/sea interfaces | Non-floating plastic materials | |
| EN ISO 18830:2016 [144] | Plastic materials | BOD | ||
| ISO 22404:2019 [145] | Marine sediments | Non-floating plastic materials | ||
| EN ISO 17556:2019 [146] | Soil | Natural and/or synthetic polymers, copolymers or mixtures thereof. Plastic materials containing additives Water-soluble polymers | Released CO2 | |
| Group 5—Simulation test methods and success criteria for demonstrating degradation under relevant environmental conditions | ||||
| OECD LD 307 [147] | Degradation half-life < 180 days | Soil | Non-volatile organic substance | Half-life (monitoring of the test substance concentration in the medium) |
| OECD LD 308 [148] | Aquatic sediments | |||
| OECD LD 309 [149] | Degradation half-life < 60 days | Surface water | ||
| Constitute | Active Molecule | Chemical Family | References |
|---|---|---|---|
| Water | / | / | / |
| Insecticide | Tefluthrin | Pyrethroid | Ferracini et al. [180]; Agatz and Brown [181]. |
| Thiamethoxam Clothianidin | Neonicotinoid | Agatz and Brown [181]; Suganthi et al. [182]. Agatz and Brown [181]; Klatt et al. [183]. | |
| Nematicide | Abamectin | Avermectin | Clifton et al. [184]. |
| Pasteuria Nishizawae | Bacteria | ||
| Fungicide | Metalaxyl Metalaxyl-M | Acylamine | Chaudhari et al. [185]; Sharma and Madhavan [186]. |
| Fludioxonil | Phenylpyrrole | Alvarez et al. [187]; Panozzo et al. [188]. | |
| Sedaxane Penflufen | Pyrazole-carboxamides | Panozzo et al. [188]; Zhang et al. [189]. Panozzo et al. [188]; Jayaweera and Ray [190]. | |
| Prothioconazole Triticonazole Difenoconazole Tebuconazole | Triazole | Sjarpe et al. [191]; Hakki et al. [192]. Sjarpe et al. [191]; Ye et al. [193]. Mongiano et al. [194]; Hysing and Wiik [195]. Ye et al. [193]; Nampeera et al. [196]. | |
| Trifloxystrobin Pyraclostrobin | Strobilurin | Ye et al. [193]; Gireesha et al. [197]. Hakki et al. [192]; Choudhary et al. [198]. | |
| Captan | Phthalimide | Hakki et al. [192]; Kurilova and Bushneva [199]. | |
| Abamectin | Avermectin | Kumar et al. [200]; Bagri et al. [201]. | |
| Corvid repellent | Ziram | Kumar et al. [200]. | |
| Microorganisms | Growth-promoting bacteria | Bacillus Pseudomonas Azospirillum Azotobacter | Sjarpe et al. [191]; Camacho et al. [179]. Paravar et al. [202]. |
| Fungi | Rhizobia Trichoderma | Rocha et al. [203]. | |
| Fertilizers | Zinc | Rasmussen and Boawn [204]. | |
| Nitrates Molybdenum Cobalt | Lana et al. [205]. | ||
| Additives | Petroleum-based oils Natural or modified vegetable oils Surfactants Rheological agents | Chester L. Foy [206]. | |
| Seed-coating agents | Adhesive polymers | Methylcellulose Carboxymethylcellulose Gum arabic | Hathcock and Dernoeden [207]. Abdelzaher et al. [208]. Zhang et al. [209]. |
| Adhesive formulations | |||
| Biostimulants | Microorganisms | Capo et al. [210]. | |
| Algae extracts | Supraja et al. [211]. | ||
| Lignocellulosic extracts | Mutlu-Durak and Kutman [212]; Campobenedetto et al. [213]. | ||
| Trace elements | Campobenedetto et al. [213]. | ||
| Plant hormones | Pereira et al. [214]. |
| Seed | Threshold Value |
|---|---|
| Maize | 0.75 g/100,000 seeds |
| Rapeseed | 0.50 g/100,000 seeds |
| Sugar beet | 0.25 g/100,000 seeds |
| Sunflower | 0.40 g/100,000 seeds |
| Cereals | 4 g/100,000 seeds |
| Carrots, endives | 0.1 g/100,000 seeds |
| Onions | 0.2 g/100,000 seeds |
| Sweet corn | 0.75 g/100,000 seeds |
| Green and seed beans | 0.4 g/100,000 seeds |
| Fodder peas | 0.2 g/100,000 seeds |
| Cotton | 6 g/100,000 seeds |
| Function | Examples | References |
|---|---|---|
| Water | / | / |
| Adhesive polymer(s) | Polyvinyl acetate dispersion, styrene acrylate copolymer dispersion, ethylene acrylic copolymer dispersion | US 2018/0325104 A1 [244] AU 2015276335 B2 [223] |
| Flow agent(s) | Wax-based compounds: carnauba wax, paraffin wax, polyethylene wax, beeswax, polypropylene wax | US 2018/0325104 A1 [244] AU 2015276335 B2 [223] |
| Surfactant(s) | Non-ionics: Sugar alcohols, fatty alcohols, alkylphenols, or ethoxylated, propoxylated glucose, PEG, PPG Glycerol esters, sorbitan, pentaerythritol, sorbitol, sucrose Alkylamines, ethoxylated fatty amines, alkanolamides Anionics: Sulfates, phosphate, PEG sulfonate, PPG Carboxylic acids and copolymers of carboxylic acids, sulfates, sulfonic acids, phosphates (lignin sulfonate, alkylaryl sulfonates) Cationics: Amino acid derivatives Alkylammonium salts Amphoterics: Betaine-type amides | US 2018/0325104 A1 [244] |
| Pigment paste/pigments | Monoazo organic pigments (Pigment Red 112, 2, 48:2, Yellow 74, Orange 5), phthalocyanines (Pigment Blue 15:3, Green 36), oxazine (Violet 23), carbon (Black 7), TiO2 (White 6) | US 2018/0325104 A1 [244] |
| Dye(s) | Anthraquinone, triphenylmethane, phthalocyanine and derivatives, and diazonium salts | AU 2015276335 B2 [223] |
| Opacity agent(s) | CaCO3 | AU 2015276335 B2 [223] |
| Anti-foaming agent(s) | PEG, glycerol, mineral oils, silicone-based oils (PDMS), polyethers, polyacrylates | US 2018/0325104 A1 [244] |
| Rheological agent(s)/stabilizer(s) | Agar, carboxymethyl cellulose, carrageenan, chitin, fucoidan, ghatti gum, gum arabic, karaya gum, laminarin, locust bean gum, pectin, alginate, guar gum, xanthan gum, diutan gum, and gum tragacanth, bentonite clays, HEUR thickeners (hydrophobically modified ethoxylated urethane), HASE thickeners (hydrophobically modified alkaline swellable emulsion) and polyacrylates. | US 2018/0325104 A1 [244] |
| Antifreeze agent(s) | Ethylene glycol, propylene glycol | US 2018/0325104 A1 [244] |
| Preservative | Biocides: MIT (2-methyl-4-isothiazolin-3-one), BIT (1,2-benzisothiazolin-3-one), CIT (5-Chloro-2-methyl- 4-isothiazolin-3-one), Bronopol | US 2018/0325104 A1 [244] |
| pH regulator | Citric acid, NaOH |
| Polymer(s) | Function | Natural (N)/Soluble in Water (S)/Biodegradable (B) | References | Performance Factor Measured |
|---|---|---|---|---|
| Polysaccharides | ||||
| Methylcellulose | Film-forming | Not N, S, ND | Samal et al. [273]; Rocha et al. [203]; Javed et al. [177]; | Physiologic quality of seeds |
| Ethylcellulose | Not N, S, ND | Samal et al. [273] | ||
| Carboxymethylcellulose | Not N, S, not B according to OECD 301E [274] | Rocha et al. [203] Abdelzaher et al. [208] | ||
| Hydroxypropyl cellulose | Not N, S, not B according to OECD 301D [275] | Samal et al. [273] | ||
| Sodium alginate + Ca(NO3)2 | N, S, B according to OECD TG 301F [276] | de Castro et al. [277] | ||
| Chitosan | Not N, not S, B according to OECD 301B [278] | Rocha et al. [203] | ||
| Chitosan lignosulfonate | Not N, not S, ND | Thobunluepop [279] | ||
| Gum arabic | N, S, ND | Zhang et al. [209]; Javed et al. [177]; Rocha et al. [203] | ||
| Pre-gelatinized starch | N, S, B according to OECD TG 301B [280] | Accinelli et al. [227] | Seed dust-off and physiologic quality of seeds | |
| Pulullan | N, S, ND | AU 2015276335 B2 [223] | Drying speed, seed dust-off, flow ability, physiologic quality of seeds | |
| Guar gum | Absorbent | N, S, B according to OECD TG 301 F [281] | Chen et al. [282] | Seed dust-off, flow ability, physiologic quality of seeds |
| Hydroxypropylguar | Not N, S, ND | |||
| Denatured starch + chitin + glycerol (0.2% w/w) | Film-forming | N, S, ND | Accinelli et al. [249] | Seed dust-off; physiological quality of seeds |
| Proteins | ||||
| Soy flour | Film-forming | N, not S, ND | Javed et al. [177] | Physiologic quality of seeds |
| Zein | N, not S, ND | AU 2015276335 B2 [223] | Drying speed, seed dust-off, flow ability, physiologic quality of seeds | |
| Casein | N, not S, ND | |||
| Gelatin | N, not S, ND | |||
| Soy protein isolate + Starch + arabic gum | N, not S, ND | Accinelli et al. [228] | Seed dust-off, physiological quality of seeds | |
| Silk fibroin/trehalose inner layer + pectin/caboxymethylcellulose outer layer | Film-forming + absorbent | N, not S, ND | Zvinavashe et al. [283] | Physiological quality of seeds |
| Synthetic polymers | ||||
| Polyvinylpyrrolidone | Film-forming | Not N, S, not B according to OECD TG 302B [284] | Halecky et al. [169]; Samal et al. [273]; Kimmelshue et al. [285] | Seed dust-off, physiological quality of seeds |
| Polyvinyl alcohol | Not N, S, B according to OECD TG 302B [286] | Javed et al. [177]; Halecky et al. [169] | Seed dust-off, physiological quality of seeds | |
| Polyacrylamide (PAM) | Absorbent | Not N, S, ND | Amirkhani et al. [243]; Su et al. [287] | Flow ability, physiological quality of seeds |
| PAM with graphite (PAM + G) | Not N, S, ND | Amirkhani et al. [243] | ||
| Acrylamide and sodium acrylate copolymer | Not N, S, ND | Su et al. [287] | Physiological quality of seeds | |
| Acrylamide and potassium acrylate copolymer | Not N, S, not B according to OCDE TG 302B [288] | Su et al. [287] | ||
| Sodium polyacrylate | Not N, S, ND | Su et al. [287] | ||
| Others | ||||
| Lignin/polyethylene glycol copolymer | Film-forming | Not N, not S, ND | Gu et al. [216] | Release of active ingredients |
| Shellac | N, not S, ND | AU 2015276335 B2 [223] | Drying speed, seed dust-off, flow ability, physiologic quality of seeds | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langlet, R.; Valentin, R.; Morard, M.; Raynaud, C.D. Transitioning to Microplastic-Free Seed Coatings: Challenges and Solutions. Polymers 2024, 16, 1969. https://doi.org/10.3390/polym16141969
Langlet R, Valentin R, Morard M, Raynaud CD. Transitioning to Microplastic-Free Seed Coatings: Challenges and Solutions. Polymers. 2024; 16(14):1969. https://doi.org/10.3390/polym16141969
Chicago/Turabian StyleLanglet, Rozenn, Romain Valentin, Marie Morard, and Christine Delgado Raynaud. 2024. "Transitioning to Microplastic-Free Seed Coatings: Challenges and Solutions" Polymers 16, no. 14: 1969. https://doi.org/10.3390/polym16141969







