Structure, Property Optimization, and Adsorption Properties of N,N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly (AA)-Based Hydrogels
2.3. Characterization of PAA Hydrogel
2.4. Measurement of Solubility, Water Loss Rate, and Hygroscopicity
2.5. Mechanical Testing of Hydrogels
2.6. Applied Research on the Adsorption of Lead Ions
3. Results
3.1. FTIR Analysis
3.2. Nitrogen Isothermal Adsorption–Desorption Analysis (BET) and Pore Structure Analysis
3.3. SEM Analysis
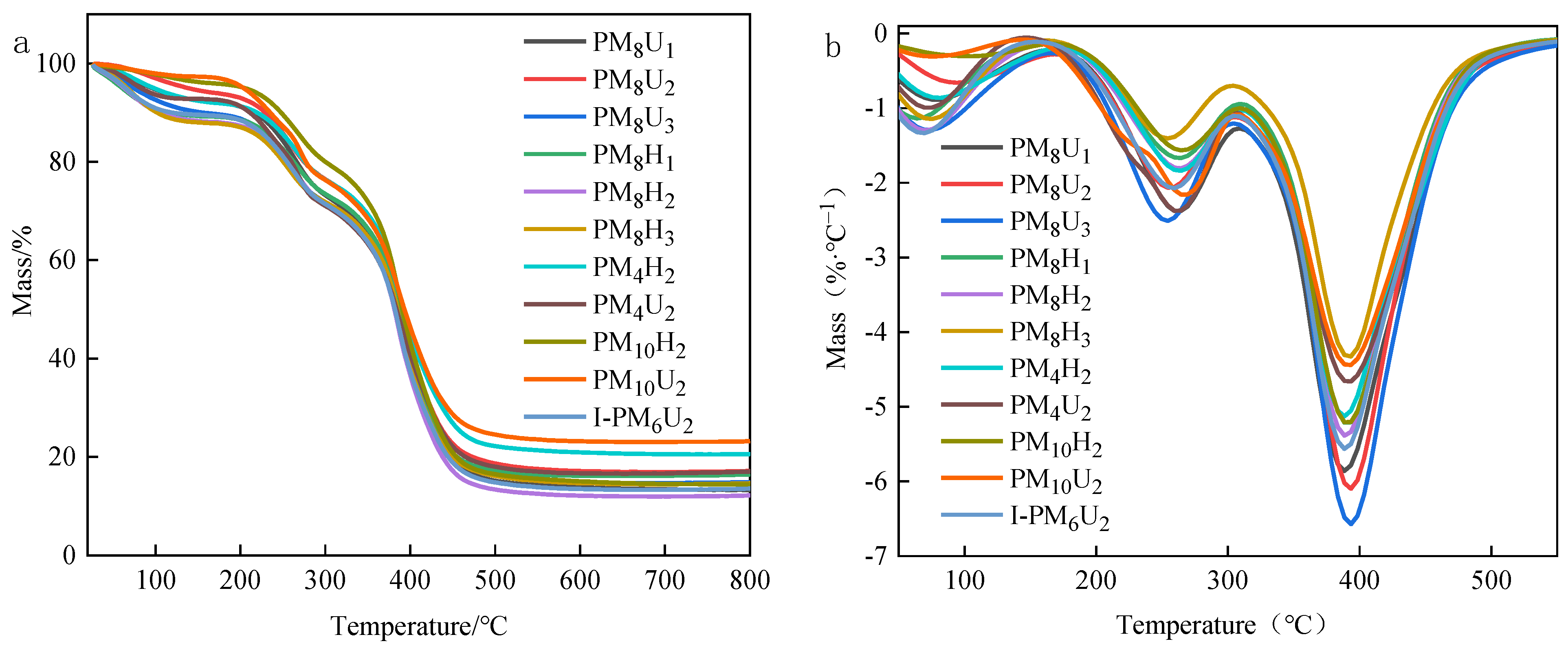
3.4. Thermal Stability Testing
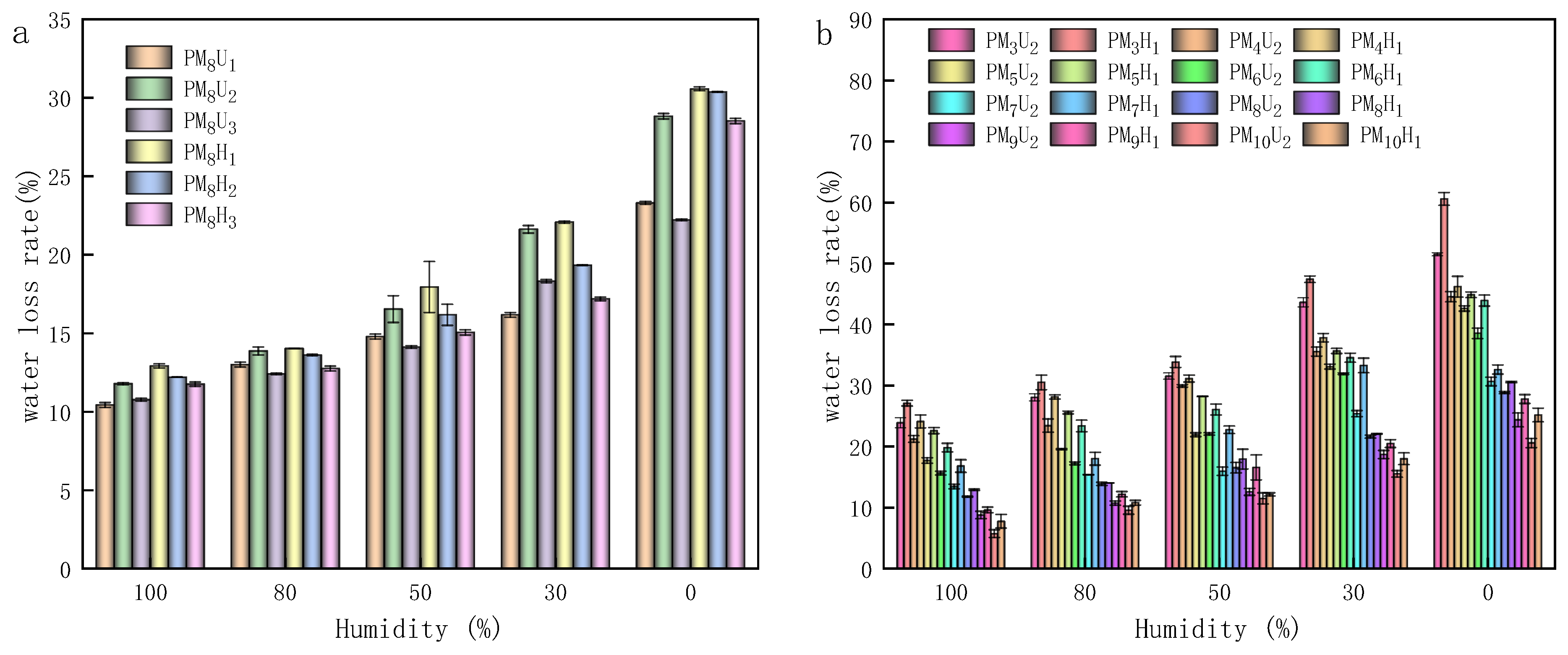
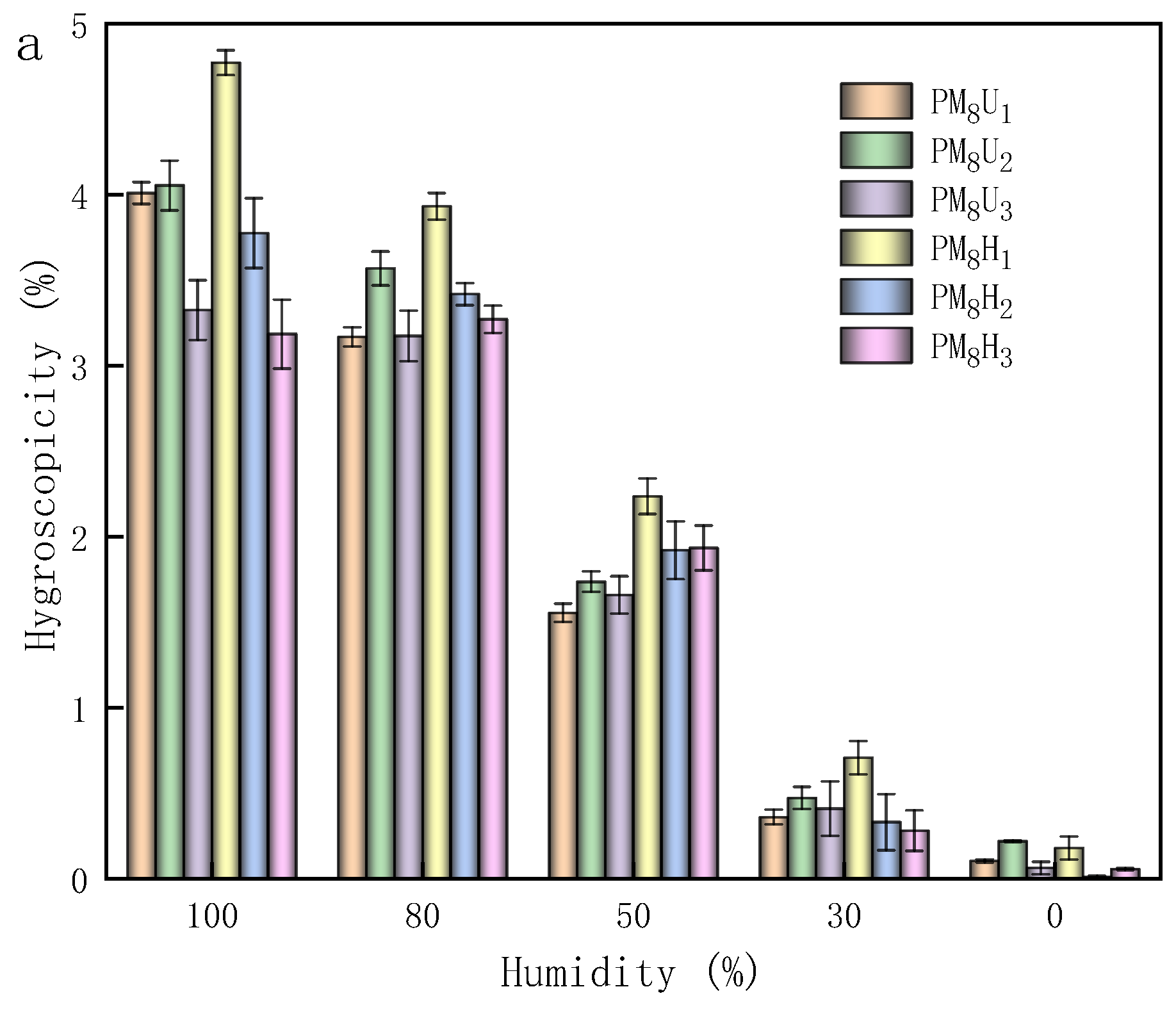
3.5. Solubility, Water Loss Rate, and Hygroscopicity of PAA Hydrogels
3.6. Mechanical Properties of Hydrogels
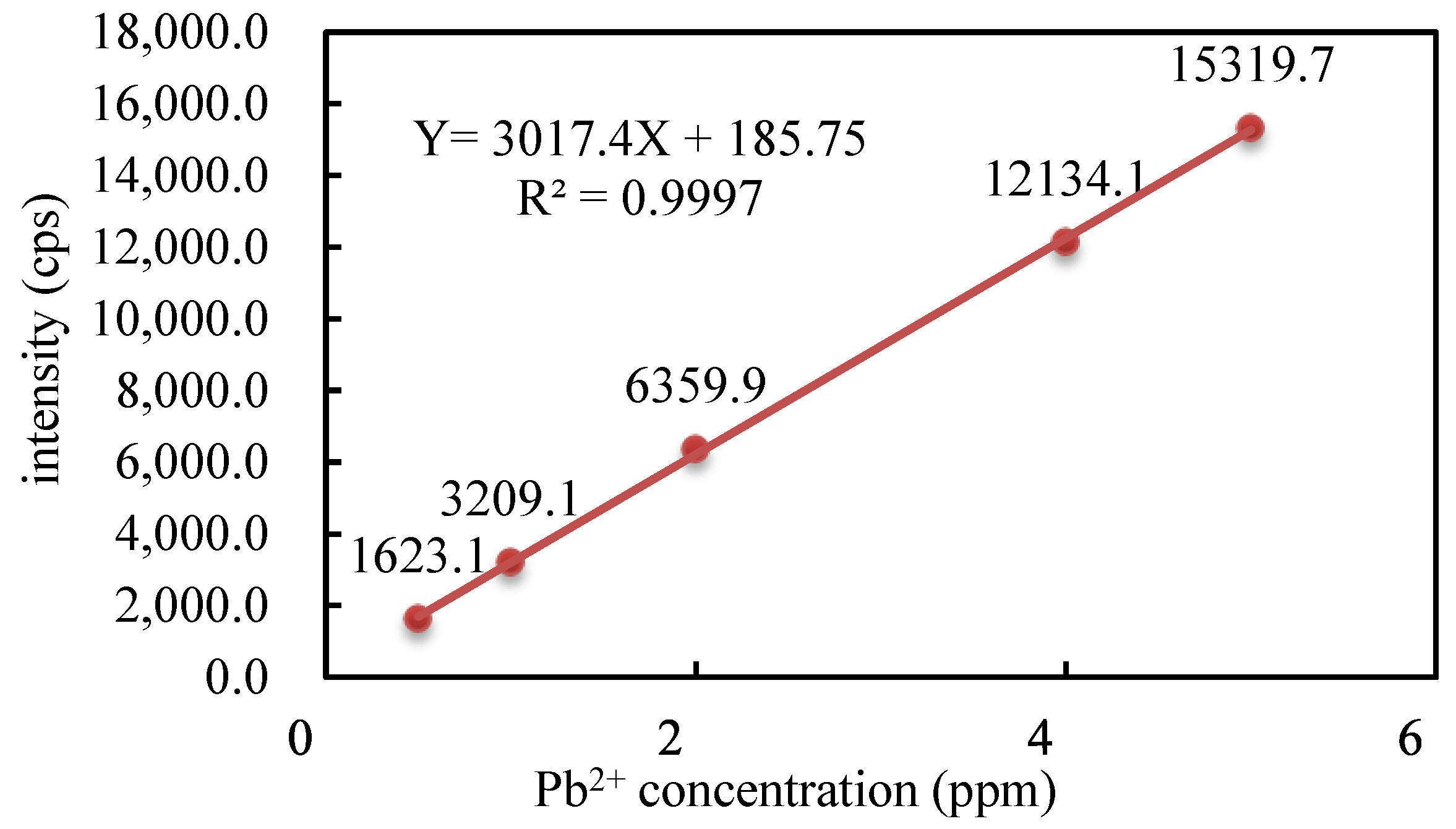
3.7. Research on the Application of PAA Hydrogel to Adsorb Lead Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Inphonlek, S.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer. Polymers 2023, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiang, P.; Zhang, P.; Duan, N.; Liu, Q.; Qin, C. Cross-Linked Polyacrylic-Based Hydrogel Polymer Electrolytes for Flexible Supercapacitors. Polymers 2024, 16, 800. [Google Scholar] [CrossRef]
- Gokmen, F.O.; Yaman, E.; Temel, S. Eco-friendly polyacrylic acid based porous hydrogel for heavy metal ions adsorption: Characterization, adsorption behavior, thermodynamic and reusability studies. Microchem. J. 2021, 65, 106357. [Google Scholar] [CrossRef]
- An, X.; Yu, J.; Yu, J.; Tahmasebi, A.; Wu, Z.; Liu, X.; Yu, B. Incorporation of biochar into semi-interpenetrating polymer networks through graft co-polymerization for the synthesis of new slow-release fertilizers. J. Clean. Prod. 2020, 272, 122731. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Q.; Liu, S.; Lin, D.; Zhao, H.; Liu, X.; Zhou, G. Research progress on antimicrobial hydrogel dressing for wound repair. Eur. Polym. J. 2023, 197, 112372. [Google Scholar] [CrossRef]
- Tan, Z.; Li, X.; Yu, C.; Yao, M.; Zhao, Z.; Guo, B.; Liang, L.; Wei, Y.; Yao, F.; Zhang, H.; et al. A self-gelling powder based on polyacrylic acid/polyacrylamide/quaternate chitosan for rapid hemostasis. Int. J. Biol. Macromol. 2023, 45, 123449. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Qin, H.; Guo, W.; Gao, P.; Xiao, H. Porous reticular CuO/ZnO/CeO2/ZrO2 catalyst derived from polyacrylic acid hydrogel system on Al2O3 foam ceramic support for methanol steam reforming microreactor. Ceram. Int. 2021, 47, 33667–33677. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Y.; Sun, X.; Liang, H. Preparation of stretchable and self-healable dual ionically cross-linked hydrogel based on chitosan/polyacrylic acid with anti-freezing property for multi-model flexible sensing and detection. Int. J. Biol. Macromol. 2021, 43, 629–637. [Google Scholar] [CrossRef]
- Mohammadi, R.; Saboury, A.; Javanbakht, S.; Foroutan, R.; Shaabani, A. Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur. Polym. J. 2021, 57, 110500. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhang, L. Ultrastretchable hydrogels with strong damping effects. Polym. J. 2024, 56, 599–607. [Google Scholar] [CrossRef]
- GB/T 16491-2022; Electronic Universal Testing Machine. National Standard of the People’s Republic of China: Beijing, China, 2022.
- Suda, A.; Kumatani, N.; Morikawa, A.; Hatanaka, M.; Iwasaki, M. Fabrication of ultrafine cerium oxide nanoparticles as an aqueous colloidal solution with single-molecular dispersant via shear agitation reactor or one-pot hydrolysis. Adv. Powder Technol. 2023, 34, 104232. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Si, S.; Yue, J. Study on the Effect of Pore Structure on Desorption Hysteresis of Deep Coking Coal under High-Temperature and High-Pressure Conditions. ACS Omega 2024, 9, 3709–3729. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, W.; Goworek, J.; Biliński, B. Pore size analysis by nitrogen adsorption and thermal desorption. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 231–237. [Google Scholar] [CrossRef]
- Virtanen, T.; Rudolph, G.; Lopatina, A.; Al-Rudainy, B.; Schagerlöf, H.; Puro, L.; Kallioinen, M.; Lipnizki, F. Analysis of membrane fouling by Brunauer-Emmet-Teller nitrogen adsorption/desorption technique. Sci. Rep. 2020, 10, 3427. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, Y.; Wang, G.; Song, J.; Hayat, U.; Liu, C.; Raza, A.; Huang, X.; Lin, H.; Wang, J.Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater. 2021, 17, 289–301. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Gu, Z.; Luo, K.; Yang, J.; Zhang, S. Multifunctional Poly(acrylic acid)/Chitosan nanoparticle network hydrogels with tunable mechanics. Appl. Mater. Today 2022, 9, 101696. [Google Scholar] [CrossRef]
- Amoozadeh, P.; Mohsen Sarrafi, A.H.; Shirkavand Hadavand, B.; Niazi, A.; Konoz, E. UV-curable hybrid hydrogels of carbon quantum dots: Synthesis, characterizations and investigation of properties and rheological behavior. Polym. Plast. Technol. Mater. 2022, 61, 2063–2072. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, J.; Fang, Y.; Yu, C.; Zhang, J.; Xue, Y.; Liu, Z.; Zhang, J.; Tang, C.; Huang, Y. Porous boron nitride nanofibers/PVA hydrogels with improved mechanical property and thermal stability. Ceram. Int. 2018, 44, 22439–22444. [Google Scholar] [CrossRef]
- Nakano, S.I.; Yamaguchi, D.; Sugimoto, N. Thermal stability and conformation of DNA and proteins under the confined condition in the matrix of hydrogels. Mol. Biol. Rep. 2018, 45, 403–411. [Google Scholar] [CrossRef]
- De, N.M.; Jin, F.X. The preparation of double network hydrogel with high mechanical properties by photopolymerization under the green LED irradiation and enhancement of wet adhesion by tannic acid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 31, 131656. [Google Scholar]
- Goestenkors, A.P.; Liu, T.; Okafor, S.S.; Semar, B.A.; Alvarez, R.M.; Montgomery, S.K.; Friedman, L.; Rutz, A.L. Manipulation of cross-linking in PEDOT:PSS hydrogels for biointerfacing. J. Mater. Chem. B 2023, 11, 11357–11371. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Gao, G.; Ren, X.; Xia, S.; Gao, Y.; Wang, Q.; Duan, L. Mechanical Property of Hydrogels Regulated by Different Ratios of Latex Particles and Hydrophobic Segments. ChemistrySelect 2018, 3, 4562–4568. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Liu, S.; Chen, K.; Feng, C.; Li, X.; Qi, J.; Luo, Y.; Liu, H.; Zhang, D. Cartilage-bone inspired the construction of soft-hard composite material with excellent interfacial binding performance and low friction for artificial joints. Friction 2022, 11, 1177–1193. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Gong, Y.; Cheng, Y.; Yang, H.; Kang, M.; Ding, H.; Lei, Z.; Wei, Y.; Huang, D. A facile method to fabricate high performance PVA/PAA-AS hydrogel via the synergy of multiple hydrogen bonding and Hofmeister effect. J. Biomater. Sci. Polym. Ed. 2022, 34, 11–15. [Google Scholar] [CrossRef]
- Gao, Q.; Li, C.; Wang, M.; Zhu, J.; Gao, C. A low-hysteresis, self-adhesive and conductive PAA/PEDOT: PSS hydrogel enabled body-conformable electronics. J. Mater. Chem. C 2023, 11, 9355–9365. [Google Scholar] [CrossRef]
- Li, C.; Tan, K.; Hua, F.; Wang, C.; Sun, M.; Wang, F.; Wang, X. Eco-friendly hydrogels serving as water carriers for improving methane hydrate formation and dissociation processes. Fuel 2024, 303, 130854. [Google Scholar] [CrossRef]
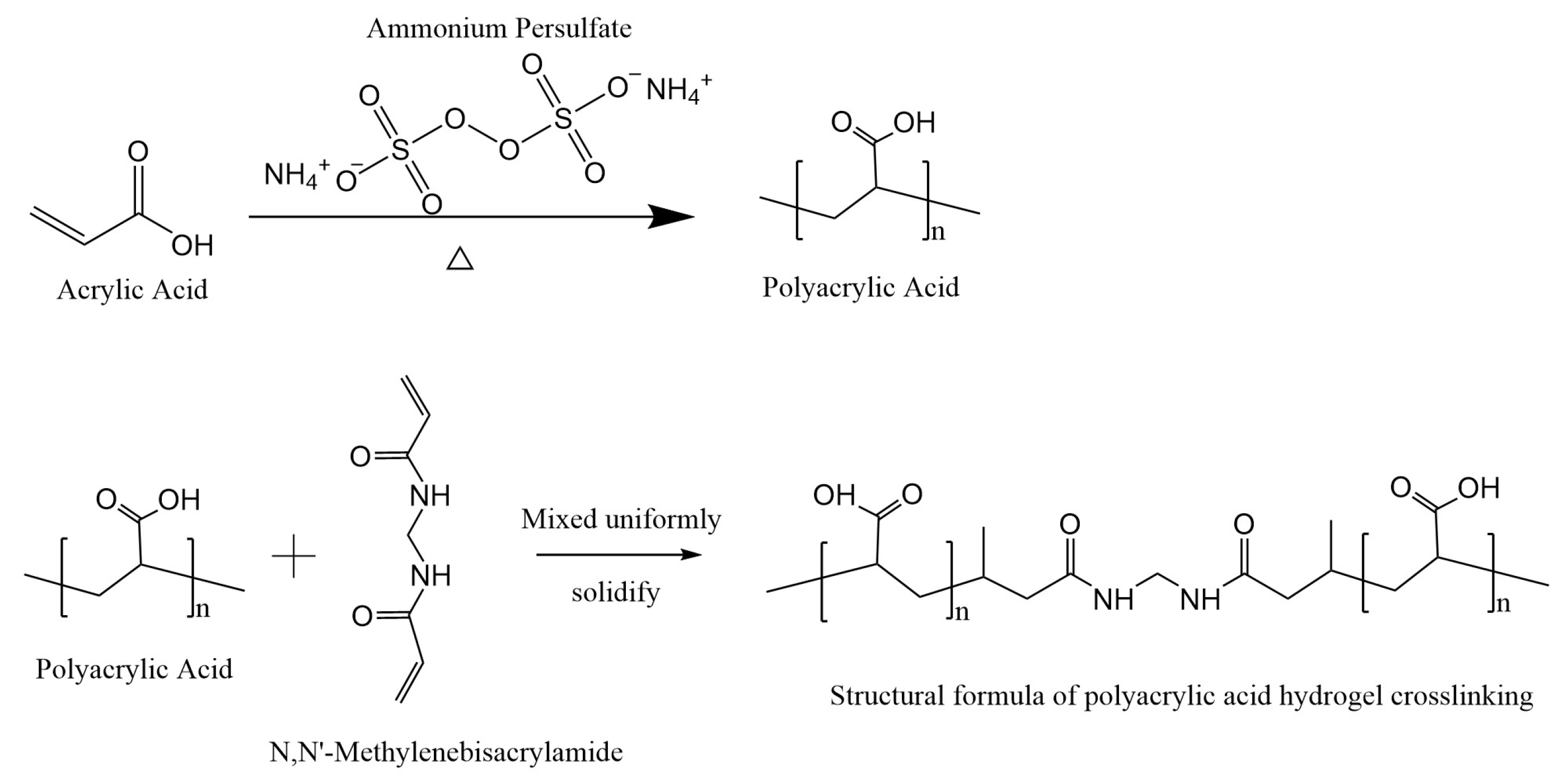
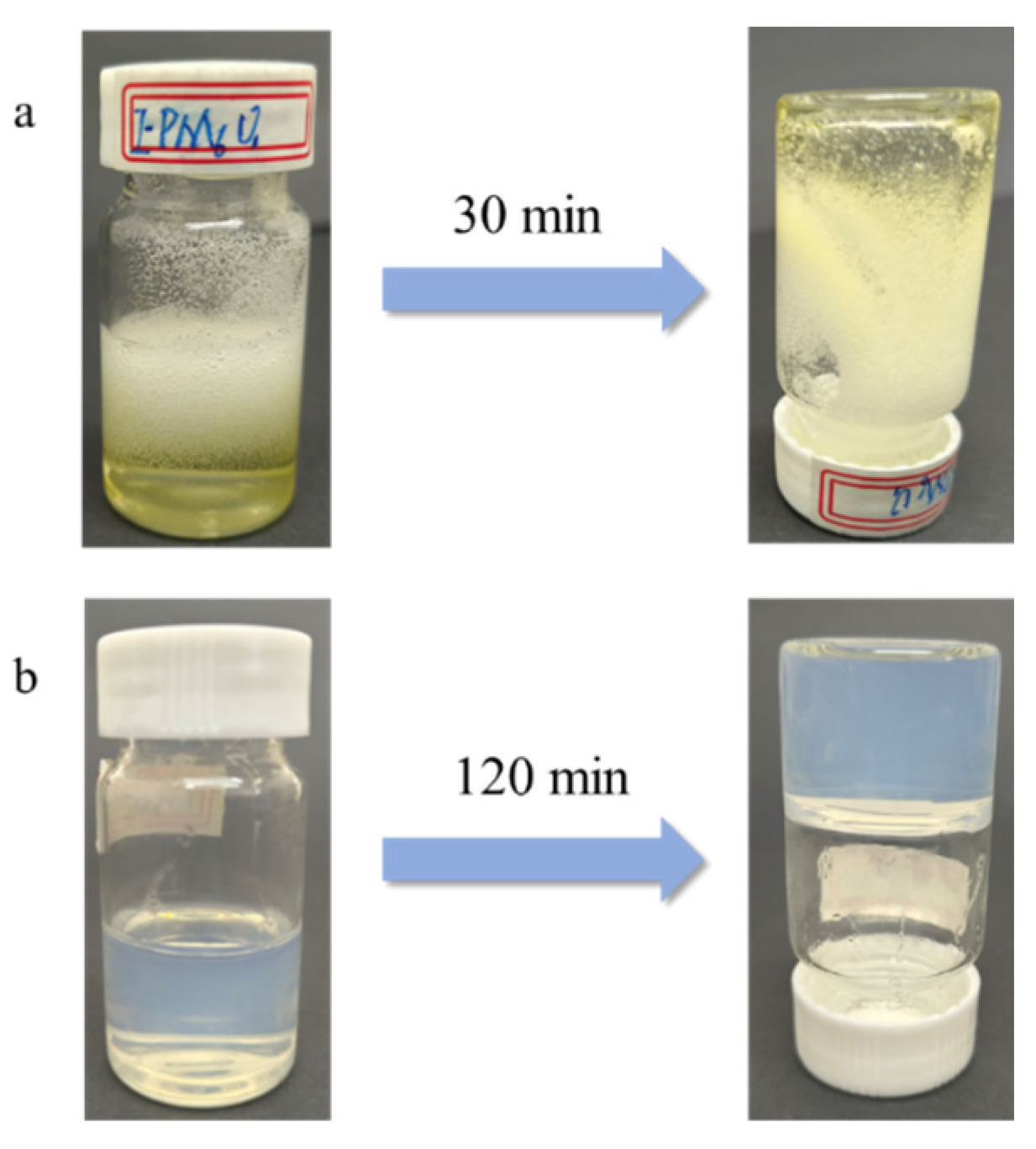
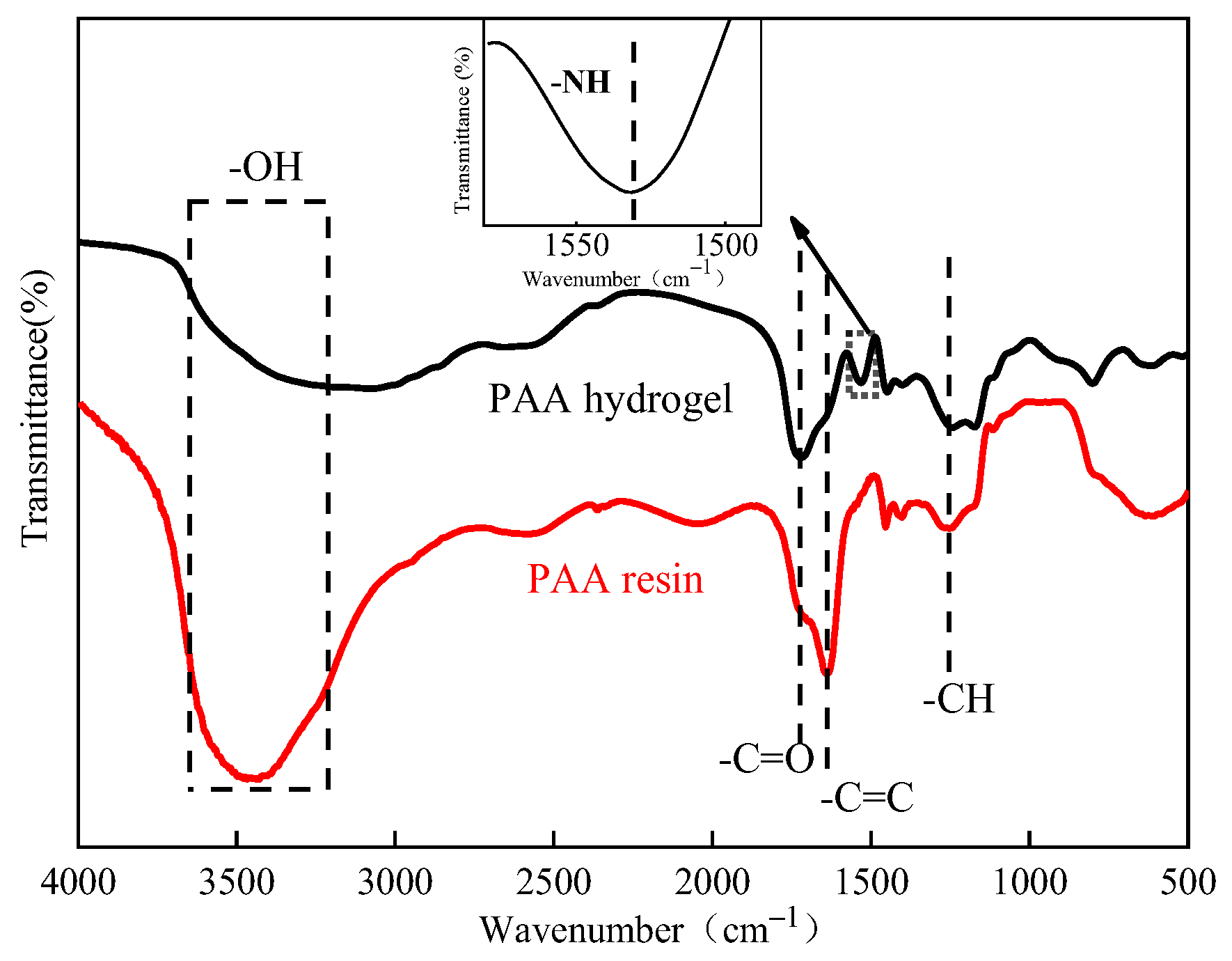


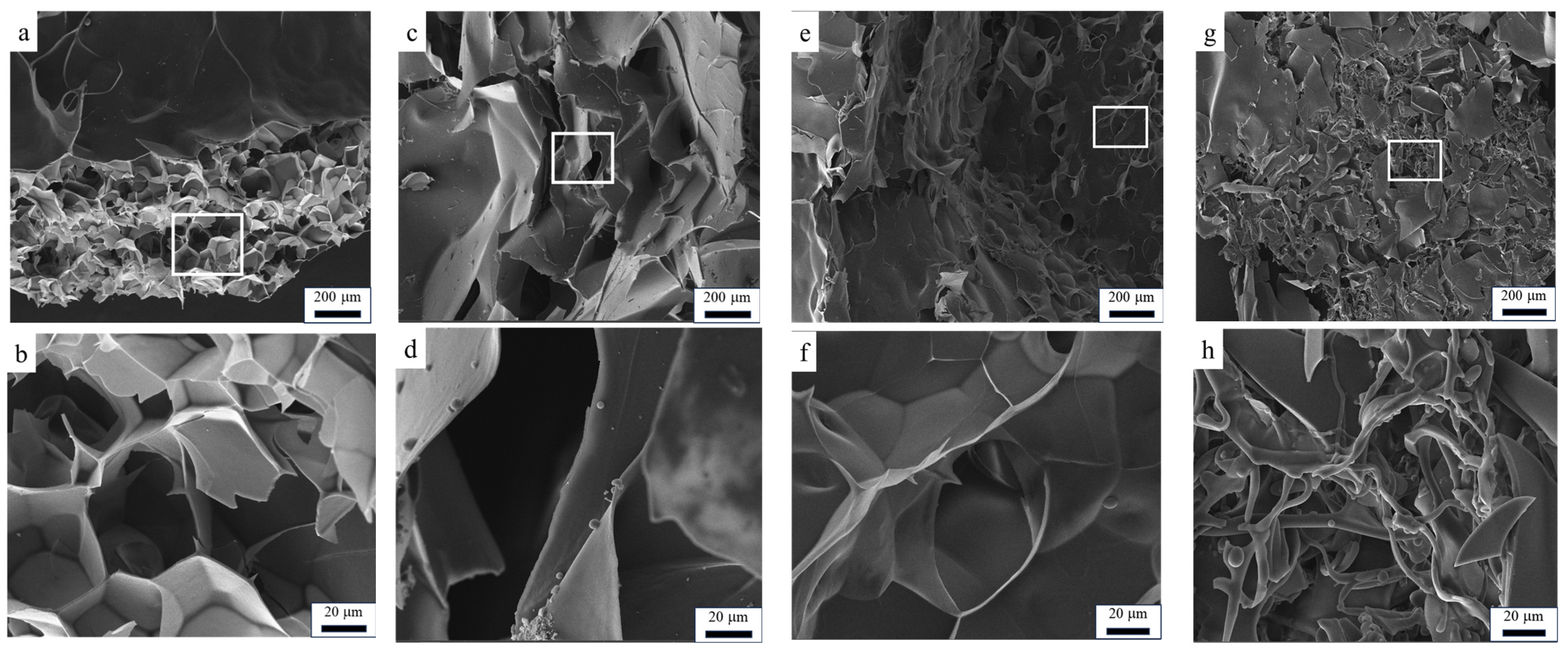



| Christen | Cross-Linking Agent Mass Ratio (%) | (g) | Hydrogel Curing Method |
|---|---|---|---|
| PM3U2 | 1.48 | 0.15 | UV-20 min |
| PM3H1 | 1.48 | 0.15 | Heat curing-2 h |
| PM4U2 | 1.96 | 0.20 | UV-20 min |
| PM4H1 | 1.96 | 0.20 | Heat curing-2 h |
| PM4H2 | 1.96 | 0.20 | Heat curing-4 h |
| PM5U2 | 2.44 | 0.25 | UV-20 min |
| PM5H1 | 2.44 | 0.25 | Heat curing-2 h |
| PM5H2 | 2.44 | 0.25 | Heat curing-4 h |
| PM6U2 | 2.91 | 0.30 | UV-20 min |
| PM6H1 | 2.91 | 0.30 | Heat curing-2 h |
| PM6H2 | 2.91 | 0.30 | Heat curing-4 h |
| PM7U2 | 3.38 | 0.35 | UV-20 min |
| PM8U1 | 3.85 | 0.40 | UV-10 min |
| PM8U2 | 3.85 | 0.40 | UV-20 min |
| PM9U2 | 4.31 | 0.45 | UV-20 min |
| PM9H1 | 4.31 | 0.45 | Heat curing-2 h |
| PM9H2 | 4.31 | 0.45 | Heat curing-4 h |
| PM10U2 | 4.76 | 0.50 | UV-20 min |
| PM10H1 | 4.76 | 0.50 | Heat curing-2 h |
| PM10H2 | 4.76 | 0.50 | Heat curing-4 h |
| I-PM6U1 | 2.91 | 0.3 + Photoinitiator I-2959 | UV-10 min |
| Sample Name | 10% Mass Loss Temperature (°C) | 50% Mass Loss Temperature (°C) | Remaining Charcoal (%) |
|---|---|---|---|
| PM8H1 | 215.4 | 384.0 | 13.3 |
| PM8H2 | 227.9 | 390.1 | 17.0 |
| PM8H3 | 143.7 | 386.5 | 14.8 |
| PM8U1 | 110.3 | 385.6 | 16.6 |
| PM8U2 | 100.2 | 380.8 | 12.2 |
| PM8U3 | 101.1 | 384.0 | 14.6 |
| PM4H2 | 217.4 | 390.1 | 20.5 |
| PM4U2 | 209.7 | 384.0 | 16.9 |
| PM10H2 | 246.0 | 390.8 | 14.5 |
| PM10U2 | 233.6 | 392.3 | 23.2 |
| I-PM6U2 | 118.6 | 380.5 | 13.6 |
| Sample Name | Elastic Modulus (kPa) | Maximum Strength (kPa) | Elongation at Break (%) |
|---|---|---|---|
| PM8H1 | 0.05 ± 0.007 | 5 ± 0.2 | 142 ± 13 |
| PM8H2 | 0.11 ± 0.02 | 8 ± 0.6 | 112 ± 12 |
| PM8H3 | 0.05 ± 0.003 | 9 ± 0.6 | 297 ± 21 |
| PM8U1 | 0.78 ± 0.05 | 45 ± 5 | 126 ± 14 |
| PM8U2 | 2.79 ± 0.14 | 135 ± 12 | 171 ± 17 |
| PM8U3 | 3.7 ± 0.19 | 130 ± 11 | 101 ± 9 |
| PM5U2 | PM5H2 | PM6U2 | PM6H2 | PM7U2 | PM7H2 | PM8U2 | PM8H2 | |
|---|---|---|---|---|---|---|---|---|
| cps | 10,352.5 | 10,266.4 | 9948.1 | 10,084.7 | 10,337.4 | 9917.1 | 10,139.9 | 9974.3 |
| Pb2+ concentration/ppm | 3.37 | 3.34 | 3.24 | 3.28 | 3.36 | 3.23 | 3.30 | 3.24 |
| AC/% | 0.42 | 1.26 | 4.38 | 3.04 | 0.57 | 4.69 | 2.50 | 4.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Qu, D.; Wang, S.; Qi, S.; Zuo, H. Structure, Property Optimization, and Adsorption Properties of N,N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions. Polymers 2024, 16, 1990. https://doi.org/10.3390/polym16141990
Zhang J, Qu D, Wang S, Qi S, Zuo H. Structure, Property Optimization, and Adsorption Properties of N,N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions. Polymers. 2024; 16(14):1990. https://doi.org/10.3390/polym16141990
Chicago/Turabian StyleZhang, Jinyu, Dezhi Qu, Shuyu Wang, Shien Qi, and Huajiang Zuo. 2024. "Structure, Property Optimization, and Adsorption Properties of N,N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions" Polymers 16, no. 14: 1990. https://doi.org/10.3390/polym16141990
APA StyleZhang, J., Qu, D., Wang, S., Qi, S., & Zuo, H. (2024). Structure, Property Optimization, and Adsorption Properties of N,N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions. Polymers, 16(14), 1990. https://doi.org/10.3390/polym16141990







