Toward the Manufacturing of a Non-Toxic High-Performance Biobased Epoxy–Hemp Fibre Composite
Abstract
1. Introduction
- (i)
- The epoxy–hardener mixture must be fluid enough to facilitate the impregnation step at a temperature for which the cross-linking remains slow, i.e., at a temperature well below the curing temperature. This condition should allow for a gel time compatible with conventional composite manufacturing processes. The cross-linking temperature should obviously be lower than the degradation temperature of natural fibres. Usually, the viscosity required for these systems should be less than 1 Pa s. for vacuum infusion.
- (ii)
- The prepolymer mixture will be selected to give strongest fibre/matrix interface by maximising the attractive interactions, i.e., weak and, if possible, covalent bonds between the polymer matrix and the biochemical components of the fibre wall. Moreover, a system compatible with the water present in the plant fibres would be desirable in order to avoid the fibre dehydration step before the manufacture of the composite.
- (iii)
- The reactivity, non-toxicity and biosourcing aspects will also be considered to select the optimal epoxy–hardener system.
- (iv)
- The chemical structures of the prepolymers should provide the cross-linked material with sufficiently high mechanical properties for structural composite applications (E (Young modulus) > 2 GPa, σR (stress at failure) > 50 MPa, Tg > 100 °C…).
2. Materials and Methods
3. Results and Discussion
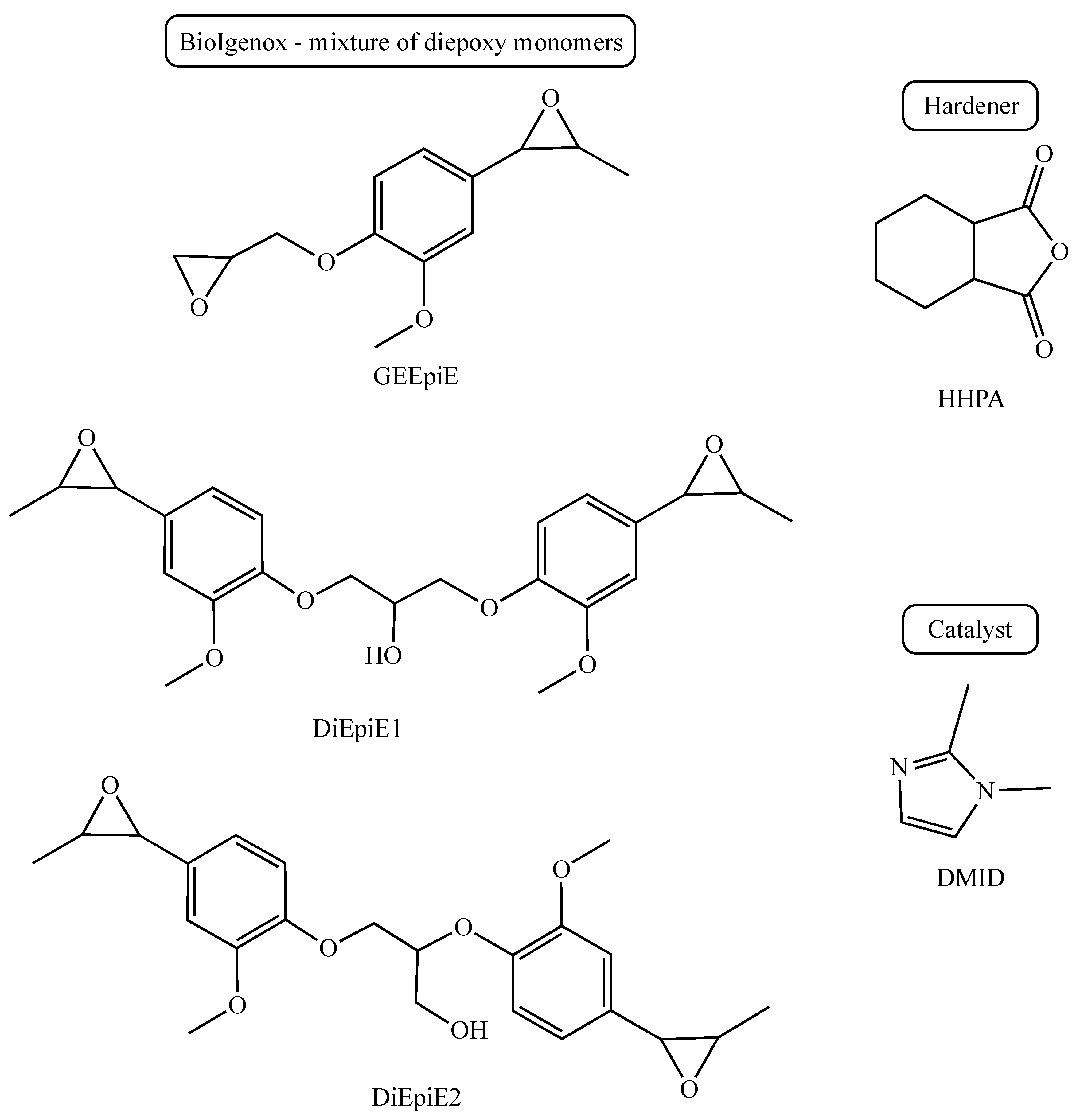
3.1. Choice of the Hardener
- (1)
- The cross-linking reaction is much less exothermic. This point is particularly interesting to make the temperature control easier during the implementation of the composite, and thus to prevent the damage to the natural fibres.
- (2)
- The final cross-linked product is less sensitive to oxidation reactions [41].
- (3)
- Lastly, the hydroxyl functions present at the fibre wall could also react with the anhydride functions, leading to the reinforcement of the fibre–matrix interface.
- (i)
- HHPA is a known solid hardener for epoxy resins. It contains a rigid ring that allows for the reinforcement of the mechanical properties of thermosets.
- (ii)
- HHPA has a low melting point (37 °C) and, in addition, a low melt viscosity (47 cps at 40 °C). Thus, it allows us to obtain a liquid mixture with the epoxy resin at a low temperature and facilitates the thermoset processing.
- (iii)
- With HHPA, the exothermic peak related to the cross-linking reaction is observed at about 100 °C, which is compatible with the use of the vegetable fibre reinforcements and limits the energy input during processing.
- (iv)
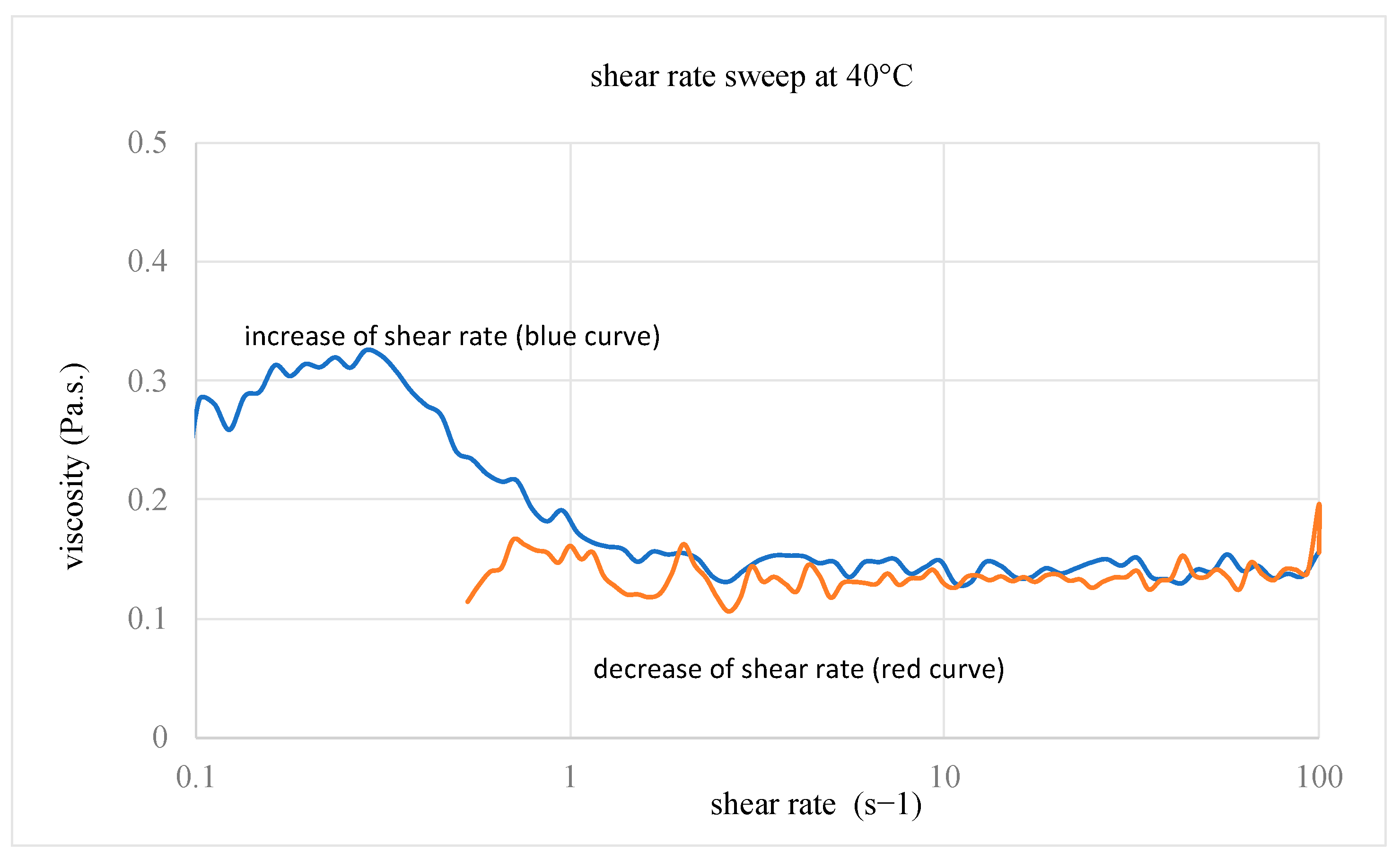
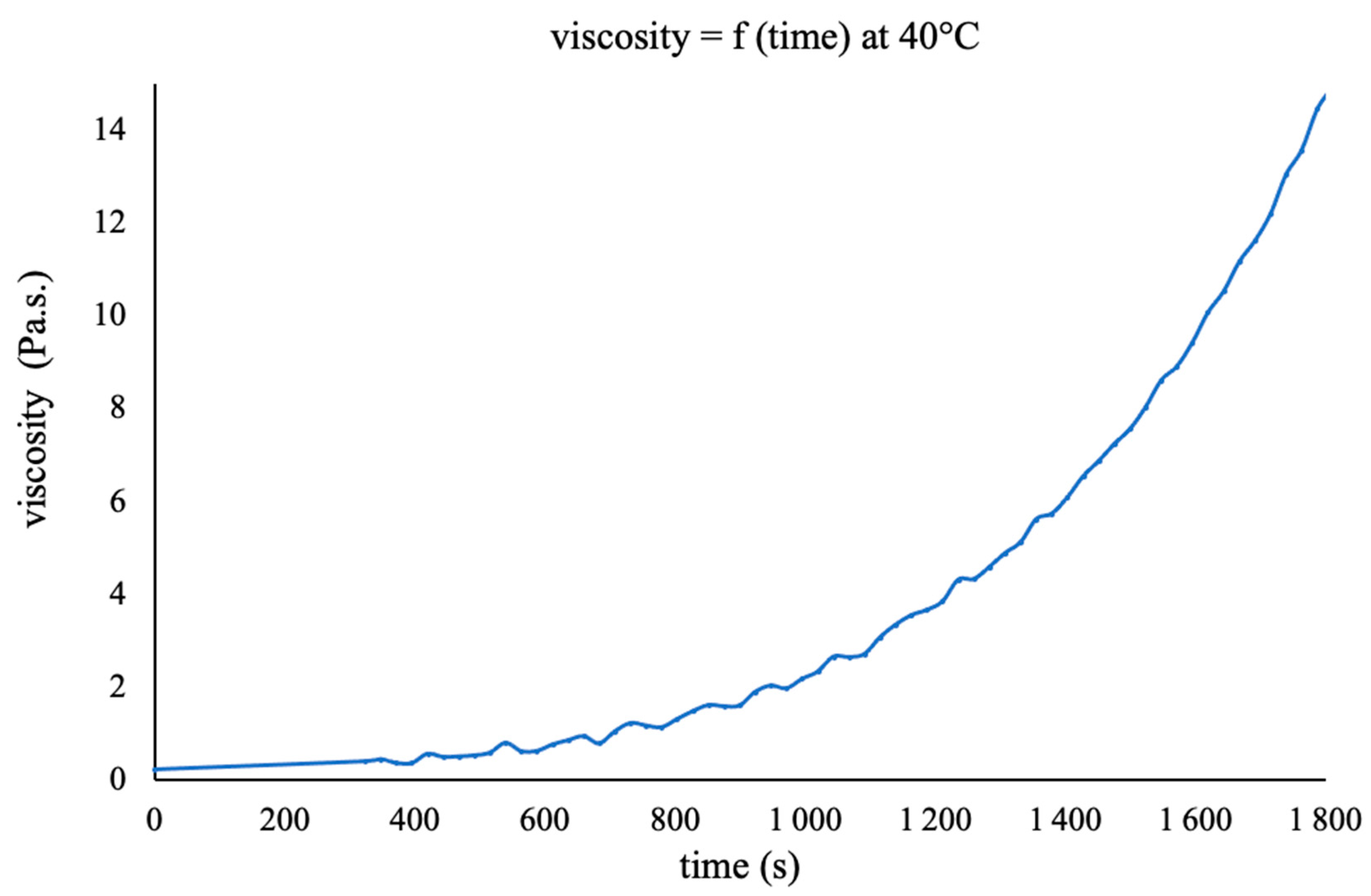
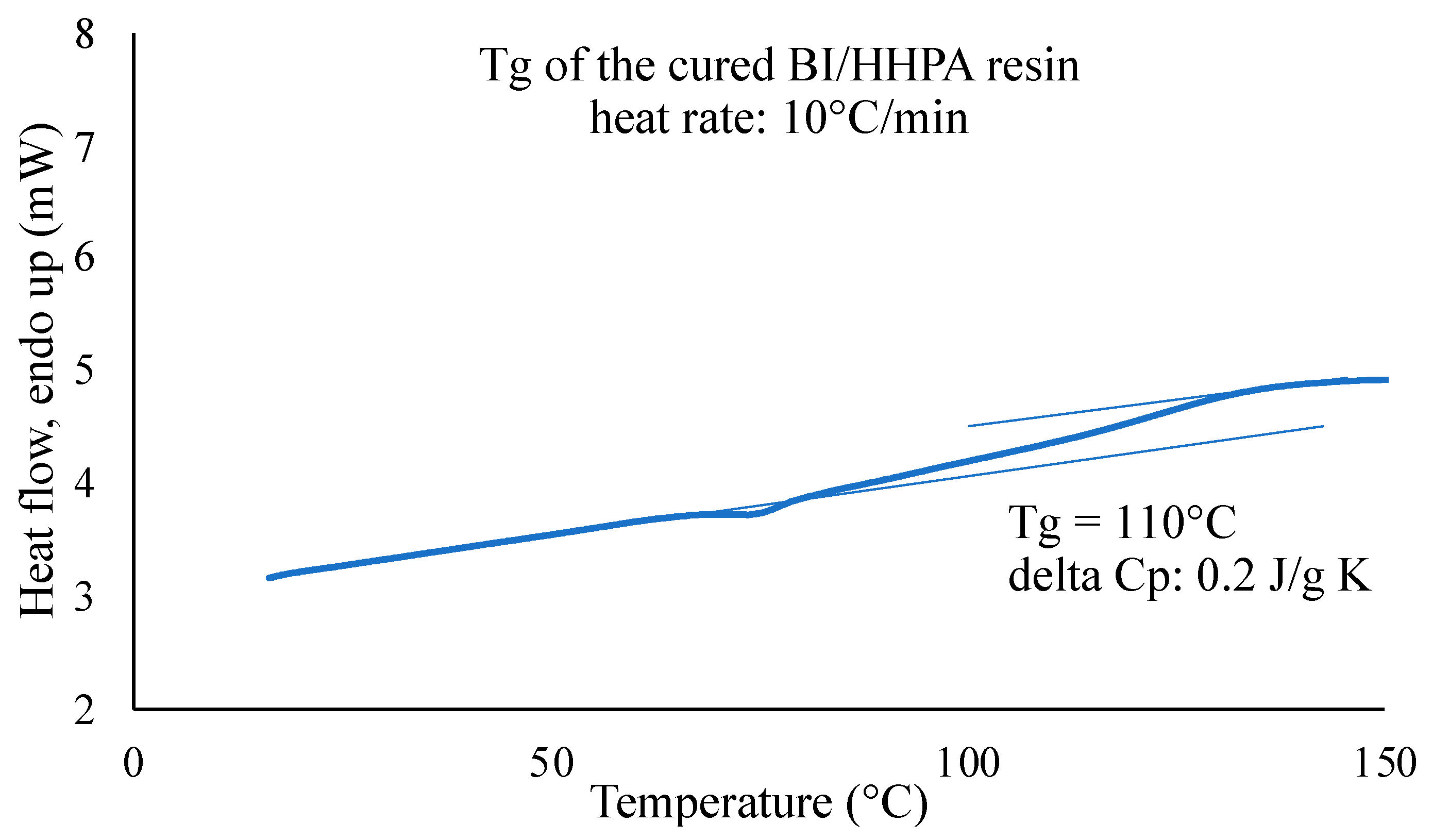
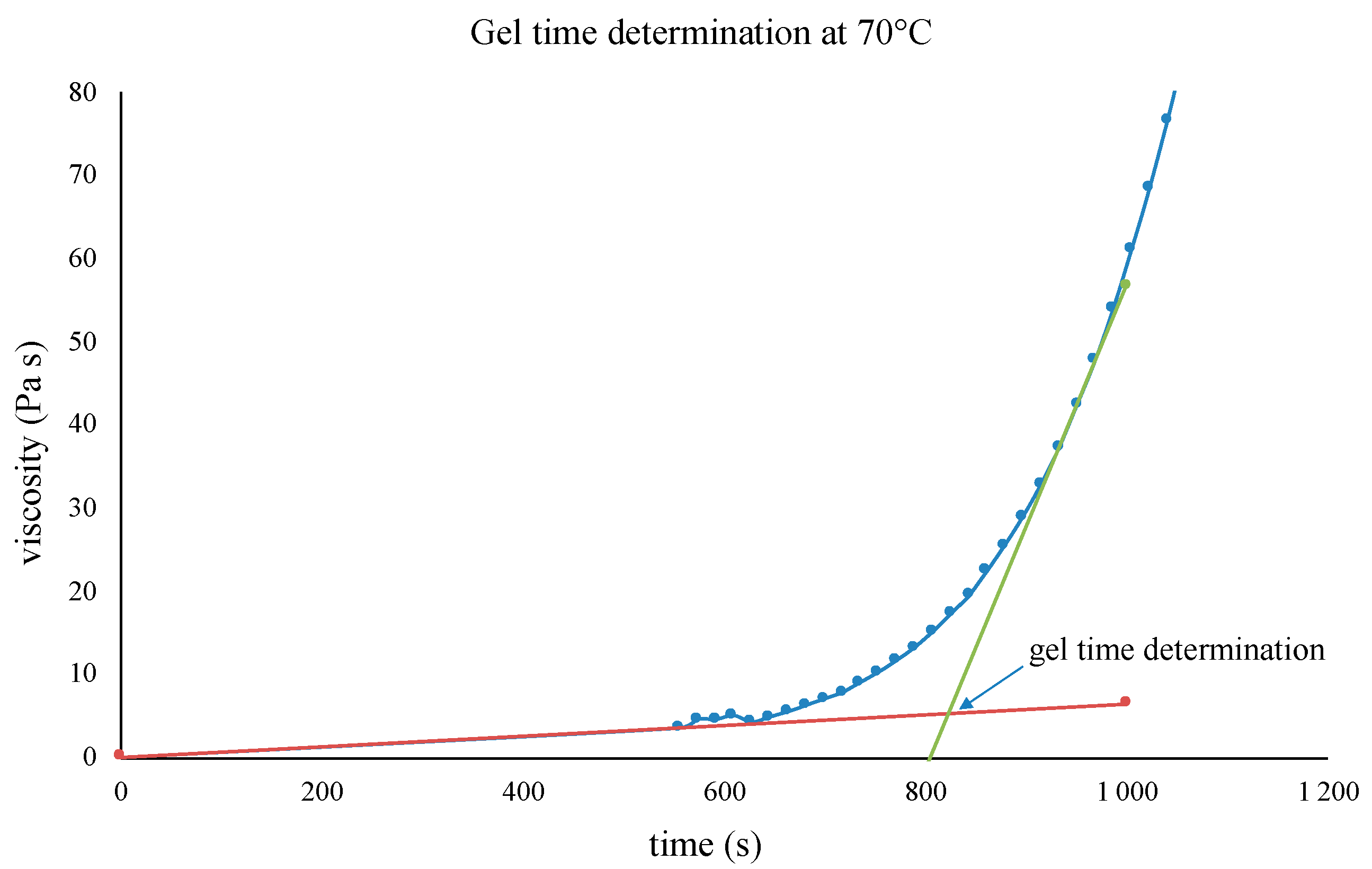
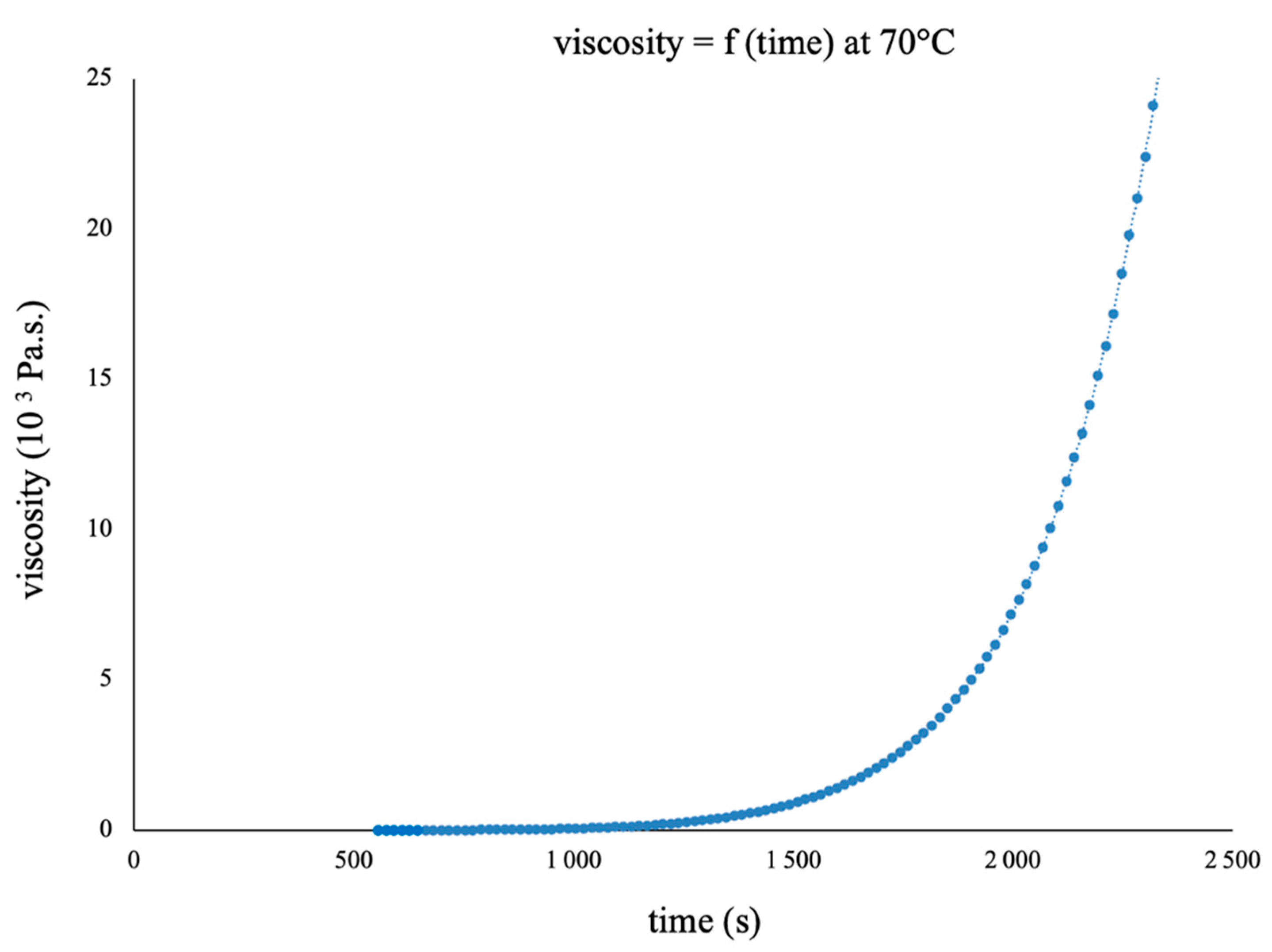
3.2. Choice of the Composite Processing: DSC Analyses and Rheological Measurements
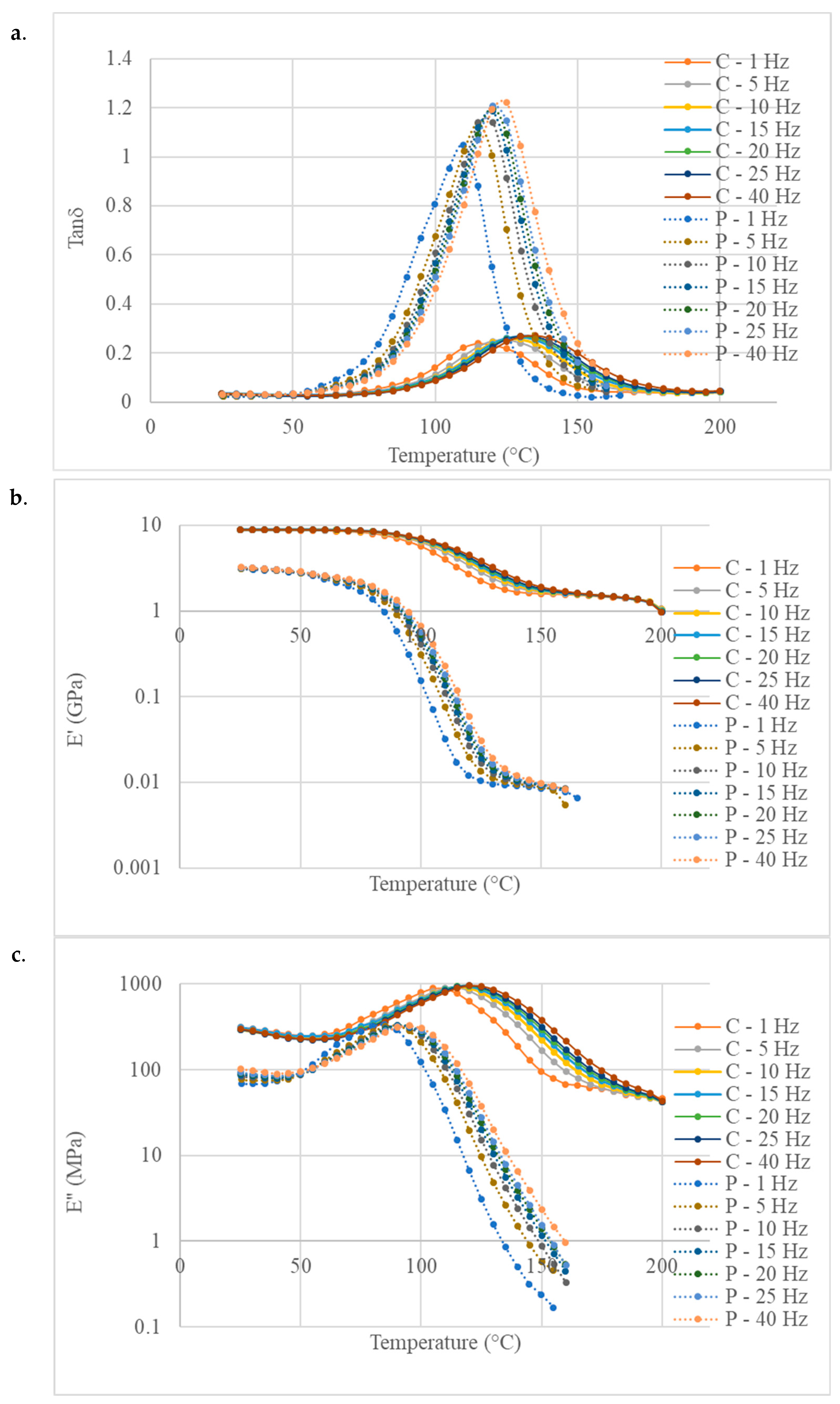
3.3. Characterisation of the Final Material
Bending Behaviour
3.4. Toxicology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Dal Pont, B.; Gigante, V.; Panariello, L.; Canesi, I.; Aliotta, L.; Lazzeri, A. Investigation of Novel Flax Fiber/Epoxy Composites with Increased Biobased Content. Polymers 2023, 15, 4030. [Google Scholar] [CrossRef] [PubMed]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Zarafshani, H.; Watjanatepin, P.; Lepelaar, M.; Verbruggen, J.; Ouagne, P.; De Luca, R.; Li, Q.; Scarpa, F.; Placet, V.; Van Acker, K. Environmental assessment of woven hemp fibre reinforced epoxy composites and potential applications in aerospace and electric scooter industries. Results Mater. 2023, 20, 100474. [Google Scholar] [CrossRef]
- Vinod, A.; Tengsuthiwat, J.; Gowda, Y.; Vijay, R.; Sanjay, M.R.; Siengchin, S.; Dhakal, H.N. Jute/Hemp bio-epoxy hybrid bio-composites: Influence of stacking sequence on adhesion of fiber-matrix. Int. J. Adhes. Adhes. 2022, 113, 103050. [Google Scholar] [CrossRef]
- Guo, Q. (Ed.) Thermosets: Structure, Properties, and Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef]
- Kloukos, D.; Pandis, N.; Eliades, T. Bisphenol-A and residual monomer leaching from orthodontic adhesive resins and polycarbonate brackets: A systematic review. Am. J. Orthod. Dentofacial. Orthol. 2013, 143, e1–e2. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione III, J.F. Recent advances in biobased epoxy resins and biobased epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 1–19. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Biobased epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame retardant biobased epoxy thermosets: A review. Compos. Part B Eng. 2019, 179, 107487. [Google Scholar] [CrossRef]
- Witthayolankowit, K.; Rakkijakan, T.; Ayub, R.; Kumaniaev, I.; Pourchet, S.; Boni, G.; Watjanatepin, P.; Zarafshani, H.; Gabrion, X.; Chevallier, H.; et al. Use of a fully biobased and non-reprotoxic epoxy polymer and woven hemp fabric to prepare environmentally friendly composite materials with excellent physical properties. Compos. Part B Eng. 2023, 258, 110692. [Google Scholar] [CrossRef]
- Hu, Y.; Nabipour, H.; Wang, X. Biobased Flame-Retardant Technology for Polymeric Materials, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Liu, K.; Madbouly, S.A.; Kessler, M.R. Biorenewable thermosetting copolymer based on soybean oil and eugenol. Eur. Polym. J. 2015, 69, 16–28. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased Heat Resistant Epoxy Resin with Extremely High Biomass Content from 2,5-Furandicarboxylic Acid and Eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Li, Z.; Wang, X. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar] [CrossRef]
- Danthu, P.; Ranoarison, K.M.; Rakotondravelo, J.C.; Michel-Dounias, I.; Tiollier, M.; Michels, T.; Normand, F.; Razafimamonjison, G.; Fawbush, F.; Jahiel, M. The clove tree Madagascar: A success story with an unpredictable future. Bois Forêt Trop. 2014, 320, 83–96. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. J. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Franco, A.; Fernandes de Souza, J.; Pinheiro do Nascimiento, P.F.; Mendes Pedroza, M.; Carvalho, L.; Rodriguez-Castellón, E.; Luque, R. Sewage Sludge-Derived Materials as Efficient Catalysts for the Selective Production of Vanillin from Isoeugenol. ACS Sustain. Chem. Eng. 2019, 7, 7519–7526. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Biobased Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, L.; Zhang, Y.; Liu, Q.; Ru, C.; Zhang, W. Novel biobased epoxy resin thermosets derived from eugenol and vanillin. Polym. Degrad. Stab. 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.W.; Wang, P.L.; Xing, W.; Song, L. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Selective Route to 2-Propenyl Aryls Directly from Wood by a Tandem Organosolv and Palladium-Catalysed Transfer Hydrogenolysis. ChemSusChem 2014, 7, 2154–2158. [Google Scholar] [CrossRef]
- Cheng, C.; Truong, J.; Barrett, J.A.; Shen, D.; Abu-Omar, M.M.; Ford, P.C. Hydrogenolysis of Organosolv Lignin in Ethanol/Isopropanol Media without Added Transition-Metal Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 1023–1030. [Google Scholar] [CrossRef]
- François, C.; Pourchet, S.; Boni, G.; Fontaine, S.; Gaillard, Y.; Placet, V. Diglycidylether of iso-eugenol: A suitable lignin-derived synthon for epoxy thermoset applications. RSC Adv. 2016, 6, 68732–68738. [Google Scholar] [CrossRef]
- François, C.; Pourchet, S.; Boni, G.; Rautiainen, S.; Samec, J.; Fournier, L. Design and synthesis of biobased epoxy thermosets from biorenewable resources. Comptes Rendus Chim. 2017, 20, 1006–1016. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef]
- Ruiz, Q.; Pourchet, S.; Placet, V.; Plasseraud, L.; Boni, G. New ecofriendly synthesized thermosets from isoeugenol based epoxy resins. Polymers 2020, 229, 229. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; le Maire, A.; Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sakai, H.; Nishigori, M.; Suyama, K.; Nawaji, T.; Ikeda, S.; Nishigouchi, M.; Okada, H.; Matsushima, A.; Nose, T.; et al. Receptor-binding affinities of bisphenol A and its next-generation analogs for human nuclear receptors. Toxicol. Appl. Pharmacol. 2019, 377, 114610. [Google Scholar] [CrossRef]
- Corbin, A.C.; Sala, B.; Soulat, D.; Ferreira, M.; Labanieh, A.R.; Placet, V. Development of quasi-unidirectional fabrics with hemp fibre: A competitive reinforcement for composite materials. J. Compos. Mater. 2021, 55, 551–564. [Google Scholar] [CrossRef]
- Grimaldi, M.; Boulahtouf, A.; Toporova, L.; Balaguer, P. Functional profiling of bisphenols for nuclear receptors. Toxicology 2019, 420, 39–45. [Google Scholar] [CrossRef]
- Theophanides, T. (Ed.) Infrared Spectroscopy-Materials Science, Engineering and Technology; National Technical University of Athens: Athens, Greece, 2012. [Google Scholar]
- Paramarta, A.; Webster, D.C. Biobased high-performance epoxy-anhydride thermosets for structural composites: The effect of composition variables. React. Funct. Polym. 2016, 105, 140–149. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Shi, Y.; Huang, S.; Zhang, W.; Chen, J.; Dong, B. Hydrogenation Catalyst, Its Preparation Method and Its Application in Preparation of Hexahydrophthalic Anhydride. Patent Number CN104785250, 22 July 2015. [Google Scholar]
- Shao, X.; Su, L.; Zhang, J.; Tian, Z.; Zhang, N.; Wang, Y. Green Production of Phthalic Anhydride from Biobased Furan and Maleic Anhydride by an Acid Resin Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 14385–14394. [Google Scholar] [CrossRef]
- Gabrion, X.; Koolen, G.; Grégoire, M.; Musio, S.; Bar, M.; Botturi, D. Influence of industrial processing parameters on the effective properties of long aligned European hemp fibres in composite materials. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106915. [Google Scholar] [CrossRef]
- Placet, V.; Cissé, O.; Boubakar, M.L. Nonlinear tensile behaviour of elementary hemp fibres. Part I: Investigation of the possible origins using repeated progressive loading with in situ microscopic observations. Compos. Part A Appl. Sci. Manufact. 2014, 56, 319–327. [Google Scholar] [CrossRef]
- Datta, C.; Basu, D.; Banerjee, A. Mechanical and dynamic mechanical properties of jute fibres–Novolac–epoxy composite laminates. J. Appl. Polym. Sci. 2002, 85, 2800–2807. [Google Scholar] [CrossRef]
- Geethamma, V.G.; Joseph, R.; Thomas, S. Short coir fibre-reinforced natural rubber composites: Effects of fibre length, orientation, and alkali treatment. J. Appl. Polym. Sci. 1995, 55, 583–594. [Google Scholar] [CrossRef]
- Pothan, L.A.; George, C.N.; John, M.J.; Thomas, S. Dynamic Mechanical and Dielectric Behavior of Banana-Glass Hybrid Fibre Reinforced Polyester Composites. J. Reinf. Plast. Compos. 2010, 29, 1131–1145. [Google Scholar] [CrossRef]
- Pothan, L.A.; Thomas, S. Polarity parameters and dynamic mechanical behaviour of chemically modified banana fibre reinforced polyester composites. Compos. Sci. Technol. 2003, 63, 1231–1240. [Google Scholar] [CrossRef]
- Warrier, A.; Godara, A.; Rochez, O.; Mezzo, L.; Luizi, F.; Gorbatikh, L. The effect of adding carbon nanotubes to glass/epoxy composites in the fibre sizing and/or the matrix. Compos. Part A Appl. Sci. Manuf. 2010, 41, 532–538. [Google Scholar] [CrossRef]
- Tajvidi, M.; Falk, R.H.; Hermanson, J.C. Effect of natural fibres on thermal and mechanical properties of natural fibre polypropylene composites studied by dynamic mechanical analysis. J. Appl. Polym. Sci. 2006, 101, 4341–4349. [Google Scholar] [CrossRef]
- Gupta, M.K.; Srivastava, R.K. Mechanical, thermal and dynamic mechanical analysis of jute fibre reinforced epoxy composite. Indian J. Fibre Text. Res. 2017, 42, 64–71. [Google Scholar]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Chen, Y.; Liu, M.; Ding, Y.; Luo, X. Water Transportation in Epoxy Resin. Chem. Mater. 2005, 17, 839–845. [Google Scholar] [CrossRef]
- Boutin, M.; Rogeon, A.; Aufray, M.; Piquet, R.; Rouilly, A. Influence of flax fibres on network formation of DGEBA/DETA matrix. Compos. Interfaces 2020, 28, 1–18. [Google Scholar]
- Harris, B.; Braddell, O.G.; Almond, D.P.; Lefebvre, C.; Verbist, J. Study of carbon fibre surface treatments by dynamic mechanical analysis. J. Mater. Sci. 1993, 28, 3353–3366. [Google Scholar] [CrossRef]
- Almond, D.P.; Harris, B.; Hallett, R.G.; Braddell, O.G. Dynamical mechanical analysis of glass-transition peaks in polymers and composites. J. Alloys Compd. 1994, 211–212, 381–384. [Google Scholar] [CrossRef]
- Garoche, C.; Grimaldi, M.; Michelin, E.; Boulahtouf, A.; Marconi, A.; Brion, F.; Balaguer, P.; Aït-Aïssa, S. Interlaboratory prevalidation of a new in vitro transcriptional activation assay for the screening of (anti-)androgenic activity of chemicals using the UALH-hAR cell line. Toxicol. In Vitro 2023, 88, 105554. [Google Scholar] [CrossRef]

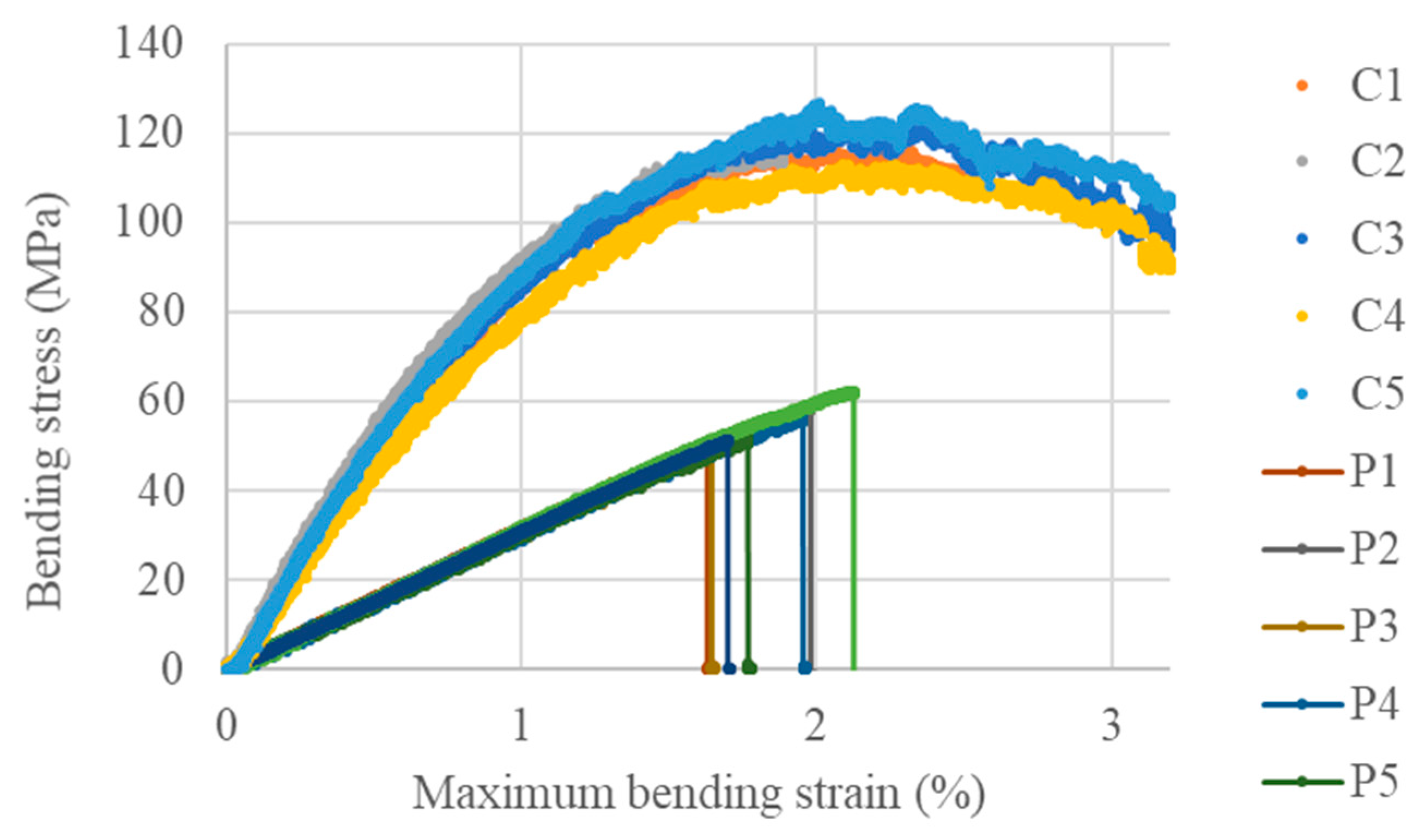
| Mean Value ± Standard Deviation | Bending Modulus (GPa) | Bending Strength (MPa) | Maximum Bending Strain (%) |
|---|---|---|---|
| Polymer (BI-HHPA-DMID) | 3.1 ± 0.08 | 54.8 ± 4.6 | 1.82 ± 0.19 |
| Composite | 10.1 ± 0.66 | 125.4 ± 15.6 | 2.64 ± 0.78 |
| Glass Transition Temperature (Tg) in °C | Polymer (BI-HHPA-DMID) | Composite |
|---|---|---|
| E′ max derivative | 100 | 115 |
| E″ peak | 85 | 110 |
| tan peak | 110 | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, G.; Placet, V.; Grimaldi, M.; Balaguer, P.; Pourchet, S. Toward the Manufacturing of a Non-Toxic High-Performance Biobased Epoxy–Hemp Fibre Composite. Polymers 2024, 16, 2010. https://doi.org/10.3390/polym16142010
Boni G, Placet V, Grimaldi M, Balaguer P, Pourchet S. Toward the Manufacturing of a Non-Toxic High-Performance Biobased Epoxy–Hemp Fibre Composite. Polymers. 2024; 16(14):2010. https://doi.org/10.3390/polym16142010
Chicago/Turabian StyleBoni, Gilles, Vincent Placet, Marina Grimaldi, Patrick Balaguer, and Sylvie Pourchet. 2024. "Toward the Manufacturing of a Non-Toxic High-Performance Biobased Epoxy–Hemp Fibre Composite" Polymers 16, no. 14: 2010. https://doi.org/10.3390/polym16142010
APA StyleBoni, G., Placet, V., Grimaldi, M., Balaguer, P., & Pourchet, S. (2024). Toward the Manufacturing of a Non-Toxic High-Performance Biobased Epoxy–Hemp Fibre Composite. Polymers, 16(14), 2010. https://doi.org/10.3390/polym16142010









