Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels
Abstract
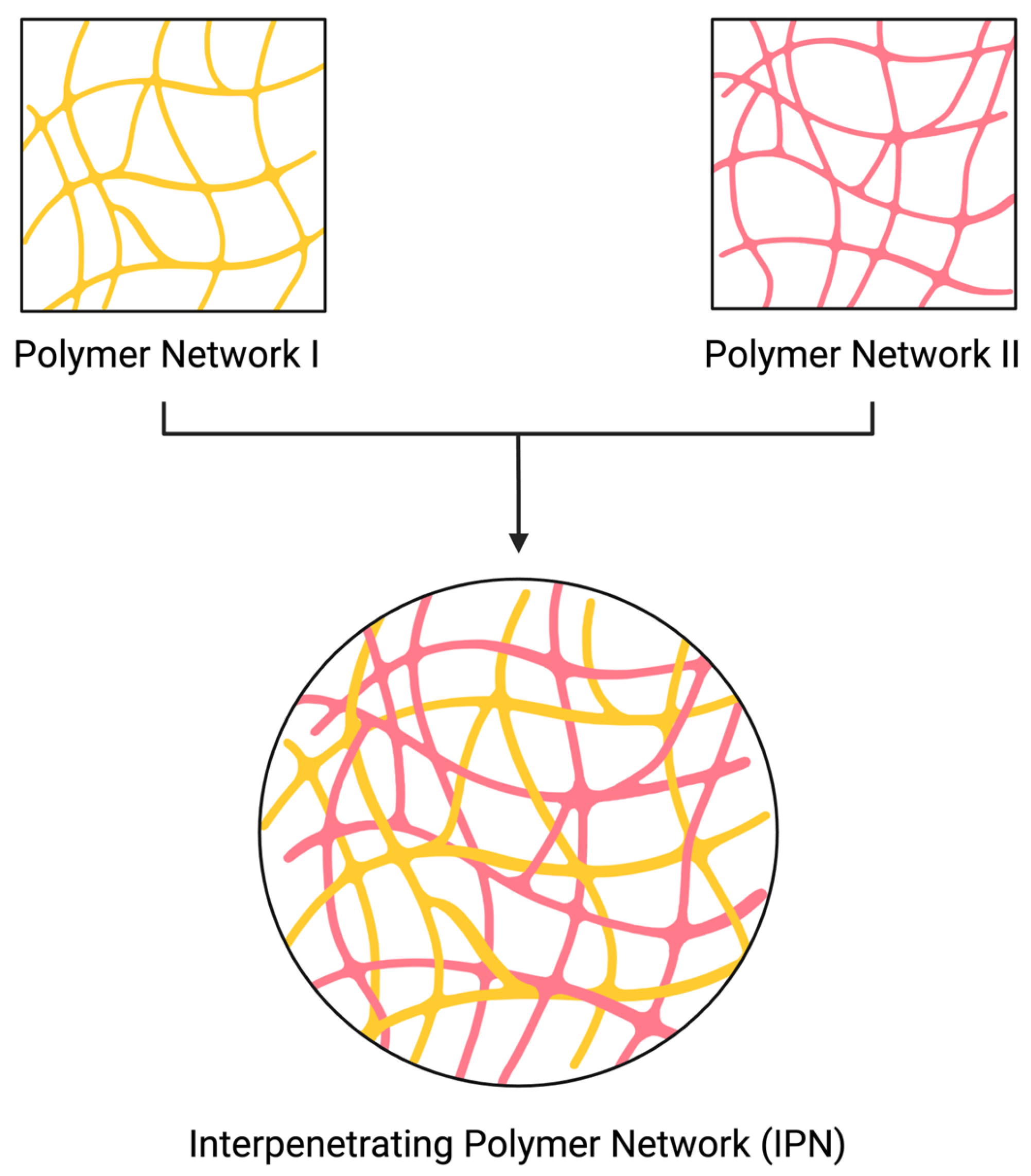
:1. Introduction
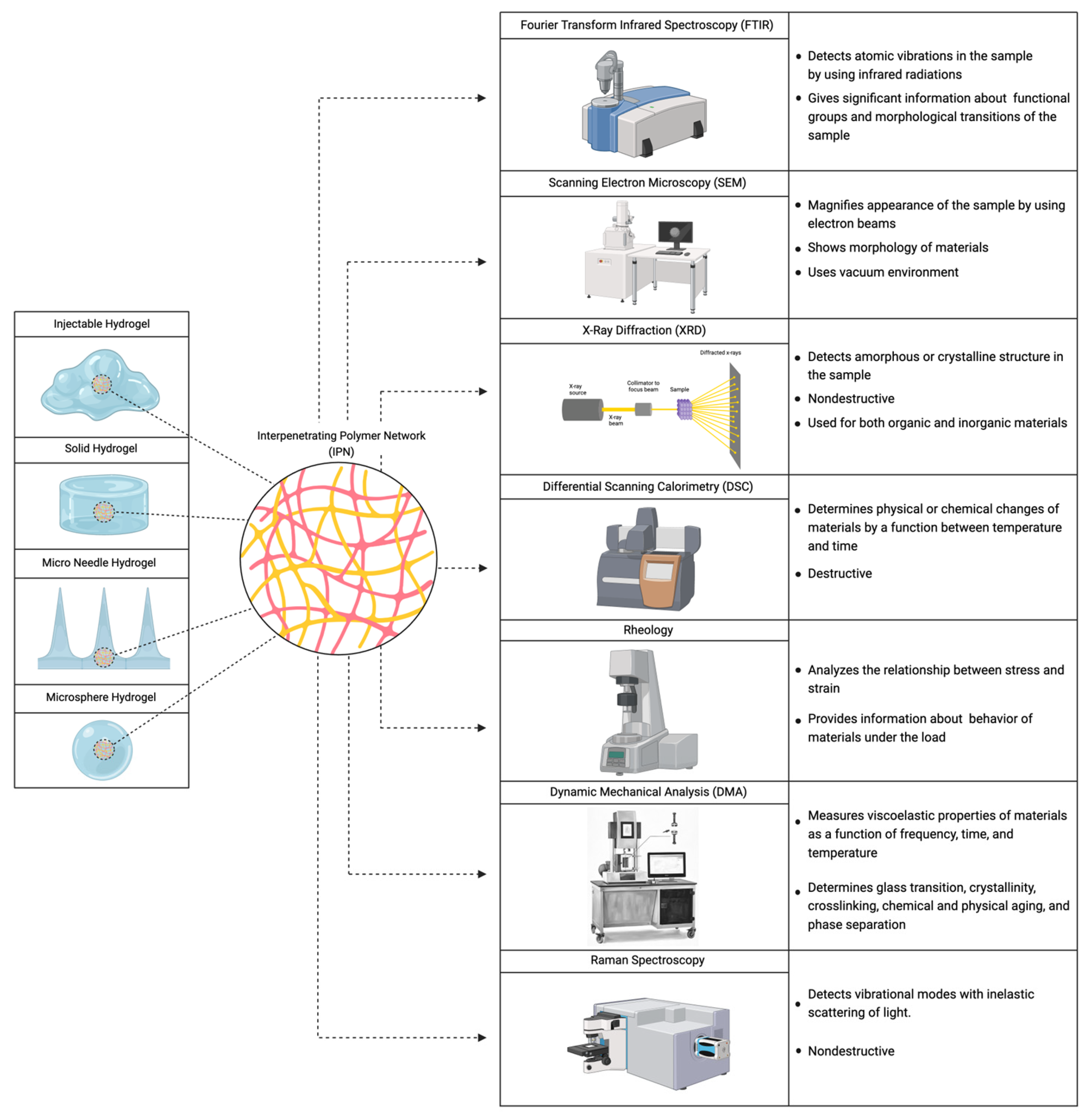
2. Characterization Methods to Determine the Formation of IPNs
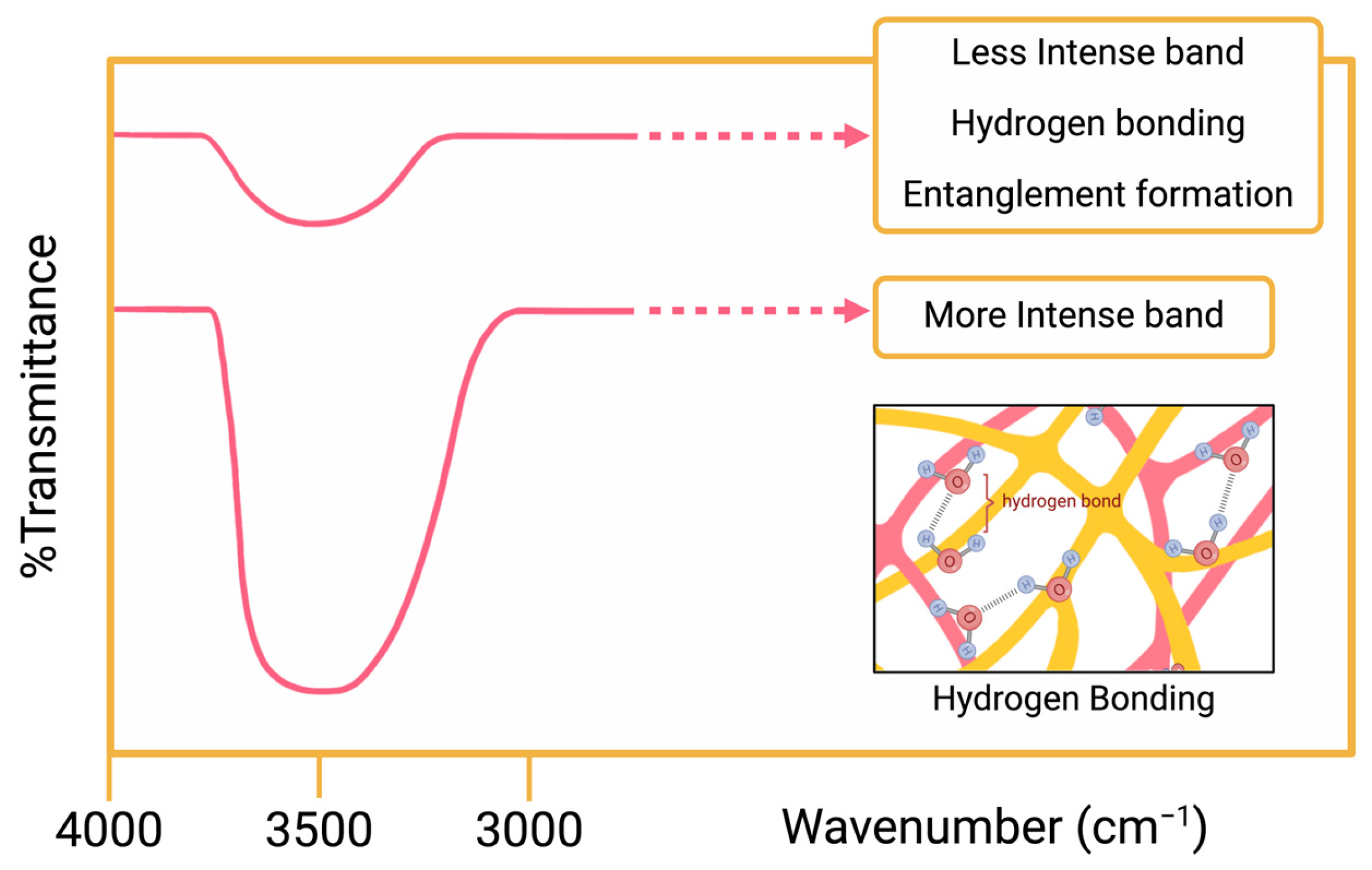
2.1. Spectroscopy Methods
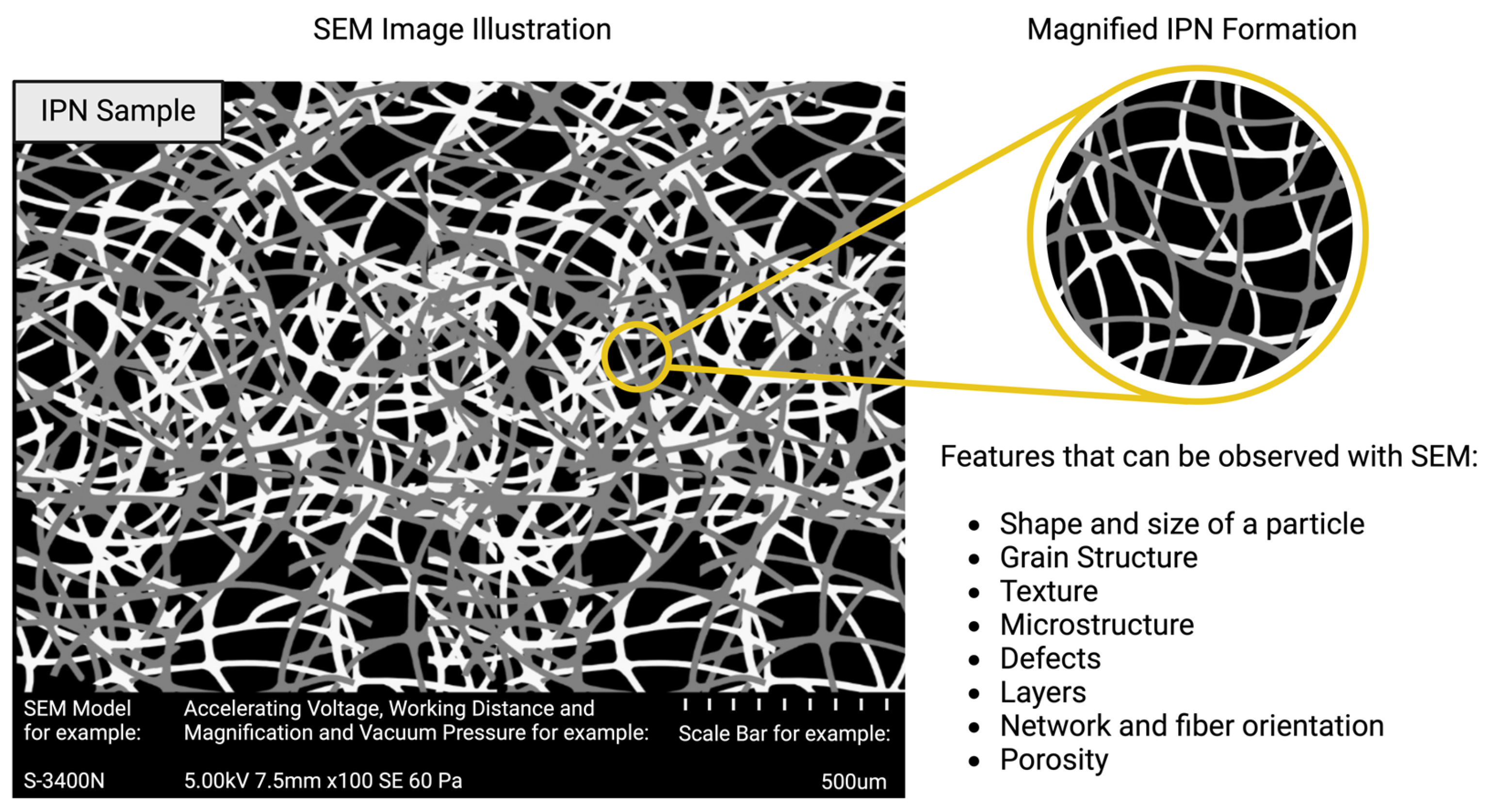
2.2. Electron Microscopies
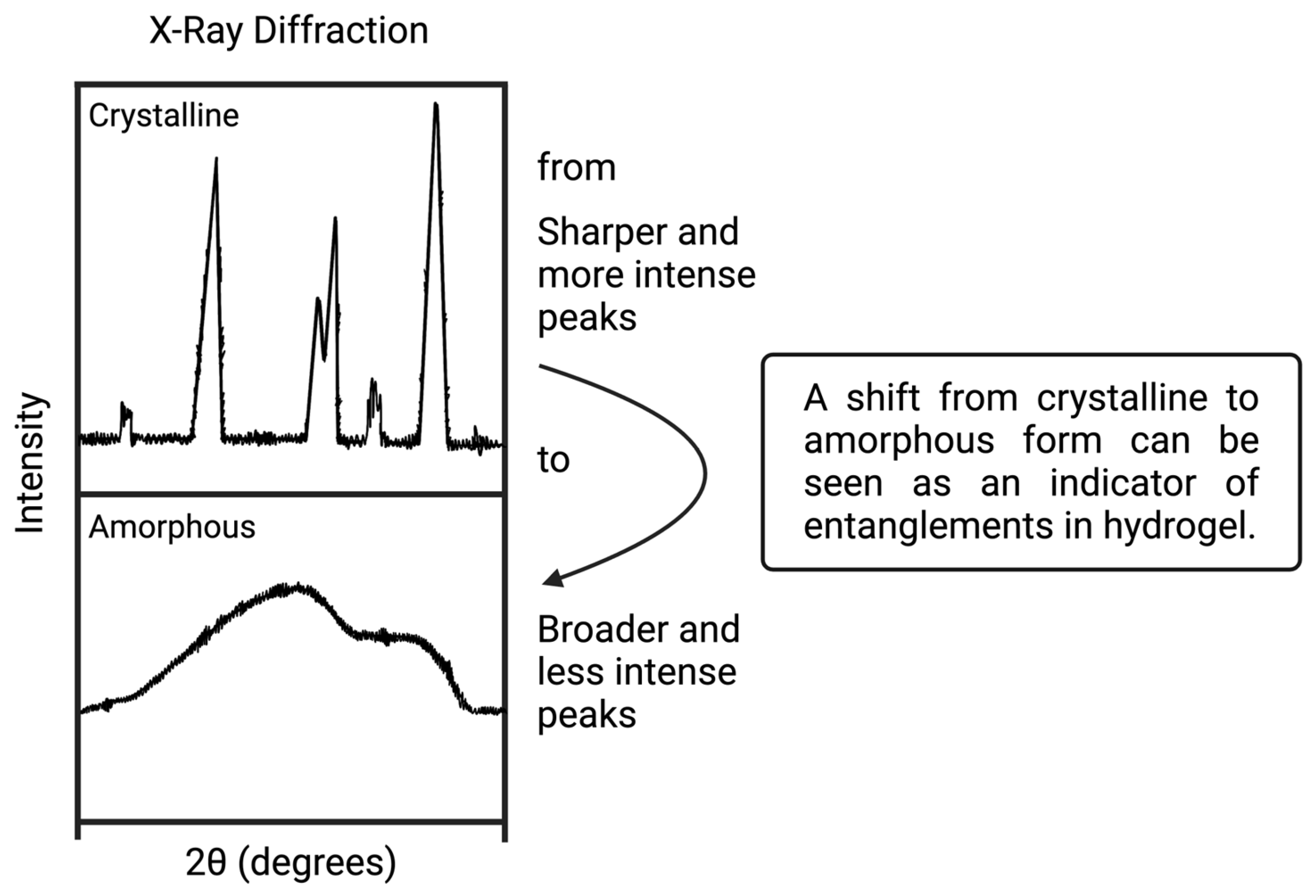
2.3. X-ray Diffraction
2.4. Differential Scanning Calorimetry
2.5. Dynamic Mechanical Analysis
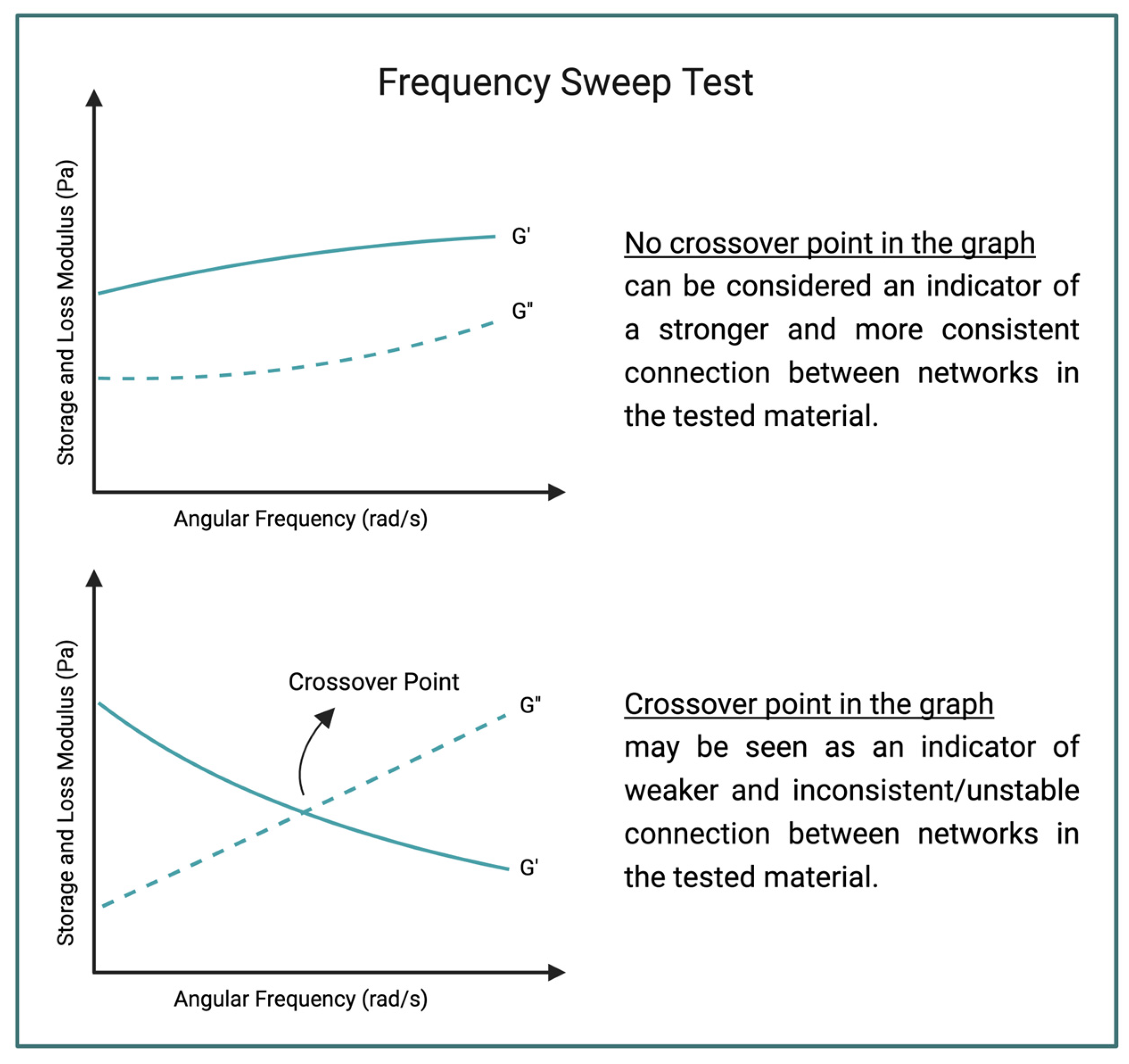
2.6. Rheology
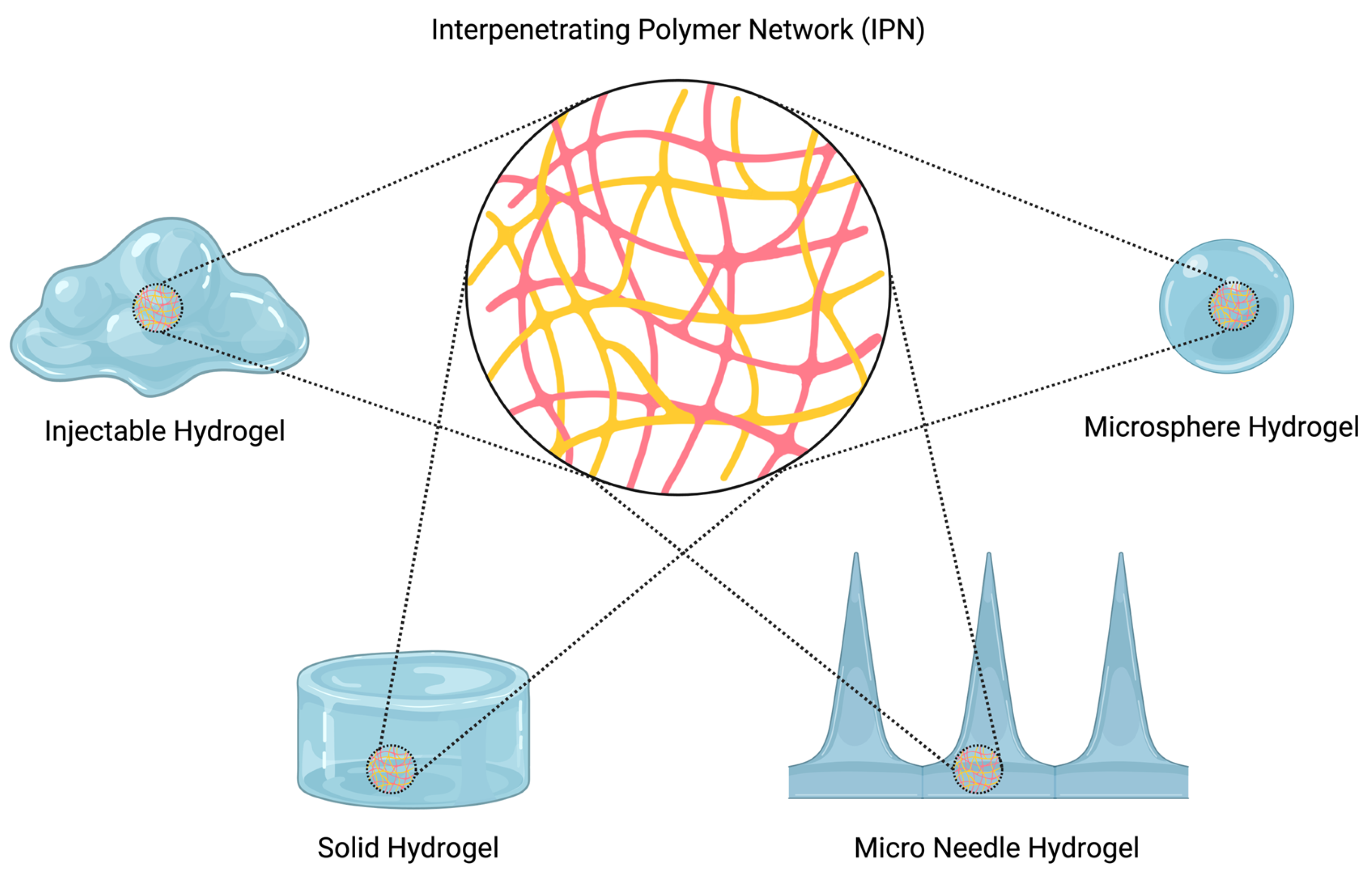
3. Studies That Investigate IPN Formation
3.1. Injectable Hydrogels
3.2. Drug Delivery Hydrogels
3.3. Hydrogels for Other Biomedical Applications
3.4. Hydrogels for Non-Biomedical Applications
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patrickios, C.S. Polymer networks: Recent developments. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Niu, Y.; Xia, Q.; Gu, M.; Yu, L.L. Interpenetrating network gels composed of gelatin and soluble dietary fibers from tomato peels. Food Hydrocoll. 2019, 89, 95–99. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, Q.; Liu, X.; Liu, F.; McClements, D.J. Double-network hydrogels: Design, fabrication, and application in foods and biomedicines. Adv. Colloid Interface Sci. 2023, 320, 102999. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Lu, W.; Zhang, Y.; Mata, A.; Fang, Y. Natural polymer-sourced interpenetrating network hydrogels: Fabrication, properties, mechanism and food applications. Trends Food Sci. Technol. 2021, 116, 342–356. [Google Scholar] [CrossRef]
- Lin, Q.; Hu, Y.; Qiu, C.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Xu, X.; Wang, J.; et al. Peanut protein-polysaccharide hydrogels based on semi-interpenetrating networks used for 3D/4D printing. Food Hydrocoll. 2023, 137, 108332. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, X.; Li, Y.; Jiang, Y.; Zhang, M.; Lu, P.; Chen, H. Synthesis and characterization of bio-based PA/EP interpenetrating network polymer as coating material for controlled release fertilizers. J. Appl. Polym. Sci. 2018, 135, 46052. [Google Scholar] [CrossRef]
- Chen, R.; Yang, S.; Liu, B.; Liao, Y. Eco-Friendly Semi-Interpenetrating Polymer Network Hydrogels of Sodium Carboxymethyl Cellulose/Gelatin for Methylene Blue Removal. Materials 2023, 16, 3385. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.; Oldal, D.G.; Peskov, M.V.; Beke, A.K.; Hardian, R.; Schwingenschlogl, U.; Szekely, G. Biobased Interpenetrating Polymer Network Membranes for Sustainable Molecular Sieving. ACS Nano 2024, 18, 7433–7443. [Google Scholar] [CrossRef] [PubMed]
- Ngwabebhoh, F.A.; Gazi, M.; Oladipo, A.A. Adsorptive removal of multi-azo dye from aqueous phase using a semi-IPN superabsorbent chitosan-starch hydrogel. Chem. Eng. Res. Des. 2016, 112, 274–288. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, T.; Chen, S.; Wang, X.; He, J.; Luo, Y. Construction and characterization of chitosan/poly (acrylamide-[2-(methacryloyloxy) ethyl] trimethylammonium chloride) double-network hydrogel with enhanced antibacterial activity. Adv. Compos. Hybrid Mater. 2023, 6, 192. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An interpenetrating alginate/gelatin network for three-dimensional (3D) cell cultures and organ bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.N.; Lee, C.S. A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv. Funct. Mater. 2020, 30, 1904156. [Google Scholar] [CrossRef]
- Cassimjee, H.; Kumar, P.; Ubanako, P.; Choonara, Y.E. Genipin-crosslinked, proteosaccharide scaffolds for potential neural tissue engineering applications. Pharmaceutics 2022, 14, 441. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Ding, Y.; Yao, Y.; Liu, Y.; Tang, J. Fe3+-Coordination mediated synergistic dual-network conductive hydrogel as a sensitive and highly-stretchable strain sensor with adjustable mechanical properties. J. Mater. Chem. B 2022, 10, 1442–1452. [Google Scholar] [CrossRef]
- Shi, X.; Deng, Z.; Zhang, P.; Wang, Y.; Zhou, G.; de Haan, L.T. Wearable optical sensing of strain and humidity: A patterned dual-responsive semi-interpenetrating network of a cholesteric main-chain polymer and a poly (ampholyte). Adv. Funct. Mater. 2021, 31, 2104641. [Google Scholar] [CrossRef]
- Song, F.; Gong, J.; Tao, Y.; Cheng, Y.; Lu, J.; Wang, H. A robust regenerated cellulose-based dual stimuli-responsive hydrogel as an intelligent switch for controlled drug delivery. Int. J. Biol. Macromol. 2021, 176, 448–458. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart microneedle fabricated with silk fibroin combined semi-interpenetrating network hydrogel for glucose-responsive insulin delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Sellamuthu, K.; Angappan, S. Formulation and Characterization of Interpenetrating Polymer Network Hydrogel Bead as Drug Carrier System for Extended Release of Sulphonyl Urea Medication. J. Pharm. Innov. 2024, 19, 2. [Google Scholar] [CrossRef]
- Varnava, C.K.; Patrickios, C.S. Polymer networks one hundred years after the macromolecular hypothesis: A tutorial review. Polymer 2021, 215, 123322. [Google Scholar] [CrossRef]
- Quirino, R.L.; Monroe, K.; Fleischer, C.H., III; Biswas, E.; Kessler, M.R. Thermosetting polymers from renewable sources. Polym. Int. 2021, 70, 167–180. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Gandhi, A.; Sen, K.K. Chitosan-Based Interpenetrating Polymer Networks: Drug Delivery Application. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2019; pp. 269–295. [Google Scholar]
- Zainudin, B.H.; Wong, T.W.; Hamdan, H. Pectin as oral colon-specific nano-and microparticulate drug carriers. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–286. [Google Scholar]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Dobrynin, A.V. Architectural code for rubber elasticity: From supersoft to superfirm materials. Macromolecules 2019, 52, 7531–7546. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Wanasinghe, S.V.; Schreiber, E.M.; Thompson, A.M.; Sparks, J.L.; Konkolewicz, D. Dynamic covalent chemistry for architecture changing interpenetrated and single networks. Polym. Chem. 2021, 12, 1975–1982. [Google Scholar] [CrossRef]
- Shah, S.Z.O.; Trengove, A.; O’Connor, A.J.; Quinn, J.F.; Kempe, K. Simultaneous Interpenetrating Polymer Networks Based on Poly(2-Oxazoline)s. Macromol. Mater. Eng. 2024, 309, 2300210. [Google Scholar] [CrossRef]
- Rajawasam, C.W.; Dodo, O.J.; Weerasinghe, M.A.S.N.; Raji, I.O.; Wanasinghe, S.V.; Konkolewicz, D.; Watuthanthrige, N.D.A. Educational series: Characterizing crosslinked polymer networks. Polym. Chem. 2024, 15, 219–247. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, J.; Johnson, J.A. Polymer networks: From plastics and gels to porous frameworks. Angew. Chem. Int. Ed. 2020, 59, 5022–5049. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Peppas, N.; Slaughter, B.V.; Kanzelberger, M.A.; Matyjaszewski, K.; Möller, M. 9.20–Hydrogels. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 385–395. [Google Scholar]
- Hou, X.; Lin, L.; Li, K.; Jiang, F.; Qiao, D.; Zhang, B.; Xie, F. Towards superior biopolymer gels by enabling interpenetrating network structures: A review on types, applications, and gelation strategies. Adv. Colloid Interface Sci. 2024, 325, 103113. [Google Scholar] [CrossRef] [PubMed]
- Dror, M.; Elsabee, M.; Berry, G. Interpenetrating polymer networks for biological applications. Biomater. Med. Devices Artif. Organs 1979, 7, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Korah, L.V.; Anilkumar, G.; Thomas, S. Hydrogels, DNA, and RNA polypeptides for the preparation of biomaterials. In Fundamental Biomaterials: Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–104. [Google Scholar]
- Silverstein, M.S. Interpenetrating polymer networks: So happy together? Polymer 2020, 207, 122929. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Aminabhavi, D.T.M. Drug delivery using interpenetrating polymeric networks of natural polymers: A recent update. J. Drug Deliv. Sci. Technol. 2021, 66, 102915. [Google Scholar] [CrossRef]
- Sperling, L.H.; Hu, R. Interpenetrating polymer networks. In Polymer Blends Handbook; Springer: Berlin/Heidelberg, Germany, 2014; pp. 677–724. [Google Scholar]
- Hernández-Ortiz, J.C.; Vivaldo-Lima, E. Crosslinking. In Handbook of Polymer Synthesis, Characterization, and Processing; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 187–204. [Google Scholar]
- Manna, S.; Manna, M.; Jana, S. Interpenetrating Polymer Network in Microparticulate Systems: Drug Delivery and Biomedical Application. In Interpenetrating Polymer Network: Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–23. [Google Scholar]
- Akovali, G.; Biliyar, K.; Shen, M. Gradient Polymers by Diffusion Polymerization. J. Appl. Polym. Sci. 1976, 20, 2419–2427. [Google Scholar] [CrossRef]
- Karabanova, L.V.; Mikhalovsky, S.V.; Lloyd, A.; Boiteux, G.; Sergeeva, L.M.; Novikova, T.I.; Lutsyk, E.D.; Meikle, S. Gradient semi-interpenetrating polymer networks based on polyurethane and poly (vinyl pyrrolidone). J. Mater. Chem. 2005, 15, 499–507. [Google Scholar] [CrossRef]
- Barker, E.D. Preclinical Evaluation of a Novel Polysaccharide Hydrogel for Local Chemotherapy Delivery to Solid Tumors; The University of Tennessee Health Science Center: Knoxville, TN, USA, 2014. [Google Scholar]
- Barszczewska-Rybarek, I.M. A New Approach to Morphology Studies on Diacrylate Polymer Networks Using X-Ray Powder Diffraction. Macromol. Chem. Phys. 2013, 214, 1019–1026. [Google Scholar] [CrossRef]
- Heß, C.; Hartmann, B.; Lechner, M.D.; Nierling, W.; Seidel, C.; Kulicke, W.M. Influence of soluble polymer residues in crosslinked carboxymethyl starch on some physical properties of its hydrogels. Starch-Stärke 2007, 59, 423–429. [Google Scholar] [CrossRef]
- Nebhani, L.; Jaisingh, A. Chemical analysis of polymers. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–116. [Google Scholar]
- Kumar, A.; Khandelwal, M.; Gupta, S.K.; Kumar, V.; Rani, R. Fourier transform infrared spectroscopy: Data interpretation and applications in structure elucidation and analysis of small molecules and nanostructures. In Data Processing Handbook for Complex Biological Data Sources; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–96. [Google Scholar]
- Cao, D.; Lv, Y.; Zhou, Q.; Chen, Y.; Qian, X. Guar gum/gellan gum interpenetrating-network self-healing hydrogels for human motion detection. Eur. Polym. J. 2021, 151, 110371. [Google Scholar] [CrossRef]
- Dodd, L.J.; Lima, C.; Costa-Milan, D.; Neale, A.R.; Saunders, B.; Zhang, B.; Sarua, A.; Goodacre, R.; Hardwick, L.J.; Kuball, M.; et al. Raman analysis of inverse vulcanised polymers. Polym. Chem. 2023, 14, 1369–1386. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Buratti, E.; Franco, S.; Ruzicka, B. Chemical-physical behaviour of microgels made of interpenetrating polymer networks of PNIPAM and poly (acrylic acid). Polymers 2021, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Appel, R.; Xu, W.; Zerda, T.W.; Hu, Z. Direct observation of polymer network structure in macroporous N-isopropylacrylamide gel by Raman microscopy. Macromolecules 1998, 31, 5071–5074. [Google Scholar] [CrossRef] [PubMed]
- Karakoç, Z.; Ketani, M.A.; Ketani, Ş. Mikroskopların çalışma mekanizması ve çeşitleri. Dicle Üniversitesi Vet. Fakültesi Derg. 2016, 1, 1–6. Available online: https://dergipark.org.tr/en/pub/duvetfd/issue/29551/317104 (accessed on 12 May 2024).
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018. [Google Scholar]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. Characterization of biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–115. [Google Scholar]
- Ul-Hamid, A. A Beginners’ Guide to Scanning Electron Microscopy; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1. [Google Scholar]
- Di Lorenzo, F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Michler, G.H.; Lebek, W. Electron microscopy of polymers. In Polymer Morphology: Principles, Characterization, and Processing; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 37–53. [Google Scholar]
- Müller, A.J.; Michell, R.M. Differential scanning calorimetry of polymers. In Polymer Morphology: Principles, Characterization, and Processing; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 72–99. [Google Scholar]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, B.; Szyryńska, N. Field-Emission Scanning Electron Microscope as a Tool for Large-Area and Large-Volume Ultrastructural Studies. Animals 2021, 11, 3390. [Google Scholar] [CrossRef] [PubMed]
- Jaya, R.P. Porous concrete pavement containing nanosilica from black rice husk ash. In New Materials in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–527. [Google Scholar]
- Rohde, M. Microscopy. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 61–100. [Google Scholar]
- Che, Y.J.; Li, D.; Liu, Y.; Yue, Z.; Zhao, J.; Ma, Q.; Zhang, Q.; Tan, Y.; Yue, Q.; Meng, F. Design and fabrication of a triple-responsive chitosan-based hydrogel with excellent mechanical properties for controlled drug delivery. J. Polym. Res. 2018, 25, 169. [Google Scholar] [CrossRef]
- Satani, H.; Kuwata, M.; Ishii, H.; Inoue, T.; Shimizu, A. Preparation of SEM hydrogel samples using a high pressure water freeze fracture method. High Press. Res. 2021, 41, 97–108. [Google Scholar] [CrossRef]
- Fleck, R.A.; Humbel, B.M. Biological Field Emission Scanning Electron Microscopy, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mayeen, A.; Mayeen, A.; Shaji, L.K.; Nair, A.K.; Kalarikkal, N. Morphological characterization of nanomaterials. In Characterization of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 335–364. [Google Scholar]
- Muscariello, L.; Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Cafiero, G.; Barbarisi, A. A critical overview of ESEM applications in the biological field. J. Cell. Physiol. 2005, 205, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Nasrazadani, S.; Hassani, S. Chapter 2—Modern analytical techniques in failure analysis of aerospace, chemical, and oil and gas industries. In Handbook of Materials Failure Analysis with Case Studies from the Oil and Gas Industry; Makhlouf, A.S.H., Aliofkhazraei, M., Eds.; Butterworth-Heinemann, Elsevier: Amsterdam, The Netherlands, 2016; pp. 39–54. [Google Scholar]
- Tamiri, T.; Zitrin, S. Explosives: Analysis. In Encyclopedia of Forensic Sciences, 2nd ed.; Siegel, J.A., Saukko, P.J., Houck, M.M., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 64–84. [Google Scholar]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar]
- Popelka, A.; Zavahir, S.; Habib, S. Morphology analysis. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–68. [Google Scholar]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. (Eds.) Chapter 6—Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–322. [Google Scholar]
- Usmani, M.A.; Khan, I.; Haque, A.; Bhat, A.H.; Mondal, D.; Gazal, U. 4—Biomass-based composites from different sources: Properties, characterization, and transforming biomass with ionic liquids. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Jawaid, M., Tahir, P.M., Saba, N., Eds.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–76. [Google Scholar]
- Goel, H.; Goel, H.; Saini, K.; Razdan, K.; Khurana, R.K.; Elkordy, A.A.; Singh, K.K. Chapter 3—In vitro physicochemical characterization of nanocarriers: A road to optimization. In Nanoparticle Therapeutics; Kesharwani, P., Singh, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 133–179. [Google Scholar]
- Tomoda, B.T.; De Moraes, M.A.; Da Silva, C.F.; Vieira, R.S. Chapter 3—Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–95. [Google Scholar]
- Singh, M.K.; Singh, A. Chapter 9—Thermal characterization of materials using differential scanning calorimeter. In Characterization of Polymers and Fibres; Singh, M.K., Singh, A., Eds.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–222. [Google Scholar]
- Ratna, D. Chapter 6—Characterization, performance evaluation and lifetime analysis of thermoset resin. In Recent Advances and Applications of Thermoset Resins, 2nd ed.; Ratna, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 503–582. [Google Scholar]
- Lu, X.; Weiss, R. Relationship between the glass transition temperature and the interaction parameter of miscible binary polymer blends. Macromolecules 1992, 25, 3242–3246. [Google Scholar] [CrossRef]
- Hammer, L.; Van Zee, N.J.; Nicolaÿ, R. Dually crosslinked polymer networks incorporating dynamic covalent bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Matricardi, P. Semi-IPNs and IPN-based hydrogels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–124. [Google Scholar]
- Chartoff, R.P.; Menczel, J.D.; Dillman, S.H. Dynamic mechanical analysis (DMA). In Thermal Analysis of Polymers: Fundamentals and Applications; Wiley Online Library: Hoboken, NJ, USA, 2009; pp. 387–495. [Google Scholar]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic mechanical analysis investigations of PLA-based renewable materials: How are they useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovářík, T.; Muzaffar, A.; Ahamed, M.B.; Pasha, S.K. Mechanical analysis of polymers. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–152. [Google Scholar]
- Dunson, D. Characterization of polymers using dynamic mechanical analysis (DMA). In EAG App Note; EAG Laboratories: Tokyo, Japan, 2017. [Google Scholar]
- Patra, S.; Ajayan, P.M.; Narayanan, T.N. Dynamic mechanical analysis in materials science: The Novice’s Tale. Oxf. Open Mater. Sci. 2021, 1, itaa001. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of dynamic mechanical analysis (DMA) for characterizing interfacial interactions in filled polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Cook, W.D.; Scott, T.F.; Quay-Thevenon, S.; Forsythe, J.S. Dynamic mechanical thermal analysis of thermally stable and thermally reactive network polymers. J. Appl. Polym. Sci. 2004, 93, 1348–1359. [Google Scholar] [CrossRef]
- Costa, C.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F. Dynamic mechanical thermal analysis of polymer composites reinforced with natural fibers. Polym. Rev. 2016, 56, 362–383. [Google Scholar] [CrossRef]
- Jones, D.S. Dynamic mechanical analysis of polymeric systems of pharmaceutical and biomedical significance. Int. J. Pharm. 1999, 179, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ayón, M.A.G.; Hoppenstedt, A.M.; López, A.Z.; Sarabia, J.B.; Claverie, A.L.; Urías, M.A. Semi-Interpenetrated Polymer Networks Based on PHEMA and Modified Chitosan as a Potential Bactericide Hydrogel for Wound-Healing. In Macromolecular Chemistry and Physics; Wiley Online Library: Hoboken, NJ, USA, 2004; p. 2400018. [Google Scholar]
- Widyatmoko, I. Sustainability of bituminous materials. In Sustainability of Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 343–370. [Google Scholar]
- Lapasin, R. Rheological characterization of hydrogels. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Jenny Stanford Publishing: Signapore, 2015; pp. 83–137. [Google Scholar]
- Danielsen, S.P.; Beech, H.K.; Wang, S.; El-Zaatari, B.M.; Wang, X.; Sapir, L.; Ouchi, T.; Wang, Z.; Johnson, P.N.; Hu, Y.; et al. Molecular characterization of polymer networks. Chem. Rev. 2021, 121, 5042–5092. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.; Wanasinghe, S.V.; Matyjaszewski, K.; Konkolewicz, D. Are RAFT and ATRP universally interchangeable polymerization methods in network formation? Macromolecules 2021, 54, 8331–8340. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Yang, H.; Hao, X.; Tang, Q.; Zhang, X. An Injectable Interpenetrating Polymer Network Hydrogel with Tunable Mechanical Properties and Self-Healing Abilities. Macromol. Chem. Phys. 2017, 218, 1700348. [Google Scholar] [CrossRef]
- Shariatzadeh, F.J.; Solouk, A.; Khoulenjani, S.B.; Bonakdar, S.; Mirzadeh, H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf. B Biointerfaces 2021, 203, 111725. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, S.; Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023, 300, 120243. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Chen, K.; Yuan, S.; Li, Y.; Huang, Y.; Wang, C.; Zhang, Y.; Liu, W.; Luo, W.; Liang, P.; et al. Gelatin nanoparticle-injectable platelet-rich fibrin double network hydrogels with local adaptability and bioactivity for enhanced osteogenesis. Adv. Healthc. Mater. 2020, 9, 1901469. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, M.S.N.; Dodo, O.J.; Rajawasam, C.W.; Raji, I.O.; Wanasinghe, S.V. Educational series: Turning monomers into crosslinked polymer networks. Polym. Chem. 2023, 14, 4503–4514. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Guo, B.; Ma, P.X. Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules 2014, 15, 3246–3252. [Google Scholar] [CrossRef] [PubMed]
- Darge, H.F.; Lee, C.Y.; Lai, J.Y.; Lin, S.Z.; Harn, H.J.; Chen, Y.S.; Tsai, H.C. Separable double-layered microneedle-based transdermal codelivery of DOX and LPS for synergistic immunochemotherapy of a subcutaneous glioma tumor. Chem. Eng. J. 2022, 433, 134062. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Development of novel interpenetrating network gellan gum-poly (vinyl alcohol) hydrogel microspheres for the controlled release of carvedilol. Drug Dev. Ind. Pharm. 2005, 31, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. pH-sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J. Bioact. Compat. Polym. 2007, 22, 342–356. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Shukla, S.; Banerjee, S.; Chowdhury, P.; Chakraborty, P.; Ghosh, A. Tailored IPN hydrogel bead of sodium carboxymethyl cellulose and sodium carboxymethyl xanthan gum for controlled delivery of diclofenac sodium. Polym.-Plast. Technol. Eng. 2013, 52, 795–805. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Cai, P.; Xiao, H. Dual-responsive IPN hydrogel based on sugarcane bagasse cellulose as drug carrier. Int. J. Biol. Macromol. 2018, 118, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sampath, U.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Tığlı, R.S.; Gümüşderelioğlu, M. Evaluation of alginate-chitosan semi IPNs as cartilage scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 699–709. [Google Scholar] [CrossRef] [PubMed]


| Author/Year | Materials | Material Form | IPN Type | Application | Properties | Characterization Methods to Determine IPN Structure |
|---|---|---|---|---|---|---|
| Darge et al., 2022 [106] | Sodium alginate and sulfobetaine methacrylate (SBMA) | Microneedles | Sequential IPN | Drug delivery |
| FTIR Mechanical Testing |
| Agnihotri & Aminabhavi, 2005 [107] | Gellan gum (GG) and poly(vinyl alcohol) (PVA) | Microspheres | IPN | Controlled drug delivery of a hypertension drug |
| FTIR DSC Mechanical Testing |
| Mohamadnia et al., 2007 [108] | Alginate and carrageenan | Beads | IPN | Drug delivery of a corticosteroid drug |
| SEM |
| Bhattacharya et al., 2013 [109] | Sodium carboxymethyl cellulose and Sodium carboxymethyl xanthan gum (SCMC-SCMXG) | Beads | IPN | Drug delivery of an anti-inflammatory drug |
| XRD |
| Wahid et al., 2019 [34] | Bacterial cellulose (BC), chitosan (CS) | Slurry | Semi IPN | Antimicrobial applications |
| FTIR XRD FESEM |
| Sampath et al., 2017 [112] | Cellulose nanocrystal (CNC) and chitosan (CS) | Gel | Semi IPN | Biomedical applications |
| FTIR |
| Pan et al., 2018 [110] | Sugar bagasse cellulose (SBC), carboxymethylcellulose (CMC) and poly(N-isopropylacrylamide) (PNIPAm) | Gel | IPN | Drug delivery |
| SEM |
| Tığlı & Gümüşderelioğlu, 2009 [113] | Alginate (Alg) and chitosan (CS) | - | Semi IPN | Cartilage scaffold |
| SEM Fluorescence microscope |
| Wang et al., 2017 [100] | p(N, N-dimethyl- acrylamide-co-4-acryloyldopamine), p(N, N-dimethylacrylamide-co-3-acrylamidopheynlboronic acid) and p(N, N-dimethylacrylamide-co-4-acryloyloxybenzaldehyde) | Gel | Semi IPN | 3D printing |
| Rheology |
| Shariatzadeh et al., 2021 [101] | Gelatin methacrylate (GelMA) and hyaluronic acid (HA) | Cryogel | Sequential IPN | Soft tissue engineering |
| FTIR |
| Cui et al., 2023 [102] | Oxidized alginate (OSA)/gelatin (Gel)/ cellulose nanofiber (CNF) | Gel | Semi IPN | Bone repair |
| FTIR |
| Mu et al., 2020 [103] | Injectable platelet-rich fibrin (iPRF) and gelatin nanoparticles (GNPs) | Gel | IPN | Bone healing |
| SEM |
| Kim et al., 2018 [111] | Hyaluronic acid (HA)/poly (N-isopropylacrylamide) (PNIPAM) | Gel | Full IPN | Transdermal drug delivery |
| Swelling ratio |
| Zhao et al., 2014 [105] | Poly(ethylene glycol) methacrylate (PEGMA)/ N-isopropylacrylamide (NIPAm)/ methacrylated alginate (ALGMA) | Gel | IPN | Release studies |
| Rheology FTIR |
| Ngwabebhoh et al., 2016 [9] | Chitosan and Starch | Gel | Semi IPN | Wastewater treatment |
| FTIR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cona, C.; Bailey, K.; Barker, E. Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels. Polymers 2024, 16, 2050. https://doi.org/10.3390/polym16142050
Cona C, Bailey K, Barker E. Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels. Polymers. 2024; 16(14):2050. https://doi.org/10.3390/polym16142050
Chicago/Turabian StyleCona, Ceren, Katherine Bailey, and Elizabeth Barker. 2024. "Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels" Polymers 16, no. 14: 2050. https://doi.org/10.3390/polym16142050
APA StyleCona, C., Bailey, K., & Barker, E. (2024). Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels. Polymers, 16(14), 2050. https://doi.org/10.3390/polym16142050






